Abstract
Schistosomiasis, a neglected tropical disease affecting hundreds of millions, is caused by parasitic flatworms of the genus Schistosoma. Treatment and control of schistosomiasis relies almost exclusively on a single drug, praziquantel (PZQ), a dangerous situation for a disease of this magnitude. Though PZQ is highly effective overall, it has drawbacks, and reports of worms showing PZQ resistance, either induced in the laboratory or isolated from the field, are disconcerting. Multidrug transporters underlie multidrug resistance (MDR), a phenomenon in which resistance to a single drug is accompanied by unexpected cross-resistance to several structurally unrelated compounds. Some of the best studied multidrug transporters are members of the ancient and very large ATP-binding cassette (ABC) superfamily of efflux transporters. ABC multidrug transporters such as P-glycoprotein (Pgp; ABCB1) are also associated with drug resistance in parasites, including helminths such as schistosomes. In addition to their association with drug resistance, however, ABC transporters also function in a wide variety of physiological processes in metazoans. In this review, we examine recent studies that help define the role of schistosome ABC transporters in regulating drug susceptibility, and in normal schistosome physiology, including reproduction and excretory activity. We postulate that schistosome ABC transporters could be useful targets for compounds that enhance the effectiveness of current therapeutics as well as for agents that act as antischistosomals on their own.
1. Introduction
Schistosomiasis is a devastating and neglected tropical disease caused by parasitic flatworms of the genus Schistosoma. It affects hundreds of millions of people worldwide [1,2]. Left untreated, schistosome infections pose a significant health burden in many parts of the world, resulting in serious and permanent damage to various organs, severe morbidity, devastating effects on childhood development and adult productivity, and, in some cases, death [1,2,3,4]. There is no vaccine, and treatment relies almost entirely on one drug (praziquantel, PZQ), a worrisome situation for a disease of this magnitude [5].
PZQ is effective against all human schistosome species, and has been used successfully in large-scale schistosomiasis control efforts in a variety of countries [6,7]. However, PZQ does have its limitations. Failure rates of PZQ in the field range as high as 30% [8,9,10], and several schistosome field and lab isolates show significantly reduced susceptibility to PZQ [reviewed by 8,11,12], perhaps a harbinger for emergence of more widespread drug resistance. Furthermore, liver-stage juvenile schistosomes (~28 days post infection) are refractory to PZQ; worms become fully susceptible to PZQ as egg production begins approximately 6 weeks following infection of the mammalian host [13,14,15,16]. A further concern is that the molecular target of PZQ has yet to be defined rigorously [17,18,19], making the prospect of emerging resistance particularly worrisome. On the other hand, even if the molecular target of PZQ were established, it is clear that other downstream factors contribute to levels of PZQ susceptibility. For example, as described above, juvenile worms are refractory to PZQ, yet PZQ nonetheless induces Ca2+-dependent contraction and paralysis similar to that observed in adult worms [20]. Unlike adults, however, juveniles recover, indicating that though the same initial receptor is likely being targeted at both stages, adaptive responses that allow parasite survival come into play in the immature, but not mature, worms.
Combination therapy represents one strategy for overcoming drug resistance and enhancing drug efficacy. Thus, current anthelmintics could be potentiated by agents targeted against different, but possibly interacting, sites of action [21,22]. One implementation of such an approach would be to target cellular components that regulate rates of drug uptake, metabolism, or efflux. ATP-dependent multidrug transporters such as P-glycoprotein (Pgp) have been advanced as particularly attractive targets of this type [23,24]. Multidrug transporters underlie multidrug resistance (MDR), a phenomenon in which resistance to a single drug is accompanied by unexpected cross-resistance to several structurally unrelated compounds. Multidrug transporters have also been implicated in drug resistance in several parasites, including helminths [reviewed in 25,26,27]
This review will examine recent evidence pertaining to the role of multidrug transporters in schistosomes and other platyhelminths. The focus will be two-fold. First, as discussed above, evidence suggests these transporters may be useful targets to potentiate PZQ (or other drug) action and perhaps overcome certain manifestations of drug resistance. However, schistosome multidrug transporters also appear to be critical components of schistosome physiology, making them potentially useful as therapeutic targets on their own. Given the high suitability of these transporters as drug targets and the ready availability of many safe and inexpensive approved drugs known to interact with (ie, inhibit) them, the targeting of schistosome multidrug transporters may provide unique opportunities for development of innovative treatment strategies.
2. Drug resistance and multidrug transporters
Cells and organisms can develop resistance to a single class of drugs via several mechanisms. These include target modification, changes in drug uptake, or altered drug metabolism or efflux. On the other hand, the principal mechanism underlying MDR is active transport of drugs out of the cell [28]. MDR is typically mediated by multidrug transporters with broad substrate specificity, though non-transport-related MDR can also occur [29,30]. Genes encoding multidrug transporters are present in all living cells [31], and comprise five major families [28,32]: the major facilitator superfamily (MFS); the resistance/nodulation/division (RND) superfamily; the small multidrug resistance (SMR) family; the multidrug and toxic compounds efflux (MATE) family; and the ATP-binding cassette (ABC) superfamily. Representative crystal structures from members of each of the five families have been solved and reported [33]. To a first approximation, different transporter types are either primary-active transporters, in which substrate translocation is coupled directly to ATP hydrolysis, or secondary-active transporters, which derive energy from the electrochemical gradient of sodium and potassium ions across the cell membrane [34]. This review will focus on the role in schistosomes of multidrug transporters such as P-glycoprotein (Pgp) that are part of the primary-active ABC protein superfamily. Bernadette Ardelli discusses the role of these transporters in nematodes in a separate article in this volume.
ABC multidrug transporters are members of the ABC protein superfamily, a sizable and ancient group of proteins. Indeed, the ABC superfamily comprises one of the largest groups of transmembrane proteins, and representatives can be found in organisms from all kingdoms of life [35,36]. ABC transporters contain nucleotide binding domains (NBDs) that bind ATP, and use the energy resulting from ATP hydrolysis to power translocation of compounds across the membrane. Some ABC transporters, seen only in prokaryotes, import compounds into the cell, while efflux ABC transporters are found in both prokaryotes and eukaryotes [36,37]. MDR results from an increased capacity for efflux of both the original drug as well as other unrelated compounds via amplification, overexpression, or modification of a subset of these ABC transporters.
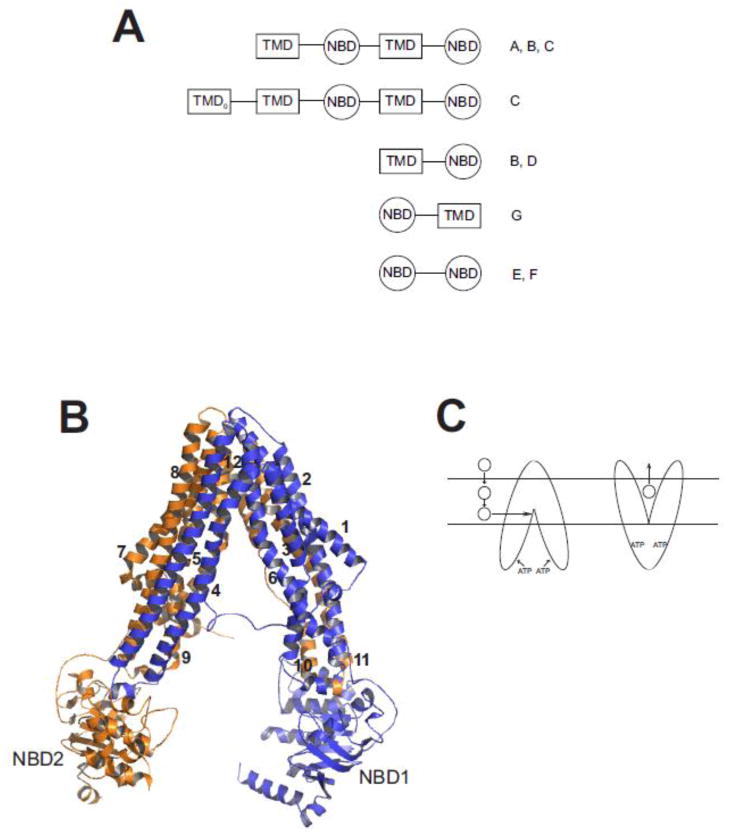
All ABC transporters have at least one cytoplasmic ATP binding cassette, a highly-conserved ATPase domain that includes a specific signature motif linked to WalkerA and WalkerB motifs that are characteristic of ATPases [38]. Those ABC transporters that contain two ATP-binding cassettes alternating with two membrane-spanning domains are considered full transporters, while those that consist of one of each of these structural features are defined as half transporters [39,40,41]. There are also variations on these basic themes, and phylogenetic analysis clusters the many different ABC transporters into eight families, designated ABCA through ABCH. ABCE and ABCF proteins typically contain two NBDs and no transmembrane domains; they are not known to exhibit any transporter function, but their NBDs are clearly derived from other ABC transporters [42]. Humans have 48 ABC transporter genes representing seven of these families (ABCA to ABCG), while Drosophila and zebrafish possess genes for members of all eight families (ABCA to ABCH) [43,44,45]. Figure 1A shows the predicted domain topology for members of the ABC transporter classes found in humans.
Fig. 1. Structure of ABC multidrug transporters.
A. Predicted domain arrangement of ABC transporters found in the human genome. Shown are the arrangement of transmembrane domains (TMD) and nucleotide binding domains (NBD) found in human ABC transporters. The TMD0 domain is found in some members of the ABCC sub-family. Letters on to the right of the cartoon designate ABC sub-families in which that predicted domain topology is found. Figure adapted from [41] and [35]. B. Homology model of SMDR2. SMDR2 was modeled against C. elegans Pgp (pdb 4F4C; [55]) using ESyPred3D (http://www.fundp.ac.be/sciences/biologie/urbm/bioinfo/esypred/) [113]. Residues at the N-terminus (1–4), linker (609–656), and C-terminus (1243–1255) were not included in the final model. The figure was generated using the program PyMOL (http://www.pymol.org/). NBD1 and NBD2 designate the nucleotide binding domains, and the numbers 1–12 indicate the helical transmembrane-spanning segments. Domain 1 is in blue; domain 2 is in orange. C. Model for binding and transport of substrate by Pgp. Substrate (circle) partitions from outside the cell into the inner leaflet of the lipid bilayer, where, according to the hydrophobic “vacuum cleaner” model, it enters the drug-binding pocket of Pgp. ATP binding to the NBDs produces a conformational change that results in presentation of the substrate to the outer leaflet or extracellular space.
The different ABC transporters have selectivity for various substrates, ranging from metabolic byproducts to physiologically significant signaling molecules such as peptides, lipids, cyclic nucleotides, and immunomodulators. A subset of these transporters (ABCB1, ABCG2, several members of the ABCC sub-family, and possibly ABCA2) have been linked to MDR [40,42]. We previously [27] identified S. mansoni genes predicted to code for ABC transporters, including several representatives of the classes associated with MDR, and a revised catalog is presented in Table 1 and outlined in more detail in section 3.1 below.
Table 1.
ABC transporter genes found in the S. mansoni and S. japonicum genomes
| Human gene family | S. mansoni | S. japonicum |
|---|---|---|
| ABCA1 | Smp_176450 | Sjp_0045330 |
| ABCA3 | Smp_165800 | |
| ABCA4 | Smp_056290 | Sjp_0045310 |
| ABCB1 (SMDR2, Pgp) | L26287, Smp_055780 | Sjp_0093230 |
| ABCB1 (other Pgp) | Smp_089200 | Sjp_0066120 |
| Smp_137080 | Sjp_0039970 | |
| Smp_170820 | ||
| ABCB6 | Smp_134890 | Sjp_0007520 |
| ABCB7 | Smp_087930 | Sjp_0035360 |
| ABCB8 (SMDR1) | L26286, Smp_063000 | Sjp_0012640 |
| ABCC1 (SmMRP1) | GU967672, Smp_171740 | Sjp_0028040 |
| ABCC1 (other MRP1) | Smp_129820 | Sjp_0105130* |
| ABCC4 | Smp_167610 | Sjp_0088380 |
| ABCC10 (MRP7) | Smp_147250 | Sjp_0094160 Sjp_0031710 |
| ABCE1 | Smp_124460 | Sjp_0011870 |
| ABCF1 | Smp_166040 | Sjp_0068000 |
| ABCF2 | Smp_040540 | |
| ABCF3 | Smp_049500 | Sjp_0010440 |
| ABCG1 | Smp_181150 | Sjp_0034670 |
| ABCG2 (BCRP) | Smp_126450 | Sjp_0087910 |
| Smp_137890 | Sjp_0067580 |
Also shows equivalent similarity to human ABCC3
Pgp is considered the prototypical eukaryotic ABC multidrug transporter (though LmrA, a bacterial ABC transporter, can complement human Pgp [46]). Pgp is a glycosylated, ATP-dependent efflux transporter associated with resistance to a broad array of compounds that include several anticancer drugs [47]. The substrate specificity of Pgp is not completely defined [35], though it is clear that Pgp preferentially transports neutral and cationic hydrophobic compounds [39]. Nonetheless, Pgp also has the capacity to transport a hydrophilic, negatively-charged drug such as methotrexate when the drug crosses the cell membrane slowly via passive diffusion (as opposed to rapid influx via a transporter) [48]. Pgp also transports or translocates significant signaling molecules such as glycolipids and phospholipids [49,50,51,52], and appears to be involved in secretion of cytokines [50,53]. The crystal structures of both mammalian [54] and Caenorhabditis elegans [55] Pgp have recently been solved. These two structures are the highest scoring “hits” when the predicted amino acid sequence of SMDR2, a S. mansoni Pgp [56,57], is searched against the PDB database. Figure 1B shows the results of homology modeling of SMDR2 using the 3.4 Å-resolution C. elegans structure (PDB ID: 4F4C) as template (analogous results were obtained using the mammalian structure as template). The derived model is quite similar overall to that of the other Pgp proteins, with an inward-facing conformation (eg, open to the cytoplasm), cytoplasmic NBDs, and the 12 predicted transmembrane helices flanking a relatively large substrate binding site that is at the level of the inner leaflet of the membrane. Though access to the binding site is available from the cytoplasm, the hydrophobic “vacuum cleaner” model (Figure 1C) posits that Pgp interacts with substrates at the inner leaflet of the membrane then, upon binding of ATP, either pumps or “flips” them to the outer leaflet of the membrane or to the extracellular medium [58,59]; recent evidence showing up to 4000-fold higher affinity of drugs in the membrane bilayer than in detergent supports this view [55].
Besides Pgp, other ABC transporter proteins associated with MDR include several of the multidrug resistance-associated proteins (MRPs; ABCCs) and the breast cancer resistance protein (BCRP) half transporter (ABCG2) [35,42]. Though the other known ABC multidrug transporters have substrate specificities that overlap somewhat with Pgp, the differences are more notable. For example, as described, Pgp preferentially transports unmodified neutral or positively-charged hydrophobic compounds, while MRP1 (ABCC1) shows a propensity for transport of organic anions and Phase II metabolic products (eg, glutathione-conjugates) likely to be found in the cytoplasm [40,60]. Interestingly, the highest affinity substrate known for MRP1 is the inflammatory mediator leukotriene C4 [61], suggesting a possible role for MRP1 and perhaps other ABC transporters in immune signaling and immunomodulation [62,63]. BCRP selectivity, though not identical to Pgp, shows substantial substrate overlap, and furthermore co-localizes with Pgp to sites such as the intestinal epithelium and the blood-brain barrier [64].
Most of the attention focused on multidrug transporters is based on their association with drug transport and MDR. As such, the major role of these transporters in normal cellular physiology is thought to be to act as a guardian, removing or excluding xenotoxins (including drugs) from cells and tissues. For example, in places such as the gut mucosa and the blood-brain barrier, Pgp is critical to preventing entry of toxins into the body and providing protection to a vital organ (eg, the brain) [35]. Indeed, the excellent safety profile of ivermectin, a macrocyclic lactone anthelmintic that is a also a substrate for Pgp, results in large part from Pgp mediating exclusion of the drug from the host central nervous system; loss or disruption of this function can lead to ivermectin-induced neurological toxicity in mammals [65,66]. Interestingly, there are sporadic reports that PZQ can have effects on molluscan and mammalian cells, and it is tempting to speculate that the high host safety profile of PZQ may in part result from a similar mechanism. Though PZQ does not appear to be a substrate of mammalian Pgp [67], it could possibly be excluded from the central nervous system by an alternative transporter such as BCRP (ABCG2), which is also found in the blood-brain barrier.
Besides their role in transporting xenobiotics, ABC multidrug transporters are also involved in intercellular signaling, as many of their high-affinity substrates are known signaling molecules [49,50,52,68,69,70]. Indeed, several lines of evidence suggest that ABC multidrug transporters play important roles in a wide variety of physiological functions [50,71]. For example, they have been implicated in regulation of apoptosis [72], immune function [63] (including transport of cytokines [50]), and tumor promotion [73], among others, though there has been some debate about whether some of these proposed activities reflect authentic functions in vivo [74]. Sarkadi and co-authors have postulated that ABC transporters comprise a “chemoimmunity defense system”, with innate and adaptive phases analogous to those found in classical immunity [75].
3. ABC multidrug transporters in schistosomes and other platyhelminths
There is increasing evidence for an association between drug resistance and ABC multidrug transporters in parasites [25,76], and, most relevant for this review, parasitic helminths [26,27,77,78]. In addition to their being implicated in modulating drug susceptibility, however, these transporters are likely also to be important components of normal parasite physiology, and we and others have obtained evidence to suggest potential roles for these transporters in schistosome excretory activity and reproduction.
3.1 Representatives of several ABC transporter classes are found in schistosomes and other platyhelminths
In 1994, Bosch et al. [56] cloned and sequenced two cDNAs (SMDR1 and SMDR2) from S. mansoni that were predicted to encode ABC transporter-like proteins. SMDR1 codes for a half-transporter, with single ATP-binding and transmembrane domains. It appears to be most closely related to ABCB8, a mitochondrial half transporter with an elusive function, but with likely involvement in protection from oxidative stress. Mammalian ABCB8 also exports doxorubicin, a cancer chemotherapeutic drug, suggesting a possible role in MDR in cancer cells [79,80]. SMDR2 codes for a Pgp (ABCB1)-like protein, with two predicted NBDs and 12 transmembrane segments. Mining of the version of the S. mansoni genome available in 2010 [81] uncovered several other ABC transporter genes [27]. Table 1 presents a revised version of that catalog, searched against the updated S. mansoni genome resources [82], and also including ABC transporters found in the S. japonicum genome [83]. Approximately 20 genes from both species are predicted to encode ABC transporters. These include other Pgp-like proteins, several sequences most similar to multidrug transporters of the ABCC class, including MRP1 (SmMRP1), and schistosome orthologues of BCRP (ABCG2). Other potentially interesting ABC transporter subfamilies are also represented. For example, the genomes contain either three (S. mansoni) or two (S. japonicum) genes that appear to be members of the ABCA family. In mammals, ABCA proteins transport a variety of physiological lipid compounds, many of which are important signaling molecules. When mutated, ABCA transporters are associated with several disease states and have also been implicated in neurodegenerative disorders such Alzheimer’s Disease [reviewed in 84,85,86]. Both S. mansoni and S. japonicum also contain ABCE-like and ABCF-like genes, but there do not appear to be genes for any members of the ABCD or ABCH sub-families in either schistosome species.
There is also evidence for ABC transporters in trematodes other that schistosomes; four ABC transporters were shown to be expressed and to have functional activity in the liver fluke Fasciola gigantica [87], and a Pgp orthologue has been described in F. hepatica [88]. Cursory scans of the Echinococcus multilocularis [89] and Schmidtea mediterranea [90] genomes indicate that representatives of the cestode and turbellarian classes of platyhelminths also contain multiple sub-families of ABC transporters.
3.2 Evidence for a potential role in drug susceptibility
Several studies are beginning to link schistosome ABC transporters with modulation of drug susceptibility. Particularly intriguing is work using fluorescent substrates of mammalian Pgp and MRP as probes for the presence of these transporters. When schistosomes are exposed to these substrates, the fluorescence localizes primarily to the excretory system of worms [91,92], suggesting that these multidrug transporters function in the excretory system of the worm. Thus, ABC multidrug transporters likely play an important role in removal of xenobiotics in schistosomes, but could also function in regulating parasite metabolism and in excreting factors that influence interactions with the host [93]. Exposure of worms to PZQ dramatically disrupts this pattern of fluorescence [94,95]. Remarkably, that disruption does not occur when an experimentally-induced S. mansoni isolate selected at the snail stage for reduced PZQ susceptibility is exposed to PZQ [96]. Thus, at the least, the distribution of schistosome ABC multidrug transporters may serve as a useful marker for reduced PZQ susceptibility, and could in fact be playing a role in development or maintenance of PZQ resistance.
Further indications of possible involvement of these transporters in modulating drug susceptibility have come from a variety of studies focusing on the transporters themselves. Early experiments showed no association between SMDR2 RNA levels and resistance to the antischistosomals oxamniquine and hycanthone [56]. However, with PZQ having become the antischistosomal drug of choice, we decided to explore whether such an association might exist for PZQ.
We found that PZQ interacts directly with S. mansoni Pgp, acting as both an inhibitor and a likely substrate of SMDR2 cDNA functionally expressed in CHO cells [57]. Others have shown that PZQ also inhibits mammalian Pgp, but, in contrast to what we found for SMDR2, it does not appear to be a substrate for mammalian Pgp [67], a potentially important difference that could perhaps be exploited. We also found that exposure of adult worms to low levels of PZQ (100 nM – 300 nM) induces a transient increase in SMDR2 and SmMRP1 levels and alters the normal distribution of anti-Pgp immunoreactivity [97,98]. Of particular note are our findings that schistosomes showing reduced PZQ susceptibility (eg, juvenile worms, worms from single-sex infections) express higher levels of SMDR2 and SmMRP1 [27,97]. The most dramatic example is found in the EE2 isolate, derived from Egyptian patients showing continued excretion of S. mansoni eggs following three doses of PZQ [99]. Worms from this isolate show ~10-fold higher levels of SMDR2 RNA and comparably higher levels of anti-Pgp immunoreactivity compared to Egyptian PZQ-sensitive worms (in contrast, SmMRP1 does not appear to be overexpressed) [97,98]. This result suggests not only a potential marker for emergence of PZQ resistance (eg, SMDR2 levels), but also a possible underlying mechanism. As noted above, PZQ appears to be a substrate for SMDR2 [57]. We hypothesize that the efflux activity of SMDR2 (and possibly other parasite multidrug transporters) may serve as a protective mechanism against PZQ by excreting the drug from schistosome cells and thereby reducing its intracellular concentration. Indeed, if some variant of the hydrophobic “vacuum cleaner” model for Pgp is correct, SMDR2 may in fact be preventing PZQ from crossing the cell membrane, translocating it to the extracellular space while it is still in the lipid bilayer. Higher levels of SMDR2 might be preventing or delaying interaction of PZQ with its target(s) more efficiently, thus effectively reducing drug susceptibility and perhaps allowing other protective mechanisms to come into play.
ABC multidrug transporters have also been suggested to play a role in drug susceptibility in other trematodes such as liver flukes. A recent paper provided evidence that an amino acid substitution in a critical region of F. hepatica Pgp appears to be associated with parasite resistance to the benzimidazole triclabendazole [88], and two of the four ABC transporters described in the liver fluke F. gigantica showed increased expression in the presence of triclabendazole in isolated cells [87].
3.3. Role in parasite physiology
A 2003 patent [100] described work showing that exposure of either S. mansoni to verapamil, or the intestinal fluke Echinostoma caproni to verapamil or nifedipine resulted in significantly reduced parasite egg production ex vivo. Both verapamil and nifedipine are mammalian L-type voltage-gated calcium channel blockers, and the authors of the patent attributed the effects of these drugs to that calcium antagonist activity. However, both drugs are also inhibitors of Pgp [101,102,103], and we have shown that both also inhibit SMDR2 [57]. Indeed, verapamil is considered one of the “classic” Pgp inhibitors,. We therefore hypothesized that perhaps the effects of these drugs on trematode egg production was dependent on their interaction with Pgp rather than with calcium channels, and tested that hypothesis using a combination of genetic and pharmacological approaches [104]. We confirmed the results reported for verapamil interference with egg production, and also showed that a variety of Pgp and MRP1 inhibitors, including the verapamil enantiomer dexverapamil, which retains activity against Pgp but not calcium channels, also disrupt egg production by worms ex vivo. We also found that knockdown of SMDR2 or SmMRP1 expression using RNA interference disrupts egg production in cultured schistosomes, further implicating these proteins as the source of the effects on parasite reproduction. Finally, we found that administration of a variety of Pgp and MRP1 inhibitors to S. mansoni-infected mice produces a significant reduction in liver egg burden and numbers of granulomas [104].
These studies suggest a key role for ABC transporters in schistosome reproduction. Further work will be required to better characterize that role, and to understand the mechanism by which interference with transporter function or expression disrupts egg production. Nonetheless, these results could eventually produce new strategies for disease treatment and control. Parasite eggs represent the primary cause of pathology in schistosomiasis and are also the agents for transmission of the disease. Indeed, our results show that the disruption of parasite egg production by Pgp inhibitors also reduces S. mansoni-induced pathology in mice; it will be interesting to determine if similar results can be obtained in humans. We suspect that therapeutic strategies that limit egg production could be of great importance in reducing schistosomiasis pathology, either alone, or in combination with another anthelmintic (eg, PZQ). Such treatments furthermore have the potential to limit the spread of PZQ resistance, should it arise in the field.
4. Conclusions and prospects for future research
Several lines of evidence are beginning to point to schistosome and possibly other platyhelminth multidrug transporters as practical drug targets that could be exploited either on their own or, more likely, in combination with current anthelmintics to enhance drug susceptibility. One might argue that a similar strategy of using Pgp inhibitors to reverse MDR in cancer chemotherapy has provided disappointing results, due in part to the complications associated with long-term/high-dose administration of these compounds [40,105,106]. However, unlike cancer treatments, the approach for schistosomiasis would entail exposure to only one or two doses of a drug efflux inhibitor in combination with PZQ, thus minimizing the risk of adverse effects. Such short-term treatments might also reduce the need for agents that act selectively against schistosome (vs. mammalian) targets. As noted, the availability of many safe, inexpensive drugs throughout the world that, in addition to targeting their primary receptors, also interact with multidrug transporters, provides the potential for adaption to such a combination strategy.
Future research efforts should be geared towards confirming whether these combination approaches are indeed feasible. One obvious set of studies would test both adult and PZQ-refractory juvenile worms for changes in PZQ potency when it is combined with inhibitors of Pgp or other multidrug transporters. Indeed, these types of experiments have shown that that co-administration of MDR reversing agents (eg, Pgp inhibitors such as verapamil) can increase susceptibility to macrocyclic lactones in a variety of drug-resistant and -sensitive nematodes [107,108,109,110,111,112]. RNA interference experiments in schistosomes to test the effects of knockdown of these transporters on PZQ sensitivity could be used to confirm the pharmacological studies, but with greater specificity. Such a genetic approach could also lead to a better understanding of the relative contribution of different transporters to PZQ susceptibility (or lack thereof). These types of experiments have potential pitfalls however, including the confounding effects of partial knockdown and off-target effects. Furthermore, the presence of multiple genes for particular ABC transporters may present a challenge for obtaining unambiguous phenotypes. Nonetheless, combined with the pharmacological studies, they could provide important insights into PZQ action, as well as practical strategies for increasing effective drug potency and possibly overcoming some forms of drug resistance. At the least, such genetic and pharmacological dissection of the properties and regulation of schistosome ABC transporters could lead to new insights into fundamental parasite physiological functions such as excretion, reproduction, and perhaps parasite-host interactions.
Research Highlights.
I provide an overview of ABC transporters in schistosomes and other platyhelminths
I survey the evidence suggesting a role for these transporters in drug resistance
I discuss the possible roles ABC transporters play in normal schistosome biology
Acknowledgments
This work was supported by NIH grant R01 AI073660 and in part by NIH grant R21 AI100505. I thank Ravi Kasinathan for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Tropica. 2003;86:125–39. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 2.King CH. Parasites and poverty: the case of schistosomiasis. Acta Tropica. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotez PJ, Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Neglected Tropical Diseases. 2009;3:e485. doi: 10.1371/journal.pntd.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illness. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- 5.Caffrey CR. Chemotherapy of schistosomiasis: present and future. Current Opinion in Chemical Biology. 2007;11:433–9. doi: 10.1016/j.cbpa.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Toure S, Zhang Y, Bosque-Oliva E, Ky C, Ouedraogo A, Koukounari A, Gabrielli AF, Bertrand S, Webster JP, Fenwick A. Two-year impact of single praziquantel treatment on infection in the national control programme on schistosomiasis in Burkina Faso. Bulletin of the World Health Organization. 2008;86:780–7. doi: 10.2471/BLT.07.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vennervald BJ, Booth M, Butterworth AE, Kariuki HC, Kadzo H, Ireri E, Amaganga C, Kimani G, Kenty L, Mwatha J, Ouma JH, Dunne DW. Regression of hepatosplenomegaly in Kenyan school-aged children after praziquantel treatment and three years of greatly reduced exposure to Schistosoma mansoni. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2005;99:150–60. doi: 10.1016/j.trstmh.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Day TA, Botros S. Drug resistance in schistosomes. In: Maule A, Marks NJ, editors. Parasitic Flatworms: Molecular Biology, Biochemistry, Immunology and Physiology. Oxfordshire, UK: CAB International; 2006. pp. 256–68. [Google Scholar]
- 9.Mutapi F, Rujeni N, Bourke C, Mitchell K, Appleby L, Nausch N, Midzi N, Mduluza T. Schistosoma haematobium treatment in 1–5 year old children: safety and efficacy of the antihelminthic drug praziquantel. PLoS Neglected Tropical Diseases. 2011;5:e1–143. doi: 10.1371/journal.pntd.0001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behbehani M, Savioli L. Report of the WHO informal consultation on monitoring of drug efficacy in the control of schistosomiasis and intestinal nematodes. World Health Organization; Geneva: 1998. [Google Scholar]
- 11.Doenhoff MJ, Pica-Mattoccia L. Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Expert Review of Anti-infective Therapy. 2006;4:199–210. doi: 10.1586/14787210.4.2.199. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Wang L, Liang YS. Susceptibility or resistance of praziquantel in human schistosomiasis: areview. Parasitology Research. 2012;111:1871–7. doi: 10.1007/s00436-012-3151-z. [DOI] [PubMed] [Google Scholar]
- 13.Aragon AD, Imani RA, Blackburn VR, Cupit PM, Melman SD, Goronga T, Webb T, Loker ES, Cunningham C. Towards an understanding of the mechanism of action of praziquantel. Molecular and Biochemical Parasitology. 2009;164:57–65. doi: 10.1016/j.molbiopara.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao SH, Catto BA, Webster LT., Jr Effects of praziquantel on different developmental stages of Schistosoma mansoni in vitro and in vivo. Journal of Infectious Diseases. 1985;151:1130–7. doi: 10.1093/infdis/151.6.1130. [DOI] [PubMed] [Google Scholar]
- 15.Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. Schistosoma mansoni: chemotherapy of infections of different ages. Experimental Parasitology. 1986;61:294–303. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- 16.Pica-Mattoccia L, Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. International Journal for Parasitology. 2004;34:527–33. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Redman CA, Robertson A, Fallon PG, Modha J, Kusel JR, Doenhoff MJ, Martin RJ. Praziquantel: an urgent and exciting challenge. Parasitology Today. 1996;12:14–20. doi: 10.1016/0169-4758(96)80640-5. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg RM. Are Ca2+ channels targets of praziquantel action? International Journal for Parasitology. 2005;35:1–9. doi: 10.1016/j.ijpara.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Current Opinion in Infectious Diseases. 2008;21:659–67. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- 20.Pica-Mattoccia L, Orsini T, Basso A, Festucci A, Liberti P, Guidi A, Marcatto-Maggi AL, Nobre-Santana S, Troiani AR, Cioli D, Valle C. Schistosoma mansoni: lack of correlation between praziquantel-induced intra-worm calcium influx and parasite death. Experimental Parasitology. 2008;119:332–5. doi: 10.1016/j.exppara.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y, Platzer EG, Bellier A, Aroian RV. Discovery of a highly synergistic anthelmintic combination that shows mutual hypersusceptibility. Proceedings of the National Academy of Sciences. 2010;107:5955–60. doi: 10.1073/pnas.0912327107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geary TG, Woo K, McCarthy JS, Mackenzie CD, Horton J, Prichard RK, de Silva NR, Olliaro PL, Lazdins-Helds JK, Engels DA, Bundy DA. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. International Journal for Parasitology. 2010;40:1–13. doi: 10.1016/j.ijpara.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Lespine A, Alvinerie M, Vercruysse J, Prichard RK, Geldhof P. ABC transporter modulation: a strategy to enhance the activity of macrocyclic lactone anthelmintics. Trends in Parasitology. 2008;24:293–8. doi: 10.1016/j.pt.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Liang XJ, Aszalos A. Multidrug transporters as drug targets. Current Drug Targets. 2006;7:911–21. doi: 10.2174/138945006778019264. [DOI] [PubMed] [Google Scholar]
- 25.Leprohon P, Legare D, Ouellette M. ABC transporters involved in drug resistance in human parasites. Essays in Biochemistry. 2011;50:121–44. doi: 10.1042/bse0500121. [DOI] [PubMed] [Google Scholar]
- 26.Lespine A, Menez C, Bourguinat C, Prichard RK. P-glycoproteins and other multidrug resistance transporters in the pharmacology of anthelmintics: prospects for reversing transport-dependent anthelmintic resistance. International Journal for Parasitology: Drugs and Drug Resistance. 2012;2:58–75. doi: 10.1016/j.ijpddr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasinathan RS, Greenberg RM. Pharmacology and potential physiological significance of schistosome multidrug resistance transporters. Experimental Parasitology. 2012;132:2–6. doi: 10.1016/j.exppara.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446:749–57. doi: 10.1038/nature05630. [DOI] [PubMed] [Google Scholar]
- 29.Pommier Y, Pourquier P, Urasaki Y, Wu J, Laco GS. Topoisomerase I inhibitors: selectivity and cellular resistance. Drug Resistance Updates. 1999;2:307–18. doi: 10.1054/drup.1999.0102. [DOI] [PubMed] [Google Scholar]
- 30.Pommier Y, Sordet O, Antony S, Hayward RL, Kohn KW. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene. 2004;23:2934–59. doi: 10.1038/sj.onc.1207515. [DOI] [PubMed] [Google Scholar]
- 31.Blackmore CG, McNaughton PA, Van Veen HW. Multidrug transporters in prokaryotic and eukaryotic cells: physiological functions and transport mechanisms. Molecular Membrane Biology. 2001;18:97–103. doi: 10.1080/09687680010030200. [DOI] [PubMed] [Google Scholar]
- 32.Paulsen IT. Multidrug efflux pumps and resistance: regulation and evolution. Current Opinion in Microbiology. 2003;6:446–51. doi: 10.1016/j.mib.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 33.van Veen HW. Structural biology: last of the multidrug transporters. Nature. 2010;467:926–7. doi: 10.1038/467926a. [DOI] [PubMed] [Google Scholar]
- 34.Ventner H, Shahi S, Balakrishnan L, Velamakanni S, Bapna A, Woebking B, van Veen HW. Similarities between ATP-dependent and ion-coupled multidrug transporters. Biochemical Society Transactions. 2005;33:1008–11. doi: 10.1042/BST20051008. [DOI] [PubMed] [Google Scholar]
- 35.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annual Review of Biochemistry. 2002;71:537–92. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 36.Dassa E, Bouige P. The ABC of ABCs: a phylogenetic and functional classification of ABC systems in living organisms. Research in Microbiology. 2001;152:211–29. doi: 10.1016/s0923-2508(01)01194-9. [DOI] [PubMed] [Google Scholar]
- 37.Saier MH, Paulsen IT. Phylogeny of multidrug transporters. Seminars in Cell & Developmental Biology. 2001;12:205–13. doi: 10.1006/scdb.2000.0246. [DOI] [PubMed] [Google Scholar]
- 38.Hyde SC, Emsley P, Hartshorn MJ, Mimmack MM, Gileadi U, Pearce SR, Gallagher MP, Gill DR, Hubbard RE, Higgins CF. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990;346:362–5. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- 39.Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–85. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- 40.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nature Reviews: Drug Discovery. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 41.Sheps JA, Ralph S, Zhao ZY, Baillie DL, Ling V. The ABC transporter gene family of Caenorhabditis elegans has implications for the evolutionary dynamics of multidrug resistance in eukaryotes. Genome Biol. 2004;5:R15. doi: 10.1186/gb-2004-5-3-r15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Research. 2001;11:1156–66. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 43.Annilo T, Chen ZQ, Shulenin S, Constantino J, Thomas L, Lou H, Stefanov S, Dean M. Evolution of the vertebrate ABC gene family: analysis of gene birth and death. Genomics. 2006;88:1–11. doi: 10.1016/j.ygeno.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Dean M, Allikmets R. Complete characterization of the human ABC gene family. Journal of Bioenergetics and Biomembranes. 2001;33:475–9. doi: 10.1023/a:1012823120935. [DOI] [PubMed] [Google Scholar]
- 45.Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annual Review of Genomics and Human Genetics. 2005;6:123–42. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- 46.van Veen HW, Callaghan R, Soceneantu L, Sardini A, Konings WN, Higgins CF. A bacterial antibiotic-resistance gene that complements the human multidrug-resistance P-glycoprotein gene. Nature. 1998;391:291–5. doi: 10.1038/34669. [DOI] [PubMed] [Google Scholar]
- 47.Kartner N, Riordan JR, Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983;221:1285–8. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- 48.de Graaf D, Sharma RC, Mechetner EB, Schimke RT, Roninson IB. P-glycoprotein confers methotrexate resistance in 3T6 cells with deficient carrier-mediated methotrexate uptake. Proceedings of the National Academy of Sciences. 1996;93:1238–42. doi: 10.1073/pnas.93.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pohl A, Lage H, Muller P, Pomorski T, Herrmann A. Transport of phosphatidylserine via MDR1 (multidrug resistance 1) P-glycoprotein in a human gastric carcinoma cell line. Biochemical Journal. 2002;365:259–68. doi: 10.1042/BJ20011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizutani T, Masuda M, Nakai E, Furumiya K, Togawa H, Nakamura Y, Kawai Y, Nakahira K, Shinkai S, Takahashi K. Genuine functions of P-glycoprotein (ABCB1) Current Drug Metabolism. 2008;9:167–74. doi: 10.2174/138920008783571756. [DOI] [PubMed] [Google Scholar]
- 51.Bosch I, Dunussi-Joannopoulos K, Wu RL, Furlong ST, Croop J. Phosphatidylcholine and phosphatidylethanolamine behave as substrates of the human MDR1 P-glycoprotein. Biochemistry. 1997;36:5685–94. doi: 10.1021/bi962728r. [DOI] [PubMed] [Google Scholar]
- 52.Aye ILMH, Singh AT, Keelan JA. Transport of lipids by ABC proteins: interactions and implications for cellular toxicity, viability, and function. Chemico-Biological Interactions. 2009;180:327–39. doi: 10.1016/j.cbi.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 53.Pendse SS, Behjadi S, Schatton T, Izawa A, Sayegh MH, Frank MH. P-glycoprotein functions as a differentiation switch in antigen presenting cell maturation. American Journal of Transplatation. 2006;6:2884–93. doi: 10.1111/j.1600-6143.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- 54.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–22. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin MS, Oldham ML, Zhang Q, Chen J. Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature. 2012;490:566–9. doi: 10.1038/nature11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bosch IB, Wang ZX, Tao LF, Shoemaker CB. Two Schistosoma mansoni cDNAs encoding ATP-binding cassette (ABC) family proteins. Molecular and Biochemical Parasitology. 1994;65:351–6. doi: 10.1016/0166-6851(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 57.Kasinathan RS, Goronga T, Messerli SM, Webb TR, Greenberg RM. Modulation of a Schistosoma mansoni multidrug transporter by the antischistosomal drug praziquantel. FASEB Journal. 2010;24:128–35. doi: 10.1096/fj.09-137091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Higgins CF, Gottesman MM. Is the multidrug transporter a flippase? Trends in Biochemical Sciences. 1992;17:18–21. doi: 10.1016/0968-0004(92)90419-a. [DOI] [PubMed] [Google Scholar]
- 59.Sharom FJ. The P-glycoprotein multidrug transporter. Essays in Biochemistry. 2011;50:161–78. doi: 10.1042/bse0500161. [DOI] [PubMed] [Google Scholar]
- 60.Gimenez-Bonafe P, Guillen Canovas A, Ambrosio S, Tortosa A, Perez-Tomas R. In: Drugs modulating MDR. Colabufo NA, editor. Kerala, India: Research Signpost; 2008. pp. 63–99. [Google Scholar]
- 61.Leier I, Jedlitschky G, Buccholz U, Cole SP, Deeley RG, Keppler D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. Journal of Biological Chemistry. 1994;269:27807–10. [PubMed] [Google Scholar]
- 62.van de Ven R, Oerlemans R, van der Heijden JW, Scheffer GL, de Gruijl TD, Jansen G, Scheper RJ. ABC drug transporters and immunity: novel therapeutic targets in autoimmunity and cancer. Journal of Leukocyte Biology. 2009;86:1075–87. doi: 10.1189/jlb.0309147. [DOI] [PubMed] [Google Scholar]
- 63.van de Ven R, Scheffer GL, Scheper RJ, de Gruijl LD. The ABC of dendritic cell development and function. Trends in Immunology. 2009;30:421–9. doi: 10.1016/j.it.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 64.Doyle L, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22:7340–58. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- 65.Mealey KL, Bentjen SA, Gay JM, Cantor GH. Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics. 2001;11:727–33. doi: 10.1097/00008571-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 66.Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP, Berns AJM, Borst P. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 67.Hayeshi R, Masimirembwa C, Mukanganyama S, Ungell AL. The potential inhibitory effect of antiparasitic drugs and natural products on P-glycoprotein mediated efflux. European Journal of Pharmaceutical Sciences. 2006;29:70–81. doi: 10.1016/j.ejps.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Quazi F, Molday RS. Lipid transport by mammalian ABC proteins. Essays in Biochemistry. 2011;50:265–90. doi: 10.1042/bse0500265. [DOI] [PubMed] [Google Scholar]
- 69.Borst P, Zelcer N, van Helvoort A. ABC transporters in lipid transport. Biochimica et Biophysica Acta. 2000;1486:128–44. doi: 10.1016/s1388-1981(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 70.Sundaram P, Echalier B, Han W, Hull D, Timmons L. ATP-binding cassette transporters are required for efficient RNA interference in Caenorhabditis elegans. Molecular Biology of the Cell. 2006;17:3678–88. doi: 10.1091/mbc.E06-03-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnstone RW, Ruefli AA, Smyth MJ. Multiple physiological functions for multidrug transporter P-glycoprotein? Trends in Biochemical Sciences. 2000;25:1–6. doi: 10.1016/s0968-0004(99)01493-0. [DOI] [PubMed] [Google Scholar]
- 72.Johnstone RW, Ruefli AA, Tainton KM, Smyth MJ. A role for P-glycoprotein in regulating cell death. Leukemia and Lymphoma. 2000;38:1–11. doi: 10.3109/10428190009060314. [DOI] [PubMed] [Google Scholar]
- 73.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nature Reviews: Cancer. 2010;10:147–56. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 74.Borst P, van Blitterswijk WJ, Borst J, Tepper AD, Schinkel AH. New physiological functions for drug-transporting P-glycoproteins? Drug Resistance Updates. 1998;1:337–9. doi: 10.1016/s1368-7646(98)80049-6. [DOI] [PubMed] [Google Scholar]
- 75.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiological Reviews. 2006;86:1179–236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 76.Jones PM, George AM. Multidrug resistance in parasites: ABC transporters, P-glycoproteins and molecular modelling. International Journal for Parasitology. 2005;35:555–66. doi: 10.1016/j.ijpara.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 77.Kerboeuf D, Blackball W, Kaminsky R, von Samson-Himmelstjerna G. P-glycoprotein in helminths: function and perspectives for anthelmintic treatment and reversal of resistance. International Journal of Antimicrobial Agents. 2003;22:332–46. doi: 10.1016/s0924-8579(03)00221-8. [DOI] [PubMed] [Google Scholar]
- 78.James CE, Hudson AL, Davey MW. Drug resistance mechanisms in helminths: is it survival of the fittest? Trends in Parasitology. 2009;25:328–35. doi: 10.1016/j.pt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 79.Zutz A, Gompf S, Schagger H, Tampe R. Mitochondrial ABC proteins in health and disease. Biochimica et Biophysica Acta. 2009;1787:681–90. doi: 10.1016/j.bbabio.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 80.Liesa M, Qiu W, Shirihai OS. Mitochondrial ABC transporters function: the role of ABCB10 (ABC-me) as a novel player in cellular handling of reactive oxygen species. Biochimica et Biophysica Acta. 2012;1823:1945–57. doi: 10.1016/j.bbamcr.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, Mashiyama ST, Al-Lazikani B, Andrade LF, Ashton PD, Aslett MA, Bartholomeu DC, Blandin G, Caffrey CR, Coghlan A, Coulson R, Day TA, Delcher A, DeMarco R, Djikeng A, Eyre T, Gamble JA, Ghedin E, Gu Y, Hertz-Fowler C, Hirai H, Hirai Y, Houston R, Ivens A, Johnston DA, Lacerda D, Macedo CD, McVeigh P, Ning Z, Oliveira G, Overington JP, Parkhill J, Pertea M, Pierce RJ, Protasio AV, Quail MA, Rajandream MA, Rogers J, Sajid M, Salzberg SL, Stanke M, Tivey AR, White O, Williams DL, Wormian J, Wu W, Zamanian M, Zerlotini A, Fraser-Liggett CM, Barrell BG, El-Sayed NM. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–8. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Protasio AV, Tsai IJ, Babbage A, Nichol S, Hunt M, Aslett MA, De Silva N, Velarde GS, Anderson TJ, Clark RC, Davidson C, Dillon GP, Holroyd NE, LoVerde PT, Lloyd C, McQuillan J, Oliveira G, Otto TD, Parker-Manuel SJ, Quail MA, Wilson RA, Zerlotini A, Dunne DW, Berriman M. A systematically improved high quality genome and transcriptome of the human blood fluke Schistosoma mansoni. PLoS Neglected Tropical Diseases. 2012;6:e1455. doi: 10.1371/journal.pntd.0001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–51. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim WS, Weickert CS, Garner B. Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. Journal of Neurochemistry. 2008;104:1145–66. doi: 10.1111/j.1471-4159.2007.05099.x. [DOI] [PubMed] [Google Scholar]
- 85.van Meer G, Halter D, Sprong H, Somerharju P, Egmond MR. ABC lipid transporters: Extruders, flippases, or flopless activators? FEBS Letters. 2006;580:1171–7. doi: 10.1016/j.febslet.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 86.Piehler AP, Ozcurumez M, Kaminski WE. A-subclass ATP-binding cassette proteins in brain lipid homeostasis and neurodegeneration. Frontiers in Psychiatry. 2012;3:17. doi: 10.3389/fpsyt.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumkate S, Chunchob S, Janvilisri T. Expression of ATP-binding cassette multidrug transporters in the giant liver fluke Fasciola gigantica and their possible involvement in the transport of bile salts and anthelmintics. Molecular and Cellular Biochemistry. 2008;317:77–84. doi: 10.1007/s11010-008-9833-2. [DOI] [PubMed] [Google Scholar]
- 88.Wilkinson R, Law CJ, Hoey EM, Fairweather I, Brennan GP, Trudgett A. An amino acid substitution in Fasciola hepatica P-glycoprotein from triclabendazole-resistant and triclabendazole-susceptible populations. Molecular and Biochemical Parasitology. 2012;186:69–72. doi: 10.1016/j.molbiopara.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 89.Olson PD, Zarowiecki M, Kiss F, Brehm K. Cestode genomics - progress and prospects for advancing basic and applied aspects of flatworm biology. Parasite Immunol. 2012;34:130–50. doi: 10.1111/j.1365-3024.2011.01319.x. [DOI] [PubMed] [Google Scholar]
- 90.Robb SM, Ross E, Sanchez Alvarado A. SmedGD: the Schmidtea mediterranea genome database. Nucleic Acids Research. 2008;36:599–606. doi: 10.1093/nar/gkm684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sato H, Kusel JR, Thornhill J. Excretion of fluorescent substrates of mammalian multidrug resistance-associated protein (MRP) in the Schistosoma mansoni excretory system. Parasitology. 2004;128:43–52. doi: 10.1017/s0031182003004177. [DOI] [PubMed] [Google Scholar]
- 92.Sato H, Kusel JR, Thornhill J. Functional visualization of the excretory system of adult Schistosoma mansoni by the fluorescent marker resorufin. Parasitology. 2002;125:527–35. doi: 10.1017/s0031182002002536. [DOI] [PubMed] [Google Scholar]
- 93.Kusel JR, McVeigh P, Thornhill JA. The schistosome excretory system: a key to regulation of metabolism, drug excretion and host interaction. Trends in Parasitology. 2009;25:353–8. doi: 10.1016/j.pt.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 94.Kusel JR, Oliveira FA, Todd M, Ronketti F, Lima SF, Mattos AC, Reis KT, Coelho PM, Thornhill JA, Ribeiro F. The effects of drugs, ions, and poly-l-lysine on the excretory system of Schistosoma mansoni. Memorias do Institute Oswaldo Cruz. 2006;101:293–8. doi: 10.1590/s0074-02762006000900046. [DOI] [PubMed] [Google Scholar]
- 95.Oliveira FA, Kusel JR, Ribeiro F, Coelho PM. Responses of the surface membrane and excretory system of Schistosoma mansoni to damage and to treatment with praziquantel and other biomolecules. Parasitology. 2006;132:321–30. doi: 10.1017/S0031182005009169. [DOI] [PubMed] [Google Scholar]
- 96.Couto FF, Coelho PM, Araujo N, Kusel JR, Katz N, Mattos AC. Use of fluorescent probes as a useful tool to identify resistant Schistosoma mansoni isolates to praziquantel. Parasitology. 2010;137:1791–7. doi: 10.1017/S003118201000065X. [DOI] [PubMed] [Google Scholar]
- 97.Kasinathan RS, Morgan WM, Greenberg RM. Schistosoma mansoni express higher levels of multidrug resistance-associated protein 1 (SmMRP1) in juvenile worms and in response to praziquantel. Molecular and Biochemical Parasitology. 2010;173:25–31. doi: 10.1016/j.molbiopara.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Messerli SM, Kasinathan RS, Morgan W, Spranger S, Greenberg RM. Schistosoma mansoni P-glycoprotein levels increase in response to praziquantel exposure and correlate with reduced praziquantel susceptibility. Molecular and Biochemical Parasitology. 2009;167:54–9. doi: 10.1016/j.molbiopara.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ismail M, Metwally A, Farghaly A, Bruce J, Tao LF, Bennett JL. Characterization of isolates of Schistosoma mansoni from Egyptian villagers that tolerate high doses of praziquantel. American Journal of Tropical Medicine and Hygiene. 1996;55:214–8. doi: 10.4269/ajtmh.1996.55.214. [DOI] [PubMed] [Google Scholar]
- 100.Walter M, Kuris A. US Patent Number 6,514,963 B2. Methods for the inhibition of egg production in trematodes. 2003
- 101.Safa AR. Photoaffinity labeling of the multidrug-resistance-related P-glycoprotein with photoactive analogs of verapamil. Proceedings of the National Academy of Sciences. 1988;85:187–91. doi: 10.1073/pnas.85.19.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang CP, Mellado W, Horwitz SB. Azidopine photoaffinity labeling of multidrug resistance-associated glycoproteins. Biochemical Pharmacology. 1988;37:1417–21. doi: 10.1016/0006-2952(88)90803-9. [DOI] [PubMed] [Google Scholar]
- 103.Cornwell MM, Pastan I, Gottesman MM. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. Journal of Biological Chemistry. 1987;262:2166–70. [PubMed] [Google Scholar]
- 104.Kasinathan RS, Morgan WM, Greenberg RM. Genetic knockdown and pharmacological inhibition of parasite multidrug resistance transporters disrupts egg production in Schistosoma mansoni. PLoS Neglected Tropical Diseases. 2011;5:e1425. doi: 10.1371/journal.pntd.0001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coley HM. Overcoming multidrug resistance in cancer: clinical studies of P-glycoprotein inhibitors. Methods in Molecular Biology. 2010;596:341–58. doi: 10.1007/978-1-60761-416-6_15. [DOI] [PubMed] [Google Scholar]
- 106.Shukla S, Wu CP, Ambudkar SV. Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges. Expert Opinion in Drug Metabolism and Toxicology. 2008;4:205–23. doi: 10.1517/17425255.4.2.205. [DOI] [PubMed] [Google Scholar]
- 107.Xu M, Molento M, Blackball W, Ribeiro P, Beech R, Prichard R. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Molecular and Biochemical Parasitology. 1998;91:327–35. doi: 10.1016/s0166-6851(97)00215-6. [DOI] [PubMed] [Google Scholar]
- 108.Molento MB, Prichard RK. Effects of the multidrug-resistance-reversing agents verapamil and CL 347,099 on the efficacy of ivermectin or moxidectin against unselected and drug-selected strains of Haemonchus contortus in jirds (Mertones unguiculatus) Parasitology Research. 1999;85:1007–11. doi: 10.1007/s004360050673. [DOI] [PubMed] [Google Scholar]
- 109.Bartley DJ, McAllister H, Bartley Y, Dupuy J, Menez C, Alvinerie M, Jackson F, Lespine A. P-glycoprotein interfering agents potentiate ivermectin susceptibility in ivermectin sensitive and resistant isolates of Teladorsagia circumcincta and Haemonchus contortus. Parasitology. 2009;136:1081–8. doi: 10.1017/S0031182009990345. [DOI] [PubMed] [Google Scholar]
- 110.Tompkins JB, Stitt LE, Morrissette AM, Ardelli BF. The role of Brugia malayi ATP-binding cassette (ABC) transporters in potentiating drug sensitivity. Parasitology Research. 2011;109:1311–22. doi: 10.1007/s00436-011-2378-4. [DOI] [PubMed] [Google Scholar]
- 111.Ardelli BF, Prichard RK. Inhibition of P-glycoprotein enhances sensitivity of Caenorhabditis elegans to ivermectin. Veterinary Parasitology. 2013;191:264–75. doi: 10.1016/j.vetpar.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 112.Demeler J, Krucken J, Algusbi S, Ramunke S, De Graef J, Kerboeuf D, Geldhof P, Pomroy WE, von Samson-Himmelstjerna G. Potential contribution of P-glycoproteins to macrocyclic lactone resistance in the cattle parasitic nematode Cooperia oncophora. Molecular and Biochemical Parasitology. 2013 doi: 10.1016/j.molbiopara.2013.01.004. in press. [DOI] [PubMed] [Google Scholar]
- 113.Lambert C, Leonard N, De Bolle X, Depiereux E. ESyPred3D: Prediction of proteins 3D structures. Bioinformatics. 2002;18:1250–6. doi: 10.1093/bioinformatics/18.9.1250. [DOI] [PubMed] [Google Scholar]