Abstract
We have shown that mice with experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis, have upregulated leptin receptor expression in reactive astrocytes of the hippocampus, a region involved in sickness behavior. Leptin can exacerbate EAE when its serum concentration is high. Although leptin receptors in astrocytes modulate leptin transport across cultured endothelial cell monolayers, it is not known how leptin transport in EAE mice is regulated. Here, we determined brain and cervical spinal cord uptake of leptin in early and recovery stages of EAE, after either intravenous delivery or in situ brain perfusion of 125I-leptin and the vascular marker 131I-albumin. While increased vascular space and general blood–brain barrier (BBB) permeability after EAE were expected, the specific saturable transport system for leptin crossing the BBB also persisted. Moreover, there was upregulation of leptin transport in hippocampus and cervical spinal cord in the early stage of EAE, shown by higher leptin uptake in these regions and by competitive inhibition with coadministered excess unlabeled leptin. We conclude that EAE induced a time- and region-specific increase of leptin transport. The results provide a link between circulating leptin and enhanced leptin signaling that may play a crucial role in disease progression.
Keywords: Leptin, EAE, Blood—brain barrier, Transport, Hippocampus
Introduction
Experimental autoimmune encephalomyelitis (EAE) is an autoimmune model for multiple sclerosis and shares cardinal features of blood—brain barrier (BBB) damage, immune cell infiltration into the central nervous system (CNS), and T cell-mediated demyelination. In the brain of EAE mice, we have shown upregulation of leptin receptor expression in reactive astrocytes in the hippocampus and hypothalamus (Wu et al. 2013). In normal mice, leptin is produced mainly by adipocytes to serve a multitude of physiological actions, including suppression of feeding behavior and modulation of immune function. Leptin acts on the immune system of EAE mice and is generally thought to promote autoimmunity by promoting the T helper (Th)-1 response (Matarese et al. 2001b) and suppressing regulatory T cells (Matarese et al. 2005).
Most of the CNS effects of leptin involve its transport across the BBB (Banks et al. 1996). All membrane-bound leptin receptors can effectively endocytose leptin and have the potential of transporting leptin across the BBB given a sufficient level of expression there (Tu et al. 2007, 2010). In endothelia–astrocyte coculture studies, we have observed that the level and subtypes of leptin receptors in astrocytes modulate leptin transport across the endothelial monolayer (Hsuchou et al. 2010). Even in an in vitro model of astrogliosis by scratch wound injury, leptin potentiates reactive gliosis and promotes inflammation (Hsuchou et al. 2012). By contrast, inhibition of astrocytic metabolic activity by fluorocitrate enhances leptin signaling in neurons after its intracerebroventricular delivery (Pan et al. 2011a). However, it is not yet known how leptin transport in the EAE mice is regulated. In this study, we tested the hypothesis that EAE induces upregulation of leptin transport across the BBB.
Material and Method
EAE Induction
Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC). SJL/J female mice of 8–10 weeks old (Jackson Laboratory, Bar Harbor, ME, USA) were used for induction of EAE. Each mouse received 100 µl of incomplete Freund's adjuvant emulsified with 500 µg of heat-killed Mycobacterium tuberculosis H37Ra (both from Difco Laboratories, Detroit, MI, USA), generating complete Freund's adjuvant (CFA), along with 80 µg of proteolipid protein fragment 139–151 (PLP139–151, AnaSpec Inc., Fremont, CA, USA). The injection was performed by the subcutaneous route in three areas in the lower flank. Control mice received CFAwithout PLP peptide. Naïve controls were also included. Pertussis toxin (200 ng/100 µl/mouse, List Biological Laboratories, Campbell, CA, USA) was injected intraperitoneally to these mice on the day of immunization (day 0) and again 48 h later (day 2). EAE was scored daily as described previously (Pan et al. 1996; Wu et al. 2010, 2013): 0, no detectable signs of weakness; 0.5, distal tail limpness, mild postural changes, or reduced locomotor activity; 1, completely limp tail; 1.5, limp tail and hind limb weakness (unsteady gait and poor grip with hind limbs); 2, unilateral partial hind limb paralysis; 2.5, bilateral hind limb paralysis; 3, complete bilateral hind limb paralysis; 3.5, complete hind limb paralysis and unilateral forelimb paralysis; 4, total paralysis of hind limbs and forelimbs; and 5, moribund or dead.
BBB Permeability Studies After Intravenous (IV) Injection of 125I-Leptin
Carrier-free recombinant mouse leptin (R&D Systems, Minneapolis, MN, USA) was radioactively labeled with 125I (PerkinElmer, Boston, MA, USA) by the chloramine-T method as described previously (Pan et al. 2008a). Bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, MO, USA) was labeled with 131I by the same method to provide a control reflecting the vascular space and general BBB permeability by paracellular and transcellular routes. The radioactively labeled proteins were purified by elution on Sephadex G-10 columns. The specific activity of 125I-leptin was 98.5 Ci/g and that of 131I-albumin was 25.7 Ci/g.
We focused on two time points in the course of EAE, termed early stage (presymptomatic phase, about day 7 after EAE induction) and late stage (early recovery phase, about day 21, 6 days after the peak EAE scores). Seven groups of mice were used for iv studies: (1) naïve mice without any treatment; (2) CFA controls at early stage; (3) CFA controls at late stage; (4) EAE at early stage; (5) EAE at late stage; (6) EAE at early stage, with coadministration of unlabeled leptin at a dose that was 200-fold more than that of the 125I-leptin; and (7) EAE at late stage, with coadministration of excess unlabeled leptin. Unequal sample size (n = 4–8 mice/group) was not intentional but mandated by inclusion of all essential controls on each experimental day. Most mice were studied simultaneously on 1 day, after being induced at different days to reduce experimental bias related to decay of the radioactively labeled tracers over time. The mice and groups were assigned randomly to avoid interference by circadian factors.
After anesthesia, each mouse received an iv bolus injection of 100 µl lactated Ringer's solution containing 125I-leptin, 131I-albumin (about 1.5 µCi each), and 1 % BSA through an exposed left jugular vein. For groups receiving 200-fold excess unlabeled leptin in addition to the radioactively labeled proteins, 3 µg of leptin/mouse was included in the 100 µl of injection solution and allowed to incubate for 1 h. Immediately after blood collection by dissection of the right carotid artery 10 min after iv injection, the mice were decapitated. The hippocampus was further dissected from the brain as a focus of the study, based on its known upregulation of astrocytic leptin receptors (Wu et al. 2013), impaired BBB functions during EAE (Zlokovic et al. 1989), and a main role in sickness behavior (Godbout et al. 2008). Cervical spinal cord was separated from the total spinal cord, as it has higher permeability than the thoracic spinal cord during the course of EAE (Pan et al. 1996) and is easy to perfuse in the parallel in situ brain perfusion study (shown below). The collected tissues were weighed and the radioactivity of 125I and 131I in the tissues and 50 µl of serum from each mouse were determined with a dual-channel program in a Wallac Wizard 1470 gamma counter. The tissue uptake was calculated [(counts per minute per gram of tissue) / (counts per minute per microliter of serum), or microliters per gram].
BBB Transport Assay by In Situ Perfusion with 125I-Leptin and 131I-Albumin
Three groups of mice were used for in-situ brain perfusion study at the early symptomatic phase or recovery phase of EAE: (1) controls that received CFA (combined from both day 9 and day 25, n = 4), (2) early stage EAE (day 9 PLP in this batch, n = 8), and (3) late stage (day 25 PLP, n = 7). The study days were delayed in comparison with those used in the iv transport assay for better cross-validation and potential to observe greater differences among the groups. After anesthesia, the mice were perfused through the left ventricle, with the right atrium severed. The descending aorta was clamped immediately below the diaphragm, ensuring that the cervical segment of the spinal cord was adequately perfused. The detailed perfusion method is discussed elsewhere (Pan et al. 1998; Pan and Kastin 2000). The perfusion was driven by a syringe pump at a speed of 2 ml/min. The modified Zlokovic's buffer (Zlokovic et al. 1986) was used as perfusate (in grams per liter: NaCl, 7.19; KCl, 0.3; CaCl2, 0.28; NaHCO3, 2.1; KH2PO4, 0.16; MgCl2 6H2O, 0.37, d-glucose, 0.99, pH 7.4, and 10 g/l of BSA added after oxygenation). The vascular space was cleared by 2-min preperfusion with perfusate only. Immediately after that, the mice were perfused with perfusate containing 125I-leptin and 131I-albumin (1,000 cpm/µl each) for 5 min, followed by 1-min postwash with perfusate only to remove residual material remaining in the vascular space. The brain and cervical spinal cord were collected after decapitation. The radioactivity of the weighed brain, cervical spinal cord, and 50 µl perfusate were measured with the dual-channel program in the gamma counter.
Statistical Analysis
Group means are represented with standard errors. Group means of tissue per serum ratio were compared by one-way analysis of variance followed by Tukey's test using IBM SPSS statistical software and Prism GraphPad software.
Results
125I-Leptin Uptake by the Brain and Spinal Cord After IV Bolus Injection
EAE symptoms were evident on day 9, peaked at days 15–16, and started to resolve thereafter. The mean EAE score was 0.125±0.09 on day 9, 1.792±0.345 on day 16, and 1.292±0.156 on day 20 (n=12), whereas mice injected with only CFA had a mean score consistently below 0.2 on each day.
When total brain was measured, the 131I-albumin uptake was not significantly different from that of the naïve controls (Fig. 1a). At the presymptomatic phase (day 7), neither the CFA control nor EAE induction induced an increase of general permeability of the BBB. There was no difference between the EAE mice receiving radioactively labeled tracer only or with inclusion of 200-fold excess of unlabeled leptin. At the recovery phase (day 21), the group of EAE mice receiving excess unlabeled leptin showed an increase of 131I-albumin uptake. Nonetheless, there was no difference between the EAE groups with or without excess unlabeled leptin.
Fig. 1.
Uptake of 131I-albumin (a) and 125I-leptin (b) in the brain 10 min after iv injection. The uptake of albumin was increased only in the group of EAE mice receiveing radioactively labeled tracers and unlabeled leptin on day 21 (*p<0.05 in comparison with the naïve control). The uptake of leptin was not increased in any group, but excess unlabeled leptin was able to inhibit the uptake in the EAE mice (*p<0.05; **p<0.01; ***p<0.005)
On either day 7 (presymptomatic) or day 21 (recovery phase), there was no significant increase of 125I-leptin uptake in the total brain 10 min after iv injection. The CFA only and EAE groups did not differ from each other. The presence of excess unlabeled leptin inhibited the uptake of 125I-leptin on both day 7 (p<0.01) and day 21 (p<0.005), indicating that the transport system for leptin to cross the BBB remained functional (Fig. 1b).
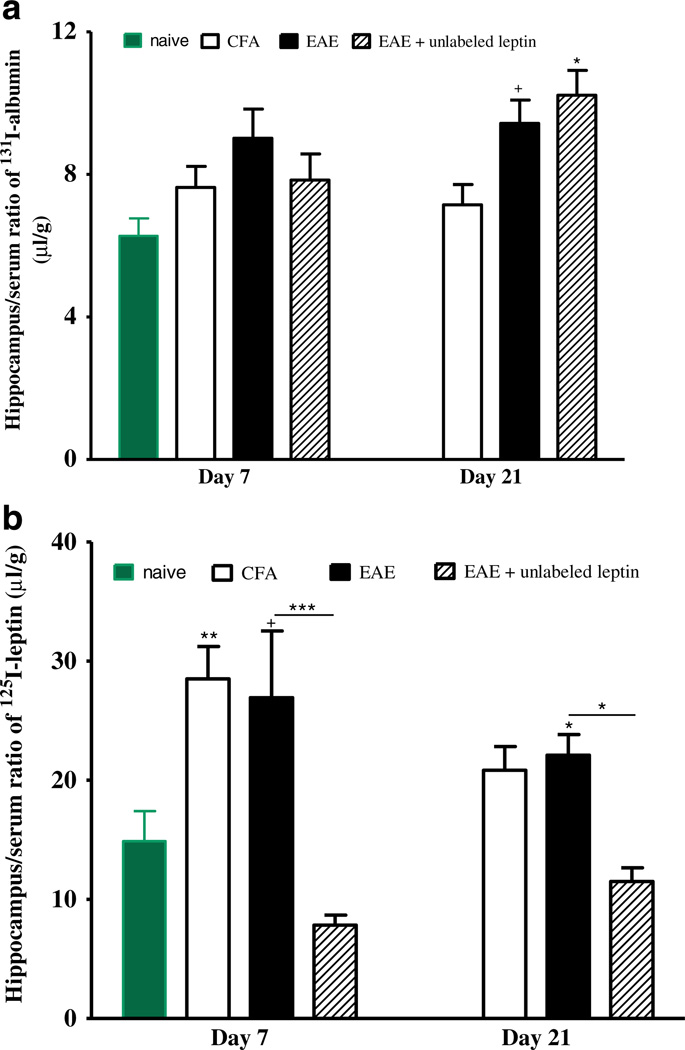
However, there were regional differences in the increase of uptake of radioactively labeled tracer. While cerebral cortex, hypothalamus, and brainstem did not show a significant increase of 131I-albumin uptake on either day, the hippocampus tended to have higher tissue/serum ratio of 131I-albumin than the naïve mice and CFA control on day 21 (p=0.07). The presence of excess unlabeled leptin did not affect the persistently high uptake of 131I-albumin (Fig. 2a).
Fig. 2.
Uptake of 131I-albumin (a) and 125I-leptin (b) in the hippocampus 10 min after iv injection. Albumin uptake tended to increase in EAE mice on day 21 (+p=0.07) and was higher in EAE mice receiving excess leptin on day 21 (*p<0.05). Leptin uptake was increased in the mice treated only with CFA on day 7, EAE mice on day 21, and tended to increase in the EAE mice on day 7 in comparison with naïve controls. The higher leptin uptake in the EAE mice remained inhibitable by excess unlabeled leptin. +p=0.09; *p<0.05; **p<0.01; ***p<0.005
It appears that CFA alone was sufficient to upregulate leptin transport from blood to the hippocampus (Fig. 2b). On day 7, there was a higher hippocampus/serum ratio of 125I-leptin in the group of mice receiving CFA when compared with the naïve control (p<0.01). The EAE group showed only a nonsignificant marginal increase (p=0.09), but the presence of excess unlabeled leptin significantly inhibited the uptake (p<0.005). On day 21, the increase was less pronounced, and excess unlabeled leptin remained effective (p<0.05).
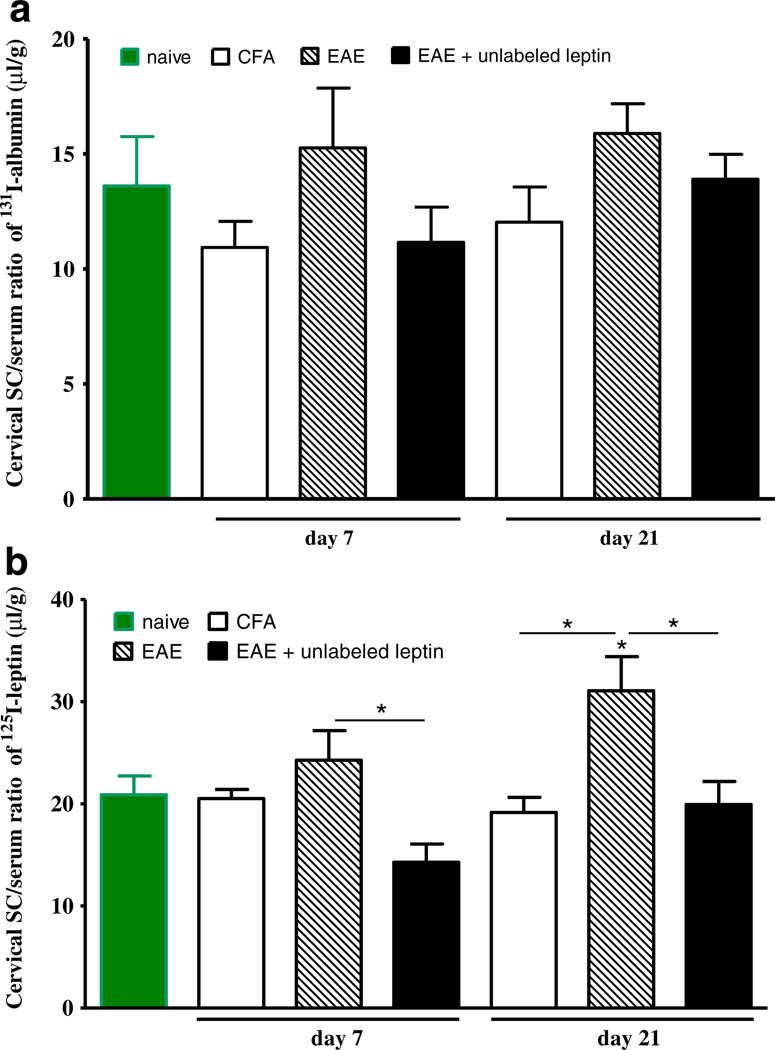
In the cervical spinal cord, the increase of albumin uptake did not reach statistical significance on either day 7 or day 21, probably influenced by the large individual variation and low statistical power. It appears that EAE induced a greater increase than only CFA on both days (Fig. 3a). The uptake of 125I-leptin showed a different pattern, and the increase from both naïve control and CFA control was significant on day 21 (p<0.05). On both days, excess unlabeled leptin was able to inhibit the uptake of 125I-leptin into the cervical spinal cord (p<0.05).
Fig. 3.
Uptake of 131I-albumin (a) and 125I-leptin (b) in the cervical spinal cord 10 min after iv injection. Albumin uptake was unchanged. Leptin uptake was increased in EAE mice on day 21 in comparison with both naïve and CFA controls. Excess unlabeled leptin inhibited the uptake of 125I-leptin in EAE mice on both days. *p<0.05
125I-Leptin Uptake by the Brain and Spinal Cord After In Situ Brain Perfusion
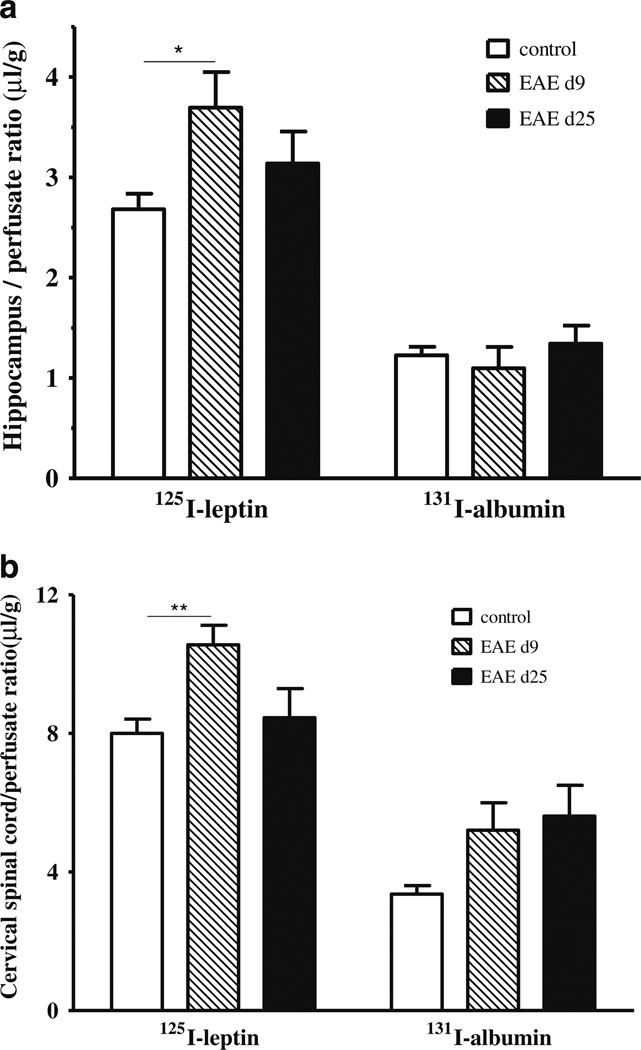
Leptin blood concentrations differ at different stages of EAE, reaching a nadir around day 20 in C57 mice immunized with myelin oligodendrocyte glycoprotein (MOG)35–55 (Sanna et al. 2003). To avoid the confounding effect of variable endogenous concentrations of leptin on assays after iv injection and CNS uptake, we performed in situ brain perfusion on separate batches of mice. Since maintenance of tissue viability is crucial for the experiment, we used a 5-min perfusion time with oxygenated perfusion buffer. This is half of the duration of the iv injection study shown above. In the hippocampus (Fig. 4a), the uptake of 131I-albumin was unchanged at 5 min of in situ brain perfusion in either the presymptomatic phase (day 9) or recovery phase (day 25). By contrast, 125I-leptin uptake was higher in the EAE mice 9 days after induction than the adjuvant only control group (p<0.05). In the cervical spinal cord (Fig. 4b), the increase of 131I-albumin did not reach significance on either day, but the uptake of 125I-albumin was higher on day 9 (p<0.01), though it had returned to baseline on day 25.
Fig. 4.
Uptake of 131I-albumin (right) and 125I-leptin (left) in the hippocampus (a) and cervical spinal cord (b) 5 min after in situ brain perfusion. In both regions, only 125I-leptin in the early phase of EAE (day 9) was increased. *p<0.05; **p<0.01
Discussion
There has been convincing evidence that leptin has biphasic roles depending on its site of action. After peripheral administration, leptin increases the susceptibility of male SJL/J mice to EAE and potentiates the symptoms of EAE in female SJL/J mice (Matarese et al. 2001b). EAE symptoms and associated autoimmunity are attenuated with antileptin antibodies or a soluble mouse leptin receptor chimera (ObR: Fc) (De Rosa et al. 2006). Mice without leptin production (ob/ob mice) are resistant to EAE (Matarese et al. 2001a). By contrast, astrocyte-specific leptin receptor knockout mice show higher EAE scores and worsened immune cell infiltration (Mishra PK, unpublished observations). As an absence of leptin signaling in astrocytes is detrimental and promotes EAE, this indicates a protective role of CNS leptin against EAE, at least as conferred by astrocytes. The difference is probably emphasized by the regulatory effects of the BBB, known to either potentiate or modulate cytokine signaling (Pan et al. 2011b) as well as control availability by way of specific transport systems. The direction of change of transport systems can include upregulation, such as tumor necrosis factor alpha (TNF) transport in EAE mice (Pan et al. 1996) and those after spinal cord injury (Pan et al. 1999, 2003a, 2003c), brain trauma (Pan et al. 2003b), stroke (Pan et al. 2006), and interleukin-15 permeation in lipopolyssachride-induced neuroinflammation (Pan et al. 2008b). Alternatively, there may be a downregulation of transport, as seen for interleukin-15 uptake in EAE mice (Hsuchou et al. 2009), or no change in many other systems. Since the regulation of leptin transport at the level of the BBB may be a crucial mediating mechanism of the differential effects of leptin in EAE, we determined how leptin uptake is regulated over the course of EAE.
In SJL/J mice, we have also observed robust upregulation of astrocytic ObR, the specific receptor for leptin, in astrocytes of the hippocampus (Wu et al. 2013). In coculture of astrocytes and endothelia flanking a Transwell membrane, the level and subtype of ObR affect the efficiency of leptin transcytosis across the BBB, with ObRb and ObRe attenuating leptin transport (Hsuchou et al. 2010). With a higher ObRb protein expression in peak disease and ObRa mRNA increased only after recovery from EAE (Wu et al. 2013), we predicted that leptin transport would be decreased and there would be an interaction with a higher paracellular as well as transcellular permeability shown by coadministered 131I-albumin. The results, however, showed that the increase of albumin uptake mainly occurred in the recovery phase of EAE, whereas leptin uptake was upregulated mainly in the early stage of EAE. The ability of excess unlabeled leptin to inhibit the uptake of 125I-leptin indicates that the transport system remained intact and functioned at a higher level. Thus, the increased uptake of leptin was not caused by increased paracellular permeability resulting from impaired BBB function. The results suggest that the upregulated astrocytic leptin receptor may not be a driving force for increased leptin transport. On the contrary, we predict that ObR expression in cerebral microvessels is upregulated in EAE, and this probably mediates increased leptin uptake in the early stage of EAE. The increased leptin transport seen after both iv delivery and in situ brain perfusion contrasts with that reported in mice challenged with lipopolysaccharide (3 mg/kg) 8–12 h later, in which leptin transport was decreased, rather than increased, and was only seen after iv injection, suggesting the presence of circulating inhibitory factors (Nonaka et al. 2004). The difference in these two models further supports the specificity of regulatory changes of leptin transport that is not simply caused by inflammation alone.
In this study, 131I-albumin was used as a reference substance for the vascular space and general BBB permeability because 131I facilitates dual channel detection in a gamma counter, and there is no perfect standard because of the different molecular sizes of reference materials. Although 131I-albumin uptake in the hippocampus was only marginally increased after iv injection in the EAE mice in the recovery phase, the d-[3H]mannitol space is higher in both early and recovery phases of in situ brain perfusion studies (Zlokovic et al. 1989). In the hippocampus of EAE mice, there is activation of neurogenesis with a higher number of double cortin-positive immature neurons, though these cells have higher expression of glial fibrillary acidic protein (GFAP), suggesting the presence of GFAP (+) neuroprogenitor cells, and perhaps greater astrocytic differentiation (Huehnchen et al. 2011). Meanwhile, there is hippocampal degeneration in EAE mice, associated with chronic microglial activation and demyelination (Ziehn et al. 2010). As leptin is known to modulate hippocampal synaptic activities (Shanley et al. 2001; Farr et al. 2006; Oomura et al. 2006), the increased leptin uptake might differentially contribute to neuronal and astrocytic signaling, thus altering synaptic activities. In all, leptin may play a role in rescuing the worsening of hippocampus-dependent learning and memory in the EAE mice (Mandolesi et al. 2010).
CFA alone induces a late-acting increase of BBB permeability, with higher CSF immunoglobulin content, similar to that seen in EAE (Walls et al. 1988). The immunologic response to M. tuberculosis, a main component of CFA, may be mainly responsible, whereas the emulsion stabilizer polyoxyethylene (10) tridecyl ether also impairs BBB functions (Namer et al. 1994). Interestingly, CFA did not increase albumin uptake in either hippocampus or cervical spinal cord but elevated leptin uptake in the hippocampus only in the presymptomatic phase. The cervical spinal cord is usually considered a vulnerable region in EAE and in patients with multiple sclerosis; however, the increase of permeability of its BBB was less pronounced than that seen in the hippocampus. Nonetheless, the leptin transport system in the cervical spinal cord was also upregulated.
In summary, by use of complementary iv injection and in situ brain perfusion approaches, we showed that EAE increased the transport of leptin into the hippocampus and cervical spinal cord. The changes were more pronounced in the early phase of EAE rather than the recovery phase and were dissociated from a subtle increase of general BBB permeability that was mainly seen in the recovery phase. The region- and time-specific increase of leptin uptake by the brain might explain the differential effects of peripheral and CNS leptin, resulting from variable cellular targets (immune cells for circulating leptin and CNS residential cells having already crossed the BBB).
Acknowledgments
Grant support is provided by the NIH (DK54880 and DK92245 to AJK, and NS 62291 to WP).
References
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- De Rosa V, Procaccini C, La CA, Chieffi P, Nicoletti GF, Fontana S, Zappacosta S, Matarese G. Leptin neutralization interferes with pathogenic T cell autoreactivity in autoimmune encephalomyelitis. J Clin Invest. 2006;116:447–455. doi: 10.1172/JCI26523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420–1425. doi: 10.1016/j.peptides.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, O'Connor J, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, Kastin AJ, Pan W. Blood-borne metabolic factors in obesity exacerbate injury-induced gliosis. J Mol Neurosci. 2012;47:267–277. doi: 10.1007/s12031-012-9734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, Kastin AJ, Tu H, Abbott NJ, Couraud P-O, Pan W. Role of astrocytic leptin receptor subtypes on leptin permeation across hCMEC/D3 human brain endothelial cells. J Neurochem. 2010;115:1288–1298. doi: 10.1111/j.1471-4159.2010.07028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, Pan W, Wu X, Kastin AJ. Cessation of blood-to-brain influx of interleukin-15 during development of EAE. J Cereb Blood Flow Metab. 2009;29:1568–1578. doi: 10.1038/jcbfm.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huehnchen P, Prozorovski T, Klaissle P, Lesemann A, Ingwersen J, Wolf SA, Kupsch A, Aktas O, Steiner B. Modulation of adult hippocampal neurogenesis during myelin-directed autoimmune neuroinflammation. GLIA. 2011;59:132–142. doi: 10.1002/glia.21082. [DOI] [PubMed] [Google Scholar]
- Mandolesi G, Grasselli G, Musumeci G, Centonze D. Cognitive deficits in experimental autoimmune encephalomyelitis: neuroinflammation and synaptic degeneration. Neurol Sci. 2010;31:S255–S259. doi: 10.1007/s10072-010-0369-3. [DOI] [PubMed] [Google Scholar]
- Matarese G, Carrieri PB, La Cava A, Perna F, De Sanna V, De Rossa R, Fontana S, Aufiero D, Zappacosta S. Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2005;102:5150–5155. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese G, Di Giacomo A, Sanna V, Lord GM, Howard JK, Di TA, Bloom SR, Lechler RI, Zappacosta S, Fontana S. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol. 2001a;166:5909–5916. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- Matarese G, Sanna V, Di Giacomo A, Lord GM, Howard JK, Blood SR, Lechler RI, Fontana S, Zappacosta S. Leptin potentiates experimental autoimmune encephalomyelitis in SJL female mice and confers susceptibility to males. Eur J Immunol. 2001b;31:1324–1332. doi: 10.1002/1521-4141(200105)31:5<1324::AID-IMMU1324>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Namer IJ, Steibel J, Poulet P, Mauss Y, Mohr M, Chambron J. The role of Mycobacterium tuberculosis in experimental allergic encephalomyelitis. Eur Neurol. 1994;34:224–227. doi: 10.1159/000117043. [DOI] [PubMed] [Google Scholar]
- Nonaka N, Hileman SM, Shioda S, Vo TQ, Banks WA. Effects of lipopolysaccharide on leptin transport across the blood—brain barrier. Brain Res. 2004;1016:58–65. doi: 10.1016/j.brainres.2004.04.066. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, Kohno D, Uramura K, Sougawa H, Yada T, Wayner MJ, Sasaki K. Leptin J Mol Neurosci facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood—brain barrier. Neuropharmcol. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Kennedy MK, Gutierrez EG, Kastin AJ. Differential permeability of the BBB in acute EAE: enhanced transport of TNF-a. Am J Physiol. 1996;271:E636–E642. doi: 10.1152/ajpendo.1996.271.4.E636. [DOI] [PubMed] [Google Scholar]
- Pan W, Csernus B, Kastin AJ. Upregulation of p55 and p75 receptors mediating TNF alpha transport across the injured blood—spinal cord barrier. J Mol Neurosci. 2003a;21:173–184. doi: 10.1385/JMN:21:2:173. [DOI] [PubMed] [Google Scholar]
- Pan W, Ding Y, Yu Y, Ohtaki H, Nakamachi T, Kastin AJ. Stroke upregulates TNFalpha transport across the blood—brain barrier. Exp Neurol. 2006;198:222–233. doi: 10.1016/j.expneurol.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, Tu H, Kastin AJ. Developmental changes of leptin receptors in cerebral microvessels: unexpected relation to leptin transport. Endocrinology. 2008a;149:877–885. doi: 10.1210/en.2007-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, Xu CL, Wu X, Bouret SG, Kastin AJ. Astrocytes modulate distribution and neuronal signaling of leptin in the hypothalamus of obese Avy mice. J Mol Neurosci. 2011a;43:478–484. doi: 10.1007/s12031-010-9470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, Yu C, Kastin AJ. Permeation of blood-borne IL15 across the blood—brain barrier and the effect of LPS. J Neurochem. 2008b;106:313–319. doi: 10.1111/j.1471-4159.2008.05390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Interactions of IGF-1 with the blood—brain barrier in vivo and in situ. Neuroendocrinology. 2000;72:171–178. doi: 10.1159/000054584. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ, Bell RL, Olson RD. Upregulation of tumor necrosis factor a transport across the blood—brain barrier after acute compressive spinal cord injury. J Neurosci. 1999;19:3649–3655. doi: 10.1523/JNEUROSCI.19-09-03649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ, McLay RN, Rigai T, Pick CG. Increased hippocampal uptake of TNFa and behavioral changes in mice. Exp Brain Res. 2003b;149:195–199. doi: 10.1007/s00221-002-1355-7. [DOI] [PubMed] [Google Scholar]
- Pan W, Stone KP, Hsuchou H, Manda VK, Zhang Y, Kastin AJ. Cytokine signaling modulates blood—brain barrier function. Curr Pharm Des. 2011b;17:3729–3740. doi: 10.2174/138161211798220918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Zhang L, Liao J, Csernus B, Kastin AJ. Selective increase in TNFa permeation across the blood—spinal cord barrier after SCI. J Neuroimmunol. 2003c;134:111–117. doi: 10.1016/s0165-5728(02)00426-5. [DOI] [PubMed] [Google Scholar]
- Sanna V, Di GA, La CA, Lechler RI, Fontana S, Zappacosta S, Matarese G. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest. 2003;111:241–250. doi: 10.1172/JCI16721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Hsuchou H, Kastin AJ, Wu X, Pan W. Unique leptin trafficking by a tailless receptor. FASEB J. 2010;24:2281–2291. doi: 10.1096/fj.09-143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Pan W, Feucht L, Kastin AJ. Convergent trafficking pattern of leptin after endocytosis mediated by ObRa—ObRd. J Cell Physiol. 2007;212:215–222. doi: 10.1002/jcp.21020. [DOI] [PubMed] [Google Scholar]
- Walls AF, Suckling AJ, Rumsby MG. Autoantibody responses in the cerebrospinal fluid of guinea pigs with chronic relapsing experimental allergic encephalomyelitis. Acta Neurol Scand. 1988;78:422–428. doi: 10.1111/j.1600-0404.1988.tb03680.x. [DOI] [PubMed] [Google Scholar]
- Wu X, Mishra PK, Hsuchou H, Kastin AJ, Pan W. Upregulation of astrocytic leptin receptor in mice with experimental autoimmune encephalomyelitis. J Mol Neurosci. 2013;49:446–456. doi: 10.1007/s12031-012-9825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Pan W, He Y, Hsuchou H, Kastin AJ. Cerebral interleukin- 15 shows upregulation and beneficial effects in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;223:65–72. doi: 10.1016/j.jneuroim.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziehn MO, Avedisian AA, Tiwari-Woodruff S, Voskuhl RR. Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab Invest. 2010;90:774–786. doi: 10.1038/labinvest.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Begley DJ, Djuricic B, Mitrovic DM. Measurement of solute transport across the blood—brain barrier in the perfused guinea-pig brain: method and application to N-methyl-aaminoisobutyric acid. J Neurochem. 1986;46:1444–1451. doi: 10.1111/j.1471-4159.1986.tb01760.x. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Skundric DS, Segal MB, Colover J, Jankov RM, Pejnovic N, Lackovic V, Mackic J, Lipovac MN, Davson H. Blood— brain barrier permeability changes during acute allergic encephalomyelitis induced in the guinea pig. Metab Brain Dis. 1989;4:33–40. doi: 10.1007/BF00999491. [DOI] [PubMed] [Google Scholar]