Abstract
The use of electroporation to facilitate gene transfer is an extremely powerful and useful method for both in vitro and in vivo applications. One of its great strengths is that it induces functional destabilization and permeabilization of cell membranes throughout a tissue leading to widespread gene transfer to multiple cells and cell types within the electric field. While this is a strength, it can also be a limitation in terms of cell-specific gene delivery. The ability to restrict gene delivery and expression to particular cell types is of paramount importance for many types of gene therapy, since ectopic expression of a transgene could lead to deleterious host inflammatory responses or dysregulation of normal cellular functions. At present, there are relatively few ways to obtain cell-specific targeting of nonviral vectors, molecular probes, small molecules, and imaging agents. We have developed a novel means of restricting gene delivery to desired cell types based on the ability to control the transport of plasmids into the nuclei of desired cell types. In this article, we discuss the mechanisms of this approach and several applications in living animals to demonstrate the benefits of the combination of electroporation and selective nuclear import of plasmids for cell-specific gene delivery.
Keywords: transfection, nuclear import, nucleus, gene delivery, trafficking, transcription, electroporation
Introduction
Electroporation uses electrical fields to create transient pores in the cell membrane that allow the entry of normally impermeable macromolecules into the cytoplasm (Escoffre et al., 2009). While this technique is used most commonly to transfer DNA to bacteria, yeast, and mammalian cells in culture, it also can be applied very effectively to living animals. In most animal studies, electroporation causes anywhere from a 20- to a 1000-fold increase in gene expression compared to DNA injection alone in normal tissues such as skin, liver, and muscle, as well as in a variety of tumors (Cemazar et al., 2009; Heller et al., 2000; Lin et al., 2012; Wells et al., 2000). We and others have adapted this technique for use in the vasculature (Martin et al., 2000; Young, Benoit & Dean, 2003; Young, Zimmer & Dean, 2008), cornea (Blair-Parks, Weston & Dean, 2002), lung (Dean et al., 2003; Machado-Aranda et al., 2005; Mutlu et al., 2007; Zhou et al., 2007), heart (Aistrup et al., 2009; Hargrave et al., 2012; Marshall et al., 2010), skin (Heller et al., 2002; Heller et al., 2010), skeletal muscle (Mir et al., 1999; Mir et al., 1998), liver (Heller et al., 1996), intestine, kidney, and prostate (Dean, unpublished), among other organs. The procedure is rapid, safe, and reproducible. Perhaps most exciting about this approach is that DNA is delivered to multiple cell layers throughout the tissue, even when the DNA is not injected into the tissue. Thus, when we bathe an organ such as the kidney in plasmid solution and apply the electric field, gene expression of the transferred transgene can be detected throughout the kidney and is not obstructed by the capsule. Similarly, when we deliver plasmid to the lungs via the airways, gene expression is detected in multiple cell layers throughout the lung, while delivery of DNA via the vasculature and subsequent application of electric fields to the lung results in a similar distribution of gene expression.
While such universal gene delivery and expression can be extremely useful in many situations, it may also be a limitation in others. There are many circumstances in which gene delivery and/or expression is desired in a unique cell type within a tissue and where ubiquitous expression may actually be detrimental. One such example would be in the case of cytostatic gene transfer or suicide gene transfer to treat tumors. Thus, the ability to restrict gene delivery and expression becomes vital in many applications.
Cell-Specific Targeting of Genes
At present there are only four methods to limit delivery/expression of genes to specific cell types and tissues. The first is by physical delivery to a desired target organ. For example, in the case of systemic delivery of DNA-containing liposomes via the circulation, endothelial cells are predominantly transduced (and primarily those of the lung and liver microvasculature), while delivery of drugs to the airways results in delivery only to airway and alveolar epithelial cells. Thus, the only “specificity“ appears to be accessibility of the vector to the cells (Young & Dean, 2002). In terms of electroporation-mediated gene delivery, another example would be the intersection of administered DNA and the applied electric field. For example, if DNA is injected into the dermal layer of the skin, and needle electrodes are inserted into this layer, the majority of gene transfer will be here. Conversely, if DNA is injected superficially into the epidermis and non-penetrating electrodes are placed on the skin, then very little gene transfer will take place in the dermis. The second method is based on pulsing parameters and sizes, shapes, and composition of specific cells. For example, it is well known that larger cells are permeabilized at lower field strengths than smaller cells and that cell shape is extremely important in the way in which the cell responds to the electric field (Valic et al., 2003). Thus, it is possible to electroporate specific cells within a tissue if they are sufficiently different in size than other cells within the tissue. One example of this was shown for adipocytes in mouse adipose tissue. Since lipid-laden adipocytes are much larger (>5-fold) than other cells in adipose tissue, they can be electroporated preferentially. Indeed, when a series of seven 20 msec pulses at 0.5 kV/cm were administered to suprascapular white adipose tissue in mice, 99% of cells expressing the delivered transgene were adipocytes, which made up only 16% of the cells within the tissue (Granneman et al., 2004). The third method is to incorporate ligands or antibodies for cell surface receptors that are expressed preferentially on one or more cell type. One example of this is to attach RGD peptides to liposomes, polymers, or viral capsids to target to vb3 integrins which are overexpressed on activated endothelial cells during tumor-induced angiogenesis (Temming et al., 2005). However, vb3 is expressed at lesser levels on all endothelial cells and many other cell types as well, indicating that this approach is not always as “cell-specific” as one would hope. The fourth, and most stringent, way to limit expression (but not drug delivery) that has been used is to employ cell-specific promoters to drive transcription in desired cell types. While this approach works well, it is not always perfect, since some promoters that are thought to be cell-specific based on experiments in cultured cells, may show more promiscuous expression in animals, as is clear to anyone who has tried to generate tissue-specific transgenic or conditional knockout mice.
Over the past several years, we have developed a new approach for cell-specific delivery of nonviral DNA-based vectors based on our elucidation of the mechanisms of plasmid DNA nuclear import. We have shown that the nuclear localization of plasmids in the absence of cell division is sequence-specific and requires transcription factors that bind to these sequences in the cytoplasm and facilitate the DNA-protein complex nuclear import. Moreover, we have identified several DNA sequences that mediate cell-specific DNA nuclear import based on the fact that they bind to cell-specific transcription factors present in these cell types. Using these DNA sequences, we have been able to direct gene delivery and expression to desired cell types in vitro and in living animals, using electroporation as our delivery method.
Nuclear import of DNA
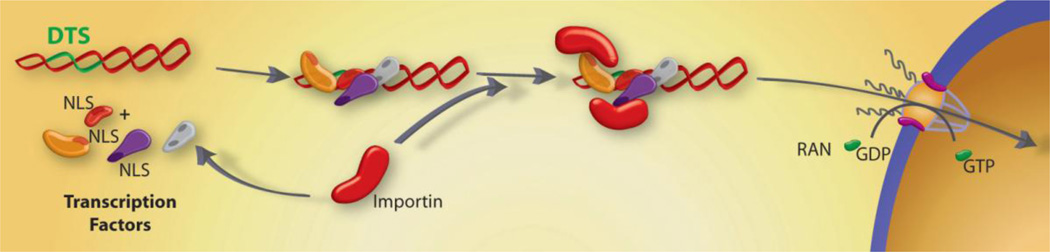
Numerous groups have demonstrated that plasmids can enter nuclei through nuclear pore complexes (NPCs) in the absence of cell division, although the efficiency of such transfection is usually much lower than in dividing cells (Dean, 1997; Dowty et al., 1995; Vacik et al., 1999). Moreover, certain DNA sequences can increase this nuclear targeting of plasmids prior to mitosis. We and others have shown that nuclear import of plasmid DNA through the NPC is a sequence-specific process, mediated by specific eukaryotic sequence elements (Dean, 1997). When delivered side-by-side, plasmids containing as little as 72 bp of the SV40 enhancer target to the nucleus of most cells within several hours whereas an isogenic plasmid lacking this 72 bp sequence remains cytoplasmic until cell division (or indefinitely if the cell is non-dividing) or eventual degradation in the cytoplasm (Fig. 1)(Dean, 1997; Dean et al., 1999). This sequence, termed the SV40 DNA nuclear targeting sequence (DTS), has been shown to mediate plasmid nuclear import in all cell lines tested, including primary cells derived from monkey, rat, mouse, hamster, chicken, and human origin, as well as in vivo (Blomberg et al., 2002; Sacramento et al., 2010; Young et al., 2003; Young et al., 2008). A major strength of many of these and other DTSs is that endogenously expressed proteins are used to coat transfected plasmid vectors with the NLSs required for import.
Figure 1. Protein-mediated plasmid nuclear import.
Transcription factors and other nuclear proteins normally enter the nucleus through the interactions between their NLSs and importin family members. However, if plasmids containing certain sequences that act as scaffolds for transcription factors and other DNA binding proteins (termed DTS, or DNA nuclear Targeting Sequences) are deposited into the cytoplasm during transfection, they can form complexes with these proteins, thereby attaching NLSs to the DNA. Some, but not all, of these NLSs may be in a conformation able to interact with importins for transport of the DNA-protein complex into the nucleus through the Nuclear Pore Complex.
The defining feature of the SV40 DTS is that it contains binding sites for a number of ubiquitously expressed mammalian transcription factors (AP1, AP2, NF- B, Oct1, TEF-1). Since transcription factors function in the nucleus, they contain NLSs for their nuclear importation. Under normal conditions, these factors would be transported into the nucleus after translation or in a regulated manner when signals activate transcription (e.g., TNF- stimulation of NF- B). In either case, a significant cytoplasmic pool of these factors exists at any given time. When plasmids carrying the SV40 DTS are delivered into the cytoplasm by any method, some of these transcription factors can bind to the DTS thereby coating a region of the plasmid with NLSs, at least some of which are oriented away from the DNA itself. These DNA-bound NLSs can be recognized by importin and transported into the nucleus via the NPC (Fig. 1)(Dean, 1997; Dowty et al., 1995; Wilson et al., 1999). Since the function of the DTS is mediated by binding of NLS-containing transcription factors, it would seem that any eukaryotic promoter or enhancer could function similarly for DNA nuclear import. Surprisingly, this is not the case and although half a dozen or so DNA nuclear targeting sequences have been identified, most promoters and enhancers, including the CMV immediate early promoter/enhancer, the Herpes TK promoter, and the RSV LTR have no import activity (Dean et al., 1999). The likely explanation for this is that the transcription factors bound to these other promoters may not present their NLSs in an orientation that is accessible to the importins.
Cell-specific DNA nuclear import sequences
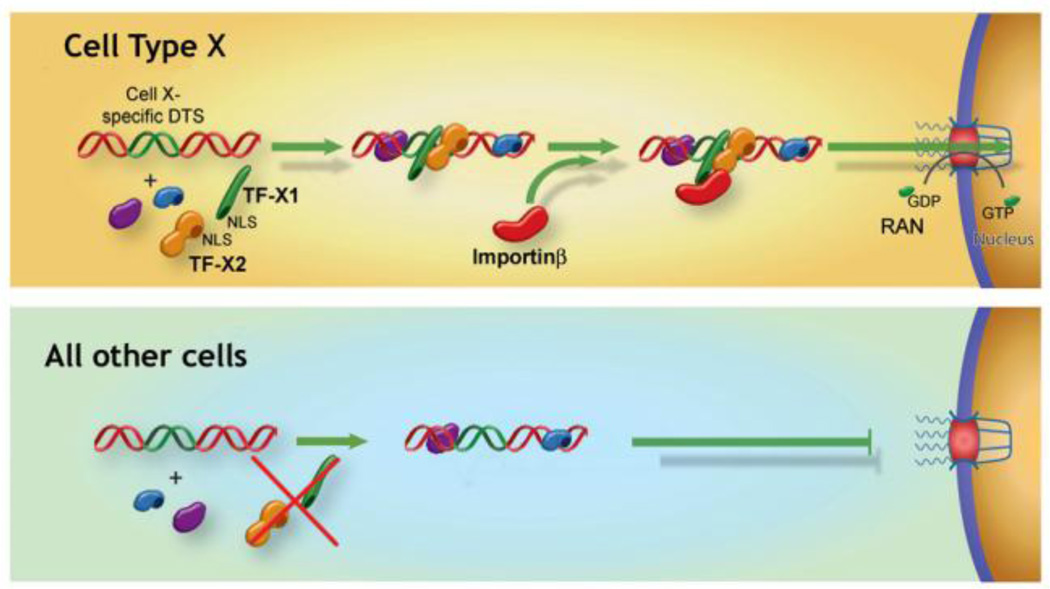
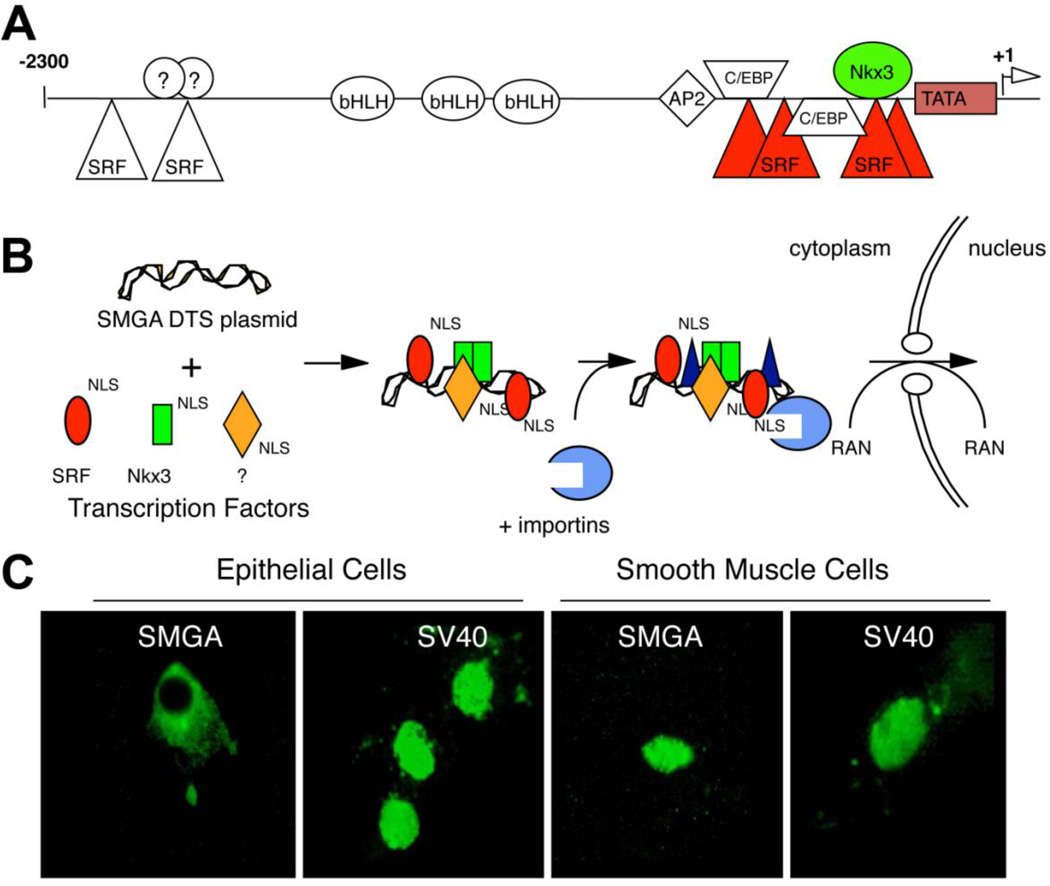
In the search for additional DNA nuclear targeting sequences, several DNA sequences were identified that promoted plasmid nuclear import in unique cell types. Since the expression of cell-specific promoters are restricted to specific cells due to the presence of a unique set of transcription factors present in those cells only, by screening promoters that are transcriptionally active only in a desired cell type, it could be possible to pull out sequences that also function for cell specific nuclear import (Fig. 2)(Miller & Dean, 2009). To date, such sequences that act in osteoblasts (Dean et al., 2006), endothelial cells (Dean, 2002), alveolar type II epithelial cells (DeGiulio & Dean, 2006) smooth muscle cells (Vacik et al., 1999; Young et al., 2008), and embryonic stem cells (Funabashi et al., 2010) have been identified. The best studied of these is the smooth muscle-specific DTS in which as little as 176 bp of the smooth muscle gamma actin (SMGA) promoter can drive nuclear import of plasmids in airway or vascular smooth muscle cells but not in other cell types. We have shown that two transcription factors that are preferentially co-expressed in smooth muscle, Nkx3.1/3.2 and SRF, are both necessary and sufficient for DNA nuclear uptake in these cells (Miller & Dean, 2008; Vacik et al., 1999). When the binding sites for these factors were mutated within the SMGA promoter, plasmids containing the mutant DTS remained in the cytoplasm of microinjected cells (Fig. 3). Similarly, when Nkx3.1/3.2 and SRF were silenced in smooth muscle cells through the use of siRNA, nuclear import of plasmids carrying the wild type SMGA promoter was abolished, again showing that these factors are necessary for DNA nuclear import (Miller & Dean, 2008). Sufficiency of these two transcription factors alone was shown by expressing the factors in bacteria, complexing the purified proteins with SMGA DTS plasmids prior to cytoplasmic microinjection, and obtaining nuclear import in non-smooth muscle cells that do not normally express these factors (Miller & Dean, 2008).
Figure 2. Model for Cell-specific DNA nuclear import.
Certain DNA nuclear Targeting Sequences have been shown to act in cell-restricted manners. In the case of certain “Cell type X” promoters, which act as Cell type X –specific DTSs, we propose that the cell-specific transcription factors, TF-X1 and TF-X2, form complexes with the plasmid leading to an importin-recognizable complex that can be localized to the nucleus. By contrast, in all other cell types that do not express one or the other of these factors, an importin-binding complex is not formed leading to greatly reduced nuclear import. We have shown that this model is valid in smooth muscle, alveolar epithelial cells, and osteoblast examples.
Figure 3. The SMGA promoter acts as a cell-specific DTS in smooth muscle cells.
A. Cartoon of the SMGA promoter showing binding sites for various transcription factors. B. Model for cell-specific DNA nuclear transport. In smooth muscle cells, the SMGA DTS binds transcription factors that are specific to smooth muscle cells, thus forming an import competent complex. C. CV1 cells, an epithelial cell line, and primary human vascular smooth muscle cells, were cytoplasmically microinjected with plasmids carrying either the SV40 DTS or the SMGA DTS and the location of the plasmids was determined 8 hours later by in situ hybridization.
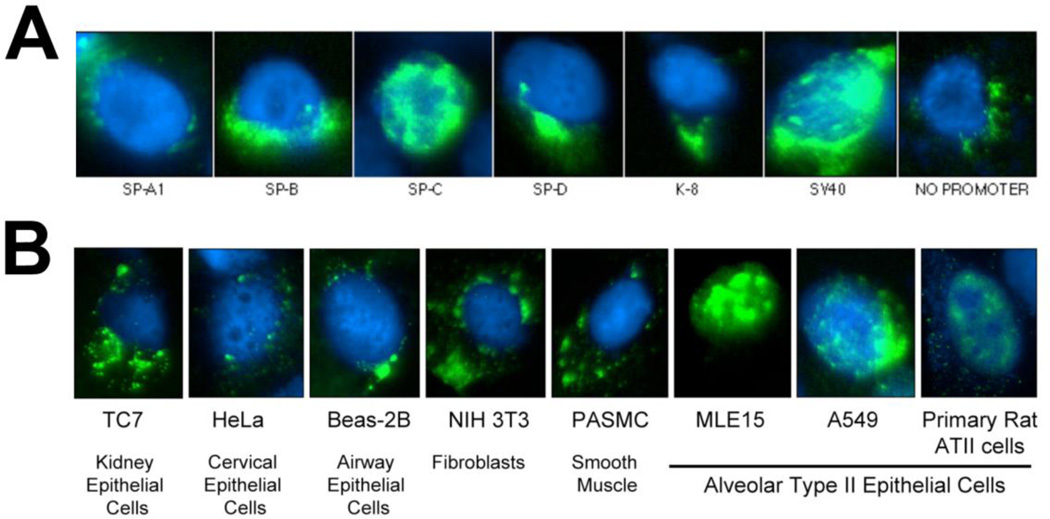
A second example of a cell-specific DTS that we have identified is the SP-C promoter which mediates DNA nuclear import in type II alveolar epithelial cells only (Degiulio, Kaufman & Dean, 2010). Six promoters that were reported in the literature to be specifically expressed (or highly enriched) in type II alveolar epithelial cells were cloned and tested for nuclear import activity. While 318 bp of the SP-C promoter showed nuclear import activity in type II cell lines, neither the SP-A, SP-B, SP-D, nor the cytokeratin 8 promoter had any import activity (Fig. 4). Further, the SP-C promoter showed nuclear import activity only in type II cells and cell lines, but in no other cell types, including epithelial cells from several different tissues (Fig. 4). As for the SMGA DTS, we identified the transcription factor binding sites that are necessary for nuclear import of the SPC DTS and found that multiple factors, including NF1, GATA-6, and TTF-1 are required for DNA nuclear import (Degiulio et al., 2010).
Figure 4. The SP-C promoter mediates cell-specific DNA nuclear import in alveolar epithelial cells.
A. A549 cells were cytoplasmically injected with plasmids containing the indicated promoters. Eight hours later, the location of the DNA was detected by in situ hybridization (green). Between 500 and 1000 cells were injected and analyzed for each DNA. B. Plasmids containing the SPC promoter were injected into the cytoplasm of the indicated cells and 8 hours later, the location of the DNA was detected by in situ hybridization (green). Between 300 and 500 cells of each type were injected and analyzed (Degiulio et al., 2010).
DNA Nuclear Targeting Sequences direct plasmid nuclear import in living animals
While such DNA sequences can be of utility for gene delivery in isolated cells, they would be much more use for cell-specific gene delivery in animals where multiple cell types coexist within any given tissue or organ. To determine the effects DNA nuclear targeting sequences on gene transfer and expression in vivo, we electroporated rat mesenteric blood vessels with isogenic plasmids that either contained or lacked the 72 bp SV40 sequence and expressed a reporter gene from the CMV promoter (which does not promote DNA nuclear import). When these plasmids are transfected into dividing populations of cells, they express their gene products at the same level (Dean et al., 1999). Vessels were electroporated with equal concentrations of either pCMV-GFP-DTS or pCMV-GFP and harvested 3 days post-transfer. A series of 8 square wave pulses were delivered, each of which was 10 msec in duration at a field strength of 200V/cm and separtated by 1 second intervals. As can be seen, plasmids carrying a nuclear targeting sequence gave high level expression, whereas those lacking a plasmid nuclear import sequence showed very little expression (Fig. 5)(Young et al., 2003). When a similar experiment was performed with luciferase-expressing plasmids, there was a 20- fold difference in levels of gene expression at day 2. We and others have obtained similar results in murine skeletal muscle by direct DNA injection with or without electroporation, confirming the universal activity of the SV40 DTS (Blomberg et al., 2002; Li et al., 2001). Further, by following DNA nuclear localization by in situ hybridization in rat mesenteric vessels, we have shown that the increased expression is due to increased nuclear targeting of the DNA (Young, Benoit & Dean, 2002). Thus, DNA nuclear import sequences function in microinjected cells, transfected cells, and living animals.
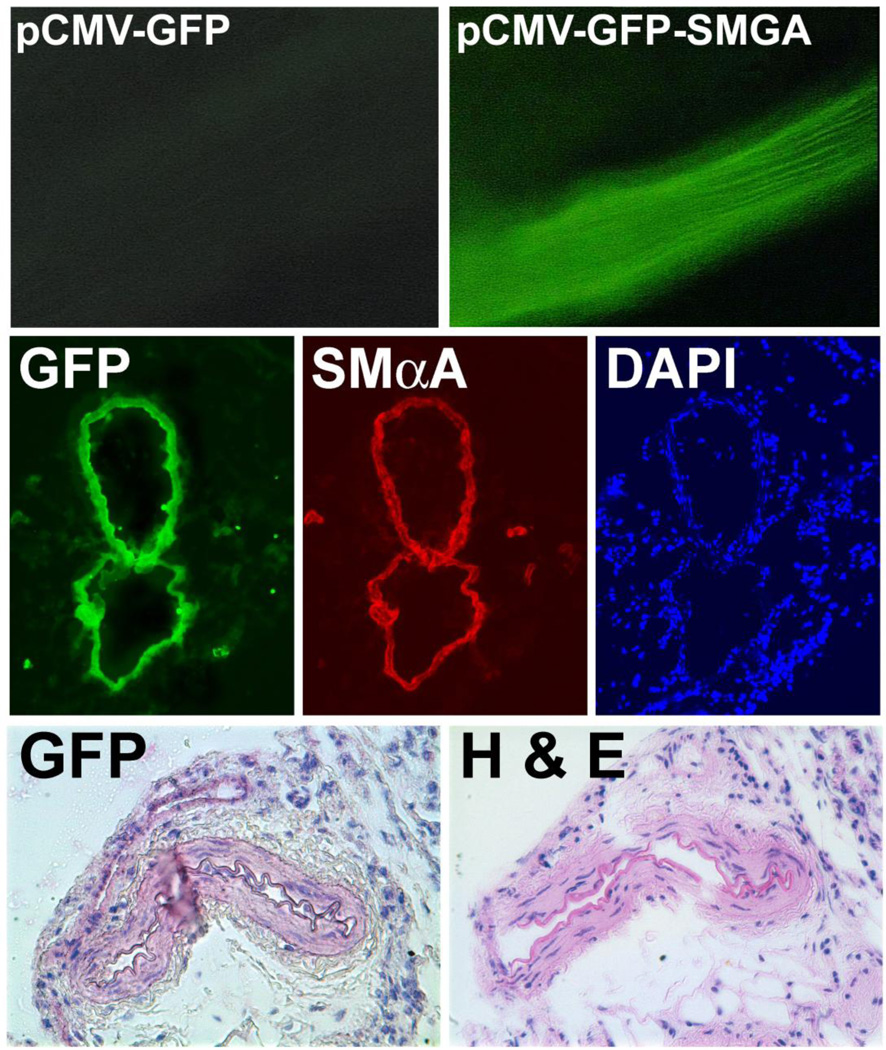
Figure 5. SMGA functions in vivo as a nuclear targeting sequence.
Rat mesenteric arteries were electroporated with pCMV-GFP-SMGA or no DNA and 2 days later harvested. GFP expression could be detected directly in whole vessels. Cryosections were immunostained with anti-GFP (green) or anti-SMαA (red) and reacted with DAPI. Paraffin sections were immunostained with anti-GFP antibodies and visualized with VectaRed or stained with H&E. In both types of thin sections (vessels from different animals), GFP expression was restricted to the smooth muscle layer (Young et al., 2008).
Cell-specific DNA nuclear import sequences also show cell-specificity in vivo. To determine whether the SMGA promoter mediates nuclear import in vivo as it does in cultured cells, we electroporated rat mesenteric vessels, using the same conditions as above, with a plasmid expressing GFP (driven from the CMV promoter) and containing the SMGA DTS downstream of the GFP gene and 2 days later stained for expression in thin sections (Fig. 5). Intact vessels receiving the pCMV-GFP-SMGA plasmid expressed GFP and in thin sections, GFP expression was restricted to the smooth muscle layer of the vessels; no expression in the adventitial cells or endothelial cells was evident (Young et al., 2008). Studies using in situ hybridization to detect transferred DNA clearly showed that the SMGA DTS was able to drive nuclear accumulation of plasmids in smooth muscle cells, but not in other cell types, demonstrating that the restriction of gene expression to smooth muscle cells was due to preferential nuclear uptake of the plasmids in these cells. These results clearly demonstrate that the SMGA promoter can be used to target plasmids to the nucleus of smooth muscle cells in the vasculature, but no other cells in the vessel wall.
Similar results have also been seen with other cell-specific DTS in the lung. When similar reporter constructs expressing GFP and containing the SP-C DTS downstream of the transgene were electroporated into the lungs of mice, we detected GFP gene expression specifically in type II pneumocytes (identified by immunofluorescent co-localization with lamellar body protein LB180) in the absence of cell division (identified by a lack of BrdU incorporation). As for mesentric vessels, the electroopration conditions were the same: 8 pulses at 200V/cm and 10 msec duration. By contrast, the SV40 DTS that acts in all cell types to promote DNA nuclear uptake, causes gene delivery and expression in multiple cell types throughout the lung, including type I and type II alveolar epithelial cells, airway epithelial cells, vascular and airway smooth muscle cells, fibroblasts, and endothelial cells. Thus, the SPC DTS directs gene transfer preferentially to type II cells and in conjunction with electroporation-mediated gene delivery, a similar cell-specific DTS-based approach can be used to deliver genes to any specific cell type.
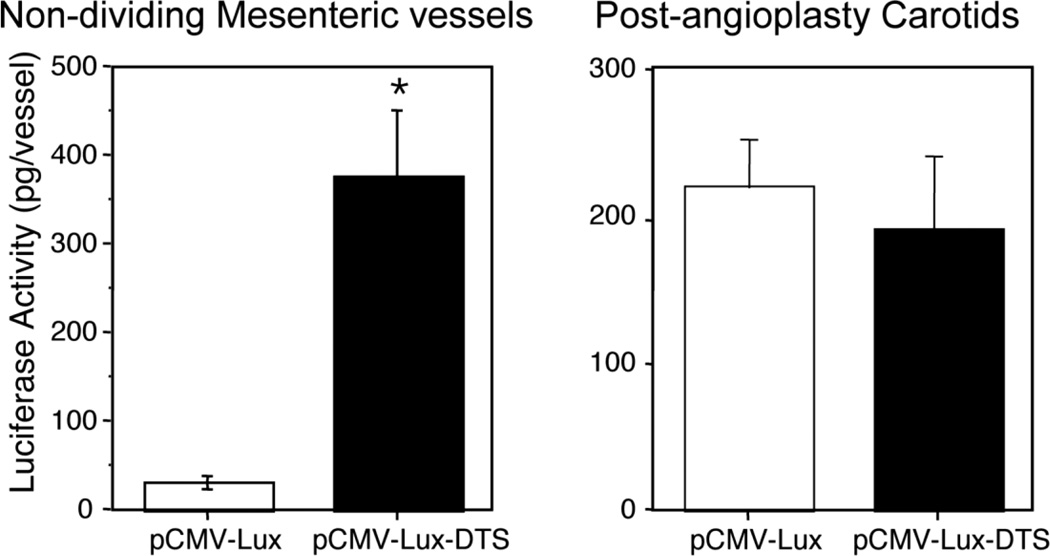
The one caveat to the use of this cell-specific DTS strategy is that the DTS only works as anticipated in cells that are not dividing. When the cell goes through mitosis, the nuclear envelope breaks down and reassembles after cell division. Thus, any plasmid that is in the cytoplasm during mitosis is no longer prevented from entering the nuclear space and when cell division is finished, plasmids wind up being trapped within the new nuclear space. In this case, a DTS is not required for nuclear uptake. Indeed, when actively dividing populations of cells are transfected with plasmids that contain or lack a DTS (either the SV40 or a cell-specific one), there is no difference in transfection efficiency between the plasmids; all plasmids can enter the nucleus equally. However, when the same plasmids are used to transfect growth-arrested cells, only plasmids containing a DTS lead to efficient gene transfer (Vacik et al., 1999; van Gaal et al., 2011). The same is seen in animals: if the tissues are actively dividing, the presence of a DTS does not provide any advantage. To demonstrate this, we carried out angioplasty on the rat carotid artery using a 2F catheter. This technique denudes endothelial cells from the artery and causes a rapid proliferation of both vascular smooth muscle cells and the remaining endothelial cells. When plasmids that express luciferase from the CMV promoter (which does not act as a DTS and cannot cause DNA nuclear import) were electroporated into the vessels at the time of injury (8 pulses at 200V/cm, 10 msec each) and assayed for gene expression three days later, no difference in levels of gene expression were seen with plasmids carrying or lacking an SV40 DTS (Fig. 6). By contrast, when the same plasmids were delivered by electroporation into non-dividing mesenteric arteries, the plasmid carrying the SV40 DTS showed almost 50-fold more expression compared to the plasmid lacking the sequence (Young et al., 2003). However, taken together, these results clearly demonstrate the utility of controlling DNA nuclear import as a means to controlling gene delivery in non-dividing tissues in vivo.
Figure 6. DNA nuclear targeting sequences work only in the absence of cell division.
Plasmids were transferred to undisturbed rat mesenteric vessels (left) or rat carotids that had undergone angioplasty immediately prior to gene delivery and gene expression was measured 2 days later.
Summary
The ability to restrict gene delivery and expression to particular cell types is of paramount importance for many types of gene therapy, since ectopic expression of a transgene could lead to deleterious host inflammatory responses or dysregulation of normal cellular functions. Coupling the high efficiency of electroporation for gene delivery and its ability to permeabilize multiple cell types within a treated tissue with the use of DNA nuclear targeting sequences contained within the transferred plasmids offers a new way to attain this goal. Since many of the DTS's identified to date are derived from cell-specific promoters, this raises the possibility of using these promoters to drive cell-specific nuclear import of the plasmids as well as directing cell-specific transcription of the delivered gene. By placing the promoter/DTS upstream of the transgene, both transcription and nuclear import can be controlled, thus ensuring cell-specificity at two levels. Taken together, this approach may see great use in the future of gene therapy.
ACKNOWLEDGEMENT
This work was supported by NIH grants R01 GM94228, HL81148, HL107331, and EB9903.
Literature Cited
- Aistrup GL, Villuendas R, Ng J, Gilchrist A, Lynch TW, Gordon D, Cokic I, Mottl S, Zhou R, Dean DA, Wasserstrom JA, Goldberger JJ, Kadish AH, Arora R. Targeted G-protein inhibition as a novel approach to decrease vagal atrial fibrillation by selective parasympathetic attenuation. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair-Parks K, Weston BC, Dean DA. Gene delivery to the cornea by plasmid injection and electroporation. J. Gene Med. 2002;4:92–100. doi: 10.1002/jgm.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg P, Eskandarpour M, Xia S, Sylven C, Islam KB. Electroporation in combination with a plasmid vector containing SV40 enhancer elements results in increased and persistent gene expression in mouse muscle. Biochem Biophys Res Commun. 2002;298:505–510. doi: 10.1016/s0006-291x(02)02486-5. [DOI] [PubMed] [Google Scholar]
- Cemazar M, Golzio M, Sersa G, Hojman P, Kranjc S, Mesojednik S, Rols MP, Teissie J. Control by pulse parameters of DNA electrotransfer into solid tumors in mice. Gene Ther. 2009;16:635–644. doi: 10.1038/gt.2009.10. [DOI] [PubMed] [Google Scholar]
- Dean DA. Import of plasmid DNA into the nucleus is sequence specific. Exp. Cell Res. 1997;230:293–302. doi: 10.1006/excr.1996.3427. [DOI] [PubMed] [Google Scholar]
- Dean DA. Nucleocytoplasmic trafficking. In: Mahato RI, editor. Pharmaceutical perspectives of nucleic acid-based therapeutics. London: Harwood Academic Publishers; 2002. pp. 229–260. [Google Scholar]
- Dean DA, Dean BS, Muller S, Smith LC. Sequence requirements for plasmid nuclear entry. Exp. Cell Res. 1999;253:713–722. doi: 10.1006/excr.1999.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level non-viral gene transfer to the lung. Gene Ther. 2003;10:1608–1615. doi: 10.1038/sj.gt.3302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DA, Stivers C, Linkhart TA, Strong DD. Sequences from the human type 1α2 procollagen promoter mediate osteoblast-specific plasmid nuclear import. Mol Ther. 2006;13:S413. [Google Scholar]
- DeGiulio JV, Dean DA. Surfactant Protein C Promoter Nuclear Import is Dependent on Binding Sites for TTF-1, GATA-6, and NFI. Am J Respir Crit Care Med. 2006;175:A113. [Google Scholar]
- Degiulio JV, Kaufman CD, Dean DA. The SP-C promoter facilitates alveolar type II epithelial cell-specific plasmid nuclear import and gene expression. Gene Ther. 2010 doi: 10.1038/gt.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowty ME, Williams P, Zhang G, Hagstrom JE, Wolff JA. Plasmid DNA entry into postmitotic nuclei of primary rat myotubes. Proc. Natl. Acad. Sci. USA. 1995;92:4572–4576. doi: 10.1073/pnas.92.10.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoffre JM, Portet T, Wasungu L, Teissie J, Dean D, Rols MP. What is (still not) known of the mechanism by which electroporation mediates gene transfer and expression in cells and tissues. Mol Biotechnol. 2009;41:286–295. doi: 10.1007/s12033-008-9121-0. [DOI] [PubMed] [Google Scholar]
- Funabashi H, Takatsu M, Saito M, Matsuoka H. Sox2 regulatory region 2 sequence works as a DNA nuclear targeting sequence enhancing the efficiency of an exogenous gene expression in ES cells. Biochem Biophys Res Commun. 2010;400:554–558. doi: 10.1016/j.bbrc.2010.08.098. [DOI] [PubMed] [Google Scholar]
- Granneman JG, Li P, Lu Y, Tilak J. Seeing the trees in the forest: selective electroporation of adipocytes within adipose tissue. Am J Physiol Endocrinol Metab. 2004;287:E574–E582. doi: 10.1152/ajpendo.00567.2003. [DOI] [PubMed] [Google Scholar]
- Hargrave B, Downey H, Strange R, Jr, Murray L, Cinnamond C, Lundberg C, Israel A, Chen YJ, Marshall W, Jr, Heller R. Electroporation-mediated gene transfer directly to the swine heart. Gene Ther. 2012 doi: 10.1038/gt.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller L, Jaroszeski MJ, Coppola D, Pottinger C, Gilbert R, Heller R. Electrically mediated plasmid DNA delivery to hepatocellular carcinomas in vivo. Gene Ther. 2000;7:826–829. doi: 10.1038/sj.gt.3301173. [DOI] [PubMed] [Google Scholar]
- Heller R, Coppola D, Pottinger C, Gilbert R, Jaroszeski MJ. Effect of electrochemotherapy on muscle and skin. Technol Cancer Res Treat. 2002;1:385–392. doi: 10.1177/153303460200100509. [DOI] [PubMed] [Google Scholar]
- Heller R, Cruz Y, Heller LC, Gilbert RA, Jaroszeski MJ. Electrically mediated delivery of plasmid DNA to the skin, using a multielectrode array. Hum Gene Ther. 2010;21:357–362. doi: 10.1089/hum.2009.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R, Jaroszeski M, Atkin A, Moradpour D, Gilbert R, Wands J, Nicolau C. In vivo gene elctroinjection and expression in rat liver. FEBS Lett. 1996;389:225–228. doi: 10.1016/0014-5793(96)00590-x. [DOI] [PubMed] [Google Scholar]
- Li S, MacLaughlin FC, Fewell JG, Gondo M, Wang J, Nicol F, Dean DA, Smith LC. Muscle-specific enhancement of gene expression by incorporation of the SV40 enhancer in the expression plasmid. Gene Therapy. 2001;8:494–497. doi: 10.1038/sj.gt.3301419. [DOI] [PubMed] [Google Scholar]
- Lin F, Shen X, Kichaev G, Mendoza JM, Yang M, Armendi P, Yan J, Kobinger GP, Bello A, Khan AS, Broderick KE, Sardesai NY. Optimization of electroporation-enhanced intradermal delivery of DNA vaccine using a minimally invasive surface device. Hum Gene Ther Methods. 2012;23:157–168. doi: 10.1089/hgtb.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Aranda D, Adir Y, Young JL, Briva A, Budinger GRS, Yeldandi A, Sznajder JI, Dean DA. Gene transfer of the Na+,K+-ATPase b1 subunit using electroporation increases lung liquid clearance in rats. Am J Respir Crit Care Med. 2005;171:204–211. doi: 10.1164/rccm.200403-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WG, Jr, Boone BA, Burgos JD, Gografe SI, Baldwin MK, Danielson ML, Larson MJ, Caretto DR, Cruz Y, Ferraro B, Heller LC, Ugen KE, Jaroszeski MJ, Heller R. Electroporation-mediated delivery of a naked DNA plasmid expressing VEGF to the porcine heart enhances protein expression. Gene Ther. 2010;17:419–423. doi: 10.1038/gt.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JB, Young JL, Benoit JN, Dean DA. Gene transfer to intact mesenteric arteries by electroporation. J Vasc Res. 2000;37:372–380. doi: 10.1159/000025753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Dean DA. Cell-specific nuclear import of plasmid DNA in smooth muscle requires tissue-specific transcription factors and DNA sequences. Gene Ther. 2008;15:1107–1115. doi: 10.1038/gt.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Dean DA. Tissue-specific and transcription factor-mediated nuclear entry of DNA. Adv Drug Deliv Rev. 2009;61:603–613. doi: 10.1016/j.addr.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud J-M, Delaere P, Branellec D, Schwartz B, Scherman D. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc. Natl. Acad. Sci. USA. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir LM, Bureau MF, Rangara R, Schwartz B, Scherman D. Long-term, high level in vivo gene expression after electric pulse-mediated gene transfer into skeletal muscle. C R Acad Sci III. 1998;321:893–899. doi: 10.1016/s0764-4469(99)80003-1. [DOI] [PubMed] [Google Scholar]
- Mutlu GM, Machado-Aranda D, Norton JE, Bellmeyer A, Urich D, Zhou R, Dean DA. Electroporation-mediated gene transfer of the Na+,K+-ATPase rescues endotoxin-induced lung injury. Am J Respir Crit Care Med. 2007;176:582–590. doi: 10.1164/rccm.200608-1246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacramento CB, Moraes JZ, Denapolis PM, Han SW. Gene expression promoted by the SV40 DNA targeting sequence and the hypoxia-responsive element under normoxia and hypoxia. Braz J Med Biol Res. 2010;43:722–727. doi: 10.1590/s0100-879x2010007500064. [DOI] [PubMed] [Google Scholar]
- Temming K, Schiffelers RM, Molema G, Kok RJ. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist Updat. 2005;8:381–402. doi: 10.1016/j.drup.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Vacik J, Dean BS, Zimmer WE, Dean DA. Cell-specific nuclear import of plasmid DNA. Gene Therapy. 1999;6:1006–1014. doi: 10.1038/sj.gt.3300924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valic B, Golzio M, Pavlin M, Schatz A, Faurie C, Gabriel B, Teissie J, Rols MP, Miklavcic D. Effect of electric field induced transmembrane potential on spheroidal cells: theory and experiment. Eur Biophys J. 2003;32:519–528. doi: 10.1007/s00249-003-0296-9. [DOI] [PubMed] [Google Scholar]
- van Gaal EV, Oosting RS, van Eijk R, Bakowska M, Feyen D, Kok RJ, Hennink WE, Crommelin DJ, Mastrobattista E. DNA nuclear targeting sequences for non-viral gene delivery. Pharm Res. 2011;28:1707–1722. doi: 10.1007/s11095-011-0407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JM, Li LH, Sen A, Jahreis GP, Hui SW. Electroporation-enhanced gene delivery in mammary tumors. Gene Ther. 2000;7:541–547. doi: 10.1038/sj.gt.3301141. [DOI] [PubMed] [Google Scholar]
- Wilson GL, Dean BS, Wang G, Dean DA. Nuclear import of plasmid DNA in digitonin-permeabilized cells requires both cytoplasmic factors and specific DNA sequences. J. Biol. Chem. 1999;274:22025–22032. doi: 10.1074/jbc.274.31.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JL, Benoit JN, Dean DA. Effect of a DNA nuclear targeting sequence on gene transfer and expression of plasmids in the intact vasculature. Gene Ther. 2002 doi: 10.1038/sj.gt.3302021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JL, Benoit JN, Dean DA. Effect of a DNA nuclear targeting sequence on gene transfer and expression of plasmids in the intact vasculature. Gene Ther. 2003;10:1465–1470. doi: 10.1038/sj.gt.3302021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JL, Dean DA. Non-Viral Gene Transfer Strategies for the Vasculature. Microcirculation Res. 2002;9:35–50. doi: 10.1038/sj/mn/7800120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JL, Zimmer WE, Dean DA. Smooth muscle-specific gene delivery in the vasculature based on restriction of DNA nuclear import. Exp Biol Med. 2008;233:840–848. doi: 10.3181/0712-RM-331. [DOI] [PubMed] [Google Scholar]
- Zhou R, Norton JE, Zhang N, Dean DA. Electroporation-mediated transfer of plasmids to the lung results in reduced TLR9 signaling and inflammation. Gene Ther. 2007;14:775–780. doi: 10.1038/sj.gt.3302936. [DOI] [PMC free article] [PubMed] [Google Scholar]