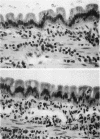
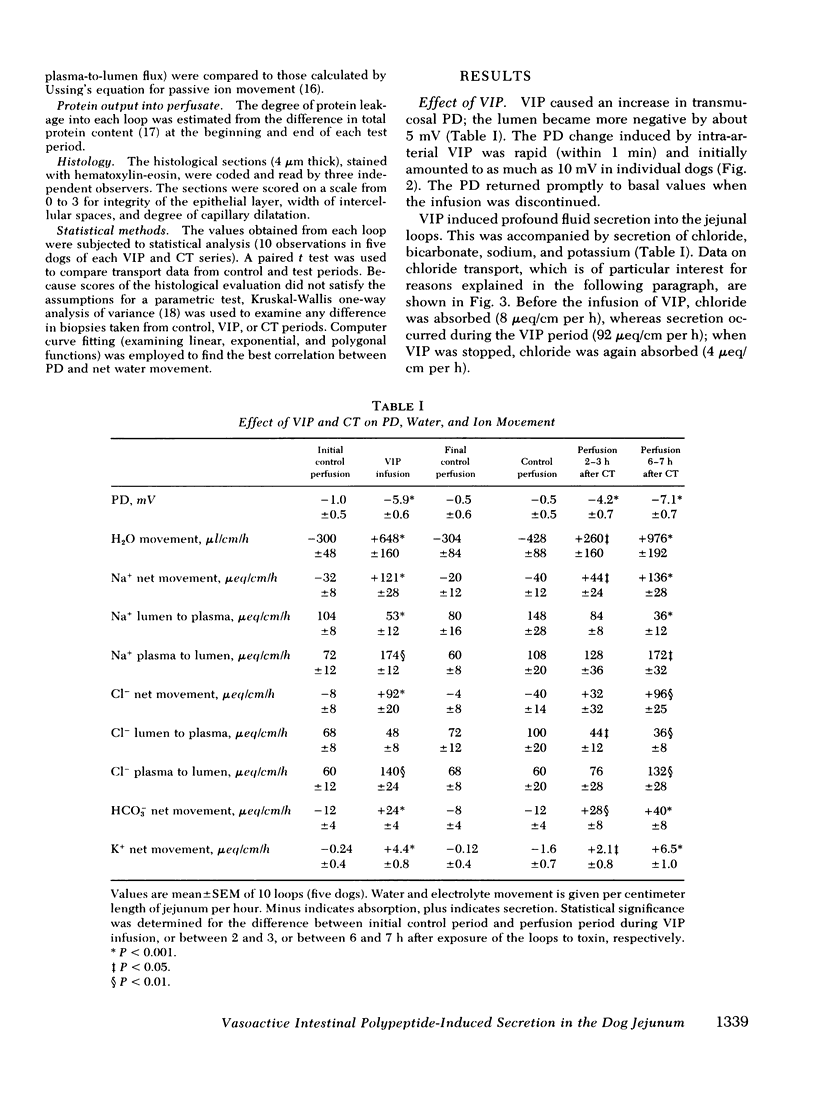
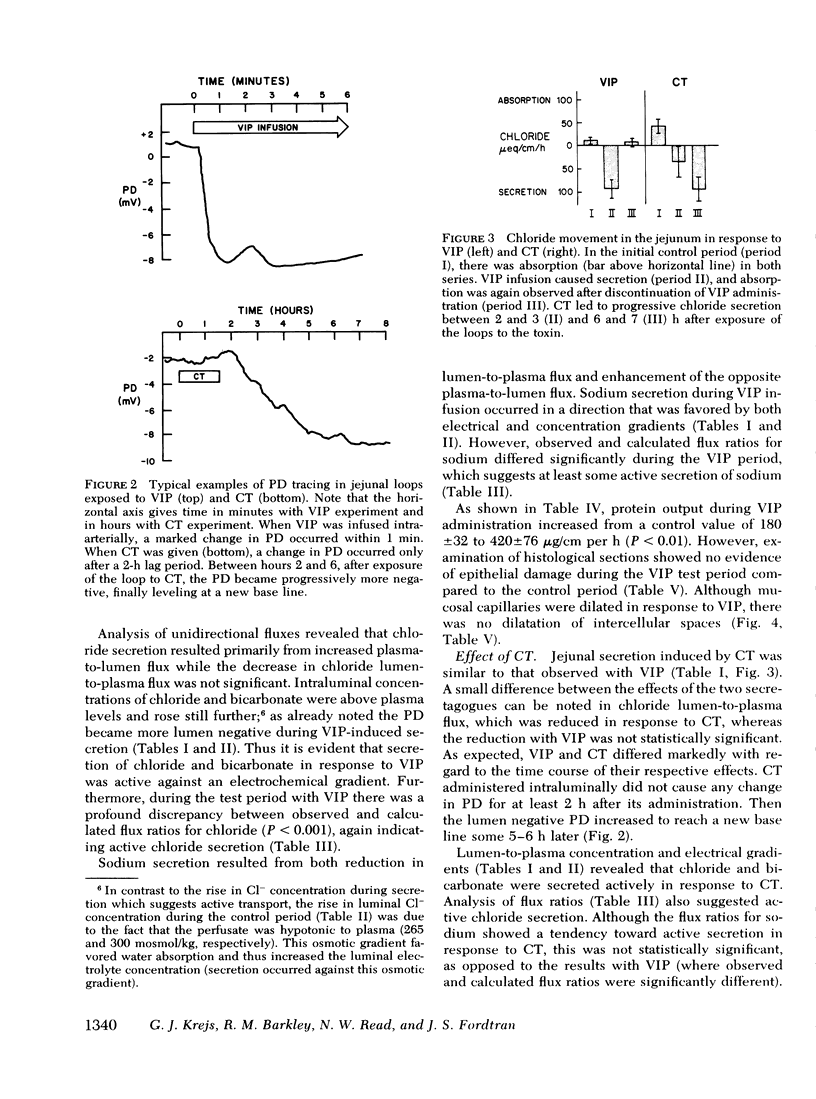
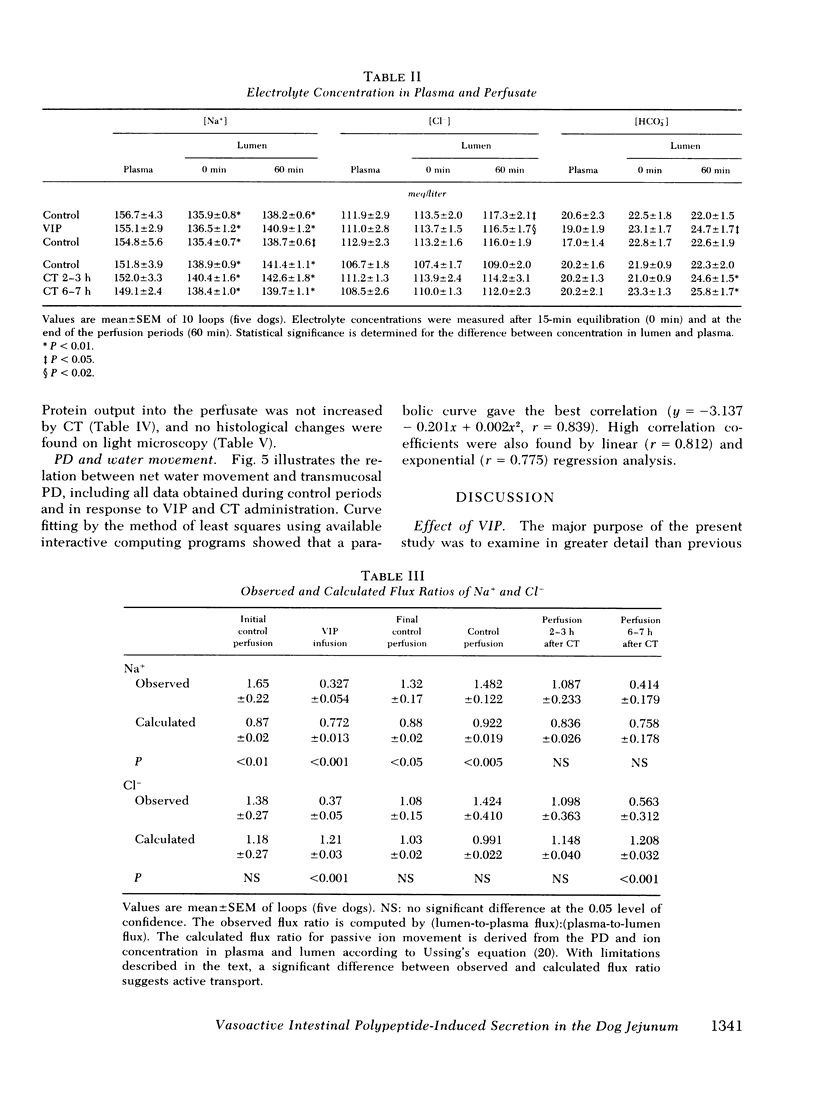
Abstract
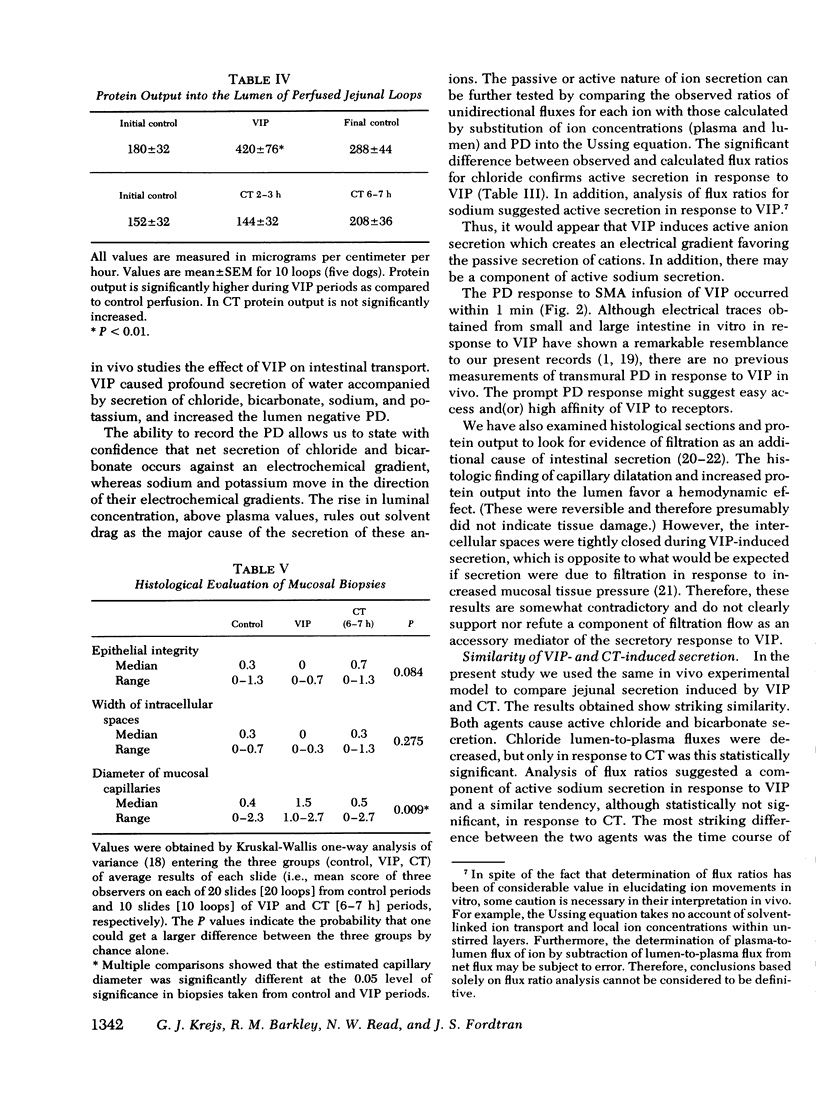
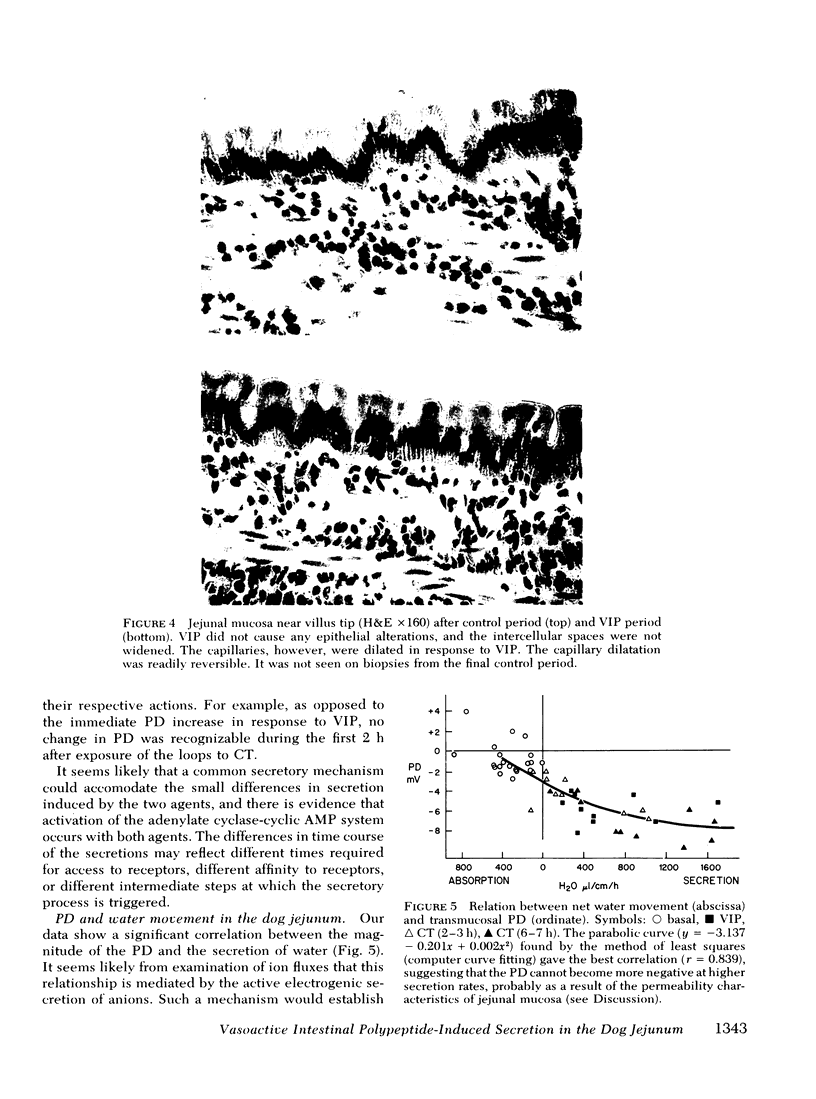
The effect of vasoactive intestinal polypeptide (VIP) on intestinal water and electrolyte transport and transmucosal potential difference was investigated in the dog jejunum in vivo and compared to secretion induced by cholera toxin. Isolated jejunal loops were perfused with a plasma-like electrolyte solution. VIP (0.08 μg/kg per min) was administered directly into the superior mesenteric artery by continuous infusion over 1 h. From a dye dilution method, it was estimated that a mean plasma VIP concentration of 12,460 pg/ml reached the loops. VIP caused secretion of water and electrolytes; for example, chloride: control, 8 μeq/cm per h absorption; VIP, 92 μeq/cm per h secretion. A marked increase in transmucosal potential difference (control, −1.0 mV; VIP, −5.9 mV, lumen negative) occurred within 1 min after starting VIP infusion. Analysis of unidirectional fluxes showed increased plasma-to-lumen flux of sodium and chloride and decreased lumen-to-plasma flux of sodium. Chloride and bicarbonate were actively secreted against an electrochemical gradient. Although sodium secretion occurred down an electrochemical gradient, flux ratio analysis suggested a component of active sodium secretion. VIP caused a slight increase in protein output into the loops; light microscopy revealed capillary dilatation and closed intercellular spaces. The effect of VIP was readily reversible. Except for the delayed onset of secretion, the effect of cholera toxin was qualitatively similar to VIP; however, capillary dilatation and increased protein output were not noted with cholera toxin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGER E. Y., STEELE J. M. The calculation of transfer rates in two compartment systems not in dynamic equilibrium. J Gen Physiol. 1958 Jul 20;41(6):1135–1152. doi: 10.1085/jgp.41.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbezat G. O. Stimulation of intestinal secretion by polypeptide hormones. Scand J Gastroenterol Suppl. 1973;22:1–21. [PubMed] [Google Scholar]
- Coupar I. M. Stimulation of sodium and water secretion without inhibition of glucose absorption in the rat jejunum by vasoactive intestinal peptide (VIP). Clin Exp Pharmacol Physiol. 1976 Nov-Dec;3(6):615–618. doi: 10.1111/j.1440-1681.1976.tb00643.x. [DOI] [PubMed] [Google Scholar]
- DiBona D. R., Chen L. C., Sharp G. W. A study of intercellular spaces in the rabbit jejunum during acute volume expansion and after treatment with cholera toxin. J Clin Invest. 1974 May;53(5):1300–1307. doi: 10.1172/JCI107677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORDTRAN J. S., LEVITAN R., BIKERMAN V., BURROWS B. A., INGELFINGER F. J. The kinetics of water absorption in the human intestine. Trans Assoc Am Physicians. 1961;74:195–206. [PubMed] [Google Scholar]
- Field M. Intestinal secretion. Gastroenterology. 1974 May;66(5):1063–1084. [PubMed] [Google Scholar]
- Finkelstein R. A., Fujita K., LoSpalluto J. J. Procholeragenoid: an aggregated intermediate in the formation of choleragenoid. J Immunol. 1971 Oct;107(4):1043–1051. [PubMed] [Google Scholar]
- Krejs G. J., Seelig L. L., Fordtran J. S. Effect of protonated 2,4,6-triaminopyrimidine, a tight junction blocker, on intestinal transport in dog ileum in vivo. Gastroenterology. 1977 Apr;72(4 Pt 1):685–691. [PubMed] [Google Scholar]
- LEVITAN R., FORDTRAN J. S., BURROWS B. A., INGELFINGER F. J. Water and salt absorption in the human colon. J Clin Invest. 1962 Sep;41:1754–1759. doi: 10.1172/JCI104634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Makhlouf G. M. Distinct mechanisms for stimulation of intestinal secretion by vasoactive intestinal peptide (VIP) and glucagon. Gastroenterology. 1977 Feb;72(2):368–369. [PubMed] [Google Scholar]
- Moore W. L., Jr, Bieberdorf F. A., Morawski S. G., Finkelstein R. A., Fordtran J. S. Ion transport during cholera-induced ileal secretion in the dog. J Clin Invest. 1971 Feb;50(2):312–318. doi: 10.1172/JCI106496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Greenough W. B., 3rd, Carpenter C. C., Jr Vibrio cholerae enterotoxin and its mode of action. Bacteriol Rev. 1971 Mar;35(1):1–13. doi: 10.1128/br.35.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. W., Binder H. J., Curran P. F. Active electrolyte secretion stimulated by choleragen in rabbit ileum in vitro. Am J Physiol. 1973 Oct;225(4):781–787. doi: 10.1152/ajplegacy.1973.225.4.781. [DOI] [PubMed] [Google Scholar]
- Powell D. W. Intestinal conductance and permselectivity changes with theophylline and choleragen. Am J Physiol. 1974 Dec;227(6):1436–1443. doi: 10.1152/ajplegacy.1974.227.6.1436. [DOI] [PubMed] [Google Scholar]
- Racusen L. C., Binder H. J. Alteration of large intestinal electrolyte transport by vasoactive intestinal polypeptide in the rat. Gastroenterology. 1977 Oct;73(4 Pt 1):790–796. [PubMed] [Google Scholar]
- Read N. W., Smallwood R. H., Levin R. J., Holdsworth C. D., Brown B. H. Relationship between changes in intraluminal pressure and transmural potential difference in the human and canine jejunum in vivo. Gut. 1977 Feb;18(2):141–151. doi: 10.1136/gut.18.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. E., Lust W. D., Sircar B., Goldberg N. D. Elevated concentration of adenosine 3':5'-cyclic monophosphate in intestinal mucosa after treatment with cholera toxin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):851–856. doi: 10.1073/pnas.67.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C. J., Kimberg D. V., Sheerin H. E., Field M., Said S. I. Vasoactive intestinal peptide stimulation of adenylate cyclase and active electrolyte secretion in intestinal mucosa. J Clin Invest. 1974 Sep;54(3):536–544. doi: 10.1172/JCI107790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W. Action of cholera toxin on fluid and electrolyte movement in the small intestine. Annu Rev Med. 1973;24:19–23. doi: 10.1146/annurev.me.24.020173.000315. [DOI] [PubMed] [Google Scholar]
- Yablonski M. E., Lifson N. Mechanism of production of intestinal secretion by elevated venous pressure. J Clin Invest. 1976 Apr;57(4):904–915. doi: 10.1172/JCI108367. [DOI] [PMC free article] [PubMed] [Google Scholar]