Introduction
The cornea is the major refractive element of the adult eye. It consists primarily of three layers: an outer layer containing an epithelium, a middle stromal layer consisting of a collagen-rich extracellular matrix (ECM) interspersed with keratocytes and an inner layer of endothelial cells (Fig 1). There also is a population of transient bone marrow derived cells, monocytes (macrophages) and dendritic cells that reside in the cornea. The stroma comprises 90% of the thickness of the cornea. It consists of dense, regularly packed collagen fibrils arranged as orthogonal layers or lamellae. The corneal stroma is unique in having a homogeneous distribution of small diameter 25–30 nm fibrils that are regularly packed within lamellae. It was hypothesized, based on the physical properties of light, that this lattice-like structure produces minimal light scattering and therefore, transparency (Maurice 1957; Benedek 1971; Farrell et al. 1973).
Figure 1.
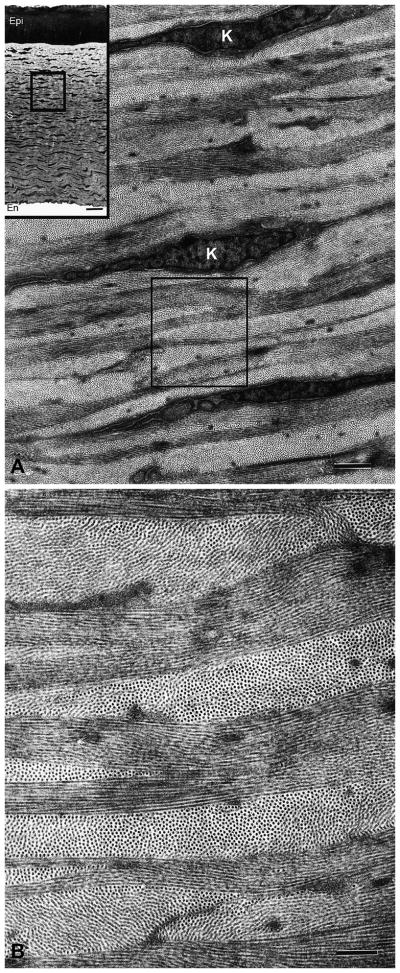
Transmission electron micrographs of cornea structure. The cornea is limited by an outer epithelium, and an inner endothelium (low magnification inset in A). (A) The stroma makes up more than 90% of the corneal thickness and contains keratocytes (K) orientated parallel to the corneal surface and found between the stromal lamellae. (B) Enlargement of area in rectangle in A. The lamellae are composed of small diameter collagen fibrils with regular packing. Adjacent layer are at approximately right angles to one another forming an orthogonal lattice.
The unique size and spacing of the collagen fibrils in the cornea stroma has suggested corneal stromal collagen may have unusual attributes and interactions associated with its assembly into small diameter, highly organized fibrils. However, there were several lines of evidence that indicated other components of the stromal ECM also were involved in reducing light scattering. There was a correlation between the appearance of metachromatic staining of the glycosaminoglycans in stromal ECM and appearance of the highly organized collagen lattice during the acquisition of corneal transparency in the developing embryo (Coulombre and Coulombre 1958). There was also a loss of the metachromatic staining in opaque corneal wounds (Dunnington and Smelser 1958) that coincided with a reduction in the levels of corneal keratan sulfate (Anseth 1961), a glycosaminoglycan determined later to be a component of some proteoglycans. These observations generated considerable interest in characterizing the collagens and proteoglycans in the corneal stroma and determining their roles in the formation of a transparent ECM.
Collagen
All collagens are trimers, each composed of one, two or three distinct gene products. There are 28 known types of collagens that constitute a diverse family of glycoproteins (Gordon and Hahn). All collagens have a triple helical domain. The triple helical domains require proline for alpha helix formation and glycine at every third residue for packing into a triple helix. In addition, collagens contain two unique amino acids, hydroxyproline and hydroxylysine. Hydroxyproline is important in triple helix stability and hydroxylysine in glycosylation (see (Canty and Kadler 2005) for review). The fibril forming types (collagen types I, II, III, V, XI, XXIV and XXVII) are rod-like collagens with extensive triple helical domains (ca. 300 nm) containing 990 to 1020 amino acids. It is now clear that these collagens co-assemble into banded fibrils in tissues. Collagen is synthesized in the RER as procollagen, where each of the 3 polypeptide chains have a globular domain at the N- and C-termini. The procollagen molecule is secreted into the ECM where specific proteases at the cell surface remove the N- and C-terminal non-helical, globular ends. The resulting collagen molecules can then laterally associate in a staggered array to form collagen fibrils of various lengths and diameters. Lysyl oxidase then converts the amino groups on some of the lysine residues in the collagen polypeptide chain to aldehydes that react with amino groups on lysines in other chains to form covalent crosslinks.
Collagen molecules that have not been crosslinked can be extracted from tissues using dilute acid and they will self assemble in vitro into collagen fibrils that are similar to those seen in vivo (Williams et al. 1978). This in vitro fibrillogenesis model system has been extensively used to determine the role that different collagen and proteoglycan types have on fibril assembly.
Proteoglycans
All proteoglycans consist of a core protein with one or more covalently attached glycosaminoglycan (GAG) side chains (for review see (Iozzo 1998)). Proteoglycans were originally named and grouped according to the type of GAG chain attached to the core protein. They have since also been grouped into families based homologous sequences of amino acids in their core protein that confer a particular activity. Most proteoglycans now fit into one of three major families: ones that either 1) intercalate into plasma membranes, 2) bind to hyaluronan or 3) modulate collagen fibril formation. Hyaluronan is a GAG but it is not attached to a core protein.
There is an incredible diversity of proteoglycans. Core proteins range in size from 20–450 kD and regions of the core protein often show homology to motifs contained in globular-type proteins. The GAG side chains are repeating disaccharides with sulfate esters and can be as large as 70kD. There are three types of GAG side chains: chondroitin/dermatan sulfate (CS/DS), keratan sulfate (KS) and heparan sulfate (HS). Both CS/DS and HS chains are attached to serine residues on the core protein that are followed by a glycine residue and the presence of a flanking cluster of acidic residues will favor HS attachment on that serine. KS chains are primarily attached to asparagines that are within one residue of a serine or a threonine.
The core protein is synthesized in the rough endoplasmic reticulum and the GAG side chains are added to the core protein in the Golgi. The proteoglycans are then secreted into the ECM. Corneal proteoglycans, like most other proteoglycans, interact strongly with other components in the ECM and can only be quantitatively extracted and in an intact form by denaturing solvents (Rada et al. 1993).
Stromal ECM Composition
The ECM of the corneal stroma consists primarily of collagen with lesser amounts of proteoglycans. The major fibril-forming collagens of the adult corneal stromal ECM are types I and V (Birk et al. 1986). The collagen fibrils of the corneal stroma are heterotypic fibrils: both of these 2 collagen types are present in each collagen fibril. Type V is involved in initiating fibril assembly (Wenstrup et al. 2004; Wenstrup et al. 2006) and regulating fibril diameter (Birk et al. 1990).
There are four proteoglycans in the adult corneal stromal ECM: decorin (Li et al. 1992), lumican (Blochberger et al. 1992; Kao et al. 2006), keratocan (Corpuz et al. 1996; Chakravarti 2006) and mimecan (Funderburgh et al. 1997), a gene product that had previously been named Proteoglycan-Lb (Shinomura and Kimata 1992) and osteoglycin (Madisen et al. 1990). The core proteins, but not their GAG side chains, of lumican and decorin have been shown to inhibit collagen fibril-formation and reduce collagen fibril diameter using the in vitro collagen fibril forming assays (Rada et al. 1993). Decorin and lumican made as recombinant products have been shown to act on different phases of fibril growth, interact with different regions of the collagen molecule and serve to stabilize the collagen fibril once formed (Neame et al. 2000). The roles of collagen type V, lumican and decorin in regulating collagen fibril growth have been confirmed by inactivating these genes in mice (Fig 2).
Figure 2.
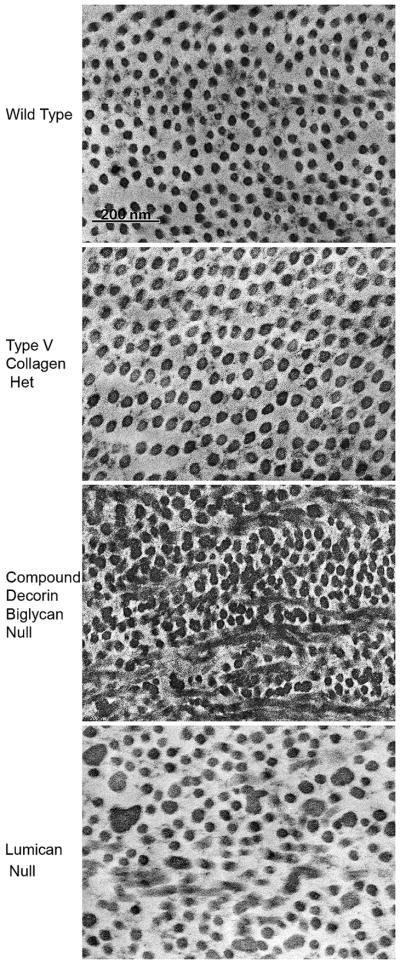
Stromal fibril structure. The wild type mouse cornea has small diameter collagen with regular packing consistent with corneal transparency. Type V collagen nucleates initial fibril assembly. Reduction in the concentration of type V collagen by half in the heteterozygous Col5a1 mouse results in half the nucleation sites. Therefore, fewer collagen fibrils with larger diameters are found in the stroma of this mouse model. Decorin is the major class I leucine-rich proteoglycan in the cornea. A targeted deletion of decorin and blocking the functional compensation by closely related biglycan in the compound decorin/biglycan mouse model results in a sever disruption in the regulation of fibril assembly. A target deletion of lumican a class II leucine-rich proteoglycan alters regulation of fibril assembly primarily in the posterior stroma.
The corneal proteoglycans have a similar or homologous core protein structure and are members of the small leucine-rich type proteoglycan gene family. They contain, as their major structural feature, ~9 leucine-rich regions that are homologous in structure, similar in length and are equally spaced within the central region of the core protein. The polypeptide chain of this region of the core protein is coiled into a tight spiral with a leucine-rich region located every 360 degrees, thereby aligning all the leucine-rich regions on one surface of the coil and the non-leucine-rich regions on the opposite surface of the coil. It is thought that it is these aligned leucine-rich regions that interact with the collagen molecules to regulate fibril formation. The majority of the asparagines that are attachment sites for keratan sulfate chains in this region of the core protein are located on the non-leucine-rich surface (Dunlevy et al. 1998).
The core proteins of the corneal stromal proteoglycans are also similar in size, 35-40kD. Decorin has a single 55–60kD chondroitin/dermatan sulfate chain while lumican, keratocan and osteoglycin/mimican have 2–3 keratan sulfate chains of 10–15kD each (Hassell et al. 1979; Midura and Hascall 1989; Dunlevy et al. 1998). The overall size of these proteoglycans are then, uniformly small enough to fit in the spaces between the collagen fibrils and regulate their spacing (Cintron et al. 1978; Hassell et al. 1983; Chakravarti et al. 1998; Liu et al. 2003; Beecher et al. 2005).
Defective proteoglycan synthesis has been shown to result in blindness in humans by disrupting the organization of the collagen fibrils. There are two human inherited corneal dystrophies that are due to faulty keratan sulfate proteoglycan production. Mutations in the gene that codes for the core protein of keratocan causes cornea plana (Pellegata et al. 2000) and mutations in a gene that codes for one of the sulfotransferases that puts sulfate esters on keratan sulfate causes macular corneal dystrophy (Hassell et al. 1980; Midura et al. 1990; Hayashida et al. 2006; Musselmann and Hassell 2006). The presence of sulfate groups on the keratan sulfate chains is essential for the function of the proteoglycan. The sulfate groups on GAG chains of the proteoglycans bind water and at the hydration levels of the normal cornea, the chondroitin/dermatan sulfate chains are fully hydrated while the keratan sulfate chains are not and this suggests that the keratan sulfate acts as a reserve for hydration (Bettelheim and Plessy 1975). The sulfate esters on keratan sulfate are also important for maintaining the solubility of the proteoglycan in an aqueous environment [for review on keratan sulfate see (Funderburgh 2000)]. In addition, a human congenital stromal corneal dystrophy, where cloudy corneas develop shortly after birth, is associated with a mutation in the gene for decorin (Bredrup et al. 2005; Rodahl et al. 2006).
Thus, the coordinated synthesis of several different collagen types and the core proteins of several different leucine-rich type proteoglycans as well as posttranslational modifications of the collagens and the proteoglycans are required to produce collagen fibrils with the size and spacing needed for corneal stromal transparency.
Keratocytes
Keratocytes have a compact cell body with numerous cytoplasmic lamellapodia, that gives them a dendritic-like morphology, and are interconnected in a three dimensional network by these lamellapodia (Poole et al. 1993; Hahnel et al. 2000). The compact cell body minimizes the surface area of the keratocyte exposed to light and this probably serves to reduce light scattering while their processes provides for cell-cell communications. Light scattering by the keratocytes is also reduced by the presence of the crystalline proteins (high levels of enzymes, such as aldehyde dehydrogenase and transketolase) in their cytoplasm, in the same way the crystalline proteins reduce light scattering in other ocular cells in the visual axis, such as the epithelial cells of the lens and the epithelium of the cornea [for review see (Jester 2008)]. The lens, a structure composed primarily of epithelial cell, is completely dependent upon the crystalline proteins for transparency. Disruption of the crystalline proteins in the lens results in an opacity, cataract.
ECM Production During Stromal Development
The formation of the cornea has been extensively studied in the chicken and the rabbit (Coulombre and Coulombre 1958; Toole and Trelstad 1971; Cintron et al. 1983; Funderburgh et al. 1986; Cornuet et al. 1994; Quantock and Young 2008). The ectoderm, remaining after the formation of the lens placode, becomes the epithelium of the cornea. Neural crest cells (Table 1) migrate into the space between the corneal epithelium and the lens placode and form the endothelium of the cornea. Other neural crest cells migrate into the space between the corneal epithelium and corneal endothelium and become keratoblasts (Table 1) (Fig 3). The keratoblasts proliferate and synthesize high levels of hyaluronan to form an embryonic corneal stroma ECM (Coulombre and Coulombre 1958; Toole and Trelstad 1971; Cintron et al. 1983). This ECM contains extensive voids, presumably filled with hyaluronan and water, and only occasional collagen fibrils (Cintron et al. 1983). The core protein of lumican and keratocan can be detected at this stage, but their GAG chains are not sulfated and they are essentially glycoproteins (Cornuet et al. 1994). The cornea is actually thicker at this time than it will be at hatching or birth and it has achieved only 40% of its final transparency (Coulombre and Coulombre 1958).
Table 1.
Corneal Stromal Cells, Derivation and Phenotype
| Cell Type | Cell Derivation | Phenotype |
|---|---|---|
| Neural Crest Cells |
Neural Ectoderm During neural tube closure |
They migrate throughout the developing embryo and contribute to the development of a number of different organs including the eye. The corneal stroma and endothelium are neural crest derivatives |
| Keratoblasts |
Neural Crest Cells That migrate between the corneal epithelium and endothelium during development |
They proliferate and produce a hyaluronan-rich ECM containing sparse collagen fibrils |
| Keratocytes | Keratoblasts | They replace the hyaluronan-rich ECM with an ECM consistent with transparency containing densely packed collagen fibrils and proteoglycans during late embryonic and post natal development |
| Quiescent Keratocytes | Keratocytes | They have a low level of biosynthetic activity to maintain the ECM produced by keratocytes |
| Hypercellular Myofibroblasts | Keratocytes/Quiescent Keratocytes | They contain α smooth muscle actin, but produce only low levels of ECM resulting in densely packed cells in a sparse ECM |
| Myofibroblasts | Hypercellular Myofibroblasts | They contain α smooth muscle actin and produce high level of ECM, but containing components such as hyaluronan that are not normally present resulting in an ECM inconsistent with transparency |
| Wound Fibroblasts |
Hypercellular Myofibroblasts Possibly Myofibroblasts |
They produce an ECM of densely packed fibrils and proteoglycans that restores transparency |
| Corneal Fibroblasts | Keratocytes/Quiescent Keratocytes | Result from cell culture in media containing fetal bovine serum. A varying proportion contain α smooth muscle actin |
Figure 3.
Corneal stromal development. Neural crest derived keratoblasts invade the space between the developing corneal epithelium and endothelium, proliferate, and produce an extensive hyaluronan (HA) rich ECM. The keratoblasts then differentiate into keratocytes and replace the HA rich ECM with a collagen fibril/proteoglycan rich ECM that is transparent.
The keratoblasts in the embryonic stroma then differentiate into keratocytes (Table 1) (Fig. 3). These cells continue to proliferate, although at a lower level, but now synthesize high levels of collagens and keratan sulfate proteoglycans that replace the hyaluronan/water-rich ECM with the densely packed collagen fibril-type ECM seen in adult corneas (Cintron et al. 1983; Funderburgh et al. 1986; Cornuet et al. 1994; Young et al. 2007). Keratoblasts and keratocytes have similar levels of mRNA for lumican, but mRNA levels for keratocan are actually higher in the keratoblasts (Dunlevy et al. 2000). This suggests that the synthesis of the keratan sulfate proteoglycan core proteins are regulated at the translational level. Biglycan, another member of this leucine-rich gene family, is also expressed at this time where it acts in concert with decorin to regulate fibril diameter growth (Zhang et al. 2009). When the production of the fibril-type ECM is complete, the embryonic cornea achieves 100% of its final transparency (Coulombre and Coulombre 1958). It is the synthesis of this collagen-fibril, keratan sulfate proteoglycan rich ECM by the keratocytes that causes stratification and allows the cornea to achieve full transparency.
Corneal Growth During Post-Natal Development
Although the collagen fibrils in the chicken corneas are completely compacted at hatching, they are not at birth of mice (Fig 4). Furthermore, the keratocytes in the corneal stroma continue to proliferate and produce ECM, although at a lower rate than during late embryonic development and this causes the cornea to continue to grow in diameter during the post-natal peroid. This growth was shown in rabbit corneas to occur as a result of uniform expansion of the cornea (Davison and Galbavy 1985). However, keratocytes in the central region of the stroma of chicken corneas proliferate and produce ECM at a higher rate than those in the periphery of the cornea and it has been suggested that this differential growth rate between the center and periphery may produce the refractive shape of the cornea (Rada et al. 1996).
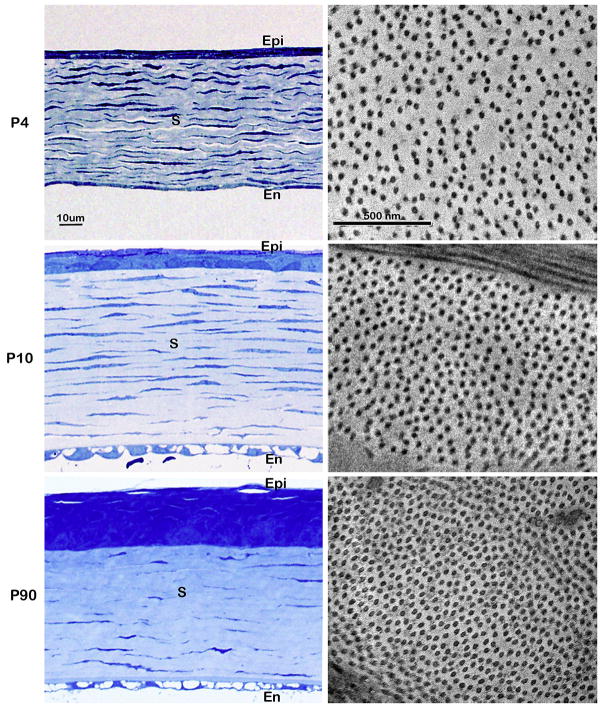
Figure 4.
Corneal post-natal development. Light microscopy (Left column) and transmission electron microscopy (right column) of the developing mouse cornea at post-natal (P) day 4, 10 and 90. Four days after birth (P4) the cornea is cellular and hydrated with less regularly packed collagen fibrils (P4). The stroma reaches it maximum thickness around P10 and then begins to compact. The mature cornea (P90) has thinned and the fibrils are regularly packed.
Keratocytes isolated from the cornea stroma by collagenase digestion and cultured in serum free media retain their low, in vivo level of proliferation (Jester et al. 1996; Beales et al. 1999). Extracts of cornea stroma from 1-year-old cows were shown to stimulate the proliferation of bovine keratocytes in serum free culture (Musselmann et al. 2003). Both IGF-II and IGF-I stimulate proliferation and collagen synthesis by bovine keratocytes in serum free culture (Hassell et al. 2008; Musselmann et al. 2008; Etheredge et al. 2009) and IGF-II was identified as the mitogen present in the stromal extract (Musselmann et al. 2008). Keratocytes from 6-week-old rabbits proliferate and produce collagen in serum free culture at slightly higher levels than keratocytes from 1-year-old cows and this difference in biosynthetic activity was shown to be due to the IGF-II produced by the rabbit keratocytes and secreted into the media during culture (Kane et al. 2009). Analysis of mRNA isolated from mouse keratocytes show that 4-day-old newborn mice contain 17-fold and 7-fold higher levels of message for IGF-I and IGF-II, respectively, than 60-day-old mice (Kane et al. 2009). Taken together, these data suggest that the IGF family of growth factors may play an important role in regulating normal post-natal corneal stromal growth.
ECM Production During Stromal Wound Healing
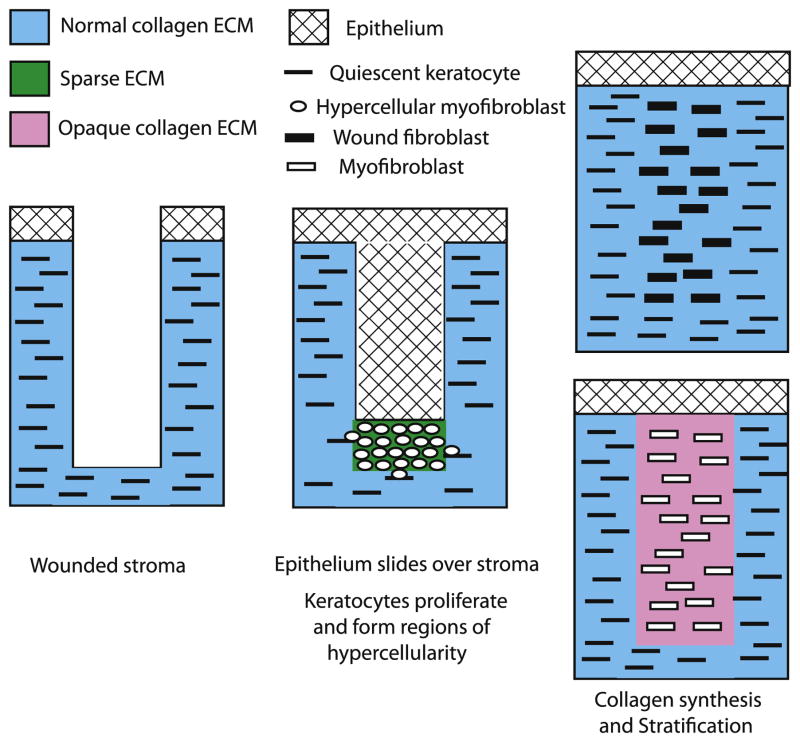
Although the keratocytes proliferate and are biosynthetically active during embryonic and post-natal development, they exhibit a relatively low level of activity in the adult cornea and are considered quiescent keratocytes (Table 1) (Jester et al. 1994; Muller et al. 1995; Zieske et al. 2001). However, the quiescent keratocytes can become active again after the cornea is injured (Fig 5). When an incisional wound through the epithelium into stroma occurs, the keratocytes damaged during wounding undergo apoptosis (Helena et al. 1998; Zieske et al. 2001). The epithelium adjacent to the wound site loses its hemidesmosome mediated attachment to the basement membrane and migrates/slides over the exposed stroma. Some of the remaining viable keratocytes are activated to proliferate (Hanna et al. 1989; Del Pero et al. 1990; Zieske et al. 2001), but they produce relatively little ECM and, as a result, form regions of hypercellularity between the recently migrated epithelium and the stroma (Lee et al. 1982; Lance et al. 1988; Sundarraj et al. 1998). The sparse ECM produced by these cells does, however, contain keratocan and lumican with keratan sulfate chains (Sundarraj et al. 1998). The cytoplasm of the hypercellular cells contains α smooth muscle actin (Jester et al. 1995), a protein involved in wound contraction and considered a marker for myofibroblasts. This suggests that the hypercellular cells seen during early wound healing are hypercellular myofibroblasts (Table 1), a form of myofibroblasts that produces a low level of ECM.
Figure 5.
Healing in a corneal stromal incisional wound. The corneal epithelium loses it hemidesmosome attachment to the basement membrane and migrates over the wound site. The quiescent keratocytes are activated to proliferate and produce α smooth muscle actin but synthesize only low levels of ECM. The resulting hypercellular myofibroblasts accumulate in regions under the epithelium. The hypercellular myofibroblasts then become either wound fibroblasts that produce a normal collagen fibril containing ECM that restores transparency or become myofibroblasts that produce a light-scattering collagen fibril containing ECM that is opaque.
The hypercellular myofibroblasts can then become myofibroblasts (Table 1), which contain α smooth muscle actin (Jester et al. 1995) and produce high levels of collagen, hyaluronan, and biglycan but only low levels of keratan sulfate proteoglycans to form a disorganized and opaque ECM or alternatively, they can become wound fibroblasts (Table 1) which, like the keratocytes in the developing cornea, produce high levels of collagen as well as keratocan and lumican with keratan sulfate chains to form the highly organized normal ECM that restores transparency (Cintron and Kublin 1977; Cintron et al. 1978; Hassell et al. 1983; Maguen et al. 1997; Funderburgh et al. 1998; Ljubimov et al. 1998; Sundarraj et al. 1998; Dawson et al. 2005). Since both wound fibroblasts and myofibroblasts make collagen and stratify, collagen would appear to be a necessary component for stratification. Transparency also can be restored to the disorganized and opaque ECM produced by myofibroblasts by turnover and the production of an organized ECM (Cintron et al. 1978; Hassell et al. 1983). In this case, the myofibroblasts would have to become or be replaced by wound fibroblasts.
Thus, based on the levels of proliferation and ECM production, stromal wound healing can be divided into an initial hypercellular phase, that primarily consists of proliferation followed by a stratification phase that primarily consists of collagen and proteoglycan synthesis. Furthermore, there appears to be three repair phenotypes: the hypercellular myofibroblast, the wound fibroblast and the myofibroblast.
A Comparison of Development and Wound Healing
Stromal development consists of an initial proliferation and hydration phase that is followed by a dehydration and collagen/proteoglycan synthesis phase. Stromal repair also consists of an initial proliferation phase but without hydration and this results in hypercellularity. This may be due, in part, to the ability of the endothelium of the adult cornea to pump salt and water out of the stroma to maintain a critical level hydration level. In addition, the hypercellularity in stromal repair can be followed by one of two different collagen/proteoglycan phases: a collagenous one where a normal-type ECM is made by wound fibroblasts and a fibrocollagenous one where a scar-type ECM is made by myofibroblasts. Stromal repair also can have an inflammation phase that is not seen in stromal development.
Keratocyte Activation
Keratocytes can be easily isolated from cornea by mincing the corneas into small fragments, digesting the tissue fragments with collagenase to release the keratocytes and collecting the keratocytes by centrifugation. Despite this trauma, the keratocytes that survive and attach to culture dishes in serum free medium retain their native, quiescent phenotype (Jester et al. 1996; Beales et al. 1999). This suggests that the physical abuse the keratocytes receive during isolation does not activate them to increase their biosynthetic activity.
There is considerable evidence that growth factors activate the keratocytes to repair the stroma (for other reviews see (Li and Tseng 1995; Lim et al. 2003)). These growth factors could come from 5 different sources. (1) Extracts of the cornea stroma have been shown to stimulate the proliferation of keratocytes in culture (Yue et al. 1986; Musselmann et al. 2003) and IGF-II was identified as a component in the stromal extract that can stimulate quiescent keratocytes to proliferate and produce ECM (Musselmann et al. 2008). This suggests that the stroma may be preloaded with IGF’s that can then activate the keratocytes remaining after apoptosis to become one of the repair keratocyte phenotypes. (2) FGF-2, TGF-β, and PDGF have been shown to be in the tear fluid and, because of the incisional wound through the epithelial barrier, they would be able to penetrate into the stroma and act on keratocytes (Tuominen et al. 2001; Zhou et al. 2007). (3) PDGF and TGF-β can be produced by the transient population of macrophages present in the cornea and by other inflammatory cells, such as neutrophils and lymphocytes that invade the stroma within hours after wounding (Hong et al. 2001; Brissette-Storkus et al. 2002; Chinnery et al. 2008; Stapleton et al. 2008). (4) The corneal epithelium, which has migrated over the stroma, has been shown to produce FGF-2 and TGF-β that can be released into the stroma to act on the keratocytes (Stramer et al. 2003; Gan et al. 2005). (5) The action of TGF-β on keratocytes can cause the keratocytes themselves to produce CTGF and FGF-2 that can then act on the keratocytes (Kay et al. 1998; Lim et al. 2003).
Proliferation and ECM Synthesis by Keratocytes In Vitro
While there are many different cell types that make the growth factors, it is the quiescent keratocyte that respond to the growth factors and make the new stromal ECM during wound healing. Because quiescent keratocytes isolated from the cornea stroma by collagenase digestion retain their quiescent phenotype in vitro when cultured in serum free media (Jester et al. 1996; Beales et al. 1999), they can be used to determine the effect that different growth factors have on the proliferation of keratocytes and on the synthesis of collagens and proteoglycans by keratocytes. While not every growth factor has been tested, a pattern has emerged from the studies that have been done (Funderburgh et al. 2001; Funderburgh et al. 2003; Musselmann et al. 2005; Musselmann et al. 2006; Guo et al. 2007a; Hassell et al. 2008; Musselmann et al. 2008; Etheredge et al. 2009; Kane et al. 2009). A recent study, that compared FGF-2, IGF-I and TGF-β, showed that while FGF-2 stimulated the highest level of cell proliferation it did not stimulate collagen synthesis and may have even inhibited collagen synthesis (Etheredge et al. 2009). In contrast, both IGF-I and TGF-β stimulated low levels of proliferation and high levels of collagen synthesis (Etheredge et al. 2009), like that shown for IGF-II and pharmacological levels of insulin (Musselmann et al. 2005; Musselmann et al. 2006; Hassell et al. 2008; Musselmann et al. 2008). FGF-2, IGF-I, IGF-II and TGF-β all stimulated proteoglycan synthesis, but TGF-β also stimulated hyaluronan, fibronectin and biglycan synthesis, which are constituents of a fibrotic ECM and induced the appearance of α smooth muscle actin (Jester et al. 1996; Funderburgh et al. 2001; Funderburgh et al. 2003; Guo et al. 2007a; Etheredge et al. 2009). Thus, TGF-β alone causes keratocytes to become myofibroblasts (Jester et al. 1996).
Spheroid Formation by Keratocytes In Vitro
Recently, it has been shown that mouse and bovine keratocytes cultured in media containing both FGF-2 and ITS (a media supplement containing pharmacological levels of insulin) will proliferate, aggregate into densely packed clusters and, can be passed and expanded without loss of keratocan production (Yoshida et al. 2005; Funderburgh et al. 2008). Bovine keratocytes cultured in FGF-2 alone can be passed but stop proliferating after one or two passages and bovine keratocytes cultured in ITS alone cannot be passed (J Hassell, unpublished). The clusters of cells induced by culture in media containing both FGF-2 and ITS also can detach from the plate and form spheroids that remain suspended in the media. These clusters of cells resemble the regions of hypercellularity seen during the early stages of wound healing. Culture in media containg FGF-2 and ITS may cause keratocytes to recapitulate in vitro some aspects of the hypercellular phase of wound healing seen in vivo and this allows them to proliferate and retain the keratocyte phenotype.
Stratification In Vitro
Keratocytes cultured in media containing fetal bovine serum (FBS) proliferate at very high levels and, although a varying proportion of them are myofibroblasts (Masur et al. 1996), they are usually called corneal fibroblasts (Table 1). Corneal fibroblasts can be easily passed and expanded, and this has made them a popular substitute for keratocytes. Corneal fibroblasts will stratify when ascorbic acid is added to the media (Hata and Senoo 1989; Saika 1992; Guo et al. 2007b). Ascorbic acid is a co-factor needed for the hydroxylation of certain proline and lysine residues in the triple helical region of collagen. Hydroxylation of these amino acids stabilizes the triple helical region, making it more resistant to proteases and increasing its affinity for other components in the ECM (Musselmann et al. 2006). Pharmacological levels of insulin will stimulate collagen synthesis by keratocytes but does not cause the cells to stratify (Musselmann et al. 2005; Musselmann et al. 2006). However, a thin layer of 3% agarose on top of keratocytes cultured in media containing insulin causes the keratocytes to stratify (Hassell et al. 2008). The agarose overlay enhanced the conversion of procollagen synthesized by the keratocytes into collagen and its subsequent assembly into fibrils associated with the cell layer (Hassell et al. 2008). Taken together, these data suggest that the assembly of collagen fibrils and deposition into a matrix is necessary for stratification. Collagen fibrils would provide attachment sites for keratocytes and are deposited between keratocytes to produce cell-separating collagen lamellae.
Candidate Growth Factors Involved in the Phases of Wound Healing
By comparing the expression of α smooth muscle actin and the production of ECM induced by the action of growth factors on keratocytes in vitro to that which occurs in vivo during wound repair, it is possible to speculate on the identity the growth factors that are responsible for each of the different repair phenotypes. A combination of two growth factors, FGF-2 and TGF-β, would be needed to induce the keratocytes to become the hypercellular myofibroblasts seen during the initial phase of wound healing. FGF-2 would stimulate high levels of proliferation and proteoglycan synthesis but would inhibit collagen synthesis; thereby producing only a sparse ECM. TGF-β would induce the synthesis of α smooth muscle actin, causing them to be myofibroblasts. IGF-I, IGF-II and TGF-β alone would likely induce the stratification phase during wound repair because they stimulate both collagen and proteoglycan synthesis in vitro. IGF-I and II, however, would likely cause the hypercellular myofibroblasts to become wound fibroblasts, producing stratification with the normal stromal ECM that restores transparency during wound healing, while TGF-β would likely cause the hypercellular myofibroblasts to become myofibroblasts, producing stratification with the opaque ECM that causes blindness as a result of wound healing.
Fibroblasts
Based on the expression levels of keratocan and keratan sulfate, corneal fibroblasts differ from hypercellular myofibroblasts, wound fibroblasts and spheroids. Corneal fibroblasts make substantially reduced levels of keratocan and keratan sulfate (Beales et al. 1999; Funderburgh et al. 2003) while the hypercellular myofibroblasts and the wound fibroblasts seen in vivo (Sundarraj et al. 1998), the wound fibroblasts induced in vitro by IGF-I/II (Musselmann et al. 2005; Musselmann et al. 2008; Etheredge et al. 2009) and the spheroids (Yoshida et al. 2005; Funderburgh et al. 2008) readily make keratocan and keratan sulfate. Furthermore, corneal fibroblasts treated with TGF-β do not make detectable levels of keratocan and keratan sulfate (Funderburgh et al. 2003) while keratocytes treated with TGF-β make reduced, but detectable levels of both (Funderburgh et al. 2001; Etheredge et al. 2009). These findings indicate that corneal fibroblasts are distinct from the normal and repair phenotypes seen in vivo and those phenotypes induced by the action of growth factors on keratocytes in vitro.
Summary and Concluding Remarks
Transparency of the corneal stromal ECM is accomplished by regulation collagen fibril growth and spacing. The major collagen of the stroma is collagen type I, but collagen type V is also required to initiate assembly of this collagen into fibrils. The growth of the initial diameter of the fibril is regulated by type V/type I interactions and it is modulated and maintained by the core proteins of the proteoglycans decorin, lumican, keratocan, and biglycan that interfere with and limit the lateral association of collagen molecules during fibril diameter growth. The spacing of the collagen fibrils is determined by the small uniform size of the proteoglycans and by the hydration properties of the keratan sulfate side chains. All these gene products as well as all the genes regulating the post-translational modifications of these genes products are expressed in many other ECMs but expressed in different proportions. This suggests that keratocytes may express a unique, yet to be discovered regulator gene that coordinates the expression levels of these genes to produce a transparent ECM.
Acknowledgments
Supported by Grants EY08104 (JRH) and EY05129 (DEB) from the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anseth A. Glycosaminoglycans in corneal regeneration. Exp Eye Res. 1961;1:122–127. doi: 10.1016/s0014-4835(61)80017-1. [DOI] [PubMed] [Google Scholar]
- Beales MP, Funderburgh JL, Jester JV, Hassell JR. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Invest Ophthalmol Vis Sci. 1999;40:1658–1663. [PubMed] [Google Scholar]
- Beecher N, Carlson C, Allen BR, Kipchumba R, Conrad GW, Meek KM, Quantock AJ. An x-ray diffraction study of corneal structure in mimecan-deficient mice. Invest Ophthalmol Vis Sci. 2005;46:4046–4049. doi: 10.1167/iovs.05-0325. [DOI] [PubMed] [Google Scholar]
- Benedek GB. Theory of transparency of the eye. Appl Opt. 1971;10:459–473. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- Bettelheim FA, Plessy B. The hydration of proteoglycans of bovine cornea. Biochim Biophys Acta. 1975;381:203–214. doi: 10.1016/0304-4165(75)90202-0. [DOI] [PubMed] [Google Scholar]
- Birk DE, Fitch JM, Babiarz JP, Doane KJ, Linsenmayer TF. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95 ( Pt 4):649–657. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- Birk DE, Fitch JM, Linsenmayer TF. Organization of collagen types I and V in the embryonic chicken cornea. Invest Ophthalmol Vis Sci. 1986;27:1470–1477. [PubMed] [Google Scholar]
- Blochberger TC, Vergnes JP, Hempel J, Hassell JR. cDNA to chick lumican (corneal keratan sulfate proteoglycan) reveals homology to the small interstitial proteoglycan gene family and expression in muscle and intestine. J Biol Chem. 1992;267:347–352. [PubMed] [Google Scholar]
- Bredrup C, Knappskog PM, Majewski J, Rodahl E, Boman H. Congenital stromal dystrophy of the cornea caused by a mutation in the decorin gene. Invest Ophthalmol Vis Sci. 2005;46:420–426. doi: 10.1167/iovs.04-0804. [DOI] [PubMed] [Google Scholar]
- Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43:2264–2271. [PMC free article] [PubMed] [Google Scholar]
- Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- Chakravarti S. Focus on molecules: keratocan (KERA) Exp Eye Res. 2006;82:183–184. doi: 10.1016/j.exer.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnery HR, Humphries T, Clare A, Dixon AE, Howes K, Moran CB, Scott D, Zakrzewski M, Pearlman E, McMenamin PG. Turnover of bone marrow-derived cells in the irradiated mouse cornea. Immunology. 2008;125:541–548. doi: 10.1111/j.1365-2567.2008.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintron C, Covington H, Kublin CL. Morphogenesis of rabbit corneal stroma. Invest Ophthalmol Vis Sci. 1983;24:543–556. [PubMed] [Google Scholar]
- Cintron C, Hassinger LC, Kublin CL, Cannon DJ. Biochemical and ultrastructural changes in collagen during corneal wound healing. J Ultrastruct Res. 1978;65:13–22. doi: 10.1016/s0022-5320(78)90017-5. [DOI] [PubMed] [Google Scholar]
- Cintron C, Kublin CL. Regeneration of corneal tissue. Dev Biol. 1977;61:346–357. doi: 10.1016/0012-1606(77)90304-9. [DOI] [PubMed] [Google Scholar]
- Cornuet PK, Blochberger TC, Hassell JR. Molecular polymorphism of lumican during corneal development. Invest Ophthalmol Vis Sci. 1994;35:870–877. [PubMed] [Google Scholar]
- Corpuz LM, Funderburgh JL, Funderburgh ML, Bottomley GS, Prakash S, Conrad GW. Molecular cloning and tissue distribution of keratocan. Bovine corneal keratan sulfate proteoglycan 37A. J Biol Chem. 1996;271:9759–9763. doi: 10.1074/jbc.271.16.9759. [DOI] [PubMed] [Google Scholar]
- Coulombre AJ, Coulombre JL. Corneal development. I. Corneal transparency. J Cell Physiol. 1958;51:1–11. doi: 10.1002/jcp.1030510102. [DOI] [PubMed] [Google Scholar]
- Davison PF, Galbavy EJ. Fluorescent dyes demonstrate the uniform expansion of the growing rabbit cornea. Invest Ophthalmol Vis Sci. 1985;26:1202–1209. [PubMed] [Google Scholar]
- Dawson DG, Kramer TR, Grossniklaus HE, Waring GO, 3rd, Edelhauser HF. Histologic, ultrastructural, and immunofluorescent evaluation of human laser-assisted in situ keratomileusis corneal wounds. Arch Ophthalmol. 2005;123:741–756. doi: 10.1001/archopht.123.6.741. [DOI] [PubMed] [Google Scholar]
- Del Pero RA, Gigstad JE, Roberts AD, Klintworth GK, Martin CA, L’Esperance FA, Jr, Taylor DM. A refractive and histopathologic study of excimer laser keratectomy in primates. Am J Ophthalmol. 1990;109:419–429. doi: 10.1016/s0002-9394(14)74608-2. [DOI] [PubMed] [Google Scholar]
- Dunlevy JR, Beales MP, Berryhill BL, Cornuet PK, Hassell JR. Expression of the keratan sulfate proteoglycans lumican, keratocan and osteoglycin/mimecan during chick corneal development. Exp Eye Res. 2000;70:349–362. doi: 10.1006/exer.1999.0789. [DOI] [PubMed] [Google Scholar]
- Dunlevy JR, Neame PJ, Vergnes JP, Hassell JR. Identification of the N-linked oligosaccharide sites in chick corneal lumican and keratocan that receive keratan sulfate. J Biol Chem. 1998;273:9615–9621. doi: 10.1074/jbc.273.16.9615. [DOI] [PubMed] [Google Scholar]
- Dunnington JH, Smelser GK. Incorporation of S35 in healing wounds in normal and devitalized corneas. AMA Arch Ophthalmol. 1958;60:116–129. doi: 10.1001/archopht.1958.00940080130016. [DOI] [PubMed] [Google Scholar]
- Etheredge L, Kane BP, Hassell JR. The effect of growth factor signaling on keratocytes in vitro and its relationship to the phases of stromal wound repair. Invest Ophthalmol Vis Sci. 2009;50:3128–3136. doi: 10.1167/iovs.08-3077. [DOI] [PubMed] [Google Scholar]
- Farrell RA, McCally RL, Tatham PE. Wave-length dependencies of light scattering in normal and cold swollen rabbit corneas and their structural implications. J Physiol. 1973;233:589–612. doi: 10.1113/jphysiol.1973.sp010325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburgh JL. Keratan sulfate: structure, biosynthesis, and function. Glycobiology. 2000;10:951–958. doi: 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Caterson B, Conrad GW. Keratan sulfate proteoglycan during embryonic development of the chicken cornea. Dev Biol. 1986;116:267–277. doi: 10.1016/0012-1606(86)90130-2. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Corpuz LM, Roth MR, Funderburgh ML, Tasheva ES, Conrad GW. Mimecan, the 25-kDa corneal keratan sulfate proteoglycan, is a product of the gene producing osteoglycin. J Biol Chem. 1997;272:28089–28095. doi: 10.1074/jbc.272.44.28089. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta -induced keratocytemyofibroblast transdifferentiation. J Biol Chem. 2001;276:44173–44178. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburgh JL, Hevelone ND, Roth MR, Funderburgh ML, Rodrigues MR, Nirankari VS, Conrad GW. Decorin and biglycan of normal and pathologic human corneas. Invest Ophthalmol Vis Sci. 1998;39:1957–1964. [PubMed] [Google Scholar]
- Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003;278:45629–45637. doi: 10.1074/jbc.M303292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburgh ML, Mann MM, Funderburgh JL. Keratocyte phenotype is enhanced in the absence of attachment to the substratum. Mol Vis. 2008;14:308–317. [PMC free article] [PubMed] [Google Scholar]
- Gan L, Fagerholm P, Palmblad J. Expression of basic fibroblast growth factor in rabbit corneal alkali wounds in the presence and absence of granulocytes. Acta Ophthalmol Scand. 2005;83:374–378. doi: 10.1111/j.1600-0420.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Hahn RA. Collagens. Cell Tissue Res. 339:247–257. doi: 10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, Kanter D, Funderburgh ML, Mann MM, Du Y, Funderburgh JL. A rapid transient increase in hyaluronan synthase-2 mRNA initiates secretion of hyaluronan by corneal keratocytes in response to transforming growth factor beta. J Biol Chem. 2007a;282:12475–12483. doi: 10.1074/jbc.M609280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Hutcheon AE, Melotti SA, Zieske JD, Trinkaus-Randall V, Ruberti JW. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2007b;48:4050–4060. doi: 10.1167/iovs.06-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnel C, Somodi S, Weiss DG, Guthoff RF. The keratocyte network of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea. 2000;19:185–193. doi: 10.1097/00003226-200003000-00012. [DOI] [PubMed] [Google Scholar]
- Hanna KD, Pouliquen Y, Waring GO, 3rd, Savoldelli M, Cotter J, Morton K, Menasche M. Corneal stromal wound healing in rabbits after 193-nm excimer laser surface ablation. Arch Ophthalmol. 1989;107:895–901. doi: 10.1001/archopht.1989.01070010917041. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Cintron C, Kublin C, Newsome DA. Proteoglycan changes during restoration of transparency in corneal scars. Arch Biochem Biophys. 1983;222:362–369. doi: 10.1016/0003-9861(83)90532-5. [DOI] [PubMed] [Google Scholar]
- Hassell JR, Kane BP, Etheredge LT, Valkov N, Birk DE. Increased stromal extracellular matrix synthesis and assembly by insulin activated bovine keratocytes cultured under agarose. Exp Eye Res. 2008;87:604–611. doi: 10.1016/j.exer.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell JR, Newsome DA, Hascall VC. Characterization and biosynthesis of proteoglycans of corneal stroma from rhesus monkey. J Biol Chem. 1979;254:12346–12354. [PubMed] [Google Scholar]
- Hassell JR, Newsome DA, Krachmer JH, Rodrigues MM. Macular corneal dystrophy: failure to synthesize a mature keratan sulfate proteoglycan. Proc Natl Acad Sci U S A. 1980;77:3705–3709. doi: 10.1073/pnas.77.6.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata R, Senoo H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J Cell Physiol. 1989;138:8–16. doi: 10.1002/jcp.1041380103. [DOI] [PubMed] [Google Scholar]
- Hayashida Y, Akama TO, Beecher N, Lewis P, Young RD, Meek KM, Kerr B, Hughes CE, Caterson B, Tanigami A, Nakayama J, Fukada MN, Tano Y, Nishida K, Quantock AJ. Matrix morphogenesis in cornea is mediated by the modification of keratan sulfate by GlcNAc 6-O-sulfotransferase. Proc Natl Acad Sci U S A. 2006;103:13333–13338. doi: 10.1073/pnas.0605441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998;39:276–283. [PubMed] [Google Scholar]
- Hong JW, Liu JJ, Lee JS, Mohan RR, Woods DJ, He YG, Wilson SE. Proinflammatory chemokine induction in keratocytes and inflammatory cell infiltration into the cornea. Invest Ophthalmol Vis Sci. 2001;42:2795–2803. [PubMed] [Google Scholar]
- Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- Jester JV. Corneal crystallins and the development of cellular transparency. Semin Cell Dev Biol. 2008;19:82–93. doi: 10.1016/j.semcdb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994;35:730–743. [PubMed] [Google Scholar]
- Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505–516. [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Barry PA, Cavanagh HD. Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing. Invest Ophthalmol Vis Sci. 1995;36:809–819. [PubMed] [Google Scholar]
- Kane BP, Jester JV, Huang J, Wahlert A, Hassell JR. IGF-II and collagen expression by keratocytes during postnatal development. Exp Eye Res. 2009 doi: 10.1016/j.exer.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao WW, Funderburgh JL, Xia Y, Liu CY, Conrad GW. Focus on molecules: lumican. Exp Eye Res. 2006;82:3–4. doi: 10.1016/j.exer.2005.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay EP, Lee MS, Seong GJ, Lee YG. TGF-beta s stimulate cell proliferation via an autocrine production of FGF-2 in corneal stromal fibroblasts. Curr Eye Res. 1998;17:286– 293. doi: 10.1076/ceyr.17.3.286.5212. [DOI] [PubMed] [Google Scholar]
- Lance SE, Capone A, Jr, SundarRaj N, Roat MI, Thoft RA. Diamond burring and surgical keratectomy. Morphologic comparison in the rabbit. Arch Ophthalmol. 1988;106:830–834. doi: 10.1001/archopht.1988.01060130900049. [DOI] [PubMed] [Google Scholar]
- Lee RE, Davison PF, Cintron C. The healing of linear nonperforating wounds in rabbit corneas of different ages. Invest Ophthalmol Vis Sci. 1982;23:660–665. [PubMed] [Google Scholar]
- Li DQ, Tseng SC. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163:61–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- Li W, Vergnes JP, Cornuet PK, Hassell JR. cDNA clone to chick corneal chondroitin/dermatan sulfate proteoglycan reveals identity to decorin. Arch Biochem Biophys. 1992;296:190–197. doi: 10.1016/0003-9861(92)90562-b. [DOI] [PubMed] [Google Scholar]
- Lim M, Goldstein MH, Tuli S, Schultz GS. Growth factor, cytokine and protease interactions during corneal wound healing. Ocul Surf. 2003;1:53–65. doi: 10.1016/s1542-0124(12)70128-3. [DOI] [PubMed] [Google Scholar]
- Liu CY, Birk DE, Hassell JR, Kane B, Kao WW. Keratocan-deficient mice display alterations in corneal structure. J Biol Chem. 2003;278:21672–21677. doi: 10.1074/jbc.M301169200. [DOI] [PubMed] [Google Scholar]
- Ljubimov AV, Alba SA, Burgeson RE, Ninomiya Y, Sado Y, Sun TT, Nesburn AB, Kenney MC, Maguen E. Extracellular matrix changes in human corneas after radial keratotomy. Exp Eye Res. 1998;67:265–272. doi: 10.1006/exer.1998.0511. [DOI] [PubMed] [Google Scholar]
- Madisen L, Neubauer M, Plowman G, Rosen D, Segarini P, Dasch J, Thompson A, Ziman J, Bentz H, Purchio AF. Molecular cloning of a novel bone-forming compound: osteoinductive factor. DNA Cell Biol. 1990;9:303–309. doi: 10.1089/dna.1990.9.303. [DOI] [PubMed] [Google Scholar]
- Maguen E, Alba SA, Burgeson RE, Butkowski RJ, Michael AF, Kenney MC, Nesburn AB, Ljubimov AV. Alterations of corneal extracellular matrix after multiple refractive procedures: a clinical and immunohistochemical study. Cornea. 1997;16:675–682. [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci U S A. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice DM. The structure and transparency of the cornea. J Physiol. 1957;136:263–286. doi: 10.1113/jphysiol.1957.sp005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midura RJ, Hascall VC. Analysis of the proteoglycans synthesized by corneal explants from embryonic chicken. II. Structural characterization of the keratan sulfate and dermatan sulfate proteoglycans from corneal stroma. J Biol Chem. 1989;264:1423–1430. [PubMed] [Google Scholar]
- Midura RJ, Hascall VC, MacCallum DK, Meyer RF, Thonar EJ, Hassell JR, Smith CF, Klintworth GK. Proteoglycan biosynthesis by human corneas from patients with types 1 and 2 macular corneal dystrophy. J Biol Chem. 1990;265:15947–15955. [PubMed] [Google Scholar]
- Muller LJ, Pels L, Vrensen GF. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995;36:2557–2567. [PubMed] [Google Scholar]
- Musselmann K, Alexandrou B, Kane B, Hassell JR. Maintenance of the keratocyte phenotype during cell proliferation stimulated by insulin. J Biol Chem. 2005;280:32634–32639. doi: 10.1074/jbc.M504724200. [DOI] [PubMed] [Google Scholar]
- Musselmann K, Hassell JR. Focus on molecules: CHST6 (carbohydrate sulfotransferase 6; corneal N-acetylglucosamine-6-sulfotransferase) Exp Eye Res. 2006;83:707–708. doi: 10.1016/j.exer.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Musselmann K, Kane B, Alexandrou B, Hassell JR. Stimulation of collagen synthesis by insulin and proteoglycan accumulation by ascorbate in bovine keratocytes in vitro. Invest Ophthalmol Vis Sci. 2006;47:5260–5266. doi: 10.1167/iovs.06-0612. [DOI] [PubMed] [Google Scholar]
- Musselmann K, Kane BP, Alexandrou B, Hassell JR. IGF-II is present in bovine corneal stroma and activates keratocytes to proliferate in vitro. Exp Eye Res. 2008;86:506–511. doi: 10.1016/j.exer.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselmann K, Kane BP, Hassell JR. Isolation of a putative keratocyte activating factor from the corneal stroma. Exp Eye Res. 2003;77:273–279. doi: 10.1016/s0014-4835(03)00160-x. [DOI] [PubMed] [Google Scholar]
- Neame PJ, Kay CJ, McQuillan DJ, Beales MP, Hassell JR. Independent modulation of collagen fibrillogenesis by decorin and lumican. Cell Mol Life Sci. 2000;57:859–863. doi: 10.1007/s000180050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegata NS, Dieguez-Lucena JL, Joensuu T, Lau S, Montgomery KT, Krahe R, Kivela T, Kucherlapati R, Forsius H, de la Chapelle A. Mutations in KERA, encoding keratocan, cause cornea plana. Nat Genet. 2000;25:91–95. doi: 10.1038/75664. [DOI] [PubMed] [Google Scholar]
- Poole CA, Brookes NH, Clover GM. Keratocyte networks visualised in the living cornea using vital dyes. J Cell Sci. 1993;106 ( Pt 2):685–691. doi: 10.1242/jcs.106.2.685. [DOI] [PubMed] [Google Scholar]
- Quantock AJ, Young RD. Development of the corneal stroma, and the collagen-proteoglycan associations that help define its structure and function. Dev Dyn. 2008 doi: 10.1002/dvdy.21579. [DOI] [PubMed] [Google Scholar]
- Rada JA, Cornuet PK, Hassell JR. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp Eye Res. 1993;56:635–648. doi: 10.1006/exer.1993.1081. [DOI] [PubMed] [Google Scholar]
- Rada JA, Fini ME, Hassell JR. Regionalized growth patterns of young chicken corneas. Invest Ophthalmol Vis Sci. 1996;37:2060–2067. [PubMed] [Google Scholar]
- Rodahl E, Van Ginderdeuren R, Knappskog PM, Bredrup C, Boman H. A second decorin frame shift mutation in a family with congenital stromal corneal dystrophy. Am J Ophthalmol. 2006;142:520–521. doi: 10.1016/j.ajo.2006.03.064. [DOI] [PubMed] [Google Scholar]
- Saika S. Ultrastructural effect of L-ascorbic acid 2-phosphate on cultured keratocytes. Cornea. 1992;11:439–445. doi: 10.1097/00003226-199209000-00014. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Kimata K. Proteoglycan-Lb, a small dermatan sulfate proteoglycan expressed in embryonic chick epiphyseal cartilage, is structurally related to osteoinductive factor. J Biol Chem. 1992;267:1265–1270. [PubMed] [Google Scholar]
- Stapleton WM, Chaurasia SS, Medeiros FW, Mohan RR, Sinha S, Wilson SE. Topical interleukin-1 receptor antagonist inhibits inflammatory cell infiltration into the cornea. Exp Eye Res. 2008;86:753–757. doi: 10.1016/j.exer.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Sundarraj N, Fite D, Belak R, Sundarraj S, Rada J, Okamoto S, Hassell J. Proteoglycan distribution during healing of corneal stromal wounds in chick. Exp Eye Res. 1998;67:433–442. doi: 10.1006/exer.1998.0540. [DOI] [PubMed] [Google Scholar]
- Toole BP, Trelstad RL. Hyaluronate production and removal during corneal development in the chick. Dev Biol. 1971;26:28–35. doi: 10.1016/0012-1606(71)90104-7. [DOI] [PubMed] [Google Scholar]
- Tuominen IS, Tervo TM, Teppo AM, Valle TU, Gronhagen-Riska C, Vesaluoma MH. Human tear fluid PDGF-BB, TNF-alpha and TGF-beta1 vs corneal haze and regeneration of corneal epithelium and subbasal nerve plexus after PRK. Exp Eye Res. 2001;72:631–641. doi: 10.1006/exer.2001.0999. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Brunskill EW, Bell SM, Chervoneva I, Birk DE. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Davidson JM, Phillips CL, Pfeiffer BJ, Menezes DW, Chervoneva I, Birk DE. Murine model of the Ehlers-Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J Biol Chem. 2006;281:12888–12895. doi: 10.1074/jbc.M511528200. [DOI] [PubMed] [Google Scholar]
- Williams BR, Gelman RA, Poppke DC, Piez KA. Collagen fibril formation. Optimal in vitro conditions and preliminary kinetic results. J Biol Chem. 1978;253:6578–6585. [PubMed] [Google Scholar]
- Yoshida S, Shimmura S, Shimazaki J, Shinozaki N, Tsubota K. Serum-free spheroid culture of mouse corneal keratocytes. Invest Ophthalmol Vis Sci. 2005;46:1653–1658. doi: 10.1167/iovs.04-1405. [DOI] [PubMed] [Google Scholar]
- Young RD, Gealy EC, Liles M, Caterson B, Ralphs JR, Quantock AJ. Keratan sulfate glycosaminoglycan and the association with collagen fibrils in rudimentary lamellae in the developing avian cornea. Invest Ophthalmol Vis Sci. 2007;48:3083–3088. doi: 10.1167/iovs.06-1323. [DOI] [PubMed] [Google Scholar]
- Yue BY, Hsieh P, Baum JL. Effects of corneal extracts on rabbit corneal stromal cells in culture. Invest Ophthalmol Vis Sci. 1986;27:14–19. [PubMed] [Google Scholar]
- Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE. Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem. 2009;284:8888–8897. doi: 10.1074/jbc.M806590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Beuerman RW, Huang L, Barathi A, Foo YH, Li SF, Chew FT, Tan D. Proteomic analysis of rabbit tear fluid: Defensin levels after an experimental corneal wound are correlated to wound closure. Proteomics. 2007;7:3194–3206. doi: 10.1002/pmic.200700137. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Guimaraes SR, Hutcheon AE. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp Eye Res. 2001;72:33–39. doi: 10.1006/exer.2000.0926. [DOI] [PubMed] [Google Scholar]