Abstract
In the late 60th - early 70th of the last century Prof. IP Lapin (June 26, 1930 – August 23, 2012) suggested that “intensification of central serotoninergic processes is a determinant of the thymoleptic (mood elevating) component” while “activation of noradrenergic processes is responsible for psychoenergetic and motor-stimulating component of the clinical antidepressant effect”. He suggested that in depression cortisol-inducible activation of liver enzyme, tryptophan 2,3-dioxygenase (TDO), shunted “metabolism of tryptophan away from serotonin production towards kynurenine production” leading to serotonin deficiency. He was the first to suggest and discover that kynurenine and its metabolites affect brain functions, and propose the role of neurokynurenines in pathogenesis of depression and action mechanisms of antidepressant effect. Further major developments of serotonin-kynurenine hypothesis include the discovery of antidepressant and cognition-enhancing effects of post-serotonin metabolite, N-acetylserotonin, an agonist to tyrosine kinase B(TrkB) receptors of brain derived neurotrophic factor. The discovery of indoleamine 2,3–dioxygenase (IDO), another rate-limiting enzyme of TRY – KYN metabolism, located in brain and inducible by pro-inflammatory cytokines, suggested the link between depression and aging/aging-associated medical (e.g., insulin resistance, obesity, cardiovascular), psychiatric (e.g., vascular cognitive impairment) and other disorders associated with chronic inflammation (e.g., hepatitis virus C, psoriasis) disorders.
A.Historical perspective
A1.Serotoninhypothesis
The discovery of the tricyclic antidepressants by R. Kuhn [1] in 1958 stimulated the research of the biological causes of depression and action mechanisms of antidepressant effect. Most researchers by the 1960s considered the noradrenaline involvement in both pathogenesis of depression and action mechanisms of antidepressants proposed in 1965 [2,3]. The serotonin hypothesis of depression was championed only byvery few psychiatrists, such as A. Coppen in England. Heoriginally suggested that “low” serotonin (but not noradrenaline) causes depression whileclinical antidepressant effect is mediated by the increase of serotonin (but not of noradrenaline) [4].
I. Lapin and his co-workers were the first to demonstrate that imipramine potentiated the central effects of serotonin (but not of noradrenaline)injected into the amygdala of cats [5,6]. To further investigate the role of serotonin in the effects of antidepressants, I. Lapin used frog (whose brain contains mostly serotonin but not noradrenaline) to observe behavioral effects specific for serotonin(in rodents serotonin effects are “masked” by noradrenaline). He found that behavioral effects of serotonin in frog were potentiated by tertiary (predominantly serotonin uptake inhibitors) (e.g., imipramine) but not by secondary (predominantly noradrenaline uptake inhibitors) (e.g., desipramine) tricyclic antidepressants[7,8].Considering that predominant clinical effect of tertiary tricyclic antidepressants is improvement of mood while that of secondary tricyclics is psychomotor stimulation, I. Lapin suggested that “intensification of central serotoninergic processes is a determinant of the thymoleptic (mood elevating) component” while “activation of noradrenergic processes isresponsible for psychoenergetic and motor-stimulating component of the clinical antidepressant effect”[9].A. Carlsson and his research team arrived to the same conclusion based on their finding that tertiary (but not secondary) tricyclic antidepressants attenuated the depletion of intraneuronal brain serotonin stores caused by 4-methyl-alpha-ethyl-meta-tyramine [10].
A.2.Tryptophan shunt towards formation of kynurenineas a cause of serotonin deficiency
As to the role of serotonin in the pathogenesis of depression, Prof. Lapin proposed that serotonin deficiency in depression was caused by the “shunt” of tryptophan(TRP) metabolism from formation of serotonin (along the methoxyindole pathway of TRP metabolism) towards production of kynurenine(KYN). The shunt was suggested to be caused by the activation of the rate-limiting enzyme of TRP – KYN pathway, TRP 2,3dioxygenase(TDO), activated by cortisol.Since production of cortisol by adrenal gland is inhibited by serotonin in brain (`amygdaloid complex”), it was proposed that serotonin deficiency resulted indis-inhibition of brain-adrenal axis, and, consequently, inincreased production of cortisol, the super-induction of TDO, and shifting of TRP metabolism from serotonin to KYN pathways, i.e., “vicious cycle” sustaining serotonin deficiency [9].(This 1969 Lancet paper was recognized as a Citation Classic).
A.3.Kynurenine hypothesis
I. Lapin formulated a new, “kynurenine”, hypothesis of depression [11].The “kynurenine” hypothesis (in difference with “serotonin” hypotheses) [4,9,10] concentrated on the role of elevated KYN and its derivatives for pathogenesis of depression and action mechanisms of antidepressive effect. He suggested that KYN and its derivatives [that were in late 60thconsidered only as intermediate products of nicotinamide adenine dinucleotide (NAD) formation from TRP] have their own biological activity [11]. I. Lapin discovered that KYN and its derivatives participate in regulation of blood pressure [12,13], body temperature [11], seizure activity [14 – 19], and anxiety [20, 21]. He introduced the term “neurokynurenines” (NEKY) and suggested that NEKY are the “participants of depression” and the“common neurochemical links of stress and anxiety” [11, 22].
It is noteworthy that I. Lapin found that kynurenine, quinolinic acid and 3-hydroxykynurenine exerted an anxiogenic while KYNA exerted anxiolitic activity in the standard animal models of anxiety [21]. He discovered the antagonistic relationships (e.g., pro-convulsive VS anti-convulsive and anxiogenic VS anxiolitic) between kynurenic acid (KYNA) and major N-methyl-D-aspartate (NMDA) agonists (e.g., quinolinic and picolinic acids [21]. (According to current understanding,KYNA is NMDA and alpha7nACh antagonist while most of the rest of KYN derivatives are NMDA agonists [23]. Since KYN attenuated serotonin effects (including activation of serotonin uptake by blood platelets), I. Lapin suggested that increased formation of NEKY might contribute to the mechanisms of resistance to antidepressants [24].
Thus, the studies of I Lapin and his research team initiated in the late 60-th – early 70-th of the last century established 1).Association of serotonin with mood elevating (thymoleptic) component of clinical antidepressant effect [9]; 2).Shunt of TRP metabolism towards formation of KYN causing deficiency of serotonin [9]; and 3). Role of NEKY in pathogenesis of depression and anxiety and action mechanisms of antidepressants [11,22].
B.Recent developments
B1.” N-acetylation” of serotonin hypothesis
B1a.Biological activities of N-acetylserotonin
Under physiological conditions only about 1 – 5% of TRY is metabolized to 5-HT [25]. Up-regulation of TRY – KYN metabolism affects not only TRP – KYN but methoxyindoles pathway as well due to competition for TRP as a common substrate for both pathways. The first steps of the methoxyindoles pathway of TRP metabolism includes formation of serotonin that served as a substrate for melatonin biosynthesis via formation of N-acetylserotonin (NAS). Therefore, deficiency of serotonin caused by TRP shunt to KYN productionmight impact not only the functions of serotonin receptors but formation of NAS and melatonin. Melatonin biosynthesis follows circadian pattern in many species [26], including humans [27,28], and its deficiency contributes to disturbances of sleep and circadian rhythms in depression [29]. The first step of melatonin biosynthesis from serotonin is formation of NAScatalyzed by serotonin-N-acetyltransferase (SNAT)[30]. For many years NAS was considered only as an intermediate product (and immediate precursor) of melatonin biosynthesis from serotonin. G. Brown and his team were the first to suggest that NAS may have a role in the central nervous system distinct from that of being a precursor for melatonin. This hypothesis was based on the immunohistochemical identification of NAS in specific brain areas separate from melatonin and serotonin (e.g., brain stem, cerebellum and hippocampus, and within the reticular formation nuclei and motor nuclei of the brain stem); on the inhibitory action of NAS on glutamate induced firing of pyramidal cells; and on its analgesic effect (separate from melatonin and serotonin) [31–34].
B1b.NAS and antidepressant effect
We were the first to observeantidepressant-like effect of NAS in a mice tail-suspension test [35]. NAS antidepressant-like effectwas confirmed in a rat forced swim test [36]. Literature data and our studies revealed NAS involvement in action mechanisms of antidepressants. Selective tricyclic antidepressants and serotonin uptake inhibitors increased SNAT mRNA expression [37]. Our in vivo and in vitro experiments revealed that acute administration of irreversible (clorgyline) and reversible (brofaromine, beflaxotone, moclobemide) selective monoamine oxidase (MAO) type A inhibitors (but not of highly selective MAO-B inhibitors) suppressed MAO-A activity and stimulated pineal NAS biosynthesis. The effect of MAO-A inhibitors is strain (spontaneously hypertensive rats > Fisher344N >Wistar Kyoto > Sprague-Dawley) and gender (male > female) dependent. Considering that MAO-A but not MAO-B inhibitors exert a clinically antidepressant effect, we suggested that stimulation of NAS biosynthesis contributes to the antidepressant effect of MAO-A inhibitors. Antidepressant and selective MAO-A inhibitor, clorgyline, stimulates rat pineal NAS biosynthesis by preserving MAO-A substrates (serotonin and NA) from deamination. Reversible MAO-A inhibitors, brofaromine and beflaxotone, stimulated pineal NAS production as well [38 – 41]. We reported that antidepressant, methylene blue, and other blue dyes, the selective MAO-A inhibitors [42] stimulate pineal NAS production [43]. Acute administration of MAO-B type inhibitor, deprenyl (not exerting an antidepressant effect), did not stimulate rat pineal NAS biosynthesis. However, chronic (6 months) injections of low dose of deprenyl, an irreversible MAO-B inhibitor, inhibits MAO-A and stimulated pineal NAS production but only during the dark phase [44,45].
The role of MAO inhibition in NAS metabolism might be further supported by the study of the effect of bioprecursor of MAO inhibitor on rat pineal NAS. Serotonin conversion into NAS (and melatonin) occurs mostly (but not exclusively) in the pineal gland located in the brain but outside of blood brain barrier (BBB). The bioprecursor amino acid (MDL 72394) is converted into the irreversible selective MAO-A inhibitor (MDL 72392) by aromatic L-amino acid decarboxylase (AADC). Pretreatment with carbidopa, a peripheral AADC inhibitor, which does not penetrate BBB, prevents the liberation of the MAO-A inhibitor outside the BBB and results in exclusive inhibition of brain MAO-A. We found that systemic administration of MDL 72394 (0.5 mg/kg) stimulated rat pineal NAS and melatonin biosynthesis. Carbidopa, in a dose-dependent manner, attenuated or completely prevented MDL-induced stimulation of NAS and melatonin biosynthesis in the pineal gland located outside of BBB) [46].
B1c.Possible mechanisms of the antidepressant effect of NAS
B1c1. NAS and QR2/MT3
Melatonin 3 type (MT-3) receptor has higher affinity to NAS than to melatonin, and was identified as the same protein as quinonereductase 2 (QR2) detoxifying and antioxidant enzyme [47]. We found that QR2/MT3 agonist 5-methoxycarbonylamino-N-acetyltryptamine (5MCA-NAT) decreased, while the QR2/MT3 antagonist prazosin increased the duration of immobility in the tail suspension test in C57BL/6 mice. Prazosin, in a dose that did not affect the duration of immobility, attenuated the antidepressant-like effect of NAS and 5MCA-NAT [48]. It is noteworthy that QR2/MT3 mediated effects appeared to be specific for antidepressant-like action of NAS since 5MCA-NAT did not affect NAS-induced protection against LPS toxicity [49].
B1c2.NAS as agonist to TrkB receptors
The most important step in establishing NAS biological activity independent from serotonin and melatonin is a recent discovery that NAS (but not serotonin or melatonin) is an agonist to tyrosine kinase B(TrkB) (but not TrkA or TrkC) receptors of brain derived neurotrophic factor (BDNF) [36,50]. Recent review indicates that activation of TrkB by NAS might contribute to antidepressant effects of NAS (and MAO-A inhibitors) [50]. Discovery of the TrkB-mediated antidepressant effect of NAS is in line with the hypothesis that loss of BDNF is directly involved in the pathophysiology of depression, and that its restoration may underlie the therapeutic efficacy of antidepressant treatment [51]. NAS-induced TrkB stimulation might be involved in several other than antidepressants effects of NAS reported by our team: cognition-enhancing [52], antihypertensive [53], prolongation of life span[54, 55], prevention of pathological opening of the mitochondrial permeability transition pores [56], protection from beta-amyloid toxicity [52] and inhibition of lipid peroxidation [57 –59].
B1d. NAS and “memory peptide”
Cognitive impairment is a common feature of depression. The involvement of NAS in cognition-enhancing effects was described by Satake& Morton [60] but went mostly underappreciated [61]. The idea that memories could be transferred from one organism to another by administration of a “trained” donor brain to a naive recipient seized both scientific and public attention in the 1960's and early 1970's[62]. One of the “memory peptide” candidates was identified in the brain extracts of rats trained with shock to avoid dark (hence, “scotophobin”) [63]. Scotophobin was found to be an effective inhibitor (KI50, 6 × 10(−7) M) of purified bovine HIOMT, the enzyme, converting NAS in to melatonin. The finding that scotophobin inhibits HIOMT suggested that NAS is a mediator of the memory-enhancing effect of scotophobin. Further experiments confirmed that scotophobin action required intact pineal, and that NAS induced dark avoidance in goldfish, as did S-adenosyl-homocysteine, another HIOMT inhibitor [60].
Therefore, the significance of TRP shunt towards KYN production and away from serotonin formation proposed by I.Lapin in 1969for pathogenesis of depression might not be limited to a creating of serotonin deficiency but resulting in deficiency of NAS as well [64].
B2. Kynurenine pathway
B2a.Tryptophan – kynurenine metabolism
The major non-protein route of TRP metabolism is formation of N-formyl-kynurenine with ensuing production of KYN, catalyzed by rate-limiting enzymes: inflammation-inducible indoleamine 2,3-dioxygenase (IDO) or stress and/or substrate-inducible TRP 2,3-dioxygenase (TDO) [25].
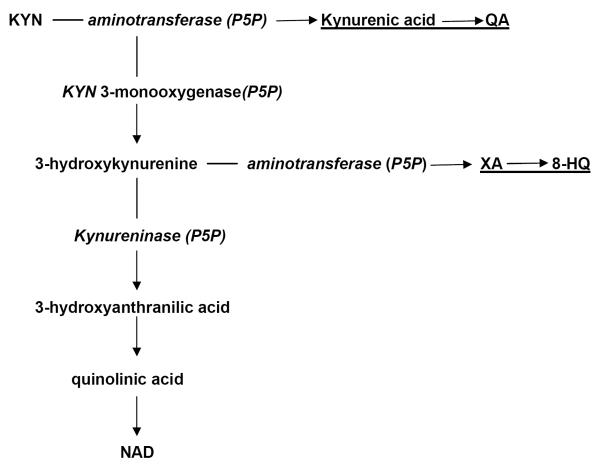
KYN, in its turn, serves as a substrate for two post-KYN pathways: 1). formation of KYNA, catalyzed by rate-limiting enzyme, KYN aminotransferase (KAT). and 2).formation of 3-hydroxyKYN (3-HK) catalyzed by KYN 3-monooxygenase (KMO) (Fig.2) [23].
Fig. 2.
Post-kynurenine metabolism and diabetogenic KYN derivatives.
Abbreviations: KYNA – kynureninc acid; XA – xanthurenic acid;P5P - pyridoxal 5'-phosphate;8-HQ - 8-Hydroxyquinaldic acid; QA - quinaldic acid; NAD - nicotinamide adenine dinucleotide.
The first step of 3-HK – NAD pathway, formation of 3-hydroxyantranilic acid, is catalyzed by kynureninase. Among the intermediate metabolites of KYN – NAD pathway are NMDA agonists (quinolinic and picolinic acids), free radical generator, 3-hydroxyanthranilic acid, and inducers of T-cell apoptosis and lipid peroxydation (KYN and 3-HK) [23]. One of the major metabolite of this pathway, 3-HK, is a potential neurotoxin in several neurodegenerative disorders [65]. Quinolinic and picolinic acids exerted anxiogenic effects in animal models [11, 21] probably, due to effect on benzodiazepines receptors [66].
B2b.Kynurenines and chronic inflammation
One of the limitations of serotonin – kynurenine hypothesis proposed by Lapin was the notion of TDO exclusive location in the liver excluding the possibility of formation of NEKY in the brain. (Recently TDO was found in the brain and suggested to be involved in the pathogenesis of schizophrenia [23]). The question was whether NEKY can penetrate BBB (some but not all, e.g., KYNA, do). The major breakthrough was achieved by the discovery of another enzyme converting TRP into KYN, indoleamine 2,3-dioxygenase, IDO [67]. IDO is located in brain (including micro- and astro-glia and dendrites), macrophages, kidney but not in liver; and has a broader substrate specificity than TDO [23].
Discovery of IDO contributed to the understanding of pathogenesis of depression as a side-effect of IFNG treatment of melanoma and hepatitis C virus patients [68]. Clinical and experimental studies revealed that development of depression (clinically almost identical to major depressive disorder) during the IFN-alpha treatment was mediated by IFN-induced up-regulated formation of NEKY [68]. Predisposition to the development of such a depression was associated with the presence of high producer allele (T) of IFNG (+874) T/A polymorphic gene that encodes the formation of IFNG protein (i.e., pro-inflammatory cytokine - inducer of IDO) [69].
B2c. TRP – KYN pathway as a link between depression and aging/aging-associated disorders
We suggested that up-regulation of TRP – KYN pathway is one of the mechanisms common for aging and aging-associated medical and psychiatric disorders (AAMPD) [70–72].
B2c1.Depression as aging-associated disorder
Aging is associated with up-regulation of TRP – KYN metabolism.[73].The increased di-oxygenation of mitochondrial TRP to N-formylKYN was consistently present among conserved biomarkers across ageing models in five species [74]. Literature and our studies indicate that genetically or pharmacologically induced inhibition of KYN formation from TRP prolonged life span in Drosophila model[75–79]. There are, at least, two possible mechanisms of aging-associated up-regulation of KYN formation from TRP: 1). Increase of cortisol production that induces TDO [73, 80]; and 2). Activation of IDO induced by pro-inflammatory cytokines (e.g., IFNG) due to aging-associated chronic inflammation [81].It is noteworthy that, besides IDO, IFNG stimulates key enzymes of KYN – NAD pathway, kynurenine mono-oxygenase and kynureninase macrophages involved in formation of NMDA agonists [23, 25].Aging-associated increase of incidence of depression and anxiety might be mediated by increased formation of NEKY and serotonin/NAS deficiency.Therefore, TRP–KYN is a common pathway for mediating genetic and environmental impacts in aging and major depressive disorder[82]
B2c2.Depression and insulin resistance
Many studies reported the association between insulin resistance (IR) (or diabetes type 2) and depression [83]. Some of these studies suggested that depression leads to diabetes. Thus, the prospective study of depressed patients without diabetes at the entry of the study observed the 65% increased risk of development of diabetes (mostly type 2) in clinically depressed patients [84]. The major diabetogenic derivative of KYN is xanthurenic acid (XA) formed from 3-HK. XA is further converted into diabetogenic8-Hydroxyquinaldic acid (8-HQ) [85] (Fig.2).KYNA is further converted into diabetogenicquinaldic acid (QA) [86]. Diabetogenic effects of XA, KYNA, QA and 8-HQ was demonstrated in clinical and experimental studies:XA induced experimental diabetes in rats;urine XA concentrations were higher in type 2 diabetes patients than in healthy subjects; XA may contribute to the development of IR by formation of chelate complexes with insulin (XA-In) with 49% lower activity in comparison with pure insulin;and XA may exert a toxic effect in isolated pancreatic islets because of formation of complexes with Zn++-ions in β-cells [87 –91].
XA, KYNA and their derivatives, QA and 8-HQ, inhibit pro-insulin synthesis in isolated rat pancreatic islets [92], and insulin release from rat pancreas [93).
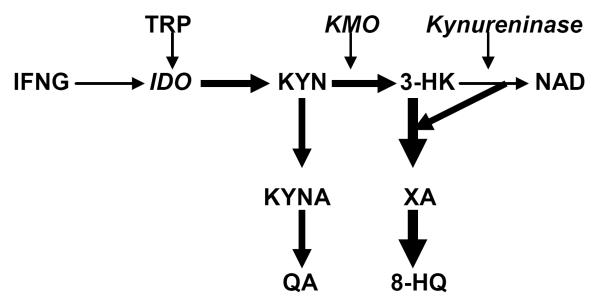
The key enzymes of post-KYN metabolism, KAT and kynureninaserequirepyridoxal 5'-phosphate(P5P) (an active metabolite of vitamin B6). The latter enzyme is particularly sensitive to dietary vitamin B-6 restriction [94]. P5P deficiency-induced Inhibition of kynureninase, induced by P5P deficiency, blocks 3-HK – NAD pathway and shifts 3-HK metabolism from production of NAD to production of XA (Fig.3)[94].
Fig.3.
Vitamin B6 deficiency-induced shift of post-KYN metabolism towards formation of diabetogenic KYN derivatives..
Abbreviations.TRP – tryptophan; IDO – indoleamine 2,3,-dioxygenase; KMO- KYN 3-monooxygenase;P5P - pyridoxal 5'-phosphate; KYN – kynurenine; 3OHKYN – 3-hydroxyKYN; QUIN – quinolinic acid; XA – xanthurenic acid; 3-HAA - 3-hydroxyanthranilic acid;NAD - nicotinamide adenine dinucleotide.
Depression is characterized by up-regulated production of XA [95] and P5P deficiency [96]. Thus, P5P deficiency might shift up-regulated TRP – KYN metabolism from formation of NAD to formation of diabetogenic KYN derivatives (e.g. XA and KYNA). Increased formation of diabetogenic KYN derivatives might contribute to increased incidence of IR (and type 2 diabetes) in depressed patients.
B2d.Kynurenines and oxidative stress
The major pro-inflammatory cytokine, IFNG, activates nitric oxide synthase (NOS), the key enzyme of NO biosynthesis from arginine. It was reported that this effect is mediated by kynurenines, quinolinic and picolinic acids [97].Concurrently with IDO activation, IDO transcriptionally induces guanine triphosphate cyclohydrolase (GTPCH), the key enzyme of pteridines biosynthesis, that results in increased formation of BH2 (and its stable metabolite, neopterin) and in decreased production of BH4, the obligatory cofactor of NOS [98]. Neopterin plasma concentrations strongly (r=0.65) and highly significantly (p=0.001) positively correlate with plasma KYN [99], and (negatively) with plasma P5P and IR [100]. We suggested that IFNG-induced concurrent up-regulation of production of kynurenines and BH2 leads to formation of an “inflammation cascade” that combines up-regulated activity of NOS (by kynurenines) with deficient availability of BH4 as NOS cofactor [70,71]. Such a combination results in an uncoupling of NOS and, consequently, in shifting of arginine metabolism away from the formation of NO and towards production of reactive oxygen species (ROS) such as superoxide anion and hydrogen peroxide [101,102]. Superoxide is a substrate for formation of one of the most aggressive free radicals, peroxynitrite (70,71). Therefore, up-regulation of TRP –KYN metabolism contributes to formation of both ROS and reactive nitrogen species, the major propagators of oxidative stress,
Peroxynitrite (as well as 3-HK and 3-hydroxyanthranilic acids, and neopterin and other pteridines), triggers phospholipase A2 – arachidonic acid – cyclooxygenase 2 - arachidonate 5-lipoxygenase metabolic pathway resulting in the increased production of inflammatory factors: prostaglandins, via activation of cycloxygenase (COX) and leucotrienes, via activation of arachidonate 5-lipoxygenase (5-LOX) [103]. The pathway of the inflammatory enzyme, 5-LOX, was suggested as a putative common mechanism that is affected by aging and may link depression and atherosclerosis [104].
Conclusions
Serotonin-kynurenine hypothesis of depression and action mechanisms of antidepressant effect proposed by I. Lapin dramatically improved our understanding of pathogenesis of depression and links between depression and medical conditions associated with chronic inflammation (including aging). KYN pathway of TRP metabolism could be considered as a new target for prevention and treatment of depression, and aging/aging-associated medical (e.g., IR, obesity, cardiovascular), psychiatric (e.g., vascular cognitive impairment) and other disorders associated with chronic inflammation (e.g., HCV infection [68, 69, 105], psoriasis [106, 107]) disorders.
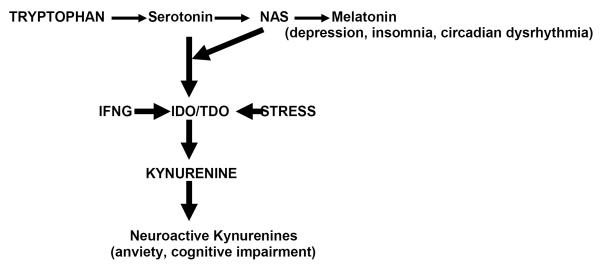
Fig.1.
Non-protein pathways of tryptophan metabolism and shunt to kynurenine formation in depression.
NAS – N-acetylserotonin; IFNG – interferon-gamma; IDO – indoleamine 2,3-dioxygenase; TDO – 2,3-dioxygenase tryptophan
Acknowledgments
G. F. Oxenkrug is recipient of NIH Grant MH083220.
Footnotes
Author declared no conflict of interest.
References
- [1].Kuhn R. The treatment of depressive states with G 22355 (imipramine hydrochloride) Am J Psychiatry. 1958;115:459–64. doi: 10.1176/ajp.115.5.459. [DOI] [PubMed] [Google Scholar]
- [2].Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–22. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- [3].Bunney WE, Jr, Davis JM. Norepinephrine in depressive reactions. Arch Gen Psychiatry. 1965;13:483–94. doi: 10.1001/archpsyc.1965.01730060001001. [DOI] [PubMed] [Google Scholar]
- [4].Coppen AJ. Depressed states and indolealkylamines. AdvPharmacol. 1968;6(Pt B):283–91. doi: 10.1016/s1054-3589(08)60328-2. [DOI] [PubMed] [Google Scholar]
- [5].Vakhing VA, Allikmets LH, Lapin IP. The development of vomiting under the influence of microinjections of serotonin into the hypothalamus, septum and amygdala of cats having previously received imipramine] BiullEkspBiol Med. 1968;66:48–51. [PubMed] [Google Scholar]
- [6].Allikmets LH, Vahing VA, Lapin IP. Dissimilar influences of imipramine, benactyzine and promazine on effects of micro-injections of noradrenaline, acetylcholine and serotonin into the amygdala in the cat. Psychopharmacologia. 1969;15:392–403. doi: 10.1007/BF00403714. [DOI] [PubMed] [Google Scholar]
- [7].Lapin IP, Oxenkrug GF, Osipova SV, Uskova NV. The frog as a subject for screening thymoleptic drugs. J Pharm Pharmacol. 1970;22:781–2. doi: 10.1111/j.2042-7158.1970.tb08429.x. [DOI] [PubMed] [Google Scholar]
- [8].Oxenkrug GF, Lapin IP. Effect of dimethyl and monomethyl tricyclic antidepressants on central 5-hydroxytryptamine processes in the frog. J Pharm Pharmacol. 1971;23:971–2. doi: 10.1111/j.2042-7158.1971.tb09905.x. [DOI] [PubMed] [Google Scholar]
- [9].Lapin IP, Oxenkrug GF. Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet. 1969;1:32–39. doi: 10.1016/s0140-6736(69)91140-4. [DOI] [PubMed] [Google Scholar]
- [10].Carlsson A, Corrodi H, Fuxe K, Hökfelt T. Effect of antidepressant drugs on the depletion of intraneuronal brain 5-hydroxytryptamine stores caused by 4-methyl-alpha-ethyl-meta-tyramine. Eur J Pharmacol. 1969;5:357–66. doi: 10.1016/0014-2999(69)90113-7. [DOI] [PubMed] [Google Scholar]
- [11].Lapin IP. Kynurenines as probable participants of depression. PharmakopsychiatrNeuropsychopharmakol. 1973;6:273–9. doi: 10.1055/s-0028-1094391. [DOI] [PubMed] [Google Scholar]
- [12].Lapin IP. Depressor effect of kynurenine and its metabolites in rats. Life Sci. 1976;19:1479–83. doi: 10.1016/0024-3205(76)90091-6. 1976. [DOI] [PubMed] [Google Scholar]
- [13].Oxenkrug GF. Kynurenine and hypotension: Historic perspectives. Critical Care Medicine. 2012;40:2006. doi: 10.1097/CCM.0b013e31824e1e3b. [DOI] [PubMed] [Google Scholar]
- [14].Lapin IP. Convulsions and tremor in immature rats after intraperitoneal injection of kynurenine and its metabolites. Pharmacol Res Commun. 1978;10:81–4. doi: 10.1016/s0031-6989(78)80065-4. [DOI] [PubMed] [Google Scholar]
- [15].Lapin IP. Stimulant and convulsive effects of kynurenines injected into brain ventricles in mice. J Neural Transm. 1978;42:37–43. doi: 10.1007/BF01262727. [DOI] [PubMed] [Google Scholar]
- [16].Lapin IP. Effect of kynurenine and quinolinic acid on the action of convulsants in mice. PharmacolBiochemBehav. 1980;13:17–20. [PubMed] [Google Scholar]
- [17].Lapin JP. Kynurenines and seizures. Epilepsia. 1981;22:257–65. doi: 10.1111/j.1528-1157.1981.tb04108.x. [DOI] [PubMed] [Google Scholar]
- [18].Milaśius AM, Grinevićius KK, Lapin IP. Effect of quinolinic acid on wakefulness and sleep in the rabbit. J Neural Transm Gen Sect. 1990;82:67–73. doi: 10.1007/BF01244835. [DOI] [PubMed] [Google Scholar]
- [19].Lapin IP, Mirzaev SM, Ryzov IV, Oxenkrug GF. Anticonvulsant activity of melatonin against seizures induced by quinolinate, kainate, glutamate, NMDA, and pentylenetetrazole in mice. J Pineal Res. 1998;24:215–8. doi: 10.1111/j.1600-079x.1998.tb00535.x. [DOI] [PubMed] [Google Scholar]
- [20].Orlikov AB, Prakhye IB, Ryzov IV. Kynurenine in blood plasma and DST in patients with endogenous anxiety and endogenous depression. Biol Psychiatry. 1994;36:97–102. doi: 10.1016/0006-3223(94)91189-4. [DOI] [PubMed] [Google Scholar]
- [21].Lapin IP. Antagonism of kynurenic acid to anxiogens in mice. Life Sci. 1998;63:231–6. doi: 10.1016/s0024-3205(98)00404-4. [DOI] [PubMed] [Google Scholar]
- [22].Lapin IP. Neurokynurenines (NEKY) as common neurochemical links of stress and anxiety. AdvExp Med Biol. 2003;527:121–5. doi: 10.1007/978-1-4615-0135-0_14. [DOI] [PubMed] [Google Scholar]
- [23].Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ]24].Lapin IP. Antagonism of nicotinic acid and other kynurenines to antidepressants: one of the probable reasons of the therapy-resistance depression? Act Nerv Super. 1974;16:260–1. [PubMed] [Google Scholar]
- [25].Oxenkrug GF. Genetic and hormonal regulation of the kynurenine pathway of tryptophan metabolism: new target for clinical intervention in vascular dementia, depression and aging Ann. N Y AcadSci. 2007;1122:35–49. doi: 10.1196/annals.1403.003. [DOI] [PubMed] [Google Scholar]
- [26].Míguez JM, Recio J, Sánchez-Barceló E, Aldegunde M. Changes with age in daytime and nighttime contents of melatonin, indoleamines, and catecholamines in the pineal gland: a comparative study in rat and Syrian hamster. J Pineal Res. 1998;25:106–15. doi: 10.1111/j.1600-079x.1998.tb00547.x. [DOI] [PubMed] [Google Scholar]
- [27].Oxenkrug GF, Anderson GF, Dragovic L, Blaivas M, Riederer P. Circadian rhythms of human pineal melatonin, related indoles, and beta adrenoreceptors: post-mortem evaluation. J. Pineal Res. 1990;9:1–11. doi: 10.1111/j.1600-079x.1990.tb00688.x. [DOI] [PubMed] [Google Scholar]
- [28].Skene DJ, Swaab DF. Melatonin rhythmicity: effect of age and Alzheimer's disease. ExpGerontol. 2003;38:199–206. doi: 10.1016/s0531-5565(02)00198-5. [DOI] [PubMed] [Google Scholar]
- [29].Zhdanova IV, Tucci V. Melatonin, Circadian Rhythms, and Sleep. Curr Treat Options Neurol. 2003;5:225–229. doi: 10.1007/s11940-003-0013-0. [DOI] [PubMed] [Google Scholar]
- [30].Weissbach H, Redfield BG, Axelrod J. Biosynthesis of melatonin: enzymic conversion of serotonin to N-acetylserotonin. BiochimBiophysActa. 1960;43:352–3. doi: 10.1016/0006-3002(60)90453-4. [DOI] [PubMed] [Google Scholar]
- [31].Brown GM, Pulido O, Grota LJ, Niles LP. N-Acetylserotonin in the central nervous system. ProgNeuropsychopharmacolBiol Psychiatry. 1984;8:475–80. doi: 10.1016/0278-5846(84)90003-4. [DOI] [PubMed] [Google Scholar]
- [32].Bubenik GA, Brown GM, Grota LG. Differential localization of N-acetylated indolealkylamines in CNS and the Harderian gland using immunohistology. Brain Res. 1976;118:417–427. doi: 10.1016/0006-8993(76)90309-7. [DOI] [PubMed] [Google Scholar]
- [33].Psarakis S, Brown GM, Grota LJ. Analgesia induced by N-acetylserotonin in the central nervous system. Life Sci. 1988;42:1109–1116. doi: 10.1016/0024-3205(88)90567-x. [DOI] [PubMed] [Google Scholar]
- [34].Pulido O, Brown GM, Grota LJ. B-adrenergic regulation of N-acetylserotonin (NAS) synthesis in the rat cerebellum. Life Sci. 1983;33:1081–9. doi: 10.1016/0024-3205(83)90664-1. [DOI] [PubMed] [Google Scholar]
- [35].Prakhie IV, Oxenkrug GF. The effect of nifedipine, Ca++ antagonist on activity of MAO inhibitors, N-acetylserotonin and melatonin in the mouse tail suspension test. J Neuropsychopharmac. 1988;1:35–40. doi: 10.1017/S1461145798001096. [DOI] [PubMed] [Google Scholar]
- [36].Jang SW, Liu X, Pradoldej S, Tosini G, Chang Q, Iuvone PM, Ye K. N-acetylserotonin activates TrkB receptor in a circadian rhythm. ProcNatlAcadSci U S A. 2010;107:3876–3881. doi: 10.1073/pnas.0912531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Uz T, Manev H. Chronic fluoxetine administration increases the serotonin N-acetyltransferase messenger RNA content in rat hippocampus. Biol Psychiatry. 1999;45:175–9. doi: 10.1016/s0006-3223(98)00032-8. [DOI] [PubMed] [Google Scholar]
- [38].Oxenkrug GF, McCauley R, McIntyre IM, Filipowicz C. Selective inhibition of MAO-A but not MAO-B activity increases rat pineal melatonin. J Neural Transm. 1985;61:265–70. doi: 10.1007/BF01251917. [DOI] [PubMed] [Google Scholar]
- [39].Oxenkrug GF. The acute effect of monoamine oxidase inhibitors on serotonin conversion to melatonin. In: Sandler M, Coppen A, Harnett S, editors. 5-hydroxytryptamine in Psychiatry: A spectrum of Ideas. Oxford University Press; Oxford New York Tokyo: 1991. pp. 98–109. [Google Scholar]
- [40].Oxenkrug GF, McIntyre IM, McCauley R, Yuwiler A. The effect of selective MAO inhibitors on rat pineal melatonin synthesis in vitro. J. Pineal Res. 1988;5:99–109. doi: 10.1111/j.1600-079x.1988.tb00772.x. [DOI] [PubMed] [Google Scholar]
- [41].Oxenkrug GF. Antidepressive and antihypertensive effects of MAO-A inhibition: role of N-acetylserotonin (a review) Neurobiology. 1999;7:213–224. [PubMed] [Google Scholar]
- [42].Sablin S, Ransay RR. Difference spectra for inhibitor binding to monoamine oxidases. BiochemSoc Trans. 1995;23:457S. doi: 10.1042/bst023457s. [DOI] [PubMed] [Google Scholar]
- [43].Oxenkrug GF, Sablin SO, Requintina PJ. Effect of methylene blue and related redox dyes on monoamine oxidase activity; rat pineal content of N-acetylserotonin, melatonin, and related indoles; and righting reflex in melatonin-primed frogs. Ann N Y AcadSci. 2007;1122:245–52. doi: 10.1196/annals.1403.017. [DOI] [PubMed] [Google Scholar]
- [44].Oxenkrug GF, Requintina PJ, Correa RM, Yuwiler A. The effect of 6-months l-deprenyl administration on pineal MAO-A and MAO-B activity and on the content of melatonin and related indoles in aged female Fisher 344N rats. J Neural TransmSuppl. 1994;41:249–52. doi: 10.1007/978-3-7091-9324-2_32. [DOI] [PubMed] [Google Scholar]
- [45].Oxenkrug GF. Anti-Aging Effect of (−) Deprenyl and Inhibition of Lipid Peroxidation by N-acetylserotonin and Mating (a mini-review) In: Torok T, Klebovich L, editors. Monoamine Oxidase inhibitors and their role in neurotransmission (drug development) Medicina Publishing House Co.; Budapest: 2004. pp. 309–319. [Google Scholar]
- [46].Oxenkrug GF, Requintina PJ, Yuwiler A, Palfreyman MG. The acute effect of the bioprecursor of the selective brain MAO-A inhibitor, MDL 72392, on rat pineal melatonin biosynthesis. J Neural Transm. 1994;41(Suppl):377–9. doi: 10.1007/978-3-7091-9324-2_50. [DOI] [PubMed] [Google Scholar]
- [47].Boutin JA, Marcheteau E, Hennig P, et al. MT3/QR2 melatonin binding site does not use melatonin as a substrate or a co-substrate. J Pineal Res. 2008;45:524–531. doi: 10.1111/j.1600-079X.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- [48].Oxenkrug GF, Bachurin SO, Prakhie IV, Zefirov N. Quinone reductase 2 and antidepressant effect of melatonin derivatives. Ann N Y AcadSci. 2010;1199:121–124. doi: 10.1111/j.1749-6632.2009.05354.x. [DOI] [PubMed] [Google Scholar]
- [49].Requintina PJ, Oxenkrug GF. Differential effects of lipopolysaccharide on lipid peroxidation in F344N, SHR rats and BALB/C mice and protection of melatonin and NAS against Its toxicity. Ann N Y AcadSci. 2003;993:325–333. doi: 10.1111/j.1749-6632.2003.tb07540.x. [DOI] [PubMed] [Google Scholar]
- [50].Tosini G, Ye K, Iuvone PM. N-Acetylserotonin: Neuroprotection, Neurogenesis, and the Sleepy Brain. May 14, 2012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sandler M. Neurotrophins: possible role in anxiety and affective disorders. Hum PsychopharmacolClinExp. 2001;16:61–64. doi: 10.1002/hup.184. [DOI] [PubMed] [Google Scholar]
- [52].Bachurin S, Oxenkrug G, Lermontova N, et al. N-acetylserotonin, melatonin and their derivatives improve cognition and protect against b-amyloid-induced neurotoxicity, Ann. New York AcadSci. 1999;890:155–166. doi: 10.1111/j.1749-6632.1999.tb07990.x. [DOI] [PubMed] [Google Scholar]
- [53].Oxenkrug GF. N-acetylserotonin and the hypotensive effect of MAO-A inhibition (mini-review) Vopr.Med. Khim. 1998;43:522–526. (Russian) [PubMed] [Google Scholar]
- [54].Oxenkrug GF. Antidepressant effect of N-acetylserotonin in relation to aging, hypertension and cancer. In: Berstein L, editor. Hormones, age and cancer. Nauka; St.Petersburg: 2005. pp. 140–158. [Google Scholar]
- [55].Oxenkrug GF, Requintina PJ, Bachurin S. Antioxidant and anti-aging activity of N-acetylserotonin in the in vivo and in vitro models. Ann. New York AcadSci. 2001;939:190–199. doi: 10.1111/j.1749-6632.2001.tb03626.x. [DOI] [PubMed] [Google Scholar]
- [56].Bachurin SO, Shevtsova EP, Oxenkrug GF, Sablin SO. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann. N Y AcadSci. 2003;993:334–344. doi: 10.1111/j.1749-6632.2003.tb07541.x. [DOI] [PubMed] [Google Scholar]
- [57].Oxenkrug GF. Antioxidant effects of N-acetylserotonin: possible mechanisms and clinical implications. Ann. N Y AcadSci. 2005;1053:334–347. doi: 10.1196/annals.1344.029. [DOI] [PubMed] [Google Scholar]
- [58].Perianayagam MC, Oxenkrug GF, Jaber BL. Immune-modulating effects of melatonin, N-acetylserotonin, and N-acetyldopamine. Ann N Y AcadSci. 2005;1053:386–93. doi: 10.1111/j.1749-6632.2005.tb00046.x. [DOI] [PubMed] [Google Scholar]
- [59].Oxenkrug GF, Requintina PJ. N-acetyldopamine inhibits rat brain lipid peroxidation induced by lipopolysaccharide and iron. Ann. N Y Acad. Sci. 2005;1053:394–399. doi: 10.1111/j.1749-6632.2005.tb00047.x. [DOI] [PubMed] [Google Scholar]
- [60].Satake N, Morton BE. Scotophobin A causes dark avoidance in goldfish by elevating pineal N-acetylserotonin. PharmacolBiochemBehav. 1979;10:449–456. doi: 10.1016/0091-3057(79)90216-8. [DOI] [PubMed] [Google Scholar]
- [61].Setlow B. GeorgesUngar and memory transfer. J HistNeurosci. 1997;6:181–92. doi: 10.1080/09647049709525701. [DOI] [PubMed] [Google Scholar]
- [62].Ungar G, Desiderio DM, Parr W. Isolation, identification, and synthesis of a specific-behavior-inducing brain peptide. Nature. 1972;238:198–202. doi: 10.1038/238198a0. [DOI] [PubMed] [Google Scholar]
- [63].Malin DH. Synthetic scotophobin: Analysis of behavioral effects on mice. Pharm BiochemBehav. 1974;2:147–153. doi: 10.1016/0091-3057(74)90046-x. [DOI] [PubMed] [Google Scholar]
- [64].Oxenkrug G, Ratner R. N-Acetylserotonin and Aging-Associated Cognitive Impairment and Depression. Aging and Disease. 2012;3:330–338. [PMC free article] [PubMed] [Google Scholar]
- [65].Campesan S, Green EW, Breda C, Sathyasaikumar KV, Muchowski PJ, Schwarcz R, et al. The kynurenine pathway modulates neurodegeneration in a Drosophila model of Huntington's disease. CurrBiol. 2001;21:961–6. doi: 10.1016/j.cub.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lapin IP. Structure-activity relationships in kynurenine, diazepam and some putative endogenous ligands of the benzodiazepine receptors. NeurosciBiobehav Rev. 1983;7:107–18. doi: 10.1016/0149-7634(83)90013-1. [DOI] [PubMed] [Google Scholar]
- [67].Hayaishi O. Properties and function of indoleamine 2,3- dioxygenase. Properties and function of indoleamine 2,3-dioxygenase. J Biochem (Tokyo) 1976;79:13P–21P. doi: 10.1093/oxfordjournals.jbchem.a131115. [DOI] [PubMed] [Google Scholar]
- [68].Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. PharmacolTher. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Oxenkrug G, Perianayagam M, Mikolich D, Requintina P, Shick L, et al. Interferon-gamma (+874) T/A genotypes and risk of IFN-alpha-induced depression. J Neural Transm. 2011;118:271–274. doi: 10.1007/s00702-010-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Oxenkrug GF. Interferon-gamma-inducible kynurenines/pteridines inflammation cascade: implications for aging and aging-associated medical and psychiatric disorders. J Neural Transm. 2011;118:75–85. doi: 10.1007/s00702-010-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Oxenkrug GF. Interferon-gamma – Inducible Inflammation: Contribution to Aging and Aging-Associated Psychiatric Disorders. Aging and Disease. 2011;2:474–486. [PMC free article] [PubMed] [Google Scholar]
- [72].Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan-kynurenine metabolism. Ann N Y AcadSci. 2010;1199:1–14. doi: 10.1111/j.1749-6632.2009.05356.x. [DOI] [PubMed] [Google Scholar]
- [73].Dilman VM, Lapin IP, Oxenkrug GF. Serotonin and aging. In: Essman W, editor. Serotonin in health and disease. Vol. 5. Spectrum Press; NY-London: 1979. pp. 111–123. [Google Scholar]
- [74].Groebe K, Krause F, Kunstmann B, et al. Differential Proteomic Profiling of Mitochondria from Podospora An- serina, Rat and Human Reveals Distinct Patterns of Age- Related Oxidative Changes. Experimental Gerontology. 2007;42:887–898. doi: 10.1016/j.exger.2007.07.001. [DOI] [PubMed] [Google Scholar]
- [75].Kamyshev MG. Longevity and its relation to the locomotor activity in tryptophan-xanthommatin metabolic pathway mutant of Drosophila. DoklAcadNauk USSR. 1980;253:1476–80. [Google Scholar]
- [76].Oxenkrug GF. The extended life span of Drosophila melanogaster eye-color (white and vermilion) mutants with impaired formation of kynurenine. J Neural Transm. 2010;117:23–26. doi: 10.1007/s00702-009-0341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Oxenkrug G, Navrotskaya V, Vorobyova L, Summergrad P. Extension of life span of Drosophila melanogaster by the inhibitors of tryptophan – kynurenine metabolism. Fly (Austin) 2011;5:307–309. doi: 10.4161/fly.5.4.18414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Navrotskaya VV, Oxenkrug G, Vorobyova LI, Summergrad P. Berberine prolongs life span and stimulates locomotor activity of Drosophila melanogaster. Amer J Plant Sci. 2012;3:1037–1040. doi: 10.4236/ajps.2012.327123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Oxenkrug G, Navrotskaya V, Vorobyova L, Summergrad P. Minocycline Effect on Life and Health Span of Drosophila Melanogaster. Aging and Disease. 2012;3:352–359. [PMC free article] [PubMed] [Google Scholar]
- [80].Dilman VM. Age-associated elevation of hypothalamic, threshold to feedback control, and its role in development, ageing, and disease. Lancet. 1971;1:1211–9. doi: 10.1016/s0140-6736(71)91721-1. [DOI] [PubMed] [Google Scholar]
- [81].Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;11:2516–22. [PubMed] [Google Scholar]
- [82].Oxenkrug G. Tryptophan-kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: Serotonin hypothesis revisited 40 years later. Israel J Psychiatry. 2010;47:56–63. [PMC free article] [PubMed] [Google Scholar]
- [83].Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. Depression and risk for onset of type II diabetes. A prospective population-based study. Diabetes Care. 1996;19:1097–1102. doi: 10.2337/diacare.19.10.1097. [DOI] [PubMed] [Google Scholar]
- [84].Campayo A, de Jonge P, Roy JF, Saz P, de la Camara C, et al. Depressive disorder and incident diabetes mellitus: the effect of characteristics of depression. Am J Psychiatry. 2010;167:580–588. doi: 10.1176/appi.ajp.2009.09010038. [DOI] [PubMed] [Google Scholar]
- [85].Takahashi H, Price JM. Dehydroxylation of Xanthurenic Acid to 8-Hydroxyquinaldic Acid. J. Biol. Chem. 1956;233:150–153. [PubMed] [Google Scholar]
- [86].Takahashi H, Kaihara M, Price JM. The conversion of kynurenic acid to quinaldic acid by human and rats. J. Biol. Chem. 1956;223:705–708. [PubMed] [Google Scholar]
- [87].Ogasawara N, Hagino Y, Kotake Y. Kynurenine-transaminase, kynureninase and the increase of xanthurenic acid excretion. J Biochem. 1962;52:162–6. doi: 10.1093/oxfordjournals.jbchem.a127591. [DOI] [PubMed] [Google Scholar]
- [88].Kotaki Y, Ueda T, Mori T, Igaki S, Hattori M. Abnormal tryptophan metabolism and experimental diabetes by xanthurenic acid (XA) ActaVitaminolEnzymol. 1975;29:236–239. [PubMed] [Google Scholar]
- [89].Hattori M, Kotake Y, Kotake Y. Studies on the urinary excretion of xanthurenic acid in diabetics. ActaVitaminolEnzymol. 1984;6:221–228. [PubMed] [Google Scholar]
- [90].Meyramov G, Korchin V, Kocheryzkina N. Diabetogenic activity of xanturenic acid determined by its chelating properties? ActaVitaminolEnzymol. 1984;6:221–228. doi: 10.1016/s0041-1345(98)00788-x. [DOI] [PubMed] [Google Scholar]
- [91].Ikeda S, Kotake Y. Urinary excretion of xanthurenic acid and zinc in diabetes: (3). Occurrence of xanthurenic acid-Zn2+ complex in urine of diabetic patients and of experimentally-diabetic rats. ItalJ Biochem. 1986;35:232–41. [PubMed] [Google Scholar]
- [92].Noto Y, Okamoto H. Inhibition by kynurenine metabolites of proinsulin synthesis in isolated pancreatic islets. ActaDiabetolLat. 1978;15:273–82. doi: 10.1007/BF02590750. [DOI] [PubMed] [Google Scholar]
- [93].Rogers KS, Evangelista SJ. 3-Hydroxykynurenine, 3-hydroxyanthranilic acid, and oaminophenol inhibit leucine-stimulated insulin release from rat pancreatic islets. ProcSocExpBiol Med. 1985;178:275–8. doi: 10.3181/00379727-178-42010. [DOI] [PubMed] [Google Scholar]
- [94].Bender DA, Njagi EN, Danielian PS. Tryptophan metabolism in vitamin B6-deficient mice. Br J Nutr. 1990;63:27–36. doi: 10.1079/bjn19900089. 1990. [DOI] [PubMed] [Google Scholar]
- [95].Cazzulo CL, Mangoni A, Mascherpa G. Tryptophan metabolism in affective psychoses. British J Psychiatry. 1974;112:157–162. doi: 10.1192/bjp.112.483.157. [DOI] [PubMed] [Google Scholar]
- [96].Morris MS, Sakakeeny L, Jacques PF, Picciano MF, Selhub J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J Nutr. 2010;140:103–110. doi: 10.3945/jn.109.114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Melillo G, Cox DW, Biragyn A, Sheffler L. Regulation of nitric-oxide synthase mRNA expression by interferon-gamma and picolinic acid. J BiolChem. 1994;269:8128–8813. [PubMed] [Google Scholar]
- [98].Fuchs D, Avanzas P, Arroyo-Espliguero R, Jenny M, Consuegra-Sanchez L, Kaski JC. The role of neopterin in atherogenesis and cardiovascular risk assessment. Curr Med Chem. 2009;16:4644–4653. doi: 10.2174/092986709789878247. [DOI] [PubMed] [Google Scholar]
- [99].Frick B, Schroecksnadel K, Neurauter G. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin.Biochem. 2004;37:684–687. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- [100].Oxenkrug G, Tucker KL, Requintina P, Summergrad P. Neopterin, a marker of interferon-gamma-inducible inflammation, correlates with pyridoxal-5'-phosphate, waist circumference, HDL-cholesterol, insulin resistance and mortality risk in adult Boston community dwellers of Puerto Rican origin. American Journal of Neuroprotection and Neuroregeneration. 2011;3:48–52. doi: 10.1166/ajnn.2011.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Neurauter G, Schröcksnadel K, Scholl-Bürgi S, Sperner-Unterweger B, Schubert C, et al. Chronic Immune Stimulation Correlates with Reduced Phenylalanine Turnover. Current Drug Metabolism. 2008;9:622–627. doi: 10.2174/138920008785821738. [DOI] [PubMed] [Google Scholar]
- [102].Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J BiolChem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- [103].Qu T, Uz T, Manev H. Inflammatory 5-LOX mRNA and protein are increased in brain of aging rats. Neurbiology of Aging. 2000:647–652. doi: 10.1016/s0197-4580(00)00167-6. [DOI] [PubMed] [Google Scholar]
- [104].Stewart JC, Janicki DL, Muldoon MF, Sutton-Tyrrell K, Kamarck TW. Negative emotions and 3-year progression of subclinical atherosclerosis. Arch Gen Psychiatry. 2007;64:225–233. doi: 10.1001/archpsyc.64.2.225. [DOI] [PubMed] [Google Scholar]
- [105].Oxenkrug GF, Requintina PJ, Mikolich DL, Ruthazer R, Viveiros K, Lee H, Summergrad P. Neopterin as a marker of response to antiviral therapy in hepatitis C virus patients. Hepat Res Treat. 2012;2012:619609. doi: 10.1155/2012/619609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Calandra P. Identification of tryptophan metabolites in the healthy epidermis of diabetics. ActaDiabetolLat. 1977;14:26–37. doi: 10.1007/BF02624661. [DOI] [PubMed] [Google Scholar]
- [107].Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the Risk of Diabetes Mellitus: A Systematic Review and Meta-analysis. Arch Dermatol. 2012;15:1–8. doi: 10.1001/2013.jamadermatol.406. [DOI] [PubMed] [Google Scholar]