Abstract
We have previously demonstrated that chronic plus binge ethanol feeding acts synergistically to induce liver injury in mice; however, the mechanisms underlying this phenomenon remain unclear. In the current study, we show that chronic plus binge ethanol feeding synergistically upregulated the hepatic expression of IL-1β and TNF-α and induced neutrophil accumulation in the liver compared to chronic or binge feeding alone. The in vivo depletion of neutrophils through the administration of an anti-Ly6G antibody markedly reduced chronic-binge ethanol feeding-induced liver injury. Real-time PCR analyses revealed that hepatic E-selectin expression was upregulated 10-fold, whereas the expression of other neutrophil infiltration-related adhesion molecules (such as P-selectin, ICAM-1, and VCAM-1) was slightly upregulated or downregulated in this chronic-binge model. The genetic deletion of E-selectin prevented chronic-binge ethanol-induced hepatic neutrophil infiltration and the elevation of serum transaminases without affecting ethanol-induced steatosis. In addition, E-selectin-deficient mice showed reduced hepatic expression of several proinflammatory cytokines, chemokines, and adhesion molecules compared to wild-type mice after chronic-binge ethanol feeding. Finally, the expression of E-selectin was highly upregulated in human alcoholic fatty livers but not in alcoholic cirrhosis.
Conclusions
Chronic-binge ethanol feeding upregulates the expression of proinflammatory cytokines, followed by the induction of E-selectin. Elevated E-selectin plays an important role in hepatic neutrophil infiltration and injury in mice induced by chronic-binge feeding, and may also contribute to the pathogenesis of early stages of human alcoholic liver disease.
Keywords: Adhesion molecules, alcoholic liver injury, inflammation
Introduction
More than 95% of heavy drinkers develop fatty liver, but only 20–40% develop steatohepatitis and progress to cirrhosis and hepatocellular carcinoma.1, 2 The underlying mechanisms that render some individuals more susceptible to severe forms of alcoholic liver disease (ALD) are not clear, and many risk factors may be involved.1–3 These potential risk factors include sex, obesity, dietary factors, smoking, and non-sex-linked genetic factors.1–3 Additionally, several epidemiologic studies have suggested that the drinking pattern also significantly influences the pathogenesis of ALD in humans.4–7 For example, drinking outside mealtime, the consumption of multiple types of drinks, and a mixed drinking pattern were significantly associated with an increased risk of ALD.6, 7 However, the mechanisms through which drinking pattern affects ALD pathogenesis remain largely unknown. Previously, we have demonstrated that chronic ethanol feeding for 10 days plus a single binge dose of ethanol delivered by gavage synergistically elevated serum levels of alanine aminotransferase (ALT), induced steatosis, and upregulated the expression of proinflammatory cytokines compared to chronic or gavage ethanol feeding alone.8 This pattern of chronic-binge feeding reproduces closely the drinking behaviors in many heavy drinkers, which may be associated with an increased risk of ALD.6, 7, 9, 10 In the current study, we further characterized liver inflammation in this mouse model of chronic-binge feeding. Our results showed that chronic-binge feeding synergistically induced hepatic neutrophil infiltration but not macrophage infiltration compared to chronic or binge feeding alone.
Neutrophil infiltration is a hallmark of alcoholic hepatitis11–13 and has been shown to correlate with the severity of alcoholic hepatitis.14 Feeding rats with a diet including ethanol also increased hepatic neutrophil infiltration, and the depletion of neutrophils reduced liver injury in ethanol-fed rats.15 This suggests that neutrophil infiltration likely contributes to hepatocellular damage, possibly by killing hepatocytes via the production of oxidative stress and proteases.16 However, the mechanisms through which neutrophils are recruited to the liver during alcoholic liver injury remain incompletely understood. Neutrophil recruitment is controlled by a multi-step adhesion cascade that involves multiple adhesion molecules and their ligands, which are expressed on endothelial cells and neutrophils, respectively.17–19 These include the endothelium-expressed molecules E-selectin (SELE) and P-selectin (SELP), vascular cell adhesion molecule 1 (VCAM-1), and intercellular adhesion molecule 1 (ICAM-1) and the neutrophil-expressed proteins L-selectin (CD62L) and E- and P-selectin ligands, including E-selectin ligand-1 (ESL-1), P-selectin glycoprotein ligand-1 (PSGL-1), and CD44.17, 18 To examine the mechanisms underlying the synergistic effects of chronic-binge feeding on neutrophil infiltration, we evaluated the expression of an array of adhesion molecules and their ligands. Our results revealed that among these factors, E-selectin had the highest induction (up to 10-fold) in the liver after chronic-binge ethanol feeding.
E-selectin, also called CD62 antigen-like family member E (CD62E), endothelial-leukocyte adhesion molecule 1 (ELAM-1), or leukocyte-endothelial cell adhesion molecule 2 (LECAM2), is an adhesion molecule that is specifically expressed on activated endothelial cells.20 Through interacting with an array of ligands, including ESL-1, CD44, and PSGL-1, E-selectin plays a critical role in the transition from slow rolling to arrest and the promotion of the efficient transendothelial migration and activation of neutrophils.17, 20, 21 In the present paper, we demonstrated that the genetic disruption of E-selectin abolished chronic-binge ethanol-induced neutrophil infiltration, liver injury, and inflammation, suggesting that E-selectin plays a critical role in inducing neutrophil recruitment, liver inflammation, and injury after chronic-binge feeding.
Materials and Methods
Mice and Ethanol Feeding Protocols
E-selectin-deficient (SELE−/−) mice and wild-type (WT) controls (C57BL/6J) were purchased from the Jackson Laboratory (Bar Harbor, ME). All animal experiments were approved by the NIAAA Animal Care and Use Committee. Eight- to twelve-week-old female mice were subjected to one of three different feeding protocols. (1) Chronic feeding, in which mice were initially fed the control Lieber-DeCarli diet (Bio-Serv, Frenchtown, NJ) ad libitum for 5 days to acclimatize them to a liquid diet. Then, the mice were allowed free access to the ethanol Lieber-DeCarli diet (Bio-Serv) containing 5% (vol/vol) ethanol for 10 days, and control-fed groups were pair-fed with an isocaloric control diet; (2) Binge feeding, in which mice were gavaged with a single dose of ethanol (5 g/kg body weight) or isocaloric dextrin-maltose in the early morning and sacrificed 9 hours later; and (3) Chronic-binge feeding,8, 22 in which mice were fed the control or ethanol Lieber-DeCarli diet for 10 days as described for the chronic feeding group. On day 11, ethanol-fed and pair-fed mice were gavaged in the early morning with a single dose of ethanol (5 g/kg body weight) or isocaloric dextrin-maltose, respectively, and sacrificed 9 hours later. WT and SELE−/− mice exhibited comparable daily alcohol intakes. The mean body weights were similar in all groups at the end of the experiments.
Statistical Analysis
The results are expressed as the means ± SEM of 5–21 mice per group. Group comparisons were performed using unpaired t-test or one-way ANOVA followed by Tukey’s multiple comparison test. Correlations were analyzed using Spearman’s rank correlation test. P<0.05 was considered statistically significant.
Additional methods are described in supporting materials
Results
Chronic-Binge Feeding Synergistically Induces Liver Injury and Inflammation in Mice
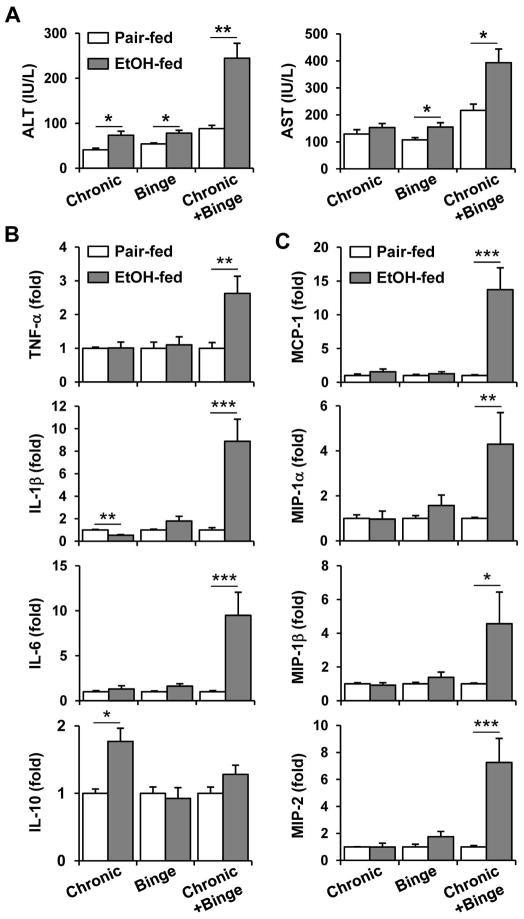
We previously demonstrated that chronic-binge feeding caused significant liver injury that peaked at 9 hours after gavage in C57BL/6 mice and that serum ALT and AST levels were higher in female than male mice.8 Consequently, we used female mice in this study to further explore the mechanisms underlying the synergistic effect of chronic-binge feeding on liver injury. Alone, ethanol feeding for 10 days (chronic) or a single gavage of ethanol (binge) induced only a mild elevation of serum ALT and AST in female C57BL/6J mice (Fig. 1A). In contrast, as expected, chronic-binge ethanol feeding resulted in more severe liver injury, as indicated by much higher serum ALT and AST levels (Fig. 1A). Furthermore, chronic-binge feeding but not 10-day chronic feeding or single ethanol gavage alone upregulated the hepatic expression of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6, and the chemokines MCP-1, MIP-1α, MIP-1β, and MIP-2 (Figs. 1B–C). Interestingly, the hepatic expression of IL-10 was upregulated after chronic feeding alone but not after binge or chronic-binge feeding.
Fig. 1.
Comparison of liver injury and inflammation induced by chronic, binge and chronic-binge feeding. C57BL6/J mice were subjected to chronic, binge or chronic-binge feeding. (A) Liver injury was assessed by measuring serum ALT and AST levels. (B, C) Liver mRNA levels of cytokines (B) and chemokines (C) were analyzed by real-time PCR. *P<0.05, **P<0.01, ***P<0.001.
Chronic alcohol consumption is known to induce CYP2E1, deplete glutathione, and increase the production of reactive oxygen species and oxidative stress in the liver. We hypothesized these changes may contribute to the increased susceptibility of 10-day alcohol-fed mice to binge-induced liver injury. Therefore, we investigated the effect of 10-day alcohol feeding on these parameters. Feeding mice with an ethanol diet for 10 days markedly induced CYP2E1 protein expression, but did not affect hepatic mRNA expression of NADPH oxidase subunits (e.g. Cyba, Cybb, Ncf1, and Ncf2), hepatic levels of malondialdehyde and glutathione (supporting Figs. 1A–D). These results indicate that chronic ethanol feeding for 10 days is sufficient to upregulate liver CYP2E1 protein expression but not oxidative stress. Such upregulated CYP2E1 likely contributes to the increased susceptibility of 10-day ethanol-fed mice to binge-induced liver injury.
Chronic-binge Feeding Induces Hepatic Neutrophil Infiltration in Mice
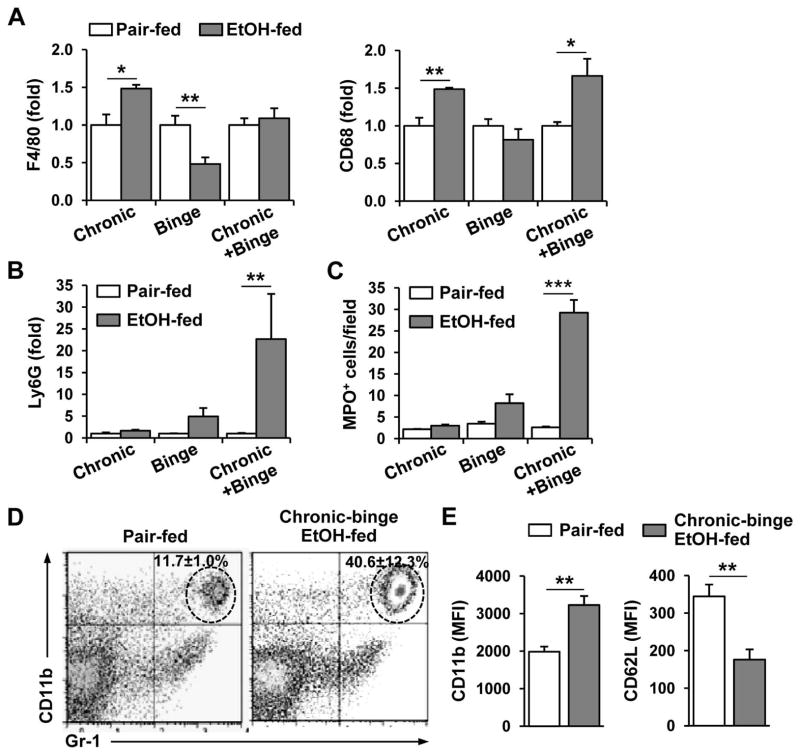
The above data show that expression of MCP-1, MIP-1α/β, and MIP-2, which are potent chemoattractants for monocytes and/or neutrophils, was upregulated after chronic-binge feeding. Therefore, we analyzed the hepatic expression of markers for these inflammatory cells in the ethanol-fed mice. As shown in Fig. 2A, the hepatic gene expression levels of the macrophage marker F4/80 and the monocyte/macrophage marker CD68 were increased after chronic ethanol feeding. After gavage of a single dose of ethanol, the gene expression of F4/80 was decreased, while CD68 was not altered. This is consistent with previous findings that short-term ethanol feeding increased apoptosis of F4/80-positive Kupffer cells.23 The expression of the F4/80 and CD68 genes was unchanged and slightly increased, respectively, after chronic-binge feeding. In contrast, chronic-binge feeding but not chronic or single ethanol gavage alone strongly upregulated the hepatic expression of the neutrophil marker Ly6G (Fig. 2B). Immunohistochemical staining for myeloperoxidase (MPO) further confirmed that a large number of MPO+ neutrophils had infiltrated the livers of chronic-binge-fed mice compared to chronic or binge ethanol-fed mice (Fig. 2C and supporting Fig. 2). Interestingly, most of neutrophils were distributed throughout zone 1 to zone 3 and appeared to infiltrate into the liver parenchyma (supporting Figs. 2A–B). A small number of inflammatory foci was also observed in the livers of chronic-binge-fed mice (supporting Figs. 2C–D). Flow cytometric analyses also confirmed higher percentages of neutrophils in the livers of chronic-binge-fed mice than in pair-fed mice (Fig. 2D). Furthermore, as illustrated in Fig. 2E, neutrophils isolated from the livers of chronic-binge-fed mice exhibited enhanced cell surface expression of CD11b but reduced surface expression of CD62L compared to those isolated from their pair-fed controls. Because neutrophil activation is associated with the upregulation of CD11b 24, 25 and downregulation of CD62L expression,25 therefore, the results in Fig. 2E suggest liver-infiltrating neutrophils are activated in response to chronic-binge feeding.
Fig. 2.
Comparison of the hepatic inflammatory cell recruitment induced by chronic, binge, and chronic-binge feeding. C57BL6/J mice were subjected to chronic, binge or chronic-binge feeding. (A, B) Liver mRNA levels of monocyte/macrophage (A, F4/80 and CD68) and neutrophil (B, Ly6G) markers were analyzed by real-time PCR. (C) Liver sections were stained for MPO and the number of MPO+ cells per ×100 field was counted. (D, E) Hepatic leukocytes were isolated and analyzed by flow cytometry. Panel D: Representative flow cytometry data for hepatic CD11b+Gr-1high neutrophil infiltration; Panel E: Mean fluorescence intensity of the cell surface levels of CD11b and CD62L on neutrophils. Neutrophil activation is associated with the upregulation of CD11b and downregulation of CD62L expression. *P<0.05, **P<0.01, ***P<0.001.
Neutrophil Depletion Reduces Chronic-Binge Feeding-Induced Liver Injury
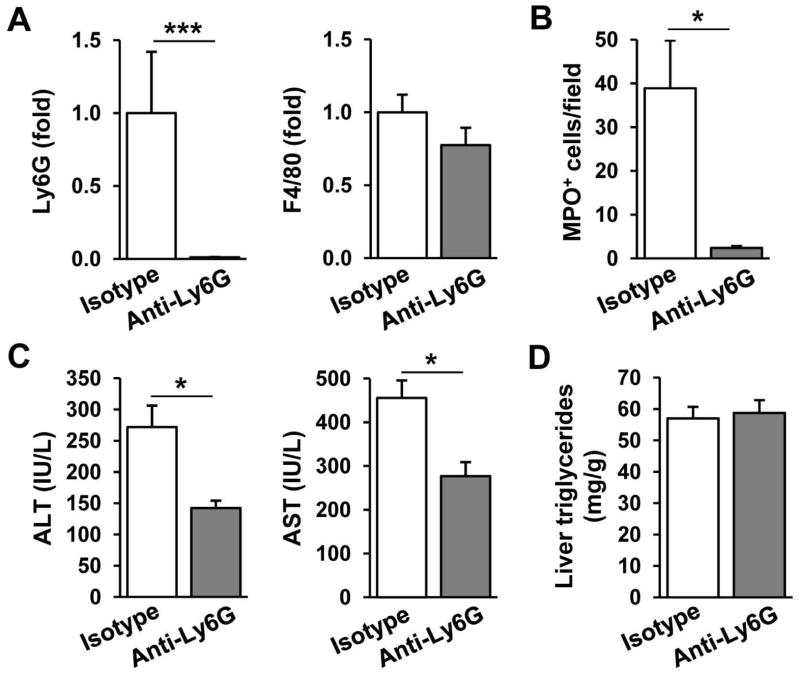
We next investigated whether the observed increase in hepatic neutrophil infiltration contributed to chronic-binge feeding-induced liver injury by depleting neutrophils. Figure 3A shows that injection of anti-Ly6G antibody, which has been shown to deplete specifically neutrophils,26 reduced hepatic expression of Ly6G mRNA by 90% without affecting hepatic expression of F4/80 mRNA in the chronic-binge-fed mice. Depletion of neutrophils was further confirmed by immunohistochemistry analyses showing that the number of MPO+ cells was reduced by 90% after anti-Ly6G antibody treatment (Fig. 3B). Finally, anti-Ly6G antibody treatment markedly reduced serum ALT and AST levels but did not affect hepatic triglyceride levels (Figs. 3C–D). These data suggest that liver-infiltrating neutrophils participated in chronic-binge feeding-induced liver injury but not steatosis.
Fig. 3.
The effect of neutrophil depletion on chronic-binge feeding-induced liver injury. C57BL6/J mice were subjected to chronic-binge feeding and were injected intravenously with 200μg and 100μg of anti-Ly6G or isotype control antibodies 24h and 4h before ethanol gavage, respectively. Mice were sacrificed 9 hours post gavage. (A) Liver mRNA levels of neutrophil (Ly6G) and macrophage (F4/80) markers were analyzed by real-time PCR. (B) Liver sections were stained for MPO and the number of MPO+ cells per ×100 field was counted. (C) Liver injury was assessed by measuring serum ALT and AST levels. (D) Steatosis was quantified by measuring hepatic triglyceride levels. *P<0.05, *** P<0.001.
Chronic-binge Feeding Upregulates Hepatic E-Selectin Expression in Mice
To explore the mechanisms underlying the chronic-binge-mediated induction of neutrophil infiltration into the liver, the hepatic expression of several adhesion molecules was examined. As illustrated in Fig. 4A, chronic-binge feeding resulted in the highest fold induction of E-selectin (10-fold), whereas the expression of SELP and ICAM-1 was only upregulated by approximately 2-fold, and the expression of VCAM-1 was actually downregulated after chronic-binge feeding. Interestingly, hepatic E-selectin was not upregulated in the chronic or gavage alone groups (Fig. 4B). In addition, the expression levels of several E-selectin ligands (such as ESL-1 and CD44) and the enzymes involved in E-selectin ligand biosynthesis (such as FucT-IV) were significantly, albeit less than 2-fold, higher in the chronic-binge feeding groups than in the pair-fed groups (Supporting Fig.3). Finally, hepatic expression levels of E-selectin and Ly6G were positively correlated in chronic-binge-fed mice (Fig. 4C).
Fig. 4.
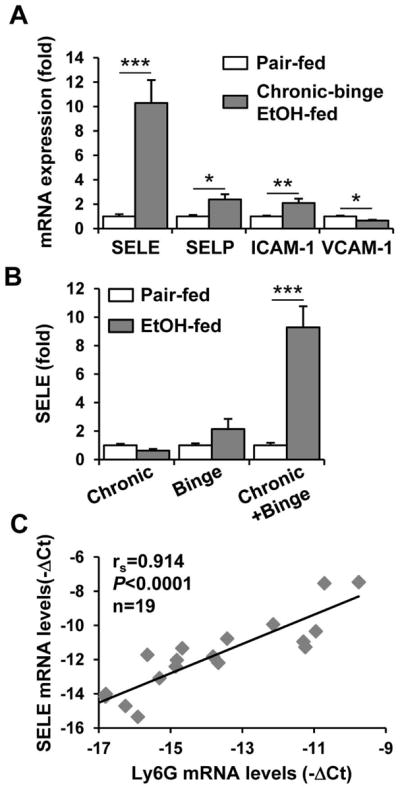
Hepatic E-selectin expression is upregulated by chronic-binge feeding and positively correlates with neutrophil infiltration. (A) Real-time PCR analyses of hepatic mRNA expression of adhesion molecules from chronic-binge-fed or pair-fed mice. (B) Real-time PCR analyses of hepatic E-selectin mRNA from chronic-, binge-, or chronic-binge-fed mice. *P<0.05, **P<0.01, ***P<0.001. (C) The correlation between the hepatic mRNA levels of E-selectin and Ly6G from pair-fed and chronic-binge-fed mice was analyzed using the Spearman rank correlation test.
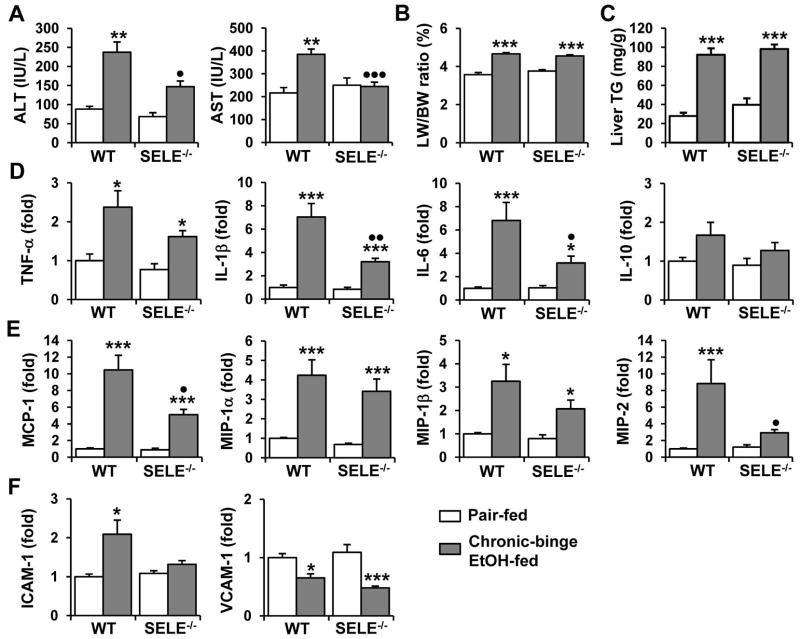
E-Selectin Deficiency Reduces the Hepatic Neutrophil Infiltration Induced by Chronic-Binge Feeding
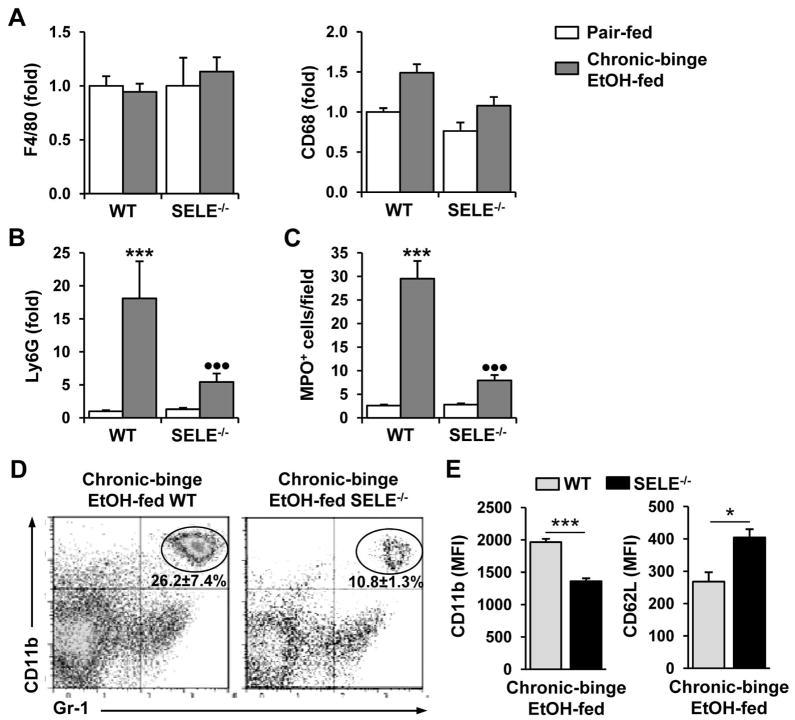
To test whether E-selectin was involved in the hepatic neutrophil recruitment induced by chronic-binge feeding, WT and SELE−/− mice were subjected to chronic-binge feeding. As shown in Fig. 5A, E-selectin deficiency did not alter hepatic expression of F4/80 or CD68 in pair-fed or ethanol-fed mice. In contrast, hepatic expression of the neutrophil marker Ly6G was strongly induced in ethanol-fed WT but not SELE−/− mice (Fig. 5B). Immunohistochemical staining confirmed that a lower number of MPO+ neutrophils infiltrated the livers of chronic-binge-fed SELE−/− mice than those of WT mice (Fig. 5C and supporting Fig. 4). Flow cytometric analyses (Figure 5D) showed that the percentage of neutrophils was significantly lower in the livers of chronic-binge-fed SELE−/− mice compared to WT mice. Finally, the cell surface expression of CD11b was lower whereas that of CD62L was higher in the liver neutrophils from chronic-binge-fed SELE−/− mice compared to those of WT mice (Fig. 5E), suggesting that chronic-binge-induced neutrophil activation is attenuated in SELE−/− mice.
Fig. 5.
Chronic-binge feeding-induced hepatic neutrophil recruitment is decreased in SELE−/− mice. WT and SELE−/− mice were subjected to chronic-binge ethanol or pair feeding. (A, B) Real-time PCR analyses of hepatic mRNA levels of monocyte/macrophage (A, F4/80 and CD68) and neutrophil (B, Ly6G) markers. (C) Liver sections were stained for MPO and the number of MPO+ cells per ×100 field was counted. ***P<0.001 for EtOH-fed vs. corresponding pair-fed; •••P<0.001 for EtOH-fed SELE−/− vs. EtOH-fed WT. (D, E) Hepatic leukocytes were isolated and analyzed by flow cytometry. Panel D: Representative flow cytometry data of hepatic neutrophil infiltration; Panel E: Mean fluorescence intensity of cell surface levels of CD11b and CD62L. *P<0.05 and ***P<0.001.
E-Selectin Deficiency Protects Mice from Chronic-Binge Feeding-Induced Liver Injury and Inflammation
We then investigated whether the reduced hepatic neutrophil infiltration observed in SELE−/− mice was associated with decreased liver injury. Indeed, chronic-binge feeding-associated elevation of serum ALT and AST levels was prevented in chronic-binge-fed SELE−/− mice compared to WT mice (Fig. 6A). However, the level of hepatomegaly was comparable between chronic-binge-fed WT and SELE−/− mice (Fig. 6B). Further analyses revealed that micro- and macrovesicular steatosis, and hepatic triglyceride content were similar in both groups of mice (Fig. 6C and supporting Fig. 5). Next, we evaluated the effect of E-selectin deficiency on the chronic-binge feeding-induced hepatic expression of inflammatory mediators. Although similar levels of TNF-α, MIP-1α, and MIP-1β mRNAs (Figs. 6D–E) were upregulated in the livers of ethanol-fed WT and SELE−/− mice, the induction of IL-1β, IL-6, MCP-1, and MIP-2 (Figs. 6D–E) was significantly blunted in the livers of SELE−/− mice. Furthermore, the chronic-binge feeding-induced hepatic expression of the adhesion molecule ICAM-1 was prevented in SELE−/− mice (Fig. 6F). The expression of VCAM-1 was similarly decreased in both groups after chronic-binge feeding (Fig. 6F). Collectively, these results indicate that SELE−/− mice were resistant to chronic-binge feeding-induced hepatocellular damage and inflammation but not steatosis compared to WT mice.
Fig. 6.
Chronic-binge feeding-induced liver injury and inflammation are attenuated in SELE−/− mice. WT and SELE−/− mice were subjected to chronic-binge ethanol or pair feeding. (A, B) Liver injury was assessed by measuring serum ALT and AST levels (A) and liver-to-body weight ratios (B). (C) Steatosis was quantified by measuring hepatic triglyceride levels. (D–F) Liver mRNA levels of cytokines (D), chemokines (E), and adhesion molecules (F) were analyzed by real-time PCR. *P<0.05, **P<0.01, ***P<0.001 for EtOH-fed vs corresponding pair-fed; P<0.05, P<0.01 for EtOH-fed SELE−/− vs. EtOH-fed WT.
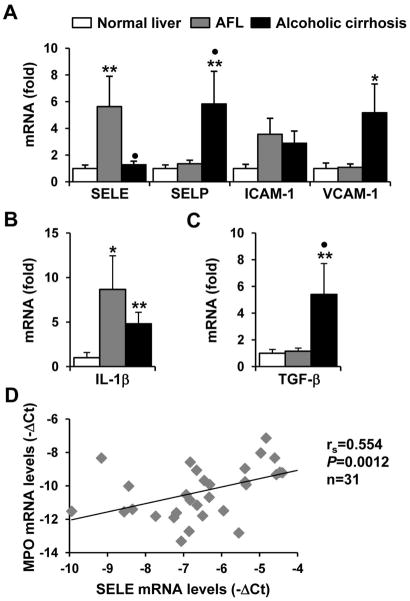
Hepatic expression of E-selectin is upregulated in patients with alcoholic fatty liver (AFL) but not with alcoholic cirrhosis
The expression of E-selectin and other adhesion molecules in normal human liver, AFL, and alcoholic cirrhosis samples was examined. As illustrated in Fig. 7A, expression of E-selectin was markedly upregulated in AFL but not in cirrhosis samples compared to normal livers. In contrast, hepatic expression of SELP and VCAM-1 was increased in alcoholic cirrhosis but not in AFL. Expression of ICAM-1 was comparable in these three groups. In addition, as expected, TGF-β expression was markedly elevated in cirrhosis samples but not in AFL samples; whereas expression of IL-1β was upregulated in both groups (Fig. 7B). Finally, E-selectin expression levels were positively correlated with the levels of the neutrophil marker MPO in these liver samples. These data suggest that E-selectin expression is upregulated in the early stages but not the late stages of human ALD.
Fig. 7.
Hepatic expression of E-selectin is upregulated in patients with early stages of ALD. (A–C) Real-time PCR analyses of hepatic mRNA levels of adhesion molecules (A), Il-1β (B), and TGF-β (C) in normal human livers (n=10) and livers from patients with alcoholic fatty liver (AFL, n=10) and alcoholic cirrhosis (n=11). *P<0.05, **P<0.01 compared to normal livers, •P<0.05 compared to AFL (Kruskal-Wallis test followed by Dunn’s multiple comparison test). (D) Correlation between the E-selectin and MPO mRNA levels in normal, AFL, and cirrhotic livers (Spearman rank correlation test).
Discussion
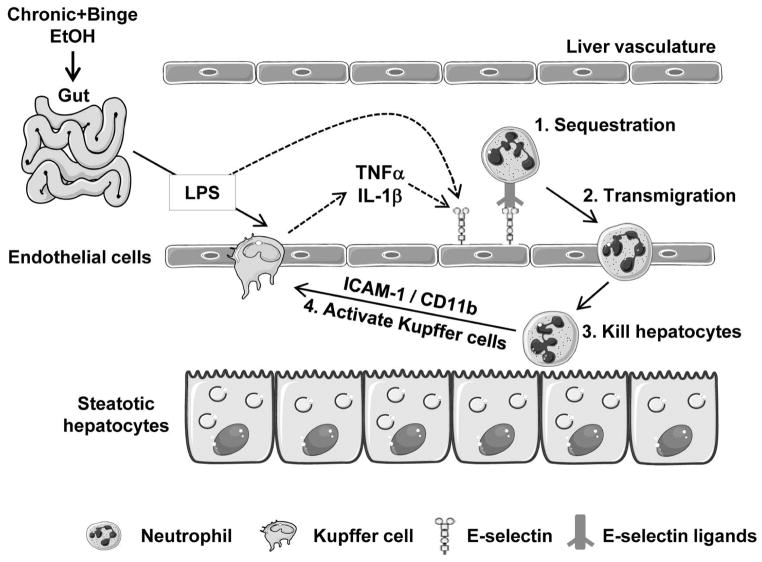
In the current study, we have demonstrated that chronic-binge feeding synergistically upregulates the hepatic expression of E-selectin, which induces hepatic neutrophil accumulation and subsequently promotes liver injury and inflammation. We have integrated all of these findings into a model (Fig. 8) depicting the important role of E-selectin in neutrophil recruitment and liver injury upon chronic-binge feeding.
Fig. 8.
A model depicting the key role of E-selectin in the pathogenesis of chronic-binge- induced liver injury. Chronic-binge ethanol consumption increases gut permeability and subsequently elevates portal LPS levels. LPS stimulates Kupffer cells to produce TNF-α and IL-1β which, together with LPS, have been shown to upregulate E-selectin on endothelial cells and may contribute to hepatic E-selectin upregulation in this chronic-binge model. E-selectin then binds to neutrophils, inducing neutrophil activation, sequestration, and transmigration. Activated neutrophils kill steatotic hepatocytes and induce Kupffer cell activation via the interaction of ICAM-1 and CD11b, thereby promoting hepatocellular necrosis and inflammation.
It has been known for many years that alcoholic hepatitis is associated with the accumulation of neutrophils in the liver;11–13 however, in mice, chronic feeding with a Lieber-DeCarli diet or the acute administration of ethanol only induces mild hepatic neutrophil infiltration. In this paper, we demonstrated that chronic-binge feeding synergistically induced neutrophil accumulation in the liver compared to chronic or binge ethanol feeding alone. Liver histology and MPO staining analyses revealed that most of neutrophils were distributed throughout zone 1 to zone 3 and only a small number of inflammatory foci was observed in the livers after chronic-binge feeding (supporting Figs. 2A–D). Interestingly, human alcoholic steatohepatitis is often associated with many inflammatory foci in the liver, which are formed due to hepatocyte necrosis and likely contribute to the removal of necrotic cells. The reason for the small number of inflammatory foci in the chronic-binge model was partly because this model represents the early stages of liver injury and is associated with mild hepatocyte injury. Moreover, we investigated the mechanisms underlying the induction of hepatic neutrophil infiltration after chronic-binge feeding by examining the expression of several adhesion molecules that are related to neutrophil recruitment. Among these molecules, we found that E-selectin had the highest level of induction in the liver after chronic-binge feeding.
Although E-selectin has been shown to play an important role in leukocyte recruitment,17, 20 it roles in hepatic neutrophil infiltration and injury are unclear. For example, several studies have reported that the blockade of E-selectin with an E-selectin neutralizing antibody inhibited leukocyte recruitment and liver injury induced by D-galactosamine (D-Gal)/ lipopolysaccharide (LPS) 27 or concanavalin A (Con A);28 but other studies have suggested that E-selectin does not contribute to liver injury in these models 29, 30 or in an LPS-induced liver injury model.31 Our findings here suggest that E-selectin is a critical factor for chronic-binge feeding-induced hepatic neutrophil recruitment and injury. First, the expression of E-selectin was positively correlated with the infiltration of neutrophils in the liver; second, SELE−/− mice had reduced neutrophil infiltration, as demonstrated by MPO staining, flow cytometric analyses, and real-time PCR analyses; and finally, SELE−/− mice had reduced liver injury. The next question is why E-selectin may not contribute to hepatic neutrophil recruitment and injury in the LPS- or Con A-induced hepatitis models29, 30 but does play a significant role in the response to chronic-binge feeding. The most likely answer is that the Con A and D-Gal/LPS models are associated with strong liver inflammation, during which many adhesion molecules other than E-selectin are highly upregulated in the liver and subsequently contribute to neutrophil recruitment. In contrast, in the chronic-binge feeding model, liver inflammation is mild, and E-selectin is dramatically upregulated (10-fold), whereas the expression levels of many other adhesion molecules are upregulated by less than 2-fold or even downregulated. Thus, E-selectin plays a critical role in neutrophil recruitment in the context of chronic-binge feeding.
Currently, the mechanism by which E-selectin is highly upregulated after chronic-binge feeding is not fully understood. E-selectin is not constitutively expressed by endothelial cells; but its expression is highly upregulated by inflammatory mediators, such as TNF-α, IL-1, and LPS.32 The hepatic expression of TNF-α and IL-1 was strongly induced after chronic-binge feeding (Fig. 1), and their expression correlated positively with the hepatic expression of E-selectin (supporting Fig. 6). This suggests that chronic-binge feeding synergistically upregulates the hepatic expression of TNF-α and IL-1, which may contribute to the induction of hepatic E-selectin.
Neutrophils have been shown to induce hepatocellular damage by generating oxidative stress and cytotoxic mediators,11, 33 but these cells also contribute to liver repair by removing necrotic debris 33–35 and promoting liver regeneration.36 Our results from the current study suggest that in the chronic-binge feeding model, neutrophils exacerbate liver injury. First, chronic-binge feeding not only induced neutrophil accumulation but also stimulated neutrophil activation (Fig. 2); second, SELE−/− mice had reduced neutrophil infiltration and reduced liver injury after chronic-binge feeding compared with wild-type mice (Fig. 5); third, mice treated with anti-Ly6G antibody were specifically depleted for neutrophils but not macrophages and were subsequently protected against liver injury induced by chronic-binge feeding (Fig. 3). Finally, chronic ethanol feeding has been shown to induce hepatocyte apoptosis.23 In chronic-binge feeding model, we observed that the number of TUNEL+ hepatocyte nuclei was only slightly but significantly elevated compared to pair-fed mice, and was comparable between the ethanol-fed WT and SELE−/− mice (supporting Fig. 7) although the ethanol-fed SELE−/− mice had significantly lower serum ALT levels than the ethanol-fed WT mice (Fig. 6). This suggests that hepatocyte necrosis but not apoptosis was most likely the major cause of hepatocyte cell death in this acute-on-chronic alcoholic liver injury model, which is consistent with the fact of neutrophil-mediated hepatocellular oncotic necrosis.34
Although our findings suggest an important role of E-selectin in neutrophil infiltration and liver injury in the chronic-binge feeding model, the roles of E-selectin in human ALD remain unclear. By using immunohistochemistry analyses of 28 ALD patients with severe alcoholic hepatitis or cirrhosis, Adams et al. demonstrated that the E-selectin staining was generally weak in these patients except two patients with strong staining.37, 38 However, in these studies, the expression levels of E-selectin were not quantified. In the current study, by using real-time quantitative PCR, we demonstrated that the expression of E-selectin was significantly upregulated in human alcoholic fatty livers but not in alcoholic cirrhotic livers compared with normal livers although the expression of IL-1β was elevated in both groups. The reason why E-selectin is not upregulated in cirrhotic livers despite of increased inflammation may be due to the elevated TGF-β (Fig. 7), a cytokine that is known to effectively suppress the expression of E-selectin in endothelial cells.39 Taken together, E-selectin may play a role in promoting neutrophil infiltration and liver injury in the early stages of ALD without fibrosis.
Supplementary Material
Acknowledgments
We wish to thank Dr. Philippe Gual (INSERM, U1065, Equipe 8, Complications Hépatiques de l’Obésité, Nice, France) for great suggestions and helpful discussion during this study.
This work was supported by the intramural program of NIAAA, NIH.
Abbreviations
- ALD
alcoholic liver disease
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ESL-1
E-selectin ligand-1
- ICAM-1
intercellular adhesion molecule 1
- MPO
myeloperoxidase
- PSGL-1
P-selectin glycoprotein ligand-1
- SELE
E-selectin
- SELE−/− mice
E-selectin-deficient mice
- SELP
P-selectin
- VCAM-1
vascular cell adhesion molecule 1 (VCAM-1)
Footnotes
No conflicts of interest exist for any of the authors.
References
- 1.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Shea RS, Dasarathy S, McCullough AJ Practice Guideline Committee of the American Association for the Study of Liver D, Practice Parameters Committee of the American College of G. Alcoholic liver disease. Hepatology. 2010;51:307–28. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 3.Anstee QM, Daly AK, Day CP. Genetics of alcoholic and nonalcoholic fatty liver disease. Semin Liver Dis. 2011;31:128–46. doi: 10.1055/s-0031-1276643. [DOI] [PubMed] [Google Scholar]
- 4.Li TK. Quantifying the risk for alcohol-use and alcohol-attributable health disorders: present findings and future research needs. J Gastroenterol Hepatol. 2008;23 (Suppl 1):S2–8. doi: 10.1111/j.1440-1746.2007.05298.x. [DOI] [PubMed] [Google Scholar]
- 5.Hatton J, Burton A, Nash H, Munn E, Burgoyne L, Sheron N. Drinking patterns, dependency and life-time drinking history in alcohol-related liver disease. Addiction. 2009;104:587–92. doi: 10.1111/j.1360-0443.2008.02493.x. [DOI] [PubMed] [Google Scholar]
- 6.Bellentani S, Saccoccio G, Costa G, Tiribelli C, Manenti F, Sodde M, Saveria Croce L, Sasso F, Pozzato G, Cristianini G, Brandi G. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut. 1997;41:845–50. doi: 10.1136/gut.41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stranges S, Freudenheim JL, Muti P, Farinaro E, Russell M, Nochajski TH, Trevisan M. Differential effects of alcohol drinking pattern on liver enzymes in men and women. Alcohol Clin Exp Res. 2004;28:949–56. doi: 10.1097/01.alc.0000128229.23396.42. [DOI] [PubMed] [Google Scholar]
- 8.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin–22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56 (Suppl 1):S39–45. doi: 10.1016/S0168-8278(12)60005-1. [DOI] [PubMed] [Google Scholar]
- 10.Choi G, Runyon BA. Alcoholic hepatitis: a clinician’s guide. Clin Liver Dis. 2012;16:371–85. doi: 10.1016/j.cld.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Jaeschke H. Neutrophil-mediated tissue injury in alcoholic hepatitis. Alcohol. 2002;27:23–7. doi: 10.1016/s0741-8329(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 12.Bautista AP. Neutrophilic infiltration in alcoholic hepatitis. Alcohol. 2002;27:17–21. doi: 10.1016/s0741-8329(02)00206-9. [DOI] [PubMed] [Google Scholar]
- 13.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–69. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 14.Dominguez M, Miquel R, Colmenero J, Moreno M, Garcia-Pagan JC, Bosch J, Arroyo V, Gines P, Caballeria J, Bataller R. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–50. doi: 10.1053/j.gastro.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 15.Bautista AP. Chronic alcohol intoxication induces hepatic injury through enhanced macrophage inflammatory protein-2 production and intercellular adhesion molecule-1 expression in the liver. Hepatology. 1997;25:335–42. doi: 10.1002/hep.510250214. [DOI] [PubMed] [Google Scholar]
- 16.Ramaiah SK, Jaeschke H. Hepatic neutrophil infiltration in the pathogenesis of alcohol-induced liver injury. Toxicol Mech Methods. 2007;17:431–40. doi: 10.1080/00952990701407702. [DOI] [PubMed] [Google Scholar]
- 17.Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011;32:461–9. doi: 10.1016/j.it.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaeschke H, Smith CW. Cell adhesion and migration. III. Leukocyte adhesion and transmigration in the liver vasculature. Am J Physiol. 1997;273:G1169–73. doi: 10.1152/ajpgi.1997.273.6.G1169. [DOI] [PubMed] [Google Scholar]
- 19.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–72. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Chase SD, Magnani JL, Simon SI. E-selectin ligands as mechanosensitive receptors on neutrophils in health and disease. Ann Biomed Eng. 2012;40:849–59. doi: 10.1007/s10439-011-0507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruchaud-Sparagano MH, Drost EM, Donnelly SC, Bird MI, Haslett C, Dransfield I. Potential pro-inflammatory effects of soluble E-selectin upon neutrophil function. Eur J Immunol. 1998;28:80–9. doi: 10.1002/(SICI)1521-4141(199801)28:01<80::AID-IMMU80>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Bertola A, Mathews S, Ki S, Wang H, Gao B. Mouse chronic plus binge ethanol feeding model (the NIAAA model) Nature Protocols. 2013 doi: 10.1038/nprot.2013.032. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen JI, Roychowdhury S, McMullen MR, Stavitsky AB, Nagy LE. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology. 2010;139:664–74. 674, e1. doi: 10.1053/j.gastro.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–63. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- 25.Taieb J, Mathurin P, Elbim C, Cluzel P, Arce-Vicioso M, Bernard B, Opolon P, Gougerot-Pocidalo MA, Poynard T, Chollet-Martin S. Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: effectof corticosteroids. J Hepatol. 2000;32:579–86. doi: 10.1016/s0168-8278(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 26.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 27.Lawson JA, Burns AR, Farhood A, Lynn Bajt M, Collins RG, Smith CW, Jaeschke H. Pathophysiologic importance of E- and L-selectin for neutrophil-induced liver injury during endotoxemia in mice. Hepatology. 2000;32:990–8. doi: 10.1053/jhep.2000.19068. [DOI] [PubMed] [Google Scholar]
- 28.Morikawa H, Hachiya K, Mizuhara H, Fujiwara H, Nishiguchi S, Shiomi S, Kuroki T, Kaneda K. Sublobular veins as the main site of lymphocyte adhesion/transmigration and adhesion molecule expression in the porto-sinusoidal-hepatic venous system during concanavalin A-induced hepatitis in mice. Hepatology. 2000;31:83–94. doi: 10.1002/hep.510310115. [DOI] [PubMed] [Google Scholar]
- 29.Wolf D, Hallmann R, Sass G, Sixt M, Kusters S, Fregien B, Trautwein C, Tiegs G. TNF-alpha-induced expression of adhesion molecules in the liver is under the control of TNFR1--relevance for concanavalin A-induced hepatitis. J Immunol. 2001;166:1300–7. doi: 10.4049/jimmunol.166.2.1300. [DOI] [PubMed] [Google Scholar]
- 30.Klintman D, Li X, Thorlacius H. Important role of P-selectin for leukocyte recruitment, hepatocellular injury, and apoptosis in endotoxemic mice. Clin Diagn Lab Immunol. 2004;11:56–62. doi: 10.1128/CDLI.11.1.56-62.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong J, Johnston B, Lee SS, Bullard DC, Smith CW, Beaudet AL, Kubes P. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest. 1997;99:2782–90. doi: 10.1172/JCI119468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong D, Dorovini-Zis K. Regualtion by cytokines and lipopolysaccharide of E-selectin expression by human brain microvessel endothelial cells in primary culture. J Neuropathol Exp Neurol. 1996;55:225–35. doi: 10.1097/00005072-199602000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–8. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 34.Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol. 2007;35:757–66. doi: 10.1080/01926230701584163. [DOI] [PubMed] [Google Scholar]
- 35.Selzner N, Selzner M, Odermatt B, Tian Y, Van Rooijen N, Clavien PA. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology. 2003;124:692–700. doi: 10.1053/gast.2003.50098. [DOI] [PubMed] [Google Scholar]
- 36.Taieb J, Delarche C, Paradis V, Mathurin P, Grenier A, Crestani B, Dehoux M, Thabut D, Gougerot-Pocidalo MA, Poynard T, Chollet-Martin S. Polymorphonuclear neutrophils are a source of hepatocyte growth factor in patients with severe alcoholic hepatitis. J Hepatol. 2002;36:342–8. doi: 10.1016/s0168-8278(01)00276-8. [DOI] [PubMed] [Google Scholar]
- 37.Adams DH, Burra P, Hubscher SG, Elias E, Newman W. Endothelial activation and circulating vascular adhesion molecules in alcoholic liver disease. Hepatology. 1994;19:588–94. doi: 10.1002/hep.1840190308. [DOI] [PubMed] [Google Scholar]
- 38.Adams DH, Hubscher SG, Fisher NC, Williams A, Robinson M. Expression of E-selectin and E-selectin ligands in human liver inflammation. Hepatology. 1996;24:533–8. doi: 10.1002/hep.510240311. [DOI] [PubMed] [Google Scholar]
- 39.DiChiara MR, Kiely JM, Gimbrone MA, Jr, Lee ME, Perrella MA, Topper JN. Inhibition of E-selectin gene expression by transforming growth factor beta in endothelial cells involves coactivator integration of Smad and nuclear factor kappaB-mediated signals. J Exp Med. 2000;192:695–704. doi: 10.1084/jem.192.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.