Abstract
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS). The etiology of MS is not well understood, but it is believed that myelin-specific CD4+ T cells play a central role in initiating and orchestrating CNS inflammation. In this scenario, CD4+ T cells, activated in the periphery, infiltrate the CNS, where, by secreting cytokines and chemokines, they start an inflammatory cascade. Given the central role of CD4+ T cells in CNS autoimmunity, they have been studied extensively, principally by using experimental autoimmune encephalomyelitis (EAE), an animal model of MS. In the late 1980s, CD4+ T cells, based on their cytokine production, were divided into two helper lineages, Th1 and Th2 cells. It was postulated that Th1 cells, which produce IFN-γ, mediate inflammation of the CNS in MS/EAE, while Th2 cells, which produce IL-4, have a beneficial effect in disease, because of their antagonistic effect on Th1 cells. The Th1/Th2 paradigm remained the prevailing view of MS/EAE pathogenesis until 2005, when a new lineage, Th17, was discovered. In a relatively short period of time it became apparent that Th17 cells, named after their hallmark cytokine, IL-17A, play a crucial role in many inflammatory diseases, including EAE, and likely in MS as well. The Th17 paradigm developed rapidly, initiating the debate whether Th1 cells contribute to EAE/MS pathogenesis at all, or if they might even have a protective role due to their antagonistic effects on Th17 cells. Numerous findings support the view that Th17 cells play an essential role in autoimmune CNS inflammation, perhaps mainly in the initial phases of disease. Th1 cells likely contribute to pathogenesis, with their role possibly more pronounced later in disease. Hence, the current view on the role of Th cells in MS/EAE pathogenesis can be called the Th17/Th1 paradigm. It is certain that Th17 cells will continue to be the focus of intense investigation aimed at elucidating the pathogenesis of CNS autoimmunity.
Keywords: multiple sclerosis, EAE, Th1, Th9, Th17, Treg, γδ T cell, IL-23, GM-CSF, IL-27
Introduction
Multiple sclerosis (MS) is a disease that affects the central nervous system (CNS), including the brain, spinal cord and optic nerves (1). MS is characterized by overall reduction in CNS volume, and by accumulation of immune cells mainly in the white matter of various CNS areas, although substantial number of plaques can be found in the grey matter, leading to formation of localized inflammatory foci (2). Inflammatory processes in these foci cause damage to myelin and destruction of oligodendrocytes, injury to axons and their loss, and transient impairment or permanent loss of neurologic function, resulting in disabilities of various types and severity.
The etiology of MS remains elusive, but it is clear that both genetic and environmental factors, perhaps infections, play a role in its development (3). Given that in MS the immune system damages the CNS, MS is considered to be an autoimmune disease. It is believed that CD4+ T cells specific for CNS antigens, most likely myelin components, play a pivotal role in initiation and perpetuation of MS. The prevailing view of MS pathogenesis has been largely formed by analogy to experimental autoimmune encephalomyelitis (EAE), an animal model of MS (4-6). In this model, myelin-specific CD4+ T cells that have developed in peripheral lymphoid organs infiltrate the CNS, where they encounter their cognate antigens presented by local antigen presenting cells (APC). This interaction with APCs leads to re-activation of myelin-specific CD4+ T cells, which in turn activate APCs by cell-cell contact and by secreted pro-inflammatory products, such as cytokines and chemokines. Secretion of pro-inflammatory mediators attracts various immune cells into the CNS, where they are activated and start secreting mediators that damage surrounding CNS tissue, leading to formation of lesions and eventually to neurologic deficits (7).
Th1/Th2 paradigm of MS/EAE
Given that in the prevailing view CD4+ T cells play a central role in CNS autoimmunity, a great deal of MS research has been focused on these cells. In the late 1980s, Mosman et al. (8, 9) postulated that CD4+ T cells can be divided into two helper T cell (Th) types, Th1 and Th2. Th1 cells are characterized by IFN-γ production and have a principal role in defense against intracellular pathogens, whereas Th2 cells produce IL-4 and are mainly responsible for clearance of extracellular parasites.
Analyses of immune responses that develop after immunization with myelin antigens for EAE induction revealed that they are dominated by IFN-γ+ Th1 cells and that these cells are the most abundant among CD4+ T cells found in the CNS of animals with EAE (10, 11). Furthermore, Th1, but not Th2 myelin-specific cells, were capable of inducing EAE when adoptively transferred into recipient mice (12, 13). These findings led to the conclusion that Th1 cells mediate EAE, and likely, by extension, also MS (14). This conclusion was supported by data showing abundant IFN-γ in CNS lesions of animals with EAE (15) and in active lesions of MS patients (16). At that time IFN-γ was regarded as an exclusively pro-inflammatory cytokine (17), as its numerous immunoregulatory functions were yet to be discovered. The view that Th1 cells mediate CNS inflammation by secreting IFN-γ, which activates other immune cells (i.e. monocytes, macrophages, neutrophils) (18) and drives them to damage CNS tissue, was supported by findings that, when administered to MS patients, IFN-γ exacerbated disease (19).
Additional supporting data for the Th1 paradigm came from STAT4-deficient mice, which were also resistant to EAE (20). STAT4 is a transcription factor necessary for IL-12 signaling downstream of its receptor (21). In addition, T-bet, a master transcription factor necessary for development of Th1 lineage, proved to be necessary for EAE development, as T-bet−/− mice are resistant to EAE (22).
Taken together, the aforementioned findings converged to strongly support a critical role of IL-12/Th1/IFN-γ axis in EAE/MS pathogenesis. However, it is now known that neither IFN-γ nor IL-12 is necessary for EAE development, and that both of them have a suppressive net effect on disease (23-26). The most important findings supporting the limited role of IFN-γ in disease came from the use of knock-out animal models and neutralization of IFN-γ with antibodies. Several groups demonstrated that treatment with anti-IFN-γ antibodies exacerbated EAE in various strains of mice (27-29). Observations in IFN-γ−/− and IFN-γR−/− mice confirmed that IFN-γ limits EAE (24, 25, 30-32). In the following section we review the paradigm shift from IL-12/Th1 cells to IL-23/Th17 cells in the pathogenesis of EAE.
IL-12 in EAE
One of the most convincing lines of evidence supporting the Th1 paradigm came from studies on IL-12. This heterodimeric cytokine comprising IL-12p40 and IL-12p35 subunits is produced by activated APCs; it promotes development of Th1 cells and in particular their IFN-γ expression (33, 34). Blockade of IL-12 by neutralizing anti-IL-12p40 antibodies or genetic deficiency in this molecule conferred resistance to EAE induction (35)(36-40). This was thought to result from impairment in development of anti-myelin Th1 responses and greatly reduced IFN-γ production in the absence of IL-12 bioactivity (41). Experiments based on treatment with rIL-12 yielded less consistent data on the role of IL-12 in EAE pathogenesis than those with knockout mice. rIL-12 exhibited opposite effects on EAE depending on the disease phase when treatment was initiated (41-47). Most studies agree that treatment with rIL-12 during the priming phase, before disease onset, suppresses EAE, while treatment initiated after clinical disease has already manifested, or during the chronic/remitting phase, exacerbates disease.
Our laboratory has shown that, in addition to immune system cells, CNS resident cells, both astrocytes and microglia, can produce the IL-12p40 subunit when exposed to inflammatory stimuli (LPS) (48). These cells also secreted TNF and IL-6, suggesting that CNS cells can contribute locally to exacerbation of CNS inflammation in EAE/MS by promoting anti-CNS Th1 responses via production of IL-12 and other pro-inflammatory mediators as well.
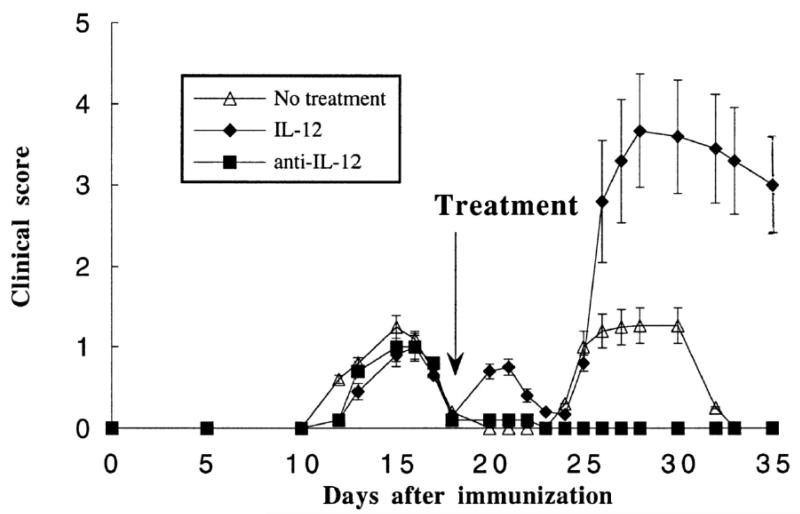
(PL/J x SJL/J)F1 mice immunized with myelin basic protein (MBP) develop relapsing-remitting EAE. We have shown that IL-12 administration induces relapses and enhances their severity and frequency (Figure 1). In agreement with these disease-promoting effects of IL-12, neutralization of IL-12 with anti-IL-12p40 Ab blocked relapses (Figure 1). This was true in the case of both spontaneous relapses and staphylococcal enterotoxin-induced relapses (49).
Figure 1.
Effect of IL-12 and of neutralizing anti-IL-12p40 mAb on the course of relapsing EAE. After recovery from the initial attack of EAE, mice were treated as indicated by the arrow. IL-12 treatment induced immediate relapses and worsening later relapses. Anti-IL-12p40 Ab prevented spontaneous relapses. Results are shown as mean × SD of clinical scores. The course of EAE in mice treated with control rat IgG overlaps completely with that of mice receiving no treatment and is not shown. (Figure first published in Journal of Immunology, 161:5097-5104, 1998, Constantinescu C et al., Antibodies against interleukin-12 prevent superantigen-induced and spontaneous relapses of experimental autoimmune encephalomyelitis. Copyright 1998. The American Association of Immunologists, Inc.)
Lack of CD40L-CD40 interaction, as in CD40-deficient mice, results in resistance to EAE. Administration of rIL-12 overcomes resistance to EAE in the absence of CD40L-CD40 interaction (46). Anti-IL-12p40 Ab prevented the reversal induced by exogenous IL-12 and protected mice from EAE. These results demonstrated that IL-12 is sufficient to overcome the CD40L blockade and suggested that induction of IL-12 by CD40L-CD40 interaction is essential for induction of EAE.
Measurement of IFN-γ and IL-4 production by MBP-stimulated lymphocytes from EAE-susceptible SJL/J and EAE-resistant BALB/c mice showed that lymphocytes of SJL/J mice produced IFN-γ and no IL-4, while lymphocytes of BALB/c mice had the opposite pattern of cytokine production (50). Neutralization of IL-12 with anti-IL-12p40 Ab protected SJL/J mice from EAE, whereas BALB/c mice that were treated with neutralizing anti-IL-4 Ab developed EAE. These findings confirmed the crucial role of IL-12 in EAE susceptibility and showed that IL-4 is important for conveying resistance in strains resistant to EAE.
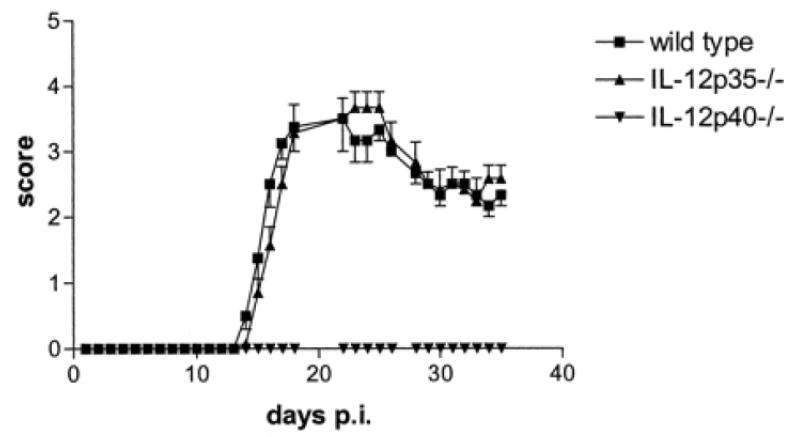
Given that IL-12 is a heterodimeric cytokine, composed of covalently bound p40 and p35 subunits forming IL-12p70, it would be expected that deficiency in either of the subunits results in the same phenotype, as IL-12p70 cannot be formed. However, p40-deficient mice were resistant to EAE, while p35-deficient mice were fully susceptible (23) (Figure 2). Typical inflammation and demyelination were observed in spinal cords of p35-deficient mice, whereas p40-deficient mice had normal spinal cords. p35-deficient mice developed somewhat weaker anti-MOG35-55 Th1 responses, with lower production of IFN-γ compared to WT mice. In contrast, p40-deficient mice developed stronger Th2 responses to MOG35-55. Microglia, CNS-infiltrating macrophages, and CD4+ T cells of p35-deficient and WT mice produced TNF, while those same cells from p40-deficient mice did not. These data suggested that a heterodimeric cytokine containing p40, other than IL-12p70, and perhaps IL-23 (p40p19 heterodimer), which had been recently cloned at that time, might play an important role in EAE.
Figure 2.
IL-12p35−/− are susceptible to EAE, while IL-12p40−/− mice are resistant. Female WT, IL-12p35−/−, and IL-12p40−/− mice were immunized with MOG35-55 in CFA. Data represent the mean clinical scores × SEM. The overall clinical score was not significantly different between WT and IL-12p35−/− mice. (Figure first published in the Journal of Immunology, 169: 7104-7110, 2002, Gran B. et al., IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: Evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. Copyright 2002. The American Association of Immunologists, Inc.)
The IL-12 receptor consists of IL-12Rβ1 and IL-12Rβ2 subunits expressed by Th1 cells (51-53). If IL-12 plays a crucial role in EAE development it would be expected that lack of its receptor would result in resistance to disease. To determine the role of IL-12Rβ1 in the development of EAE we used mice deficient in this receptor subunit. IL-12Rβ1−/− mice were completely resistant to EAE induction and exhibited Th2 skewed responses against the immunogen, MOG35-55 (54). In a co-culture of purified CD4+ T cells and APCs of MOG-immunized mice IL-12Rβ1−/− APCs drove CD4+ T cells of both WT and IL-12Rβ1−/− mice toward Th2 lineage, whereas WT APCs induced Th1 lineage. In turn, IL-12Rβ1−/− CD4+ T cells suppressed production of IFN-γ and TNF by WT APCs. Furthermore, decreased levels of IL-12p40, p35, and IL-23p19 mRNA were found in IL-12Rβ1−/− APCs. IL-18 production and IL-18Rα expression were also significantly decreased in immunized IL-12Rβ1−/− mice. We concluded that signaling involving IL-12Rβ1 drives development of encephalitogenic Th1 responses. We now know that IL-12Rβ1 is a subunit of not only IL-12R but also of IL-23R (55) and that the lack of IL-12Rβ1 in IL-12Rβ1−/− mice simultaneously prevented signaling of both IL-12 and IL-23. Hence, resistance of IL-12Rβ1−/− mice to EAE should be attributed to the lack of IL-23 signaling and defective Th17 development rather than to the lack of IL-12 signaling and impaired Th1 responses.
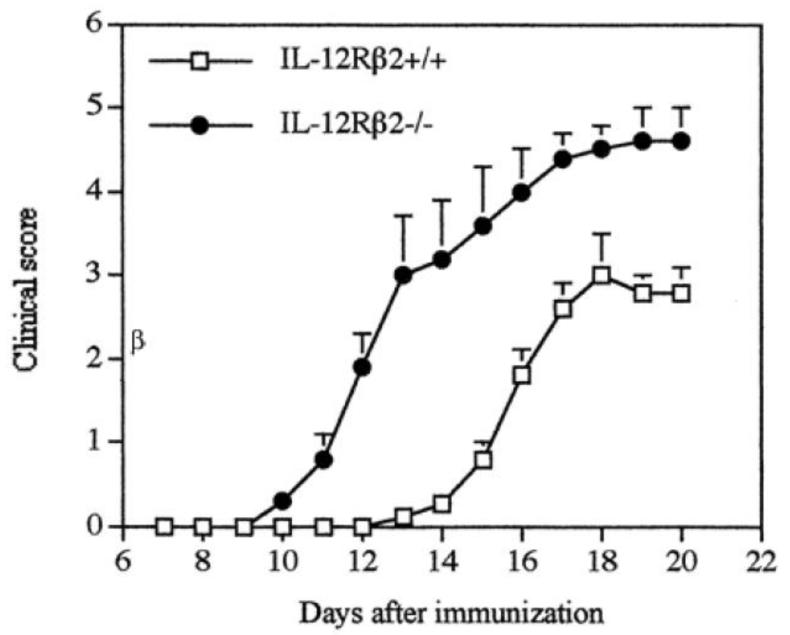
Contrary to expectations and findings in IL-12Rβ1-deficient mice, IL-12Rβ2-deficient mice were more susceptible to EAE, as characterized by earlier disease onset, more severe disease, and greater demyelination and CNS inflammation compared to WT mice (26) (Figure 3). IL-12Rβ2-deficient mice had significantly greater proliferation in response to MOG35-55 and increased production of TNF, GM-CSF, IL-17, IL-18/IL-18Rα, and NO. Furthermore, expression of IL-23p19 mRNA in spleen cells of immunized IL-12Rβ2-deficient mice was higher than in WT mice. These findings demonstrated that IL-12 is not required for EAE development.
Figure 3.
IL-12Rβ2−/− mice develop severe EAE. Female WT and IL-12Rβ2−/− mice were immunized with MOG35-55 peptide in CFA. Data represent mean clinical score × SD. The overall clinical score was significantly different between WT and IL-12Rβ2−/− mice (p < 0.001). (Figure first published in Journal of Immunology, 2003, 170(4):2153-60, Zhang G-X et al., Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. Copyright 2003. The American Association of Immunologists, Inc.)
Permanent lack of IL-12 signaling caused by genetic deficiency in either IL-12p35 or IL-12Rβ2 unequivocally demonstrated that IL-12 is not required for development of EAE (23, 26, 40, 56, 57). Most researchers have found that mice deficient in IL-12 signaling develop more severe disease compared to WT controls, demonstrating the suppressive role of IL-12 in EAE pathogenesis. Deficiency in IL-12 signaling affected IFN-γ production to a variable extent in different studies, and it remains unclear to what extent more severe EAE in IL-12-deficient mice is caused by reduced IFN-γ production versus IFN-γ-independent effects of IL-12.
These surprising findings starkly contradicted the Th1 paradigm, and remained unexplained until the discovery of IL-23 in 2000 (58) and Th17 cells in 2005. Even though major Th1 cytokines play a suppressive role in EAE, it is still believed that Th1 cells, in concert with Th17 cells, contribute to disease development and are thus a part of the Th1/Th17 paradigm that is currently the dominant view of EAE/MS pathogenesis.
IL-23 and Th17 cells in EAE/MS
IL-23 is a covalent heterodimer of IL-12p40 and IL-23p19 (58). Hence, the IL-12p40 subunit is shared between IL-12 and IL-23. Both IL-12 and IL-23 are produced by the same cell types, mainly APCs, and the relative ratio of secretion between IL-12 and IL-23 depends on the nature of stimuli that activated APCs (59, 60). In 2003, Cua et al. showed that IL-23, and not IL-12, plays an essential role in autoimmune inflammation of the CNS (57). They also proposed that IL-23 exerts its pro-encephalitogenic effect by acting on a subset of memory Th1 cells. Langrish et al. have shown that IL-23 drives development of highly encephalitogenic Th cells characterized by IL-17A production (61).
In 2005, two groups simultaneously described a new IL-17A-producing Th lineage that has been named Th17 (62, 63). This seminal discovery marked a new era in autoimmunity research, as Th17 cells proved to be a major factor in a number of autoimmune diseases, including EAE. It became clear that IL-23 promotes development of Th17 cells and stimulates their IL-17A production in a manner analogous to the role of IL-12 in development of Th1 cells and IFN-γ production. The Th1 paradigm (IL-12/Th1/IFN-γ axis) of CNS autoimmunity was rapidly replaced by the Th17 paradigm (IL-23/Th17/IL-17A axis). In this new view of the pathogenesis of CNS autoimmune inflammation, immunization with myelin antigen induces development of Th17 cells in the presence of IL-23. These myelin-specific Th17 cells traffic into the CNS, where they secrete IL-17A, which through chemokine induction attracts various immune cells, and in particular myeloid cells, into the CNS, initiating and perpetuating the inflammatory cascade. However, subsequent studies have shown that IL-17A plays a contributing, but non-essential, role in EAE, as in most studies lack of IL-17A bioactivity led to mitigated disease course and improved recovery, but did not confer resistance to disease (64-70).
GM-CSF, a crucial mediator of Th cell encephalitogenicity
Given that Th17 cells appear to play a crucial role in EAE, while neither their signature cytokine, IL-17A, nor IL-17F, IL-22 or IL-22, is required for EAE development, a search for a mediator produced by Th17 cells that endows them with encephalitogenic capacity led us to hypothesize that GM-CSF plays such a role.
GM-CSF (also known as CSF2), was initially defined by its ability to generate both granulocyte and macrophage colonies from precursor cells (71). GM-CSF can be produced by either bone-marrow-derived cells, such as T cells (72-74) and monocytes/macrophages (75), or by resident tissue cell types, including renal parenchymal cells (76), fibroblasts (77), endothelial cells (78), chondrocytes (76, 79, 80) and smooth muscle cells (81). The GM-CSF receptor (CSF2R) is a heterodimer composed of a specific ligand-binding subunit (CSF2Rα) and a common signal-transduction subunit (CSF2Rβ) (82-84), which is shared with the receptors for IL-3 and IL-5 (85-87). In addition to leukocytes, non-hematopoietic cell types (i.e. keratinocytes, smooth muscle cells, endothelial cells, epithelial cells and neurons) can also express the CSF2R and respond to GM-CSF stimulation (88-95).
Functions of GM-CSFs can be grouped into the following categories: increased cell survival and/or proliferation, differentiation, and activation (96-99). In addition to its effects on haematopoietic precursor cells, GM-CSF can promote the survival and activation of macrophages, neutrophils, basophils and eosinophils, as well as dendritic-cell maturation. Systemic administration of GM-CSF, or when its levels are increased by inflammation or infection, leads to the mobilization of monocytes and other myeloid populations from bone marrow to blood, and it primes monocytes for an increased in vitro response to other stimuli (97, 98, 100). GM-CSF can also mobilize precursors from other lineages, such as endothelial cells (101). Overall, GM-CSF can be viewed as a major regulator involved in the control of granulocyte and macrophage lineage populations at all stages of maturation.
In virtually all animal models of inflammation and autoimmunity that have been tested, GM-CSF depletion resulted in suppression of disease, which is consistent with its pro-inflammatory functions. GM-CSF has well established roles in the following diseases [reviewed in Ref. (102)]: arthritis (103, 104), autoimmune CNS inflammation (105), nephritis (76, 106), lung diseases (96, 97, 107-109), atherosclerosis and vascular injury (110, 111), cancer [reviewed in Ref. (112)], obesity (113) and type 1 diabetes mellitus (114).
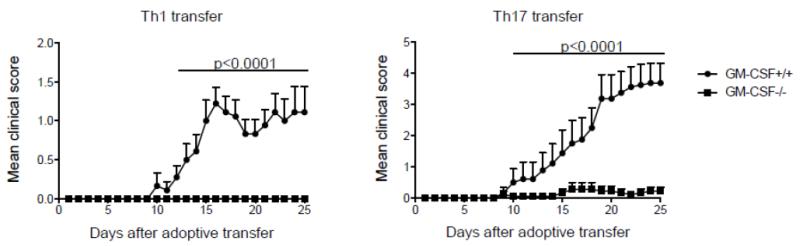
In the context of CNS autoimmunity, we have shown that encephalitogenicity of both Th1 and Th17 cells depends on their GM-CSF production (73), as Th cells deficient in GM-CSF cannot induce EAE (Figure 4). Codarri et al. made a similar observation, and in addition found that RORγt is required for production of GM-CSF by Th cells (74). However, in our studies RORγt-deficient cells, of both Th1 and Th17 lineage, produced large quantities of GM-CSF in vitro, contradicting their findings (73). The reason for this discrepancy is unclear.
Figure 4.
GM-CSF production by Th1 and Th17 cells is required for their encephalitogenicity. WT or Csf2−/− MBP(Ac1-11) TCR-transgenic splenocytes were activated with MBP(Ac1-11) in the presence of IL-12 (Th1 conditions) or TGF-β plus IL-6, anti-IFN-γ and anti-IL-4 (Th17 conditions), then allowed to ‘rest’ for 2 days in the presence of IL-2; they were then reactivated for 72 h with MBP(Ac1-11) in the presence of IL-12 (Th1 conditions) or IL-23 (Th17 conditions). Clinical scores of mice that received 5 × 106 MBP(Ac1-11)-specific WT or Csf2−/− Th1 or Th17 cells are shown. (Figure first published in: Nature Immunology, 2011 Jun;12(6):568-75. El-Behi M, et al., The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF.)
We found that IL-23 stimulates expression of GM-CSF by Th17 cells, whereas TGF-β1 suppresses it. This finding could explain the dichotomy in pathogenicity of Th17 cells, where Th17 cells stimulated with IL-23 are highly pathogenic, while TGF-β-treated Th17 cells are non-pathogenic (115). In addition, we were able to define a positive feedback loop in which IL-23 produced by APCs induces GM-CSF production by Th17 cells, which in turn stimulates IL-23 production by APCs (73). IL-1β1 is another APC-derived cytokine, in addition to IL-23, that significantly upregulates expression of GM-CSF by Th cells, making them highly pathogenic (73, 74).
IL-27, a potent regulator of Th cells
IL-27 has emerged as a potent regulator of immune responses, and in particular those mediated by Th17 cells. IL-27 can be produced by a number of cell types, but its main source appears to be activated APCs. IL-27 comprises two non-covalently bound subunits, Epstein Barr Virus-Induced gene 3 (EBI-3) and p28 (116). IL-27 signals via its heterodimeric receptor, which consists of the IL-6 receptor subunit gp130 and WSX-1 (also known as TCCR) (117, 118). By activating the Th1-driving transcription factor, T-bet, IL-27 induces IL-12Rβ2 and IFN-γ expression in naïve CD4+ T cells, thereby priming these cells for maturation into effector cells of Th1 lineage (119). IL-27 directly suppresses the development of Th17 cells (69, 120, 121) by inhibiting RORγt expression (122). IL-27 signaling activates STAT1, 3, 4 and 5; however, inhibition of Th17 development by IL-27 is mediated by STAT1 and STAT3 (69). In addition to Th17 cells, IL-27 inhibits Th2 cell development as well as Th2 cytokine production from polarized Th2 cells by down-regulation of GATA-3 and up-regulation of T-bet expression simultaneously (123). IL-27 also inhibits development of Treg cells by a STAT3-dependent mechanism (124) as well as suppresses antigen presentation functions of dendritic cells (125).
IL-27 appears to play mainly a suppressive role in cell-mediated autoimmunity, as demonstrated by its inhibitory effect on a range of animal models; however, there are studies to suggest it may also contribute to pathogenesis in some cases (61, 120, 126-129). IL-27R (WSX-1) deficient mice developed more severe EAE than WT mice, suggesting an anti-inflammatory effect of IL-27, most likely through inhibition of highly encephalitogenic Th17 cells (120).
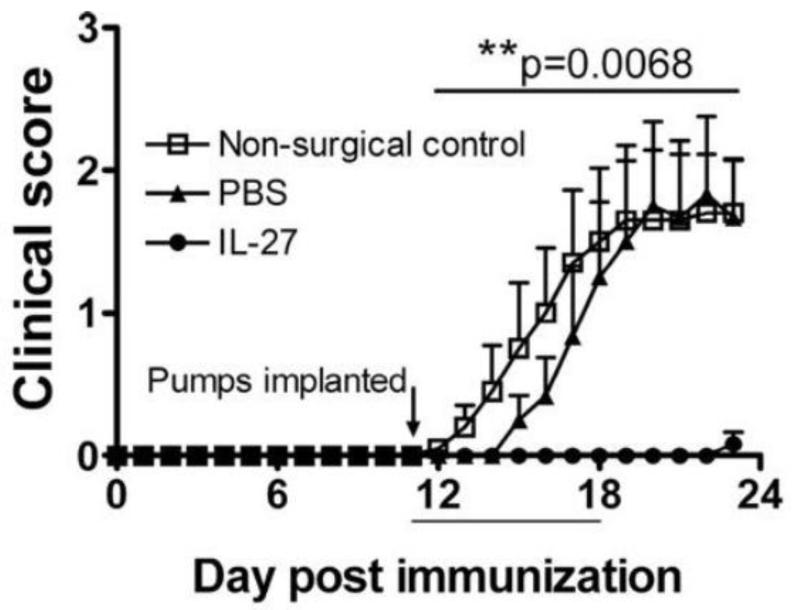
We have shown that IL-27 strongly suppresses development of Th17 cells, while inducing differentiation of IFN-γ+IL-10+ regulatory Tr1 cells (61). Exogenous IL-27 can suppress EAE development (Figure 5), but it has limited efficacy in ameliorating ongoing disease (61). This has been corroborated by another group that used exogenous IL-27 to suppress EAE (126).
Figure 5.
Exogenous IL-27 suppresses actively induced EAE. EAE was induced in C57BL/6 mice with MOG35-55 and osmotic pumps (7-day delivery capacity) containing either rmIL-27 or PBS were implanted s.c. on day 11. Mice that did not undergo surgery were also assessed. (Figure first published in Journal of Immunology 179:3268-75, 2007, Fitzgerald DC et al., Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis (EAE). Copyright 2007. The American Association of Immunologists, Inc.)
Interestingly, IL-27 has a potent regulatory effect on GM-CSF production, but only in the case of Th1 cells, while committed Th17 cells are resistant to suppression of their GM-CSF production by IL-27 (130). This finding is in agreement with our observation that committed Th17 cells exhibit little susceptibility to modulation by IL-27 (131).
Th9 cells and IL-9 in CNS autoimmunity
The momentous changes in our understanding of autoimmunity that began in 2005 with the discovery of Th17 lineage continued with the description of a novel Th9 lineage in 2008 (132, 133), named after its hallmark cytokine, IL-9. In addition to IL-9, Th9 cells can also produce relatively large quantities of IL-10, albeit with different kinetics than IL-9 (134). Th9 cells do not produce cytokines associated with other Th lineages, such as IL-4, IL-17 and IFN-γ (134-136). However, IL-9 is not exclusively produced by Th9 cells, as it can be produced by Th2, Th17 and Treg cells, as well as non-T cells (137-139).
IL-9 was discovered in the late 1980’s (140, 141) but its involvement in autoimmune CNS inflammation was described only recently when two groups published largely contradictory findings. Elyaman et al. showed that IL-9R−/− mice immunized with MOG35-55 have earlier onset and significantly more severe EAE than IL-9+/+ mice (142). IL-9 stimulated suppressive function of Tregs in vitro, which provided a mechanistic explanation for more severe EAE, as the lack of IL-9 signaling in IL-9R−/− mice could have resulted in weaker suppressive Treg function, allowing for more robust encephalitogenic responses and consequently exaggerated EAE. The same group subsequently showed that anti-IL-9 Ab treatment has no effect in EAE, at least when mice are treated with the antibody before disease onset (143). In contrast, Nowak et al. found that IL-9 has a pro-inflammatory role in EAE (144). Using approaches with neutralizing anti-IL-9 Ab, adoptive EAE, and passive EAE in IL-9R−/− mice, they showed that deficiency in IL-9 signaling delays disease onset and reduces its severity. The authors found that milder EAE in IL-9R−/− mice correlates with reduced numbers of Th17 cells and IL-6+ macrophages in the CNS, and decreased numbers of mast cells in the lymph nodes. It remains unclear why these two studies had contradictory findings.
Studies that followed, including ours, also found that neutralization of IL-9 with Ab (145) and lack of IL-9 signaling (in IL-9−/− mice) (146) attenuate EAE. Subsequently, several other studies have shown that IL-9 plays a pro-inflammatory role in EAE. Anti-IL-9 Ab treatment of IFN-γ−/− mice dramatically reduced disease severity and led to complete recovery in otherwise lethal EAE (147). The same group has also shown that adoptive transfer of 2D2 cells polarized into Th9 lineage induces EAE in recipient mice. In a study published by Zhou et al. neutralization of IL-9 with Ab also reduced EAE severity (148). Taken together, several groups, using various approaches, have shown that IL-9 significantly contributes to EAE pathogenesis, while one group concluded that IL-9 either suppresses or has no effect in EAE.
Thus far, most studies on the role of IL-9 in EAE have focused on its effects on immune cells (Tregs, mast cells, Th17 cells), but tissue cells also express IL-9R and can contribute to the effects of IL-9 in disease development or resolution. Most CNS cell types express IL-9R, including astrocytes, oligodendrocytes, microglia and oligodendrocyte progenitors, while neurons do not express the receptor [(148) and our unpublished data]. Zhou et al. have shown that IL-9 acts on astrocytes and induces production of CCL20 during EAE, which in turn attracts CCR6-expressing immune cells, such as Th17 cells, in the CNS, potentiating its inflammation (148). The importance of this pro-inflammatory mechanism is corroborated by findings that in a melanoma mouse model IL-9 inhibited tumor growth by inducing expression of CCL20 in lung epithelial cells, and promoting the recruitment of CCR6+ DCs and CCR6+CD8+ T cells (149).
To test the encephalitogenic potential of Th9 cells, Jager et al. polarized MOG-specific CD4+ T cells (2D2 cells) into Th9 lineage and injected them into naïve recipient mice. The Th9 cells induced EAE with similar kinetics and severity as Th1- and Th17-polarized 2D2 cells (150). However, each of the Th lineages mediated development of different lesion patterns, which indicates that different mechanisms dominate their pathogenic action. Interestingly, half of transferred Th9 cells isolated from the CNS at the peak of EAE produced IFN-γ, and smaller quantities of IL-9, IL-10, IL-4 and IL-17, demonstrating the high degree of Th9 lineage plasticity, similar to Th17 cells. The propensity of Th9 cells, at least when they develop in vitro and are then transferred into mice, to switch to Th1 lineage and start producing IFN-γ has also been demonstrated in the mouse model of ocular inflammation (151).
Overall, it has been clearly demonstrated that in vitro differentiated myelin-specific Th9 cells can be encephalitogenic in adoptive EAE, with potency similar to Th1 and Th17 cells. However, it remains unknown whether substantial myelin-specific Th9 responses develop after immunization with myelin antigens, and whether, if such responses develop, they are of sufficient magnitude to significantly impact EAE. This is similar to Th1 responses, as Th1 cells are encephalitogenic in adoptive EAE, but it is unclear to what extent they contribute to passive EAE induced by immunization. However, in contrast to Th9 responses, it is apparent that immunization with myelin antigens elicits strong Th1 responses, and it is therefore likely that Th1 responses contribute to EAE development. Nevertheless, there is evidence that a large proportion of myelin-specific Th1 cells are ex-Th17 cells (152), suggesting that classic Th1 cells potentially play only a minor role in EAE.
Despite some contradictory findings, the prevailing view is that IL-9 plays a pro-inflammatory role in EAE. However, it remains to be determined to what extent IL-9 is important for encephalitogenicity of Th9 cells, given that hallmark cytokines of Th1 and Th17 cells, IFN-γ and IL-17A, respectively, are not necessary for their encephalitogenicity, which might also be the case with Th9 cells and IL-9. Furthermore, given that IL-9 can be produced by Th17 cells, which are the predominant Th lineage in the CNS of animals with EAE and in acute CNS plaques of MS patients (153), it is possible that the major IL-9 source in the CNS are Th17 and not Th9 cells.
The role of Th9 cells in MS has not been investigated, and there is also a dearth of data on IL-9 in MS. One study has shown that cerebrospinal fluid of MS patients contains increased IL-9 levels (154). We found by immunostaining that a large proportion of CD4+ T cells in CNS lesions of MS patients express IL-9 (unpublished data), indicating that IL-9 may play an important role in MS pathogenesis; this, in combination with findings in EAE, warrants further studies on IL-9 in the context of autoimmune CNS inflammation.
Regulatory T cells in CNS autoimmunity
Regulatory CD4+ T cells (Tregs) have a suppressive effect on immune responses by limiting activation, proliferation, survival, and pro-inflammatory function of various immune cells including Th cells (155). It should be mentioned that other types of immune cells, including CD8+ T cells, B cells and myeloid cells, can also acquire regulatory functions similar to Tregs (156-158). The overall function of Tregs is to maintain immune homeostasis by preventing autoimmunity and by dampening or quenching anti-microbial immune responses that can harm the organism if they become exaggerated, or are unnecessary after infection has been cleared (159). Thus far, several types of Tregs have been described, differing in their developmental origin, phenotypic characteristics and mechanisms of achieving their regulatory function (155). Forkhead box protein 3 (FoxP3) is a master transcription factor that confers regulatory function to some, but not all, types of Tregs (160). Major types of Tregs are: natural Tregs (nTregs), which are FoxP3+ and develop in the thymus; inducible Tregs (iTregs), which are also FoxP3+ and develop in the periphery from naïve CD4+ T cells; IL-10-secreting Tr1 cells, which develop in the periphery and are FoxP3−; TGF-β-secreting Th3 cells; and FoxP3− IL-35-secreting iT(R)35 cells (161). In addition to FoxP3, various types of Tregs can express CD25, cytotoxic T-lymphocyte antigen 4 (CTLA4) and glucocorticoid-induced TNF receptor-related protein (GITR), but neither of these markers is exclusively expressed only by Tregs (162).
The role of Tregs in modulating autoimmune CNS inflammation has been extensively studied in EAE. One of the first observations that Tregs regulate EAE was published in 1993. The authors describe that mice recovering from EAE have a population of regulatory CD4+ T cells specific for a peptide of TCR that dominates CD4+ T cell response to myelin peptide (MBP1-11) used to induce EAE. These TCR peptide-specific Tregs suppressed responses directed against MBP1-11 peptide in vivo and protected mice against MBP-induced EAE (163). In 1994, Tonegawa’s group demonstrated that lymphocytes prevent spontaneous EAE in MBP TCR transgenic mice and raised the possibility that regulatory T cells might be responsible for the protective effect (164), an interpretation that was confirmed in a subsequent study (165). The capacity of Tregs to suppress EAE was confirmed in a study in which transfer of CD4+CD25+ T cells conferred significant protection against clinical EAE (166). Reddy et al. have shown that susceptibility to EAE is inversely correlated with natural frequency of CD4+CD25+ T cells (Tregs) specific for immunizing myelin peptide, and that depletion of Tregs renders susceptible a mouse strain that is normally resistant to EAE (167). IL-6−/− mice, which do not develop Th17 cells, have a peripheral repertoire dominated by Treg cells and are resistant to EAE induction. However, depletion of Tregs enables development of Th17 cells through the TGF-β/IL-21 pathway and renders IL-6−/− mice susceptible to EAE induction (168). Stephens et al. have shown that Treg cells raise the threshold for triggering autoreactive responses, thereby reducing the risk of autoimmune disease (169). Depletion of Tregs with anti-CD25 Ab in SJL mice immunized for EAE induction resulted in enhanced disease severity and increased mortality, while the transfer of Tregs from naïve mice reduced disease severity. However, transfer of Tregs from IL-10-deficient mice failed to suppress EAE, indicating that Tregs control EAE, at least partially, through an IL-10-dependent mechanism (170). Montero et al. also found that depletion of CD25+ Tregs enhances EAE in C57BL/6 mice and increases MOG35-55-specifc IFN-γ production by T cells (171). The majority of Tregs in the CNS of mice with EAE accumulate in the recovery phase of disease, with up to a third of CD4+ T cells in the CNS being Tregs (172-174). Depletion of Tregs precludes recovery from EAE, indicating that these cells play an important role in this process (174). However, investigators have not always been able to directly demonstrate their suppressive capacity, as Tregs failed to control CNS-derived effector T cells (173). In contrast, Tregs from the CNS of mice in the recovery phase suppressed IFN-γ, but not IL-17 production in vitro (175). It appears that, in contrast with peripheral lymphoid organs, the inflammatory environment in the CNS of animals with EAE stimulates rapid proliferation of Tregs (173, 175). An interesting observation made by Liu et al. is that neurons can induce conversion of myelin-specific autoaggressive effector Th cells into CD4+CD25+TGF-β+CTLA-4+FoxP3+ Tregs that are suppressive both in vitro and in vivo and are capable of inhibiting progression of EAE (172). Astrocytes can induce Tregs capable of suppressing autoreactive T-cell proliferation in vitro and EAE upon adoptive transfer (176). Collison et al. made an interesting observation in the study by directly comparing potency of nTregs and iT(R)35 cells (161). Adoptive transfer of 1 × 106 nTregs into mice before inducing EAE resulted in milder disease compared to controls. Mice that received iT(R)35 were completely protected from EAE, demonstrating their superior regulatory efficacy, at least in this disease. At the same time Ebi3-deficient (IL-35-deficient) iT(R)35 is entirely dependent on their IL-35 production.
Potential involvement of Tregs in pathogenesis of MS has been revealed by a finding that these cells obtained from blood of patients with RRMS have reduced suppressive capacity compared to those of healthy controls (177-181). A couple of studies found association between functional impairment of Tregs from MS patients and their lower levels of FoxP3 expression (47, 182-184). Interestingly, in more advanced stages of disease, in secondary progressive MS, Tregs had normal suppressive function and normal levels of FoxP3 expression. Furthermore, the defect in function of Tregs in RRMS patients can be corrected by therapy with IFN-γ (185, 186) and copolymer-I (187).
Most studies on the role of regulatory T cells in autoimmune CNS inflammation have focused on classic CD4+CD25+FoxP3+ Tregs, perhaps because they were the first type of Tregs to be clearly defined, and because in mice, unlike in humans, expression of FoxP3 correlates quite well with the suppressive function of T cells. Generation of FoxP3 reporter mice, which enabled less ambiguous identification of Tregs, and experiments with live Tregs provided opportunities for rapid advancement in the field. However, CD4+ T cells with regulatory function are diverse, just like various Th lineages. They differ in their phenotype (i.e. FoxP3 expression), mechanism of suppression (i.e. soluble mediators vs. cell surface molecules), phenotypic stability/plasticity and capacity to suppress inflammation.
The role of gamma delta (γδ) T cells in CNS autoimmunity
γδ T cells express on their surface TCR comprising γ and δ chains instead of the conventional α and β chains. They represent minority (up to 5%) of lymphocytes in the blood and in secondary lymphoid tissues, but are highly enriched in the skin and mucosa where they can constitute up to 50% of the T cells (188). Selection of γδ T cells in the adult thymus seems to be independent of ligand recognition by γδ TCR, and development of γδ T cells is not affected in the absence of MHC class I and II (189-191). γδ T cells have the capacity to directly respond to microbial products and to cytokines, without prior engagement of their TCR, which is reminiscent of innate immune cells. γδ T cells seem to have a more prominent role early in immune responses, when they produce large quantities of various cytokines, such as IFN-γ, TNF, IL-10, IL-17A and GM-CSF (192-197).
Studies on the role of γδ T cells in EAE have yielded contradictory results, depending on the EAE model and approaches that have been utilized. In one study, where EAE was induced in B10.PL mice by immunization with spinal cord homogenate, depletion of γδ T cells with antibody against γδ TCR resulted in earlier disease onset and disease relapse, in this otherwise monophasic model (198). Mice depleted of γδ T cell had a markedly stronger Ag-specific proliferative response of splenocytes on days 7 and 14 p.i. These findings suggest that γδ T cells play a protective role in EAE. Studies in mice on the same background (B10.PL) genetically deficient in γδ T cells yielded similar results. These mice developed a chronic disease course rather than monophasic. The presence of γδ T cells was needed to promote IFN-γ production by CD4+ and CD8+ T cells in the CNS before EAE onset, whereas lack of γδ T cells had no effect on IFN-γ production in spleen and lymph nodes (199). The same authors subsequently demonstrated that chronicity of EAE in γδ T cell-deficient mice was due to both increased proliferation of encephalitogenic T cells and reduction in their apoptosis. The regulatory function of γδ T cells was dependent on their expression of Fas ligand. Hence, the authors concluded that γδ T cells regulate EAE through Fas/Fas ligand-dependent induction of apoptosis of encephalitogenic T cells (200). In contrast to B10.PL mice, transfer of γδ T cells into C57BL/6 mice deficient for these cells did not have an effect on adoptive EAE in these mice, indicating that γδ T cells do not play a significant role in this model (201). C57BL/6 mice transgenic for MBP-specific TCR on background develop spontaneous EAE when they are simultaneously RAG−/−, whereas RAG-sufficient mice do not. Crossing of MBP TCR-specific mice with γδ T cell deficient mice did not lead to development of spontaneous EAE, indicating that these cells do not play an important role in this model (165).
In contrast to the above-described protective role or lack of significant role in EAE, a number of studies described a pathogenic role for γδ T cells in EAE. Depletion of γδ T cells with antibody in SJL mice with EAE resulted in disease amelioration, suggesting that γδ T cells contribute to disease pathogenesis (202). Application of anti- γδ T cell Ab resulted in reduction of IL-1, IL-6, TNF, lymphotoxin, and IFN-γ levels in the CNS during EAE onset. The levels of these cytokines eventually normalized, except for IFN-γ. The authors concluded that γδ T cells contribute to the pathogenesis of EAE by augmenting production of proinflammatory cytokines by cells that enter the CNS (203). Furthermore, γδ T cell-deficient C57BL/6 mice developed much reduced disease, both after immunization with MOG35-55 and after adoptive transfer of MOG35-55-specifc T cell line (204).
A subset of γδ T cells expresses IL-23R, RORγt and produce sIL-17, IL-21, and IL-22 upon exposure to IL-1β and IL-23, without activation through their TCR. γδ T cells stimulated with IL-1β and IL-23 promoted IL-17 production by CD4+ T cells and increased susceptibility to EAE (205). IL-17+IFN-γ− and IL-17+IFN- γ+ γδ T cells were found in relatively high frequency in the CNS of mice with EAE, especially at disease onset. These findings suggested that γδ T cells are an important source of IL-17 in the CNS in early EAE. IL-1β- and IL-23-activated γδ T cells promoted IL-17 production by αβ T cells, either by directly acting on the CD4+ T cells and/or by promoting cytokine production from DCs. Petermann et al. extended the above findings by discovering that IL-23R+ γδ T cells antagonize Treg cell-mediated suppression of αβ T cells (206). In addition, IL-23-stimulated γδ T cells created a cytokine milieu, independently of IL-6 and IL-21, that directly inhibited conversion of αβ T cells into Foxp3+ Treg cells. Secreted products of IL-23-activated γδ T cells suppressed generation and function of Treg, shifting the balance between Treg cells and conventional αβ T cells in favor of effector Th cells. It remains to be determined which soluble factor(s) produced by IL-23R+ γδ T cells inhibit Treg cell development and function or enable αβ T cells to resist Treg cell suppression.
Conclusions
For the past seven years Th17 cells have been the focus of intense research in immunology and especially in autoimmunity. Progress in understanding their biology and function has been rapid, and much has been discovered in a short period of time. Views on the pathogenesis of most autoimmune diseases have radically changed and paradigms that dominated research and therapeutic approaches since the 1980s have been replaced with new ones that include Th17 cells.
The discovery of Th17 cells was mainly based on findings in EAE, and subsequent progress in understanding their biology and function was largely driven by the use of this disease model. Soon after their discovery, Th17 cells took center stage in MS research, and they remain central in both basic and translational MS research. It has been demonstrated beyond any doubt that Th17 cells play a crucial role in EAE. However, pathways and mechanisms underlying their encephalitogenicity are still being discovered. Given that IL-17A plays a limited role in EAE, and that GM-CSF is also produced by Th1 cells, it is unclear which mechanism(s) employed by Th17 cells makes them uniquely encephalitogenic.
One question that has received a great deal of attention is the relative contribution of Th1 and Th17 cells to the pathogenesis of EAE and MS. Th1 cells can transfer EAE in adoptive models, which demonstrates that they can be encephalitogenic on their own, but it appears that they have no capacity to induce active EAE without the involvement of Th17 cells. Th17 cells are necessary for EAE development, but it is not clear if their contribution is sufficient or if that of Th1 cells is also required. This question became perhaps less relevant after the discovery that a great number of Th17 cells change their phenotype and transition into Th1 cells (ex-Th17 cells) during the course of EAE(152) (Figure 6). Thus, in this case the distinction between lineages has been blurred, and insistence on a division of roles that Th1 and Th17 lineages play in EAE can be immaterial, given that the same cells can have both phenotypes. An interesting question about encephalitogenicity that has not been addressed are potential differences between classic Th1 and ex-Th17 cells.
Figure 6.
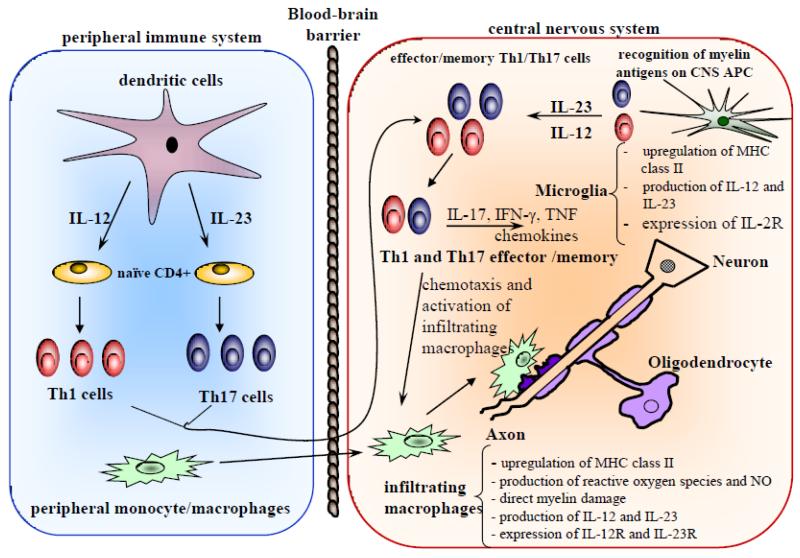
Th1/Th17 paradigm of CNS inflammatory demyelination. In the peripheral immune system, IL-12 and IL-23, produced by dendritic cells, induces the differentiation of Th1 and Th17 cells, respectively. IL-12 is not strictly required and may actually play an immunoregulatory role in development of EAE, as mice that do not produce, or cannot respond to, IL-12 develop severe EAE. Activated Th1 and Th17 cells migrate into the CNS across the blood-brain barrier. In the CNS, myelin-reactive Th cells interact with resident microglia and are reactivated upon recognition of myelin antigens. Activated effector Th cells produce cytokines and chemokines that lead to an inflammatory pathological cascade in the CNS and damage to the myelin sheath and neuronal axons. (Figure first published in Drug News & Perspectives 19(2):77-83, 2006, Touil T et al., Pathophysiology of Interleukin-23 in experimental autoimmune encephalomyelitis. Copyright © 2006 Prous Science, S.A.U. or its licensors. All rights reserved.)
The role of Th17 cells in MS has been largely inferred from animal studies, similar to the role of Th1 cells and other types of immune cells. It is believed that Th17 cells, along with Th1 cells, play a role in MS pathogenesis, which, if not crucial, is at least significant. A majority of CD4+ T cells in acute MS lesions produce IL-17A, and hence can be classified as Th17 cells (153). Certain types of MS, such as opticospinal MS, have a dominant signature of Th17-driven pathology, including a large proportion of granulocytes among CNS-infiltrating cells (207). Possibly, in different types of MS, or in different disease phases, either Th1 or Th17 cells are the main drivers of pathological processes. This might also be the case in the evolution of individual CNS lesions, with one Th lineage initiating pathology and another perpetuating it. Given the view that Th17 cells likely play an important role in MS, they have become a major focus for development of new therapeutic strategies. Thus far, no therapeutic approach that specifically targets Th17 cells or their products (i.e. IL-17A) has been clinically tested and proven useful. Nonetheless, it is certain that Th17 cells will continue to be of intense interest for therapeutic targeting in MS, and as our knowledge of these cells deepens, new approaches will be developed, hopefully with great benefit to MS patients.
Acknowledgments
We thank K. Regan for editorial assistance.
Funding
This work was supported by the National Institutes of Health (5R01NS046782 and 1U19A1082726), and the M.E. Groff Foundation.
Abbreviations
- IL
interleukin
- IFN
interferon
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- CNS
central nervous system
- EAE
experimental allergic encephalomyelitis
- APC
antigen presenting cells
- MBP
myelin basic protein
- TNF
tumor necrosis factor
- GM-CSF
granulocyte colony-stimulating factor
- NO
nitric oxide
- WT
wild-type
- Tregs
regulatory T cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. The New England journal of medicine. 2000;343(13):938–52. doi: 10.1056/NEJM200009283431307. Epub 2000/09/28. [DOI] [PubMed] [Google Scholar]
- 2.Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. The New England journal of medicine. 2006;354(9):942–55. doi: 10.1056/NEJMra052130. Epub 2006/03/03. [DOI] [PubMed] [Google Scholar]
- 3.Nylander A, Hafler DA. Multiple sclerosis. The Journal of clinical investigation. 2012;122(4):1180–8. doi: 10.1172/JCI58649. Epub 2012/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Annals of neurology. 2006;60(1):12–21. doi: 10.1002/ana.20913. Epub 2006/06/28. [DOI] [PubMed] [Google Scholar]
- 5.Kuchroo VK, Anderson AC, Waldner H, Munder M, Bettelli E, Nicholson LB. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annual review of immunology. 2002;20:101–23. doi: 10.1146/annurev.immunol.20.081701.141316. Epub 2002/02/28. [DOI] [PubMed] [Google Scholar]
- 6.Furlan R, Cuomo C, Martino G. Animal models of multiple sclerosis. Methods in molecular biology. 2009;549:157–73. doi: 10.1007/978-1-60327-931-4_11. Epub 2009/04/21. [DOI] [PubMed] [Google Scholar]
- 7.Williams KC, Ulvestad E, Hickey WF. Immunology of multiple sclerosis. Clinical neuroscience. 1994;2(3-4):229–45. Epub 1994/01/01. [PubMed] [Google Scholar]
- 8.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. Journal of immunology. 1986;136(7):2348–57. Epub 1986/04/01. [PubMed] [Google Scholar]
- 9.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, et al. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334(6179):255–8. doi: 10.1038/334255a0. Epub 1988/07/21. [DOI] [PubMed] [Google Scholar]
- 10.Ando DG, Clayton J, Kono D, Urban JL, Sercarz EE. Encephalitogenic T cells in the B10.PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cellular immunology. 1989;124(1):132–43. doi: 10.1016/0008-8749(89)90117-2. Epub 1989/11/01. [DOI] [PubMed] [Google Scholar]
- 11.Voskuhl RR, Martin R, Bergman C, Dalal M, Ruddle NH, McFarland HF. T helper 1 (Th1) functional phenotype of human myelin basic protein-specific T lymphocytes. Autoimmunity. 1993;15(2):137–43. doi: 10.3109/08916939309043888. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- 12.van der Veen RC, Kapp JA, Trotter JL. Fine-specificity differences in the recognition of an encephalitogenic peptide by T helper 1 and 2 cells. Journal of neuroimmunology. 1993;48(2):221–6. doi: 10.1016/0165-5728(93)90195-5. Epub 1993/11/01. [DOI] [PubMed] [Google Scholar]
- 13.Markiewicz K, Cholewa M, Luciak M. Influence of tobacco smoking on serum free fatty acid, triglyceride and glucose levels during physical training and post-exertional restitution. Acta medica Academiae Scientiarum Hungaricae. 1978;35(3-4):225–32. Epub 1978/01/01. [PubMed] [Google Scholar]
- 14.Olsson T. Critical influences of the cytokine orchestration on the outcome of myelin antigen-specific T-cell autoimmunity in experimental autoimmune encephalomyelitis and multiple sclerosis. Immunological reviews. 1995;144:245–68. doi: 10.1111/j.1600-065x.1995.tb00072.x. Epub 1995/04/01. [DOI] [PubMed] [Google Scholar]
- 15.Pierson E, Simmons SB, Castelli L, Goverman JM. Mechanisms regulating regional localization of inflammation during CNS autoimmunity. Immunological reviews. 2012;248(1):205–15. doi: 10.1111/j.1600-065X.2012.01126.x. Epub 2012/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Traugott U, Lebon P. Multiple sclerosis: involvement of interferons in lesion pathogenesis. Annals of neurology. 1988;24(2):243–51. doi: 10.1002/ana.410240211. Epub 1988/08/01. [DOI] [PubMed] [Google Scholar]
- 17.Chomarat P, Rissoan MC, Banchereau J, Miossec P. Interferon gamma inhibits interleukin 10 production by monocytes. The Journal of experimental medicine. 1993;177(2):523–7. doi: 10.1084/jem.177.2.523. Epub 1993/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-gamma) Current opinion in immunology. 1997;9(1):17–23. doi: 10.1016/s0952-7915(97)80154-9. Epub 1997/02/01. [DOI] [PubMed] [Google Scholar]
- 19.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37(7):1097–102. doi: 10.1212/wnl.37.7.1097. Epub 1987/07/01. [DOI] [PubMed] [Google Scholar]
- 20.Chitnis T, Najafian N, Benou C, Salama AD, Grusby MJ, Sayegh MH, et al. Effect of targeted disruption of STAT4 and STAT6 on the induction of experimental autoimmune encephalomyelitis. The Journal of clinical investigation. 2001;108(5):739–47. doi: 10.1172/JCI12563. Epub 2001/09/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schreiber RD, Darnell JE, Jr., et al. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. The Journal of experimental medicine. 1995;181(5):1755–62. doi: 10.1084/jem.181.5.1755. Epub 1995/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. The Journal of experimental medicine. 2004;200(1):79–87. doi: 10.1084/jem.20031819. Epub 2004/07/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, et al. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. Journal of immunology. 2002;169(12):7104–10. doi: 10.4049/jimmunol.169.12.7104. Epub 2002/12/10. [DOI] [PubMed] [Google Scholar]
- 24.Krakowski M, Owens T. Interferon-gamma confers resistance to experimental allergic encephalomyelitis. European journal of immunology. 1996;26(7):1641–6. doi: 10.1002/eji.1830260735. Epub 1996/07/01. [DOI] [PubMed] [Google Scholar]
- 25.Tran EH, Prince EN, Owens T. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. Journal of immunology. 2000;164(5):2759–68. doi: 10.4049/jimmunol.164.5.2759. Epub 2000/02/29. [DOI] [PubMed] [Google Scholar]
- 26.Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X, et al. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. Journal of immunology. 2003;170(4):2153–60. doi: 10.4049/jimmunol.170.4.2153. Epub 2003/02/08. [DOI] [PubMed] [Google Scholar]
- 27.Duong TT, Finkelman FD, Singh B, Strejan GH. Effect of anti-interferon-gamma monoclonal antibody treatment on the development of experimental allergic encephalomyelitis in resistant mouse strains. Journal of neuroimmunology. 1994;53(1):101–7. doi: 10.1016/0165-5728(94)90069-8. Epub 1994/08/01. [DOI] [PubMed] [Google Scholar]
- 28.Duong TT, Louis J, Gilbert JJ, Finkelman FD, Strejan GH. Effect of anti-interferon-gamma and anti-interleukin-2 monoclonal antibody treatment on the development of actively and passively induced experimental allergic encephalomyelitis in the SJL/J mouse. Journal of neuroimmunology. 1992;36(2-3):105–15. doi: 10.1016/0165-5728(92)90042-j. Epub 1992/02/01. [DOI] [PubMed] [Google Scholar]
- 29.Heremans H, Dillen C, Groenen M, Martens E, Billiau A. Chronic relapsing experimental autoimmune encephalomyelitis (CREAE) in mice: enhancement by monoclonal antibodies against interferon-gamma. European journal of immunology. 1996;26(10):2393–8. doi: 10.1002/eji.1830261019. Epub 1996/10/01. [DOI] [PubMed] [Google Scholar]
- 30.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) Journal of immunology. 1996;156(1):5–7. Epub 1996/01/01. [PubMed] [Google Scholar]
- 31.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Journal of immunology. 1996;157(8):3223–7. Epub 1996/10/15. [PubMed] [Google Scholar]
- 32.Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-gamma is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. Journal of immunology. 1999;163(10):5278–86. Epub 1999/11/24. [PubMed] [Google Scholar]
- 33.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annual review of immunology. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. Epub 1995/01/01. [DOI] [PubMed] [Google Scholar]
- 34.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(21):10188–92. doi: 10.1073/pnas.90.21.10188. Epub 1993/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonard JP, Waldburger KE, Goldman SJ. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. The Journal of experimental medicine. 1995;181(1):381–6. doi: 10.1084/jem.181.1.381. Epub 1995/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bright JJ, Du C, Coon M, Sriram S, Klaus SJ. Prevention of experimental allergic encephalomyelitis via inhibition of IL-12 signaling and IL-12-mediated Th1 differentiation: an effect of the novel anti-inflammatory drug lisofylline. Journal of immunology. 1998;161(12):7015–22. Epub 1998/12/23. [PubMed] [Google Scholar]
- 37.Heremans H, Dillen C, Groenen M, Matthys P, Billiau A. Role of endogenous interleukin-12 (IL-12) in induced and spontaneous relapses of experimental autoimmune encephalomyelitis in mice. European cytokine network. 1999;10(2):171–80. Epub 1999/07/10. [PubMed] [Google Scholar]
- 38.Ichikawa M, Koh CS, Inoue A, Tsuyusaki J, Yamazaki M, Inaba Y, et al. Anti-IL-12 antibody prevents the development and progression of multiple sclerosis-like relapsing--remitting demyelinating disease in NOD mice induced with myelin oligodendrocyte glycoprotein peptide. Journal of neuroimmunology. 2000;102(1):56–66. doi: 10.1016/s0165-5728(99)00153-8. Epub 2000/01/08. [DOI] [PubMed] [Google Scholar]
- 39.Shevach EM, Chang JT, Segal BM. The critical role of IL-12 and the IL-12R beta 2 subunit in the generation of pathogenic autoreactive Th1 cells. Springer seminars in immunopathology. 1999;21(3):249–62. doi: 10.1007/BF00812256. Epub 2000/02/10. [DOI] [PubMed] [Google Scholar]
- 40.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. The Journal of clinical investigation. 2002;110(4):493–7. doi: 10.1172/JCI15751. Epub 2002/08/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leonard JP, Waldburger KE, Schaub RG, Smith T, Hewson AK, Cuzner ML, et al. Regulation of the inflammatory response in animal models of multiple sclerosis by interleukin-12. Critical reviews in immunology. 1997;17(5-6):545–53. Epub 1997/01/01. [PubMed] [Google Scholar]
- 42.Gran B, Chu N, Zhang GX, Yu S, Li Y, Chen XH, et al. Early administration of IL-12 suppresses EAE through induction of interferon-gamma. Journal of neuroimmunology. 2004;156(1-2):123–31. doi: 10.1016/j.jneuroim.2004.07.019. Epub 2004/10/07. [DOI] [PubMed] [Google Scholar]
- 43.Cheng X, Zhao Z, Ventura E, Gran B, Shindler KS, Rostami A. The PD-1/PD-L pathway is up-regulated during IL-12-induced suppression of EAE mediated by IFN-gamma. Journal of neuroimmunology. 2007;185(1-2):75–86. doi: 10.1016/j.jneuroim.2007.01.012. Epub 2007/02/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berghmans N, Dillen C, Heremans H. Exogenous IL-12 suppresses experimental autoimmune encephalomyelitis (EAE) by tuning IL-10 and IL-5 levels in an IFN-gamma-dependent way. Journal of neuroimmunology. 2006;176(1-2):63–75. doi: 10.1016/j.jneuroim.2006.04.009. Epub 2006/06/13. [DOI] [PubMed] [Google Scholar]
- 45.Jee Y, Matsumoto Y. Two-step activation of T cells, clonal expansion and subsequent Th1 cytokine production, is essential for the development of clinical autoimmune encephalomyelitis. European journal of immunology. 2001;31(6):1800–12. doi: 10.1002/1521-4141(200106)31:6<1800::aid-immu1800>3.0.co;2-s. Epub 2001/06/01. [DOI] [PubMed] [Google Scholar]
- 46.Constantinescu CS, Hilliard B, Wysocka M, Ventura ES, Bhopale MK, Trinchieri G, et al. IL-12 reverses the suppressive effect of the CD40 ligand blockade on experimental autoimmune encephalomyelitis (EAE) Journal of the neurological sciences. 1999;171(1):60–4. doi: 10.1016/s0022-510x(99)00249-x. Epub 1999/11/24. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed Z, Baker D, Cuzner ML. Interleukin-12 induces mild experimental allergic encephalomyelitis following local central nervous system injury in the Lewis rat. Journal of neuroimmunology. 2003;140(1-2):109–17. doi: 10.1016/s0165-5728(03)00180-2. Epub 2003/07/17. [DOI] [PubMed] [Google Scholar]
- 48.Constantinescu CS, Frei K, Wysocka M, Trinchieri G, Malipiero U, Rostami A, et al. Astrocytes and microglia produce interleukin-12 p40. Annals of the New York Academy of Sciences. 1996;795:328–33. doi: 10.1111/j.1749-6632.1996.tb52684.x. Epub 1996/10/31. [DOI] [PubMed] [Google Scholar]
- 49.Constantinescu CS, Wysocka M, Hilliard B, Ventura ES, Lavi E, Trinchieri G, et al. Antibodies against IL-12 prevent superantigen-induced and spontaneous relapses of experimental autoimmune encephalomyelitis. Journal of immunology. 1998;161(9):5097–104. Epub 1998/10/30. [PubMed] [Google Scholar]
- 50.Constantinescu CS, Hilliard B, Ventura E, Wysocka M, Showe L, Lavi E, et al. Modulation of susceptibility and resistance to an autoimmune model of multiple sclerosis in prototypically susceptible and resistant strains by neutralization of interleukin-12 and interleukin-4, respectively. Clinical immunology. 2001;98(1):23–30. doi: 10.1006/clim.2000.4944. Epub 2001/01/06. [DOI] [PubMed] [Google Scholar]
- 51.Chua AO, Chizzonite R, Desai BB, Truitt TP, Nunes P, Minetti LJ, et al. Expression cloning of a human IL-12 receptor component. A new member of the cytokine receptor superfamily with strong homology to gp130. Journal of immunology. 1994;153(1):128–36. Epub 1994/07/01. [PubMed] [Google Scholar]
- 52.Chua AO, Wilkinson VL, Presky DH, Gubler U. Cloning and characterization of a mouse IL-12 receptor-beta component. Journal of immunology. 1995;155(9):4286–94. Epub 1995/11/01. [PubMed] [Google Scholar]
- 53.Gubler U, Presky DH. Molecular biology of interleukin-12 receptors. Annals of the New York Academy of Sciences. 1996;795:36–40. doi: 10.1111/j.1749-6632.1996.tb52653.x. Epub 1996/10/31. [DOI] [PubMed] [Google Scholar]
- 54.Zhang GX, Yu S, Gran B, Li J, Siglienti I, Chen X, et al. Role of IL-12 receptor beta 1 in regulation of T cell response by APC in experimental autoimmune encephalomyelitis. Journal of immunology. 2003;171(9):4485–92. doi: 10.4049/jimmunol.171.9.4485. Epub 2003/10/22. [DOI] [PubMed] [Google Scholar]
- 55.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. Journal of immunology. 2002;168(11):5699–708. doi: 10.4049/jimmunol.168.11.5699. Epub 2002/05/23. [DOI] [PubMed] [Google Scholar]
- 56.Xiao BG, Ma CG, Xu LY, Link H, Lu CZ. IL-12/IFN-gamma/NO axis plays critical role in development of Th1-mediated experimental autoimmune encephalomyelitis. Molecular immunology. 2008;45(4):1191–6. doi: 10.1016/j.molimm.2007.07.003. Epub 2007/08/19. [DOI] [PubMed] [Google Scholar]
- 57.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–8. doi: 10.1038/nature01355. Epub 2003/03/01. [DOI] [PubMed] [Google Scholar]
- 58.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–25. doi: 10.1016/s1074-7613(00)00070-4. Epub 2000/12/15. [DOI] [PubMed] [Google Scholar]
- 59.Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. The Journal of experimental medicine. 2008;205(6):1447–61. doi: 10.1084/jem.20071450. Epub 2008/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunological reviews. 2008;226:112–31. doi: 10.1111/j.1600-065X.2008.00700.x. Epub 2009/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, et al. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179(5):3268–75. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 62.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature immunology. 2005;6(11):1123–32. doi: 10.1038/ni1254. Epub 2005/10/04. [DOI] [PubMed] [Google Scholar]
- 63.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature immunology. 2005;6(11):1133–41. doi: 10.1038/ni1261. Epub 2005/10/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. Journal of immunology. 2006;177(1):566–73. doi: 10.4049/jimmunol.177.1.566. Epub 2006/06/21. [DOI] [PubMed] [Google Scholar]
- 65.Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, et al. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. The Journal of clinical investigation. 2009;119(1):61–9. doi: 10.1172/JCI35997. Epub 2008/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. The Journal of experimental medicine. 2005;201(2):233–40. doi: 10.1084/jem.20041257. Epub 2005/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. The Journal of experimental medicine. 2008;205(7):1535–41. doi: 10.1084/jem.20080159. Epub 2008/06/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tigno-Aranjuez JT, Jaini R, Tuohy VK, Lehmann PV, Tary-Lehmann M. Encephalitogenicity of complete Freund’s adjuvant relative to CpG is linked to induction of Th17 cells. Journal of immunology. 2009;183(9):5654–61. doi: 10.4049/jimmunol.0900645. Epub 2009/10/09. [DOI] [PubMed] [Google Scholar]
- 69.Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. The Journal of clinical investigation. 2006;116(5):1317–26. doi: 10.1172/JCI25308. Epub 2006/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uyttenhove C, Van Snick J. Development of an anti-IL-17A auto-vaccine that prevents experimental auto-immune encephalomyelitis. European journal of immunology. 2006;36(11):2868–74. doi: 10.1002/eji.200636662. Epub 2006/10/19. [DOI] [PubMed] [Google Scholar]
- 71.Burgess AW, Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980;56(6):947–58. Epub 1980/12/01. [PubMed] [Google Scholar]
- 72.Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178(1):39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- 73.Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nature immunology. 2011;12(6):568–75. doi: 10.1038/ni.2031. Epub 2011/04/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nature immunology. 2011;12(6):560–7. doi: 10.1038/ni.2027. Epub 2011/04/26. [DOI] [PubMed] [Google Scholar]
- 75.Hamilton JA. Coordinate and noncoordinate colony stimulating factor formation by human monocytes. J Leukoc Biol. 1994;55(3):355–61. doi: 10.1002/jlb.55.3.355. [DOI] [PubMed] [Google Scholar]
- 76.Timoshanko JR, Kitching AR, Semple TJ, Holdsworth SR, Tipping PG. Granulocyte macrophage colony-stimulating factor expression by both renal parenchymal and immune cells mediates murine crescentic glomerulonephritis. J Am Soc Nephrol. 2005;16(9):2646–56. doi: 10.1681/ASN.2004121107. [DOI] [PubMed] [Google Scholar]
- 77.Zucali JR, Dinarello CA, Oblon DJ, Gross MA, Anderson L, Weiner RS. Interleukin 1 stimulates fibroblasts to produce granulocyte-macrophage colony-stimulating activity and prostaglandin E2. J Clin Invest. 1986;77(6):1857–63. doi: 10.1172/JCI112512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bagby GC, Jr., Dinarello CA, Wallace P, Wagner C, Hefeneider S, McCall E. Interleukin 1 stimulates granulocyte macrophage colony-stimulating activity release by vascular endothelial cells. J Clin Invest. 1986;78(5):1316–23. doi: 10.1172/JCI112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leizer T, Cebon J, Layton JE, Hamilton JA. Cytokine regulation of colony-stimulating factor production in cultured human synovial fibroblasts: I. Induction of GM-CSF and G-CSF production by interleukin-1 and tumor necrosis factor. Blood. 1990;76(10):1989–96. [PubMed] [Google Scholar]
- 80.Campbell IK, Novak U, Cebon J, Layton JE, Hamilton JA. Human articular cartilage and chondrocytes produce hemopoietic colony-stimulating factors in culture in response to IL-1. J Immunol. 1991;147(4):1238–46. [PubMed] [Google Scholar]
- 81.Filonzi EL, Zoellner H, Stanton H, Hamilton JA. Cytokine regulation of granulocyte-macrophage colony stimulating factor and macrophage colony-stimulating factor production in human arterial smooth muscle cells. Atherosclerosis. 1993;99(2):241–52. doi: 10.1016/0021-9150(93)90026-q. [DOI] [PubMed] [Google Scholar]
- 82.Gearing DP, King JA, Gough NM, Nicola NA. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. Embo J. 1989;8(12):3667–76. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whetton AD, Dexter TM. Myeloid haemopoietic growth factors. Biochim Biophys Acta. 1989;989(2):111–32. doi: 10.1016/0304-419x(89)90038-3. [DOI] [PubMed] [Google Scholar]
- 84.Metcalf D. Hematopoietic regulators: redundancy or subtlety? Blood. 1993;82(12):3515–23. [PubMed] [Google Scholar]
- 85.Kitamura T, Sato N, Arai K, Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991;66(6):1165–74. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- 86.Tavernier J, Devos R, Cornelis S, Tuypens T, Van der Heyden J, Fiers W, et al. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell. 1991;66(6):1175–84. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- 87.Hercus TR, Thomas D, Guthridge MA, Ekert PG, King-Scott J, Parker MW, et al. The granulocyte-macrophage colony-stimulating factor receptor: linking its structure to cell signaling and its role in disease. Blood. 2009;114(7):1289–98. doi: 10.1182/blood-2008-12-164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hancock GE, Kaplan G, Cohn ZA. Keratinocyte growth regulation by the products of immune cells. J Exp Med. 1988;168(4):1395–402. doi: 10.1084/jem.168.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soldi R, Primo L, Brizzi MF, Sanavio F, Aglietta M, Polentarutti N, et al. Activation of JAK2 in human vascular endothelial cells by granulocyte-macrophage colony-stimulating factor. Blood. 1997;89(3):863–72. [PubMed] [Google Scholar]
- 90.Choi JK, Choi BH, Ha Y, Park H, Yoon SH, Park HC, et al. Signal transduction pathways of GM-CSF in neural cell lines. Neurosci Lett. 2007;420(3):217–22. doi: 10.1016/j.neulet.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 91.Baldwin GC, Gasson JC, Kaufman SE, Quan SG, Williams RE, Avalos BR, et al. Nonhematopoietic tumor cells express functional GM-CSF receptors. Blood. 1989;73(4):1033–7. [PubMed] [Google Scholar]
- 92.Dedhar S, Gaboury L, Galloway P, Eaves C. Human granulocyte-macrophage colony-stimulating factor is a growth factor active on a variety of cell types of nonhemopoietic origin. Proc Natl Acad Sci U S A. 1988;85(23):9253–7. doi: 10.1073/pnas.85.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bussolino F, Wang JM, Defilippi P, Turrini F, Sanavio F, Edgell CJ, et al. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature. 1989;337(6206):471–3. doi: 10.1038/337471a0. [DOI] [PubMed] [Google Scholar]
- 94.Bussolino F, Ziche M, Wang JM, Alessi D, Morbidelli L, Cremona O, et al. In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J Clin Invest. 1991;87(3):986–95. doi: 10.1172/JCI115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rivas CI, Vera JC, Delgado-Lopez F, Heaney ML, Guaiquil VH, Zhang RH, et al. Expression of granulocyte-macrophage colony-stimulating factor receptors in human prostate cancer. Blood. 1998;91(3):1037–43. [PubMed] [Google Scholar]
- 96.Bozinovski S, Jones JE, Vlahos R, Hamilton JA, Anderson GP. Granulocyte/macrophage-colony-stimulating factor (GM-CSF) regulates lung innate immunity to lipopolysaccharide through Akt/Erk activation of NFkappa B and AP-1 in vivo. J Biol Chem. 2002;277(45):42808–14. doi: 10.1074/jbc.M207840200. [DOI] [PubMed] [Google Scholar]
- 97.Bozinovski S, Jones J, Beavitt SJ, Cook AD, Hamilton JA, Anderson GP. Innate immune responses to LPS in mouse lung are suppressed and reversed by neutralization of GM-CSF via repression of TLR-4. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L877–85. doi: 10.1152/ajplung.00275.2003. [DOI] [PubMed] [Google Scholar]
- 98.Fleetwood AJ, Cook AD, Hamilton JA. Functions of granulocyte-macrophage colony-stimulating factor. Crit Rev Immunol. 2005;25(5):405–28. doi: 10.1615/critrevimmunol.v25.i5.50. [DOI] [PubMed] [Google Scholar]
- 99.Stanley ER, Chen DM, Lin HS. Induction of macrophage production and proliferation by a purified colony stimulating factor. Nature. 1978;274(5667):168–70. doi: 10.1038/274168a0. [DOI] [PubMed] [Google Scholar]
- 100.Stanley ER, Berg KL, Einstein DB, Lee PS, Pixley FJ, Wang Y, et al. Biology and action of colony--stimulating factor-1. Mol Reprod Dev. 1997;46(1):4–10. doi: 10.1002/(SICI)1098-2795(199701)46:1<4::AID-MRD2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 101.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5(4):434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 102.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8(7):533–44. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 103.Campbell IK, Rich MJ, Bischof RJ, Dunn AR, Grail D, Hamilton JA. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol. 1998;161(7):3639–44. [PubMed] [Google Scholar]
- 104.Cook AD, Braine EL, Campbell IK, Rich MJ, Hamilton JA. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (GM-CSF): requirement for GM-CSF in the effector phase of disease. Arthritis Res. 2001;3(5):293–8. doi: 10.1186/ar318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, et al. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194(7):873–82. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kitching AR, Ru Huang X, Turner AL, Tipping PG, Dunn AR, Holdsworth SR. The requirement for granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in leukocyte-mediated immune glomerular injury. J Am Soc Nephrol. 2002;13(2):350–8. doi: 10.1681/ASN.V132350. [DOI] [PubMed] [Google Scholar]
- 107.Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, et al. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173(10):6384–92. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 108.Vlahos R, Bozinovski S, Hamilton JA, Anderson GP. Therapeutic potential of treating chronic obstructive pulmonary disease (COPD) by neutralising granulocyte macrophage-colony stimulating factor (GM-CSF) Pharmacol Ther. 2006;112(1):106–15. doi: 10.1016/j.pharmthera.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 109.Yamashita N, Tashimo H, Ishida H, Kaneko F, Nakano J, Kato H, et al. Attenuation of airway hyperresponsiveness in a murine asthma model by neutralization of granulocyte-macrophage colony-stimulating factor (GM-CSF) Cell Immunol. 2002;219(2):92–7. doi: 10.1016/s0008-8749(02)00565-8. [DOI] [PubMed] [Google Scholar]
- 110.Ditiatkovski M, Toh BH, Bobik A. GM-CSF deficiency reduces macrophage PPAR-gamma expression and aggravates atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26(10):2337–44. doi: 10.1161/01.ATV.0000238357.60338.90. [DOI] [PubMed] [Google Scholar]
- 111.Shindo J, Ishibashi T, Yokoyama K, Nakazato K, Ohwada T, Shiomi M, et al. Granulocyte-macrophage colony-stimulating factor prevents the progression of atherosclerosis via changes in the cellular and extracellular composition of atherosclerotic lesions in watanabe heritable hyperlipidemic rabbits. Circulation. 1999;99(16):2150–6. doi: 10.1161/01.cir.99.16.2150. [DOI] [PubMed] [Google Scholar]
- 112.Armitage JO. Emerging applications of recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1998;92(12):4491–508. [PubMed] [Google Scholar]
- 113.Reed JA, Clegg DJ, Smith KB, Tolod-Richer EG, Matter EK, Picard LS, et al. GM-CSF action in the CNS decreases food intake and body weight. J Clin Invest. 2005;115(11):3035–44. doi: 10.1172/JCI25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol. 2007;179(6):3638–47. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- 115.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nature immunology. 2007;8(12):1390–7. doi: 10.1038/ni1539. Epub 2007/11/13. [DOI] [PubMed] [Google Scholar]
- 116.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16(6):779–90. doi: 10.1016/s1074-7613(02)00324-2. Epub 2002/07/18. [DOI] [PubMed] [Google Scholar]
- 117.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172(4):2225–31. doi: 10.4049/jimmunol.172.4.2225. Epub 2004/02/07. [DOI] [PubMed] [Google Scholar]
- 118.Villarino AV, Huang E, Hunter CA. Understanding the pro- and anti-inflammatory properties of IL-27. J Immunol. 2004;173(2):715–20. doi: 10.4049/jimmunol.173.2.715. Epub 2004/07/09. [DOI] [PubMed] [Google Scholar]
- 119.Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak TW, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170(10):4886–90. doi: 10.4049/jimmunol.170.10.4886. Epub 2003/05/08. [DOI] [PubMed] [Google Scholar]
- 120.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7(9):929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 121.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7(9):937–45. doi: 10.1038/ni1376. Epub 2006/08/15. [DOI] [PubMed] [Google Scholar]
- 122.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19(6):409–17. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yoshimoto T, Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007;179(7):4415–23. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 124.Huber M, Steinwald V, Guralnik A, Brustle A, Kleemann P, Rosenplanter C, et al. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20(2):223–34. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 125.Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J Immunol. 2007;179(10):6421–8. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 126.Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest. 2008;118(5):1680–90. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Miyazaki Y, Shimanoe Y, Wang S, Yoshida H. Amelioration of delayed-type hypersensitivity responses by IL-27 administration. Biochem Biophys Res Commun. 2008;373(3):397–402. doi: 10.1016/j.bbrc.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 128.Cao Y, Doodes PD, Glant TT, Finnegan A. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J Immunol. 2008;180(2):922–30. doi: 10.4049/jimmunol.180.2.922. [DOI] [PubMed] [Google Scholar]
- 129.Sugiyama N, Nakashima H, Yoshimura T, Sadanaga A, Shimizu S, Masutani K, et al. Amelioration of human lupus-like phenotypes in MRL/lpr mice by overexpression of IL-27R{alpha} (WSX-1) Ann Rheum Dis. 2007 doi: 10.1136/ard.2007.077537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Young A, Linehan E, Hams E, O’Hara Hall AC, McClurg A, Johnston JA, et al. Cutting edge: suppression of GM-CSF expression in murine and human T cells by IL-27. Journal of immunology. 2012;189(5):2079–83. doi: 10.4049/jimmunol.1200131. Epub 2012/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.El-behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. Journal of immunology. 2009;183(8):4957–67. doi: 10.4049/jimmunol.0900735. Epub 2009/09/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature immunology. 2008;9(12):1341–6. doi: 10.1038/ni.1659. Epub 2008/10/22. [DOI] [PubMed] [Google Scholar]
- 133.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nature immunology. 2008;9(12):1347–55. doi: 10.1038/ni.1677. Epub 2008/11/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tan C, Gery I. The unique features of Th9 cells and their products. Critical reviews in immunology. 2012;32(1):1–10. doi: 10.1615/critrevimmunol.v32.i1.10. Epub 2012/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jabeen R, Kaplan MH. The symphony of the ninth: the development and function of Th9 cells. Current opinion in immunology. 2012;24(3):303–7. doi: 10.1016/j.coi.2012.02.001. Epub 2012/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stassen M, Schmitt E, Bopp T. From interleukin-9 to T helper 9 cells. Annals of the New York Academy of Sciences. 2012;1247:56–68. doi: 10.1111/j.1749-6632.2011.06351.x. Epub 2012/01/13. [DOI] [PubMed] [Google Scholar]
- 137.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nature immunology. 2011;12(11):1071–7. doi: 10.1038/ni.2133. Epub 2011/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nowak EC, Noelle RJ. Interleukin-9 as a T helper type 17 cytokine. Immunology. 2010;131(2):169–73. doi: 10.1111/j.1365-2567.2010.03332.x. Epub 2010/08/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li H, Rostami A. IL-9: basic biology, signaling pathways in CD4+ T cells and implications for autoimmunity. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2010;5(2):198–209. doi: 10.1007/s11481-009-9186-y. Epub 2009/12/19. [DOI] [PubMed] [Google Scholar]
- 140.Hultner L, Druez C, Moeller J, Uyttenhove C, Schmitt E, Rude E, et al. Mast cell growth-enhancing activity (MEA) is structurally related and functionally identical to the novel mouse T cell growth factor P40/TCGFIII (interleukin 9) Eur J Immunol. 1990;20(6):1413–6. doi: 10.1002/eji.1830200632. [DOI] [PubMed] [Google Scholar]
- 141.Uyttenhove C, Simpson RJ, Van Snick J. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proc Natl Acad Sci U S A. 1988;85(18):6934–8. doi: 10.1073/pnas.85.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):12885–90. doi: 10.1073/pnas.0812530106. Epub 2009/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Elyaman W, Bassil R, Bradshaw EM, Orent W, Lahoud Y, Zhu B, et al. Notch receptors and Smad3 signaling cooperate in the induction of interleukin-9-producing T cells. Immunity. 2012;36(4):623–34. doi: 10.1016/j.immuni.2012.01.020. Epub 2012/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, et al. IL-9 as a mediator of Th17-driven inflammatory disease. The Journal of experimental medicine. 2009;206(8):1653–60. doi: 10.1084/jem.20090246. Epub 2009/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li H, Nourbakhsh B, Ciric B, Zhang GX, Rostami A. Neutralization of IL-9 ameliorates experimental autoimmune encephalomyelitis by decreasing the effector T cell population. Journal of immunology. 2010;185(7):4095–100. doi: 10.4049/jimmunol.1000986. Epub 2010/09/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li H, Nourbakhsh B, Cullimore M, Zhang GX, Rostami A. IL-9 is important for T-cell activation and differentiation in autoimmune inflammation of the central nervous system. European journal of immunology. 2011;41(8):2197–206. doi: 10.1002/eji.201041125. Epub 2011/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Murugaiyan G, Beynon V, Pires Da Cunha A, Joller N, Weiner HL. IFN-gamma limits Th9-mediated autoimmune inflammation through dendritic cell modulation of IL-27. Journal of immunology. 2012;189(11):5277–83. doi: 10.4049/jimmunol.1200808. Epub 2012/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou Y, Sonobe Y, Akahori T, Jin S, Kawanokuchi J, Noda M, et al. IL-9 promotes Th17 cell migration into the central nervous system via CC chemokine ligand-20 produced by astrocytes. Journal of immunology. 2011;186(7):4415–21. doi: 10.4049/jimmunol.1003307. Epub 2011/02/25. [DOI] [PubMed] [Google Scholar]
- 149.Lu Y, Hong S, Li H, Park J, Hong B, Wang L, et al. Th9 cells promote antitumor immune responses in vivo. The Journal of clinical investigation. 2012;122(11):4160–71. doi: 10.1172/JCI65459. Epub 2012/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. Journal of immunology. 2009;183(11):7169–77. doi: 10.4049/jimmunol.0901906. Epub 2009/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tan C, Aziz MK, Lovaas JD, Vistica BP, Shi G, Wawrousek EF, et al. Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. Journal of immunology. 2010;185(11):6795–801. doi: 10.4049/jimmunol.1001676. Epub 2010/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nature immunology. 2011;12(3):255–63. doi: 10.1038/ni.1993. Epub 2011/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. The American journal of pathology. 2008;172(1):146–55. doi: 10.2353/ajpath.2008.070690. Epub 2007/12/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Uzawa A, Mori M, Arai K, Sato Y, Hayakawa S, Masuda S, et al. Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult Scler. 2010;16(12):1443–52. doi: 10.1177/1352458510379247. [DOI] [PubMed] [Google Scholar]
- 155.Benoist C, Mathis D. Treg cells, life history, and diversity. Cold Spring Harbor perspectives in biology. 2012;4(9):a007021. doi: 10.1101/cshperspect.a007021. Epub 2012/09/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim HJ, Cantor H. Regulation of self-tolerance by Qa-1-restricted CD8(+) regulatory T cells. Seminars in immunology. 2011;23(6):446–52. doi: 10.1016/j.smim.2011.06.001. Epub 2011/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mauri C, Bosma A. Immune regulatory function of B cells. Annual review of immunology. 2012;30:221–41. doi: 10.1146/annurev-immunol-020711-074934. Epub 2012/01/10. [DOI] [PubMed] [Google Scholar]
- 158.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138(2):105–15. doi: 10.1111/imm.12036. Epub 2012/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lowther DE, Hafler DA. Regulatory T cells in the central nervous system. Immunological reviews. 2012;248(1):156–69. doi: 10.1111/j.1600-065X.2012.01130.x. Epub 2012/06/26. [DOI] [PubMed] [Google Scholar]
- 160.Geiger TL, Tauro S. Nature and nurture in Foxp3(+) regulatory T cell development, stability, and function. Human immunology. 2012;73(3):232–9. doi: 10.1016/j.humimm.2011.12.012. Epub 2012/01/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL-35-mediated induction of a potent regulatory T cell population. Nature immunology. 2010;11(12):1093–101. doi: 10.1038/ni.1952. Epub 2010/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. Epub 2012/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kumar V, Sercarz EE. The involvement of T cell receptor peptide-specific regulatory CD4+ T cells in recovery from antigen-induced autoimmune disease. The Journal of experimental medicine. 1993;178(3):909–16. doi: 10.1084/jem.178.3.909. Epub 1993/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78(3):399–408. doi: 10.1016/0092-8674(94)90419-7. Epub 1994/08/12. [DOI] [PubMed] [Google Scholar]
- 165.Olivares-Villagomez D, Wang Y, Lafaille JJ. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. The Journal of experimental medicine. 1998;188(10):1883–94. doi: 10.1084/jem.188.10.1883. Epub 1998/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. Journal of immunology. 2002;169(9):4712–6. doi: 10.4049/jimmunol.169.9.4712. Epub 2002/10/23. [DOI] [PubMed] [Google Scholar]
- 167.Reddy J, Illes Z, Zhang X, Encinas J, Pyrdol J, Nicholson L, et al. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(43):15434–9. doi: 10.1073/pnas.0404444101. Epub 2004/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–7. doi: 10.1038/nature05970. Epub 2007/06/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stephens LA, Gray D, Anderton SM. CD4+CD25+ regulatory T cells limit the risk of autoimmune disease arising from T cell receptor crossreactivity. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(48):17418–23. doi: 10.1073/pnas.0507454102. Epub 2005/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, et al. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. International immunology. 2004;16(2):249–56. doi: 10.1093/intimm/dxh029. Epub 2004/01/22. [DOI] [PubMed] [Google Scholar]
- 171.Montero E, Nussbaum G, Kaye JF, Perez R, Lage A, Ben-Nun A, et al. Regulation of experimental autoimmune encephalomyelitis by CD4+, CD25+ and CD8+ T cells: analysis using depleting antibodies. Journal of autoimmunity. 2004;23(1):1–7. doi: 10.1016/j.jaut.2004.05.001. Epub 2004/07/09. [DOI] [PubMed] [Google Scholar]
- 172.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nature medicine. 2006;12(5):518–25. doi: 10.1038/nm1402. Epub 2006/04/25. [DOI] [PubMed] [Google Scholar]
- 173.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nature medicine. 2007;13(4):423–31. doi: 10.1038/nm1564. Epub 2007/03/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. Journal of immunology. 2005;175(5):3025–32. doi: 10.4049/jimmunol.175.5.3025. Epub 2005/08/24. [DOI] [PubMed] [Google Scholar]
- 175.O’Connor RA, Malpass KH, Anderton SM. The inflamed central nervous system drives the activation and rapid proliferation of Foxp3+ regulatory T cells. Journal of immunology. 2007;179(2):958–66. doi: 10.4049/jimmunol.179.2.958. Epub 2007/07/10. [DOI] [PubMed] [Google Scholar]
- 176.Trajkovic V, Vuckovic O, Stosic-Grujicic S, Miljkovic D, Popadic D, Markovic M, et al. Astrocyte-induced regulatory T cells mitigate CNS autoimmunity. Glia. 2004;47(2):168–79. doi: 10.1002/glia.20046. Epub 2004/06/09. [DOI] [PubMed] [Google Scholar]
- 177.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. The Journal of experimental medicine. 2004;199(7):971–9. doi: 10.1084/jem.20031579. Epub 2004/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Feger U, Luther C, Poeschel S, Melms A, Tolosa E, Wiendl H. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clinical and experimental immunology. 2007;147(3):412–8. doi: 10.1111/j.1365-2249.2006.03271.x. Epub 2007/02/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Frisullo G, Nociti V, Iorio R, Patanella AK, Caggiula M, Marti A, et al. Regulatory T cells fail to suppress CD4T+-bet+ T cells in relapsing multiple sclerosis patients. Immunology. 2009;127(3):418–28. doi: 10.1111/j.1365-2567.2008.02963.x. Epub 2008/11/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Haas J, Hug A, Viehover A, Fritzsching B, Falk CS, Filser A, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. European journal of immunology. 2005;35(11):3343–52. doi: 10.1002/eji.200526065. Epub 2005/10/06. [DOI] [PubMed] [Google Scholar]
- 181.Venken K, Hellings N, Broekmans T, Hensen K, Rummens JL, Stinissen P. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. Journal of immunology. 2008;180(9):6411–20. doi: 10.4049/jimmunol.180.9.6411. Epub 2008/04/22. [DOI] [PubMed] [Google Scholar]
- 182.Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, et al. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123(1):79–89. doi: 10.1111/j.1365-2567.2007.02690.x. Epub 2007/09/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Huan J, Culbertson N, Spencer L, Bartholomew R, Burrows GG, Chou YK, et al. Decreased FOXP3 levels in multiple sclerosis patients. Journal of neuroscience research. 2005;81(1):45–52. doi: 10.1002/jnr.20522. Epub 2005/06/14. [DOI] [PubMed] [Google Scholar]
- 184.Venken K, Hellings N, Hensen K, Rummens JL, Medaer R, D’Hooghe MB, et al. Secondary progressive in contrast to relapsing-remitting multiple sclerosis patients show a normal CD4+CD25+ regulatory T-cell function and FOXP3 expression. Journal of neuroscience research. 2006;83(8):1432–46. doi: 10.1002/jnr.20852. Epub 2006/04/04. [DOI] [PubMed] [Google Scholar]
- 185.de Andres C, Aristimuno C, de Las Heras V, Martinez-Gines ML, Bartolome M, Arroyo R, et al. Interferon beta-1a therapy enhances CD4+ regulatory T-cell function: an ex vivo and in vitro longitudinal study in relapsing-remitting multiple sclerosis. Journal of neuroimmunology. 2007;182(1-2):204–11. doi: 10.1016/j.jneuroim.2006.09.012. Epub 2006/12/13. [DOI] [PubMed] [Google Scholar]
- 186.Korporal M, Haas J, Balint B, Fritzsching B, Schwarz A, Moeller S, et al. Interferon beta-induced restoration of regulatory T-cell function in multiple sclerosis is prompted by an increase in newly generated naive regulatory T cells. Archives of neurology. 2008;65(11):1434–9. doi: 10.1001/archneur.65.11.1434. Epub 2008/11/13. [DOI] [PubMed] [Google Scholar]
- 187.Hong J, Li N, Zhang X, Zheng B, Zhang JZ. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(18):6449–54. doi: 10.1073/pnas.0502187102. Epub 2005/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nature reviews Immunology. 2002;2(5):336–45. doi: 10.1038/nri797. Epub 2002/05/30. [DOI] [PubMed] [Google Scholar]
- 189.Lewis JM, Girardi M, Roberts SJ, Barbee SD, Hayday AC, Tigelaar RE. Selection of the cutaneous intraepithelial gammadelta+ T cell repertoire by a thymic stromal determinant. Nature immunology. 2006;7(8):843–50. doi: 10.1038/ni1363. Epub 2006/07/11. [DOI] [PubMed] [Google Scholar]
- 190.Bigby M, Markowitz JS, Bleicher PA, Grusby MJ, Simha S, Siebrecht M, et al. Most gamma delta T cells develop normally in the absence of MHC class II molecules. Journal of immunology. 1993;151(9):4465–75. Epub 1993/11/01. [PubMed] [Google Scholar]
- 191.Correa I, Bix M, Liao NS, Zijlstra M, Jaenisch R, Raulet D. Most gamma delta T cells develop normally in beta 2-microglobulin-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(2):653–7. doi: 10.1073/pnas.89.2.653. Epub 1992/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Duhindan N, Farley AJ, Humphreys S, Parker C, Rossiter B, Brooks CG. Patterns of lymphokine secretion amongst mouse gamma delta T cell clones. European journal of immunology. 1997;27(7):1704–12. doi: 10.1002/eji.1830270717. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 193.Rhodes KA, Andrew EM, Newton DJ, Tramonti D, Carding SR. A subset of IL-10-producing gammadelta T cells protect the liver from Listeria-elicited, CD8(+) T cell-mediated injury. European journal of immunology. 2008;38(8):2274–83. doi: 10.1002/eji.200838354. Epub 2008/07/16. [DOI] [PubMed] [Google Scholar]
- 194.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29(1):90–100. doi: 10.1016/j.immuni.2008.04.022. Epub 2008/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lukens JR, Barr MJ, Chaplin DD, Chi H, Kanneganti TD. Inflammasome-derived IL-1beta regulates the production of GM-CSF by CD4(+) T cells and gammadelta T cells. Journal of immunology. 2012;188(7):3107–15. doi: 10.4049/jimmunol.1103308. Epub 2012/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31(2):321–30. doi: 10.1016/j.immuni.2009.06.020. Epub 2009/08/18. [DOI] [PubMed] [Google Scholar]
- 197.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nature immunology. 2009;10(4):427–36. doi: 10.1038/ni.1717. Epub 2009/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kobayashi Y, Kawai K, Ito K, Honda H, Sobue G, Yoshikai Y. Aggravation of murine experimental allergic encephalomyelitis by administration of T-cell receptor gammadelta-specific antibody. Journal of neuroimmunology. 1997;73(1-2):169–74. doi: 10.1016/s0165-5728(96)00187-7. Epub 1997/03/01. [DOI] [PubMed] [Google Scholar]
- 199.Ponomarev ED, Novikova M, Yassai M, Szczepanik M, Gorski J, Dittel BN. Gamma delta T cell regulation of IFN-gamma production by central nervous system-infiltrating encephalitogenic T cells: correlation with recovery from experimental autoimmune encephalomyelitis. Journal of immunology. 2004;173(3):1587–95. doi: 10.4049/jimmunol.173.3.1587. Epub 2004/07/22. [DOI] [PubMed] [Google Scholar]
- 200.Ponomarev ED, Dittel BN. Gamma delta T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. Journal of immunology. 2005;174(8):4678–87. doi: 10.4049/jimmunol.174.8.4678. Epub 2005/04/09. [DOI] [PubMed] [Google Scholar]
- 201.Clark RB, Lingenheld EG. Adoptively transferred EAE in gamma delta T cell-knockout mice. Journal of autoimmunity. 1998;11(1):105–10. doi: 10.1006/jaut.1997.0180. Epub 1998/03/14. [DOI] [PubMed] [Google Scholar]
- 202.Rajan AJ, Gao YL, Raine CS, Brosnan CF. A pathogenic role for gamma delta T cells in relapsing-remitting experimental allergic encephalomyelitis in the SJL mouse. Journal of immunology. 1996;157(2):941–9. Epub 1996/07/15. [PubMed] [Google Scholar]
- 203.Rajan AJ, Klein JD, Brosnan CF. The effect of gammadelta T cell depletion on cytokine gene expression in experimental allergic encephalomyelitis. Journal of immunology. 1998;160(12):5955–62. Epub 1998/06/24. [PubMed] [Google Scholar]
- 204.Spahn TW, Issazadah S, Salvin AJ, Weiner HL. Decreased severity of myelin oligodendrocyte glycoprotein peptide 33 - 35-induced experimental autoimmune encephalomyelitis in mice with a disrupted TCR delta chain gene. European journal of immunology. 1999;29(12):4060–71. doi: 10.1002/(SICI)1521-4141(199912)29:12<4060::AID-IMMU4060>3.0.CO;2-S. Epub 1999/12/22. [DOI] [PubMed] [Google Scholar]
- 205.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31(2):331–41. doi: 10.1016/j.immuni.2009.08.001. Epub 2009/08/18. [DOI] [PubMed] [Google Scholar]
- 206.Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, et al. gammadelta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33(3):351–63. doi: 10.1016/j.immuni.2010.08.013. Epub 2010/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ishizu T, Osoegawa M, Mei FJ, Kikuchi H, Tanaka M, Takakura Y, et al. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain: a journal of neurology. 2005;128(Pt 5):988–1002. doi: 10.1093/brain/awh453. Epub 2005/03/04. [DOI] [PubMed] [Google Scholar]