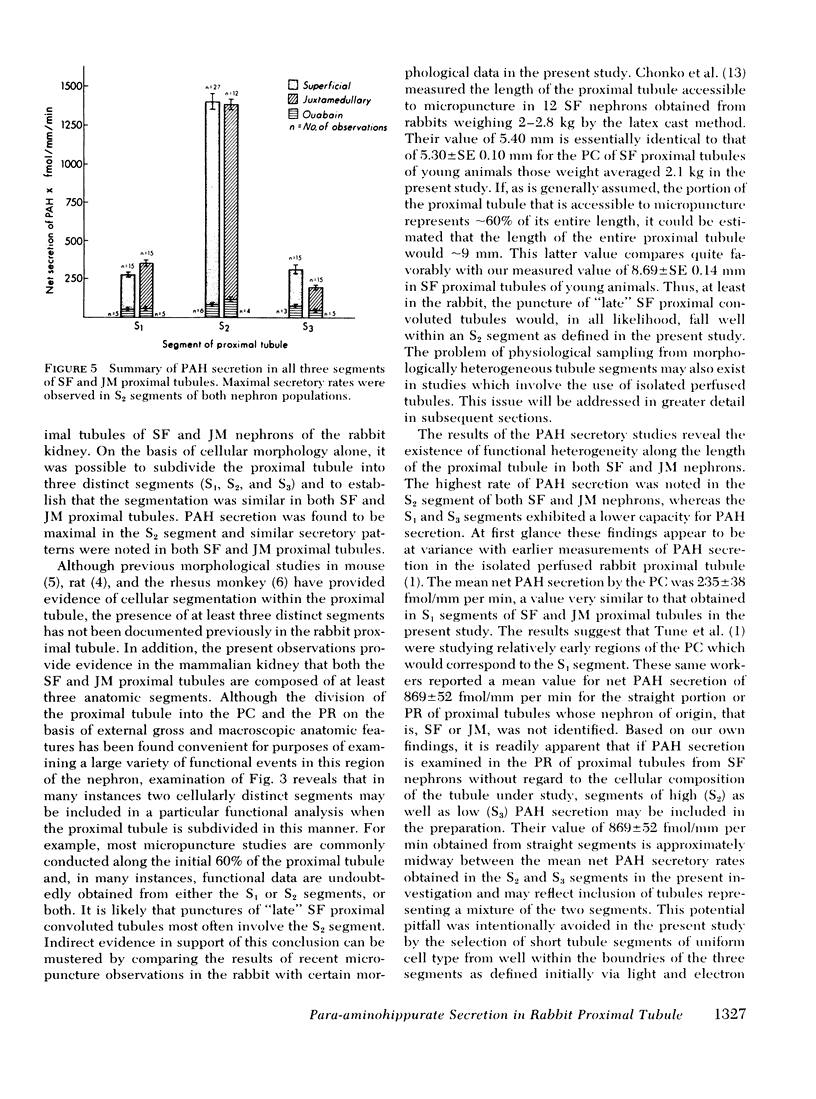
Abstract
Previous studies in the mammalian proximal tubule have suggested that para-aminohippurate (PAH) secretion is ∼threefold greater in the straight segment, or pars recta, than in the convoluted segment, or pars convoluta. However, the possibility that the site of maximal PAH secretion might be related better to particular tubule segments as identified by cell type had not been explored. In addition, the presence or absence of differences in PAH secretion between morphologically identical regions of superficial (SF) vs. juxtamedullary (JM) proximal tubules has not been examined. These issues were studied using a combination of histologic methods and measurement of [3H]PAH secretion in isolated perfused tubules. Measurements of microdissected SF and JM proximal tubules from young and adult rabbits revealed that SF proximal tubules were slightly but significantly longer than JM tubules ([young rabbits: SF, 8.69±SE 0.14 mm vs. JM, 7.97±SE 0.13 mm; P < 0.01] [adult rabbits: SF, 10.61±SE 0.28 mm; JM, 9.17±SE 0.19 mm; P < 0.001]). Light and electron microscopy revealed three sequential segments (S1, S2, and S3) along the length of SF and JM proximal tubules as defined by cell type. PAH secretion was measured in each of these three segments by the isolated perfused tubule technique. Net PAH secretion in fmol/mm per min in SF proximal tubules was: S1, 281±SE 21; S2, 1,508±SE 104; S3, 318±SE 46. Corresponding values in JM proximal tubules were 353±SE 31, 1,391±SE 72, and 188±SE 23. Net PAH secretion did not differ between comparable segments of SF and JM proximal tubules. It is concluded that differences in PAH secretion along the proximal tubule correlate best with cell type rather than the arbitrary division of the proximal tubule into pars convoluta and pars recta according to its external configuration. Evidence of functional heterogeneity between comparable segments of SF and JM proximal tubules was not observed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen F., Tisher C. C. Morphology of the ascending thick limb of Henle. Kidney Int. 1976 Jan;9(1):8–22. doi: 10.1038/ki.1976.2. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Chonko A. M., Osgood R. W., Nickel A. E., Ferris T. F., Stein J. H. The measurement of nephron filtration rate and absolute reabsorption in the proximal tubule of the rabbit kidney. J Clin Invest. 1975 Jul;56(1):232–235. doi: 10.1172/JCI108073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson H. R., Kokko J. P. Intrinsic differences in various segments of the proximal convoluted tubule. J Clin Invest. 1976 Apr;57(4):818–825. doi: 10.1172/JCI108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S., Imai M., Seldin D. W., Kukko J. P. Characteristics of salt and water transport in superficial and juxtamedullary straight segments of proximal tubules. J Clin Invest. 1975 Jun;55(6):1269–1277. doi: 10.1172/JCI108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsbach A. B. Observations on the segmentation of the proximal tubule in the rat kidney. Comparison of results from phase contrast, fluorescence and electron microscopy. J Ultrastruct Res. 1966 Oct;16(3):239–258. doi: 10.1016/s0022-5320(66)80060-6. [DOI] [PubMed] [Google Scholar]
- Sch0nheyder H. C., Maunsbach A. B. Ultrastructure of a specialized neck region in the rabbit nephron. Kidney Int. 1975 Mar;7(3):145–153. doi: 10.1038/ki.1975.22. [DOI] [PubMed] [Google Scholar]
- Tisher C. C., Clapp J. R. Intraluminal latex injection: an aid to the histological identification of renal tubules. Kidney Int. 1972 Jul;2(1):54–56. doi: 10.1038/ki.1972.69. [DOI] [PubMed] [Google Scholar]
- Tisher C. C., Rosen S., Osborne G. B. Ultrastructure of the proximal tubule of the rhesus monkey kidney. Am J Pathol. 1969 Sep;56(3):469–517. [PMC free article] [PubMed] [Google Scholar]
- Tune B. M., Burg M. B., Patlak C. S. Characteristics of p-aminohippurate transport in proximal renal tubules. Am J Physiol. 1969 Oct;217(4):1057–1063. doi: 10.1152/ajplegacy.1969.217.4.1057. [DOI] [PubMed] [Google Scholar]
- Warnock D. G., Burg M. B. Urinary acidification: CO2 transport by the rabbit proximal straight tubule. Am J Physiol. 1977 Jan;232(1):F20–F25. doi: 10.1152/ajprenal.1977.232.1.F20. [DOI] [PubMed] [Google Scholar]
- Woodhall P. B., Tisher C. C. Response of the distal tubule and cortical collecting duct to vasopressin in the rat. J Clin Invest. 1973 Dec;52(12):3095–3108. doi: 10.1172/JCI107509. [DOI] [PMC free article] [PubMed] [Google Scholar]