Abstract
The incidence of peripheral neuropathy (PN) among adults initiating antiretroviral therapy (ART) containing stavudine (d4T) versus zidovudine (ZDV) is not well described. We compared 1-year incidence between d4T- and ZDV-based regimens in adults initiating ART in a programmatic setting in Kenya. Of 1,848 adults on ART, 1,579 (85 %) initiated d4T-based and 269 (15 %) initiated ZDV-based regimens. One-year incidence of symptomatic PN per 100 person-years was 21.9 (n=236) among d4T users and 6.9 (n=7) among ZDV users (P=0.0002). D4T was associated with 2.7 greater risk of PN than ZDV (adjusted hazard ratio, 2.7, P=0.009). In settings with continued d4T use, such as Africa, the effects of d4T on PN compared to ZDV should be considered when choosing ART regimens.
Keywords: Peripheral neuropathy, Africa, Antiretroviral therapy, Toxicity, HIV
Background
Fixed-dose combination generic pills containing antiretroviral therapy (ART) are widely used in resource-limited settings to treat HIV-infected individuals (Laurent et al. 2004). Inexpensive and easy to administer, the typical fixed-dose combination in many African countries contains stavudine (d4T), lamivudine (3TC), and nevirapine and is formulated as a single pill taken twice a day (Idigbe et al. 2005). Although the long-term benefits of ART in reducing HIV-related morbidity and mortality are well established, serious adverse effects are associated with continuous ART use, and specific toxicities can often be isolated to individual drugs within a combination (Dalakas 2001). A number of adverse effects associated with mitochondrial DNA toxicity, most commonly peripheral neuropathy (PN), have been linked to d4T (Brinkman et al. 1998; Cote et al. 2002; Moyle and Sadler 1998). D4T-associated PN is typically described as a sensory neuropathy affecting the lower extremities symmetrically. While PN has been reported in 15 to 42 % of d4T users in developed countries (Cherry et al. 2009; Moyle and Sadler 1998; Scarsella et al. 2002; Simpson and Tagliati 1995), few studies have examined incidence of d4T-associated PN in sub-Saharan Africa (Forna et al. 2007; Hawkins et al. 2007; Karara et al. 2010). Studies in this setting have been limited by small sample size and varying definitions of PN. As a result, reports of PN associated with d4T range widely, from 4 to 57 % (Forna et al. 2007; Hawkins et al. 2007; Karara et al. 2010; Laurent et al. 2008; Sacktor et al. 2009; Wadley et al. 2011). To our knowledge, none has compared the incidence of PN among individuals on d4T versus ZDV, the alternative recommended by the World Health Organization (WHO) (WHO 2010).
Due to the toxic effects of d4T, WHO recommended the first-line therapy be changed from d4T- to ZDV-based regimens in 2009 (WHO November 2009). However, due to increased cost and inadequate healthcare infrastructure, the majority of HIV-infected adults in African settings continue to receive first-line regimens containing d4T (WHO et al. 2009). Continued monitoring and management of d4T-related toxicities, particularly PN, is critical as the transition to ZDV-based regimens may take years, and the availability of second-line regimens is limited in sub-Saharan Africa (Hawkins et al. 2007). The aim of this study is to compare the 1-year incidence rate and evaluate predictors of symptomatic PN among adults initiating d4T- versus ZDV-based regimens at a HIV treatment clinic in Kenya.
Patients and methods
We conducted a retrospective cohort study of HIV-infected adults initiating ART at the Coptic Hope Center for Infectious Diseases in Nairobi, Kenya between January 2005 and March 2008. HIV-infected adults were included in this study if they were at least 16 years old, had no prior history of ART use, and following enrollment initiated one of four first-line regimens: d4T/3TC/efavirenz, d4T/3TC/nevirapine, ZDV/3TC/nevirapine, or ZDV/3TC/efavirenz.
The Coptic Hope Center provides comprehensive HIV care and free ART to HIV-infected individuals meeting ART eligibility requirements according to Kenyan guidelines, as described elsewhere (Chung et al. 2009). During this time period, patients were eligible to initiate ART if CD4 count <250 cells/mm3 and/or WHO stage IV disease. The University of Washington and Kenyatta National Hospital Institutional Review Boards approved this study.
Patients receiving ART returned for routine three monthly clinic visits, during which time regular physical examinations were performed by clinical officers. Information on ART regimen, occurrence of side effects related to ART, and neuropathy-related symptoms were collected using standardized questionnaires. Diagnosis of PN was based on any patient-reported symptom of PN, in conjunction with clinical judgment of the clinical officer. More specifically, PN symptoms included report of pain, tingling, or numbness in the extremities.
Patients were excluded from analyses based on the following: (1) preexisting PN, (2) anti-tuberculosis therapy at enrollment or follow-up, (3) incomplete data on ART, and (4) pregnancy at ART initiation or follow-up. Comparisons between d4T- and ZDV-based regimens and baseline characteristics were made using Pearson χ2 and Mann–Whitney U tests. One-year incidence rate of PN was calculated by dividing the number of patients reporting first occurrence of PN in each regimen group by the total person-years of observation contributed by all patients in that regimen.
Kaplan–Meier curves were used to compare time to occurrence of first PN between regimens; log-rank test assessed differences in curves. Multivariate Cox regression models were adjusted for height, CD4 count at ART, and covariates univariately associated with PN. Patients who were lost or died during follow-up were censored at the last known visit date. Statistical analyses were conducted using SPSS 18 (Chicago, IL) and STATA 11.0 (College Station, TX).
Results
Of 1,848 patients with no prior ART use, 1,579 (85 %) received a regimen containing d4T, 3TC, and a non-nucleoside reverse transcriptase inhibitor (NNRTI), and 269 (15 %) received ZDV, 3TC, and an NNRTI. Median age at ART initiation was 37 years [interquartile range (IQR), 31–43], median CD4 count was 121 cells/μl (IQR, 59–179), and median weight was 60 kg (IQR, 53–68). Sixty-four percent of patients were female, 49 % were married, and 36 % had not completed primary education. Baseline characteristics of patients in d4T- and ZDV-based regimens were comparable, except for weight, with d4T users having lower weight at ART initiation than ZDV users [median weight (kilograms), 60 versus 62, P=0.01]. There were no significant differences in age, CD4 count, height, sex, or education between regimens.
Development of symptomatic PN within 1 year was compared between d4T and ZDV users. Adults receiving d4T were followed for a median of 349 days, while those receiving ZDV were followed for a median of 108 days. One-year incidence of PN was 21.9 [95 % confidence interval (CI), 19.3–24.9] per 100 person-years among d4T users, as compared to 6.9 (95 % CI, 3.3–14.5) per 100 person-years in ZDV users. Further, d4T users had higher risk of peripheral neuropathy than ZDV users (P=0.002, log-rank test) (Fig. 1).
Fig. 1.
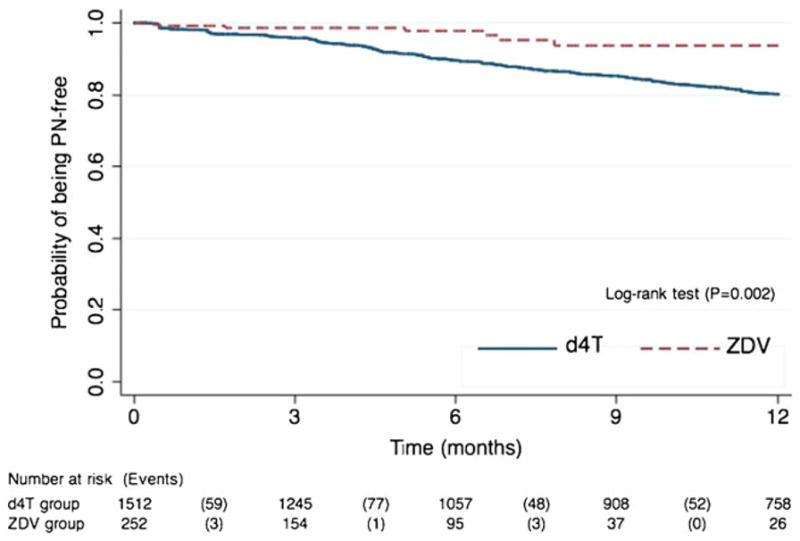
Kaplan–Meier curve illustrating the probability of remaining free of peripheral neuropathy (PN) 1-year following the initiation of ART regimen containing d4T or ZDV
Additional analysis was performed to evaluate the relationship between d4T- and ZDV-based regimens and PN (Table 1). After adjustment, 1-year risk of PN was 2.7 times higher in d4T users than ZDV users [adjusted hazard ratio (aHR), 2.72; 95 % CI, 1.28–5.80; P=0.009]. Additionally, female sex (aHR, 1.64; P=0.003), taller stature (aHR, 1.02 per centimeter; P=0.05), and older age (aHR, 1.72 per 10 years; P<0.001) at ART were predictors of PN. Among d4T users, there was no statistically significant association between PN and d4T dose (40 mg versus 30 mg; data not shown).
Table 1.
Multivariate Cox proportional hazards regression model evaluating the association between baseline characteristics and peripheral neuropathy
| Adjusted hazard ratio | 95 % confidence interval | P value | |
|---|---|---|---|
| d4T regimen (reference: ZDV) | 2.72 | (1.28–5.80) | 0.009 |
| Age (per 10 years) | 1.72 | (1.52–1.95) | <0.001 |
| Female (reference: male) | 1.64 | (1.18–2.29) | 0.003 |
| Height (cm) | 1.02 | (1.00–1.04) | 0.05 |
| Baseline weight (per 10 kg) | 1.08 | (0.97–1.21) | 0.18 |
| Baseline CD4 count (cells/μl) | 1.00 | (0.99–1.00) | 0.24 |
Discussion
In this cohort of antiretroviral-naïve adults initiating ART at a HIV treatment clinic in Kenya, we found a significantly higher 1-year incidence of symptomatic PN among users of d4T as compared to ZDV. Adults receiving d4T-based regimens were nearly three times more likely to develop PN during 1-year follow-up than adults receiving ZDV. Further, taller stature, female sex, and older age at treatment initiation were more likely to report symptoms of PN.
PN as a common adverse effect of treatment with d4T is well documented (Ellis et al. 2010; Forna et al. 2007; Karara et al. 2010; Moyle and Sadler 1998; Sacktor et al. 2009). Less established is the magnitude of difference in incidence of symptomatic PN between d4T and ZDV users presented herein (21.9 versus 6.9 per 100 person-years, respectively). Fifteen percent (236/1,579) of patients on d4T developed PN within 1-year of treatment, a finding in agreement with studies in Kenya and Uganda (Forna et al. 2007; Karara et al. 2010), however, higher than the 4 % reported in Cameroon (Laurent et al. 2008).
In recent years, evidence suggests fewer severe adverse effects associated with ZDV-based regimens (WHO November 2009). Fewer drug-related adverse effects lead to better treatment adherence, greater retention in care, and reductions in drug substitutions, ultimately allowing the preservation of first-line regimens. In light of these findings and growing concern related to d4T toxicity, WHO recommended the replacement of d4T with ZDV as first-line therapy (WHO 2010). While the change in first-line therapy has occurred quickly in Western countries, the transition in resource-limited settings has been slow due to increased costs, lack of widespread availability of ZDV, and poor healthcare infrastructure (WHO et al. 2009).
Consistent with other studies, we found that older age (Cherry et al. 2009; Forna et al. 2007; Lichtenstein et al. 2005, 2008; Wadley et al. 2011), taller stature (Cherry et al. 2009; Wadley et al. 2011), and female sex (Castelnuovo et al. 2011) were associated with 1-year risk of symptomatic PN. Neurologic disorders are known to increase with age, and thus, increases in rates of neurologic disorders observed in recent studies are in line with an aging HIV-infected population (Cherry et al. 2009; Watters et al. 2004). The relationship between taller stature and PN remains unclear. It is plausible that taller individuals with longer nerve fibers would have more exposed axon surface vulnerable to toxins (Cheng et al. 2006). Similar to a study in Uganda, our study showed that women were almost twice as likely to develop symptoms of PN than men (Castelnuovo et al. 2011). While the mechanism for this association is unknown, women may be at higher risk of mitochondrial toxicity, (Dlamini et al. 2011; Wester et al. 2007), and it has been suggested that women may not tolerate PN as well as men (Castelnuovo et al. 2011).
Our study has several strengths and limitations. Patients in this large cohort were followed longitudinally with detailed data on sociodemographic characteristics and health outcomes, including monitoring for ART-associated side effects. As this was a programmatic cohort, these findings are likely to be generalizable to ART users in African settings. Further, while underlying nutrient deficiencies may increase the likelihood of PN in this setting, our findings are comparable to those in ART-treated HIV-infected adults in developed settings. Diagnosis of PN was based on patient-reported symptoms and clinical judgment rather than clinical syndrome including physical and neurological exam. This may result in an attenuation or overestimation of our findings. Increasing awareness of d4T-associated side effects may have falsely led to more diagnoses of symptomatic PN among d4T users. However, our results are comparable to a Ugandan study reporting PN symptoms in 36 % of adults initiating stavudine-based regimens (Sacktor et al. 2009).
In summary, our findings show that incident PN risk was significantly higher among adults initiating d4T as compared to ZDV. This study underscores the lower 1-year risk of PN associated with ZDV, and reinforces recommendations to transition first-line therapy from d4T- to ZDV-based regimens. In African settings, where the transition from d4T to ZDV is an ongoing process, HIV treatment programs should consistently and intensively monitor for signs and symptoms of drug toxicity and consider the effects of d4T on PN compared to ZDV.
Acknowledgments
The Coptic Hope Center for Infectious Diseases is supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through a cooperative agreement (U62/CCU024512-04) from the US Centers for Disease Control and Prevention (CDC). Christine J. McGrath is supported by the National Institutes of Health (NIH) (TL1RR025016). Other funding sources include a K23 grant (AI065222 to Michael H. Chung), a K24 grant (HD054314 to Grace C. John-Stewart), and support by the NIH funded program, University of Washington Center for AIDS Research (CFAR) (P30 AI027757). We would like to thank the research personnel, clinic staff, and data management teams in Nairobi, Kenya and Seattle, Washington; the Coptic Hope Center for Infectious Diseases for their participation and cooperation.
Footnotes
Conflict of interest None of the authors have any financial interests or conflicts of interest relevant to this study.
Contributor Information
Christine J. McGrath, Email: mcgrathc@u.washington.edu, Department of Global Health, University of Washington, Box 359909, 325 Ninth Avenue, Seattle, WA 98104-2499, USA
Julia Njoroge, Department of Global Health, University of Washington, Seattle, USA.
Grace C. John-Stewart, Department of Global Health, University of Washington, Seattle, USA. Department of Medicine, University of Washington, Seattle, USA. Department of Pediatrics, University of Washington, Seattle, USA. Department of Epidemiology, University of Washington, Seattle, USA
Pamela K. Kohler, Department of Global Health, University of Washington, Seattle, USA
Sarah F. Benki-Nugent, Department of Medicine, University of Washington, Seattle, USA
Joan W. Thiga, Coptic Hospital, Nairobi, Kenya
Anthony Etyang, Department of Global Health, University of Washington, Seattle, USA.
Michael H. Chung, Department of Global Health, University of Washington, Seattle, USA. Department of Medicine, University of Washington, Seattle, USA. Department of Epidemiology, University of Washington, Seattle, USA
References
- Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS. 1998;12:1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- Castelnuovo B, Kiragga A, Kamya MR, Manabe Y. Stavudine toxicity in women is the main reason for treatment change in a 3-year prospective cohort of adult patients started on first-line antiretroviral treatment in Uganda. J Acquir Immune Defic Syndr. 2011;56:59–63. doi: 10.1097/QAI.0b013e3181f5bd03. [DOI] [PubMed] [Google Scholar]
- Cheng YJ, Gregg EW, Kahn HS, Williams DE, De Rekeneire N, Narayan KM. Peripheral insensate neuropathy—a tall problem for US adults? Am J Epidemiol. 2006;164:873–880. doi: 10.1093/aje/kwj281. [DOI] [PubMed] [Google Scholar]
- Cherry CL, Affandi JS, Imran D, Yunihastuti E, Smyth K, Vanar S, Kamarulzaman A, Price P. Age and height predict neuropathy risk in patients with HIV prescribed stavudine. Neurology. 2009;73:315–320. doi: 10.1212/WNL.0b013e3181af7a22. [DOI] [PubMed] [Google Scholar]
- Chung MH, Drake AL, Richardson BA, Reddy A, Thiga J, Sakr SR, Kiarie JN, Yowakim P, John-Stewart GC. Impact of prior HAART use on clinical outcomes in a large Kenyan HIV treatment program. Curr HIV Res. 2009;7:441–446. doi: 10.2174/157016209788680552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote HC, Brumme ZL, Craib KJ, Alexander CS, Wynhoven B, Ting L, Wong H, Harris M, Harrigan PR, O’Shaughnessy MV, Montaner JS. Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N Engl J Med. 2002;346:811–820. doi: 10.1056/NEJMoa012035. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Peripheral neuropathy and antiretroviral drugs. J Peripher Nerv Syst. 2001;6:14–20. doi: 10.1046/j.1529-8027.2001.006001014.x. [DOI] [PubMed] [Google Scholar]
- Dlamini J, Ledwaba L, Mokwena N, Mokhathi T, Orsega S, Tsoku M, Kowo H, Proschan M, Khabo P, Maja P, Hadigan C. Lactic acidosis and symptomatic hyperlactataemia in a randomized trial of first-line therapy in HIV-infected adults in South Africa. Antivir Ther. 2011;16:605–609. doi: 10.3851/IMP1790. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forna F, Liechty CA, Solberg P, Asiimwe F, Were W, Mermin J, Behumbiize P, Tong T, Brooks JT, Weidle PJ. Clinical toxicity of highly active antiretroviral therapy in a home-based AIDS care program in rural Uganda. J Acquir Immune Defic Syndr. 2007;44:456–462. doi: 10.1097/QAI.0b013e318033ffa1. [DOI] [PubMed] [Google Scholar]
- Hawkins C, Achenbach C, Fryda W, Ngare D, Murphy R. Antiretroviral durability and tolerability in HIV-infected adults living in urban Kenya. J Acquir Immune Defic Syndr. 2007;45:304–310. doi: 10.1097/QAI.0b013e318050d66c. [DOI] [PubMed] [Google Scholar]
- Idigbe EO, Adewole TA, Eisen G, Kanki P, Odunukwe NN, Onwujekwe DI, Audu RA, Araoyinbo ID, Onyewuche JI, Salu OB, Adedoyin JA, Musa AZ. Management of HIV-1 infection with a combination of nevirapine, stavudine, and lamivudine: a preliminary report on the Nigerian antiretroviral program. J Acquir Immune Defic Syndr. 2005;40:65–69. doi: 10.1097/01.qai.0000159516.39982.1b. [DOI] [PubMed] [Google Scholar]
- Karara MW, Okalebo FA, Oluka MN, Ombega J, Guantai AN, Osanjo GO. Comparative tolerability and efficacy of stavudine 30 mg versus stavudine 40 mg in patients on combination anti-retroviral therapy in Kenya. J AIDS HIV Res. 2010;2:24–31. [Google Scholar]
- Laurent C, Kouanfack C, Koulla-Shiro S, Nkoue N, Bourgeois A, Calmy A, Lactuock B, Nzeusseu V, Mougnutou R, Peytavin G, Liegeois F, Nerrienet E, Tardy M, Peeters M, Andrieux-Meyer I, Zekeng L, Kazatchkine M, Mpoudi-Ngole E, Delaporte E. Effectiveness and safety of a generic fixed-dose combination of nevirapine, stavudine, and lamivudine in HIV-1-infected adults in Cameroon: open-label multicentre trial. Lancet. 2004;364:29–34. doi: 10.1016/S0140-6736(04)16586-0. [DOI] [PubMed] [Google Scholar]
- Laurent C, Bourgeois A, Mpoudi-Ngole E, Ciaffi L, Kouanfack C, Mougnutou R, Nkoue N, Calmy A, Koulla-Shiro S, Delaporte E. Tolerability and effectiveness of first-line regimens combining nevirapine and lamivudine plus zidovudine or stavudine in Cameroon. AIDS Res Hum Retroviruses. 2008;24:393–399. doi: 10.1089/aid.2007.0219. [DOI] [PubMed] [Google Scholar]
- Lichtenstein KA, Armon C, Baron A, Moorman AC, Wood KC, Holmberg SD. Modification of the incidence of drug-associated symmetrical peripheral neuropathy by host and disease factors in the HIV outpatient study cohort. Clin Infect Dis. 2005;40:148–157. doi: 10.1086/426076. [DOI] [PubMed] [Google Scholar]
- Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Moorman AC, Wood KC, Holmberg SD, Brooks JT. Initiation of antiretroviral therapy at CD4 cell counts >/=350 cells/mm3 does not increase incidence or risk of peripheral neuropathy, anemia, or renal insufficiency. J Acquir Immune Defic Syndr. 2008;47:27–35. doi: 10.1097/QAI.0b013e31815acacc. [DOI] [PubMed] [Google Scholar]
- Moyle GJ, Sadler M. Peripheral neuropathy with nucleoside antiretrovirals: risk factors, incidence and management. Drug Saf. 1998;19:481–494. doi: 10.2165/00002018-199819060-00005. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Nakasujja N, Skolasky RL, Robertson K, Musisi S, Ronald A, Katabira E, Clifford DB. Benefits and risks of stavudine therapy for HIV-associated neurologic complications in Uganda. Neurology. 2009;72:165–170. doi: 10.1212/01.wnl.0000339042.96109.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarsella A, Coodley G, Shalit P, Anderson R, Fisher RL, Liao Q, Ross LL, Hernandez JE. Stavudine-associated peripheral neuropathy in zidovudine-naive patients: effect of stavudine exposure and antiretroviral experience. Adv Ther. 2002;19:1–8. doi: 10.1007/BF02850013. [DOI] [PubMed] [Google Scholar]
- Simpson DM, Tagliati M. Nucleoside analogue-associated peripheral neuropathy in human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:153–161. [PubMed] [Google Scholar]
- Wadley AL, Cherry CL, Price P, Kamerman PR. HIV neuropathy risk factors and symptom characterization in stavudine-exposed South Africans. J Pain Symptom Manage. 2011;41:700–706. doi: 10.1016/j.jpainsymman.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Watters MR, Poff PW, Shiramizu BT, Holck PS, Fast KM, Shikuma CM, Valcour VG. Symptomatic distal sensory polyneuropathy in HIV after age 50. Neurology. 2004;62:1378–1383. doi: 10.1212/01.wnl.0000120622.91018.ea. [DOI] [PubMed] [Google Scholar]
- Wester CW, Okezie OA, Thomas AM, Bussmann H, Moyo S, Muzenda T, Makhema J, van Widenfelt E, Musonda R, Novitsky V, Gaolathe T, Ndwapi N, Essex M, Kuritzkes DR, DeGruttola V, Marlink RG. Higher-than-expected rates of lactic acidosis among highly active antiretroviral therapy-treated women in Botswana: preliminary results from a large randomized clinical trial. J Acquir Immune Defic Syndr. 2007;46:318–322. doi: 10.1097/QAI.0b013e3181568e3f. [DOI] [PubMed] [Google Scholar]
- WHO. Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. 2009. [Google Scholar]
- World Health Organization, Geneva WHO. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. World Health Organization; Geneva: 2010. [PubMed] [Google Scholar]
- WHO, UNAIDS, UNICEF. Towards universal access: scaling up of priority HIV/AIDS interventions in the health sector: progress report 2009. World Health Organization; Geneva: 2009. [Google Scholar]


