Abstract
Cocaine dependence is defined by a loss of inhibitory control over drug use behaviors, mirrored by measurable impairments in laboratory tasks of inhibitory control. The current study tested the hypothesis that deficits in multiple sub-processes of behavioral control are associated with reliable neural processing alterations that define cocaine addiction. While undergoing fMRI, 38 cocaine-dependent men and 27 healthy control men performed a stop-signal task of motor inhibition. An independent component analysis (ICA) on fMRI time courses identified task-related neural networks attributed to motor, visual, cognitive and affective processes. The statistical associations of these components with five different stop-signal task conditions were selected for use in a linear discriminant analysis to define a classifier for cocaine addiction from a subsample of 26 cocaine-dependent men and 18 controls. Leave-one-out cross validation accurately classified 89.5% (39/44; chance accuracy = 26/44 = 59.1%) of subjects (with 84.6% (22/26) sensitivity and 94.4% (17/18) specificity. The remaining 12 cocaine-dependent and 9 control men formed an independent test sample, for which accuracy of the classifier was 81.9% (17/21; chance accuracy = 12/21 = 57.1%) with 75% (9/12) sensitivity and 88.9% (8/9) specificity. The cocaine addiction classification score was significantly correlated with a measure of impulsiveness as well as the duration of cocaine use for cocaine-dependent men. The results of this study support the ability of a pattern of multiple neural network alterations associated with inhibitory motor control to define a binary classifier for cocaine addiction.
Keywords: classification, fMRI, cocaine dependence, inhibitory motor control, stop-signal task
INTRODUCTION
Cocaine addiction is associated with a diminished ability to exert inhibitory control over behavior. Deficits in behavioral inhibition processes identified in laboratory studies reflect clinical diagnostic criteria for drug dependence and include longer stop-signal reaction times (Fillmore and Rush, 2002; Li et al., 2006b), greater errors of commission (Fillmore and Rush, 2002; Kaufman et al., 2003; Verdejo-Garcia et al., 2007), poor error awareness (Hester et al., 2007), and decreased post-error adaptive behavior (Hester et al., 2007; Li et al., 2006b). Neural processing alterations underlying these behavioral impairments have been separately identified by functional neuroimaging studies of cocaine-dependent individuals for response inhibition (Hester and Garavan, 2004; Kaufman et al., 2003; Li et al., 2007) and errors of commission (Kaufman et al., 2003). These studies suggest that cocaine addiction is defined by a complex set of neural processing deficits related to multiple sub-processes of the inhibitory control of behavior. We sought to test this hypothesis using the stop-signal task (Logan, 1994). A test of the ability of such deficits to define cocaine addiction is the extent to which the patterns of task-related activation reliably and accurately classify an individual as being drug-addicted versus non-drug abusing.
A common approach to the identification of group differences in brain activation involves a two sample t-test on spatial maps of statistical values representing the association of each voxel with a given task condition or contrast. Although voxel clustering thresholds are frequently applied to results, these group-level statistical analyses are performed independently for each voxel. Such univariate tests fail to account for activity in the rest of the brain that may contribute to group differences but would not independently differentiate groups. Furthermore, the tens of thousands of independent tests involved in voxel-wise analyses require extensive correction for multiple comparisons that can increase type-II error rates. The more recent application of multivariate statistical techniques for functional neuroimaging data analysis overcomes these limitations (O’Toole et al., 2007; Rowe and Hoffmann, 2006). Dimensionality reduction approaches such as principal component analysis and independent component analysis seek to attribute variance in the data to a reduced number of sources, thereby requiring fewer independent tests. Additional multivariate approaches, including support vector machines (Fu et al., 2008; LaConte et al., 2005; Mourão-Miranda et al., 2005) and linear discriminant analysis (Carlson et al., 2003; Dai et al., 2012; Ford et al., 2003), have also been successfully applied to classification and prediction for neuroimaging data. Classification analyses utilize these approaches to discriminate task conditions (Carlson et al., 2003; Mourao-Mirãnda et al., 2005) or to optimally discriminate patient groups with disorders such as Alzheimer’s disease or depression (Dai et al., 2012; Ford et al., 2003; Fu et al., 2008) to enable accurate classification of independent cases. In the context of fMRI research, the objectives of classification often focus on identifying and validating neural markers of cognitive or disease states.
The goal of this study was to define a neurophenotype of cocaine addiction based upon altered patterns of neural network activity during a motor response inhibition task. We hypothesized that cocaine addiction is associated with specific and consistent deficits in multiple neural processing networks in response to demands for inhibitory control with the expected outcome that the pattern of deficits enables the accurate classification of cocaine dependence. To test this hypothesis, 38 cocaine-dependent males and 27 healthy control males performed a stop-signal task while undergoing fMRI. We employed independent component analysis in conjunction with linear discriminant analysis to identify, in a multivariate approach, neural networks that are differentially engaged in cocaine-dependent versus healthy control men across five stop-signal task conditions.
METHODS
Subjects
Thirty-eight cocaine-dependent men [40.8 ± 8.0 (mean ± standard deviation) years of age] and 27 healthy control men [30.9 ± 7.7 (mean ± standard deviation) years of age] participated in the study. Due to sex differences in neural processing correlates of the inhibition of prepotent motor responses (Li et al., 2009; Li et al., 2006a) and sex differences in affective, cognitive and treatment aspects of cocaine addiction (Brady and Randall, 1999; Cotto et al., 2010; Najavits and Lester, 2008), we chose to focus this study on males as the sex representing the majority of cocaine abuse cases (Office of Applied Studies, 2004). Participants responded to local newspaper advertisements, flyers, and advertisements displayed in Little Rock city buses. Subjects provided informed consent to participate in the study following a thorough explanation of study procedures. This study was approved by the Institutional Review Boards at Emory University and the University of Arkansas for Medical Sciences (UAMS). All subjects were assessed for DSM-IV Axis I disorders by an experienced Masters-level psychologist using the Structured Clinical Interview for DSM-IV (SCID-1; First et al., 2007).
Inclusion/Exclusion criteria
For the Emory University site, men between the ages of 18–60 years were eligible for study participation. Cocaine-dependent men enrolled at this site had been enrolled in an abstinence-based treatment program for 2–4 weeks. At the UAMS site, cocaine-dependent males were non-treatment-seeking and between the ages of 18–45 years. All cocaine users met DSM-IV criteria for current cocaine dependence according to SCID interview. Comorbid alcohol and marijuana abuse or dependence were permitted for cocaine-dependent subjects only, enabling the generalization of results to the “real world” problem of comorbidity associated with cocaine dependence. Cocaine-dependent and healthy control subjects did not meet criteria for any other DSM-IV Axis I disorders with the exception that past histories of mood or anxiety disorders were not excluded. Recent drug use (cocaine, methamphetamine, amphetamine, opiate, and cannabis) was detected by urinalysis on the day of the fMRI scan in all study subjects. A positive test for any drug of abuse was a criterion for exclusion for healthy comparison subjects, as well as cocaine-dependent subjects from the Emory University site. Participants were free of psychotropic medication use for at least 30 days and reported no major medical disorders.
fMRI task
Subjects performed a stop-signal task while undergoing fMRI. Go stimuli were letters of the alphabet to which subjects responded by pressing a single button with the index finger of their dominant hand. The stop signal was a white square presented around the go stimulus after a short delay [“stop signal delay” (SSD)] in 75 of the 300 trials. The SSD was initially 250 ms and was performance-adjusted by 50 ms after each stop trial to achieve a successful stopping rate of approximately 50% (Aron and Poldrack, 2006). The fixed inter-trial interval was 2000 ms. Three rest periods lasting 20 seconds each were presented during the task.
Behavioral and clinical measures
Stop-signal reaction times (SSRT) were calculated as the percentile go trial reaction time corresponding to the percentage of errors of commission minus the mean SSD. To compensate for the possibility that a small percentage of successful stops were achieved by subject inattention, calculation of successful stopping rate in this equation was adjusted for the number of errors of omission on go trials (“misses”). Post-error slowing (PES) was calculated as the percent change in mean reaction time for go trials following an error of commission on stop trials relative to mean reaction for go trials not following a stop trial.
The 30-item Barratt Impulsiveness Scale (Patton et al., 1995) was administered to each subject to provide a measure of inhibitory control ability in daily functioning. Three second-order factor scores related to attentional, motor, and non-planning impulsiveness were calculated.
fMRI acquisition
Twenty-nine cocaine-dependent men and 13 control subjects were scanned at the Brain Imaging Research Center (BIRC) at UAMS with a Philips Achieva 3T MRI. Nine cocaine-dependent men and 14 control subjects were scanned in the Biomedical Imaging Technology Center (BITC) at Emory University with a Siemens Trio 3T MRI. The following parameters were used to acquire functional T2*-weighted echo-planar images (EPIs) on both scanners: 3×3×3 mm3 voxels, 34 slices, TR=2000 ms, TE=30 ms, FOV=192×192 mm2, flip angle=90°, matrix=64×64.
Anatomical images were acquired using a T1 MPRAGE sequence for the purpose of functional image registration and tissue segmentation (BIRC: matrix=256×256, 160 slices, TR=2600 ms, TE=3.02 ms, FA=8°, final resolution=1×1×1 mm3; BITC: matrix=256×240, 176 slices, TR=2300 ms, TE=3.02 ms, FA=8°, final resolution=1×1×1 mm3).
fMRI data preprocessing
Images were preprocessed in Analysis of Functional Neuroimages software (AFNI; Cox, 1996). Preprocessing steps for functional images included slice time correction, deobliquing, motion correction, despiking, alignment to the subject’s anatomical image, warping to MNI standardized space, removal of signal fluctuations in white matter and cerebral spinal fluid from voxel time courses, Gaussian smoothing at 6 mm full-width at half-maximum, and scaling to percent signal change.
ICA
A group ICA on stop-signal task fMRI time courses was performed to reduce the dimensionality of the dataset from hundreds of thousands of individual voxels to thirty independent components of activation. The ICA was conducted on all 65 subjects using the infomax algorithm in the Group ICA of fMRI Toolbox version 2 (GIFT; Calhoun et al., 2001) implemented in Matlab, solving for 30 components. The ICASSO tool within GIFT provided estimates of the iterative consistency of the component estimates (Correa et al., 2007). Of analyses performed with 20, 30, and 35 components, the 30-component ICA demonstrated the best ICASSO estimates, provided predicted canonical neural processing networks, and separated out artifacts from putative neural networks. In addition to group spatial maps, the group ICA also provides time courses associated with each of the 30 components for each subject. In this regard, components are comparable to individual voxels and can be similarly subjected to general linear model analysis. For each subject, linear regression of the task design [successful stop trials, errors of commission (failed stop trials), post-successful stop go trials, post-error go trials, go trials (not following a stop trial), and misses] convolved with the SPM hemodynamic response function and controlling for six directions of head motion identified the relationship of each of these six stop-signal task conditions to each component time course (Calhoun et al., 2001). Beta estimates for each task condition, with the exception of misses, were retained for use as variables in discriminant analysis. Estimates from components attributed to head motion, physiological noise and other artifacts were omitted from further analyses. The fifteen components contributing to discriminant analysis demonstrated anatomical correspondence to brain activations associated with stop-signal task performance (Aron and Poldrack, 2006) and to components previously defined by ICA applications to fMRI stop-signal tasks data (Congdon et al., 2010; Zhang and Li, 2012).
Discriminant Analysis
Because age and race significantly differed between the cocaine-dependent and control subjects, a subsample of 26 cocaine-dependent men and 18 controls were matched on these demographic variables and composed the training set (Table 2). Matching on these variables allowed for the development of a discriminant function for cocaine addiction that was not influenced by these factors. The remaining 12 cocaine-dependent men and 9 controls were set aside to test the ability of the discriminant function to classify an independent sample.
Table 2.
Characteristics of Training Sample
| Controls N=18 |
Cocaine N=26 |
p | |
|---|---|---|---|
| Age (years) | 34.1 (7.3) | 38.0 (7.6) | NS |
| Race | NS | ||
| African-American | 10 (55.6%) | 21 (80.8%) | |
| Caucasian | 7 (38.9%) | 5 (19.2%) | |
| Other | 1 (5.6%) | 0 (0.0%) | |
| Education | 15.2 (2.2) | 12.1 (1.1) | <0.001 |
| MRI scan site (Emory, UAMS) | 8, 10 | 6, 20 | NS |
| Lifetime Major Depressive Disorder | 3 (16.7%) | 8 (30.8%) | NS |
| Lifetime Post-Traumatic Stress Disorder | 0 | 2 (7.7%) | NS |
| Alcohol lifetime (abuse, dependence) | - | 3, 11 | - |
| Marijuana lifetime (abuse, dependence) | - | 4, 10 | - |
| Alcohol lifetime (abuse, dependence) | - | 2, 7 | - |
| Marijuana lifetime (abuse, dependence) | - | 1, 5 | - |
| Years of cocaine use | - | 11.5 (7.2) | - |
| Method (smoke, snort) | - | 19, 7 | - |
|
| |||
| Beck Depression Inventory | 3.9 (4.2) | 12.1 (10.1) | <0.001 |
|
| |||
| BIS Attentional Impulsiveness | 13.9 (3.3) | 16.7 (4.2) | 0.028 |
| BIS Motor Impulsiveness | 20.3 (4.1) | 24.7 (5.6) | 0.010 |
| BIS Nonplanning Impulsiveness | 21.4 (6.2) | 28.5 (4.6) | <0.001 |
|
| |||
| Stop Signal Reaction Time (ms) | 196 (53) | 195 (60) | NS |
| Mean Go Trial Reaction Time (ms) | 708 (238) | 752 (175) | NS |
| Mean Stop Signal Delay (ms) | 506 (233) | 546 (165) | NS |
| Post-Error Slowing (%) | 13.9 (5.2) | 8.5 (7.5) | NS |
| Successful Stop Rate (%) | 48.4 (11.6) | 51.0 (11.0) | NS |
Demographic, psychiatric, personality and stop-signal task behavioral measures for the 18 control men and 26 cocaine-dependent men in the training set. Values displayed as group mean (standard deviation) or number of observations (percentage). Significant p-values (p<0.05) corresponding with the appropriate t-test or chi-square test of group differences are reported where applicable.
Step-wise variable selection to identify the subset of beta estimates for stop-signal task conditions from components of activation that best differentiated the 26 cocaine-dependent males and 18 healthy controls of the training set was performed with the Stepdisc function in SAS 9.3. At each step, any variables in the subset no longer meeting a significance level of 0.15 (Costanza and Afifi, 1979) for differentiating between the groups (analysis of covariance), covarying for the other variables in the subset, were removed; variables meeting a significance level of 0.15, covarying for the other variables in the subset, were added. When no other variable could be added or removed, the subset of variables selected by this process was used in discriminant analyses.
A linear discriminant function was calculated with the Discrim procedure in SAS to maximize the generalized squared distance between the cocaine-dependent and control groups. Prior probabilities were set to be equal. A cocaine addiction classification score and a control group classification score were calculated for each subject by inserting the subject’s beta estimates as independent variables in the equations for the linear discriminant function. The larger of these two scores determined group allocation in classification. Leave-one-out cross validation tested the ability of the variables to correctly classify the training sample. To test the generalizability of the classifier across image acquisition sites, an additional independent cross validation was performed in which the sample from the UAMS site was used to classify the subjects from the Emory University site. Because variable selection was conducted on the training set, cross validation provides a biased estimate of classification accuracy. To obtain an unbiased estimate of classification accuracy, we tested the ability of the linear discriminant function to classify the sample of 12 cocaine-dependent men and 9 controls which were not included in either the variable selection or the computation of the discriminant function.
To explore the functional and clinical significance of the binary classifier, individual classification scores were tested in correlational analyses to explore whether the classifier was associated with task performance measures (SSRT, PES), impulsiveness (BIS), or number of years of cocaine use.
RESULTS
Subject demographics, clinical variables, and task performance variables are summarized in Table 1. Overall, cocaine-dependent subjects were older (t=−4.98, p<0.001), less educated (t=6.02, p<0.001), more likely to be African-American (χ2=16.2, p=0.001), and more likely to have been scanned at UAMS (χ2= 4.22, p=0.04) compared with controls; there was no difference between these groups in SCID diagnoses of past depression or PTSD. Cocaine-dependent and control subjects did not differ in stop-signal reaction time, go trial reaction time, stop signal delay, post-error slowing, or successful stopping rate. Table 2 provides demographic, clinical, and task performance variables for the subsample of subjects that made up the training set, for which cocaine-dependent and control men did not differ in age, race, or MRI site, but still differed in education, depression symptomatology and impulsiveness. Following careful matching of variables within the training set, the test set and training set were not matched on age for the cocaine-dependent men (training: 38.0 years; test: 46.8 years; t = 3.58, p = 0.001) or control men (training: 34.1 years; test: 24.4 years; t = 3.77, p < 0.001). However, the training and test sets of subjects did not significantly differ in race or education for either group.
Table 1.
Characteristics of Entire Sample
| Controls N=27 |
Cocaine N=38 |
p | |
|---|---|---|---|
| Age (years) | 30.9 (7.7) | 40.8 (8.0) | <0.001 |
| Race | 0.001 | ||
| African-American | 11 (40.7%) | 33 (86.8%) | |
| Caucasian | 13 (48.1%) | 5 (13.2%) | |
| Other | 3 (11.1%) | 0 (0.0%) | |
| Education | 15.4 (2.1) | 12.6 (1.4) | <0.001 |
| MRI scan site (Emory, UAMS) | 13, 14 | 9, 29 | 0.040 |
| Lifetime Major Depressive Disorder | 5 (18.5%) | 11 (29.0%) | NS |
| Lifetime Post-Traumatic Stress Disorder | 0 (0.0%) | 2 (5.3%) | NS |
| Alcohol lifetime (abuse, dependence) | - | 4, 18 | - |
| Marijuana lifetime (abuse, dependence) | - | 6, 16 | - |
| Alcohol current (abuse, dependence) | - | 2, 8 | - |
| Marijuana current (abuse, dependence) | - | 2, 7 | - |
| Years of cocaine use | - | 12.1 (6.9) | - |
| Method (smoke, snort) | - | 31, 7 | - |
|
| |||
| Beck Depression Inventory | 5.0 (6.3) | 12.9 (10.3) | <0.001 |
|
| |||
| BIS Attentional Impulsiveness | 15.0 (4.1) | 16.2 (4.2) | NS |
| BIS Motor Impulsiveness | 20.7 (4.6) | 24.5 (5.7) | 0.009 |
| BIS Nonplanning Impulsiveness | 22.3 (6.3) | 28.5 (4.8) | <0.001 |
|
| |||
| Stop Signal Reaction Time (ms) | 202 (48) | 204 (55) | NS |
| Mean Go Trial Reaction Time (ms) | 645 (240) | 741 (175) | NS |
| Mean Stop Signal Delay (ms) | 446 (234) | 534 (172) | NS |
| Post-Error Slowing (%) | 13.5 (12.9) | 8.4 (9.0) | NS |
| Successful Stop Rate | 46.5 (11.0) | 50.2 (10.7) | NS |
Demographic, psychiatric, personality and stop-signal task behavioral measures for the 27 control men and 38 cocaine-dependent men in the study. Values displayed as group mean (standard deviation) or number of observations (percentage). Significant p-values (p<0.05) corresponding with the appropriate t-test or chi-square test of group differences are reported where applicable.
MRI, magnetic resonance imaging; UAMS, University of Arkansas for Medical Sciences; ms, milliseconds; NS, not significant.
Of the thirty components identified by ICA, 15 were noise components attributed to motion and other artifacts, whereas the other 15 represented a diverse array of networks of distributed brain activation (Supplemental Figure 1), including those attributed to motor, visual, cognitive, and emotional processing based on their spatial maps. For each subject, beta estimates were obtained for the five different stop-signal task conditions (i.e., go trials, successful stop trials, post-successful stop go trials, errors of commission, post-error go trials,) for each of these 15 non-noise components (75 variables total).
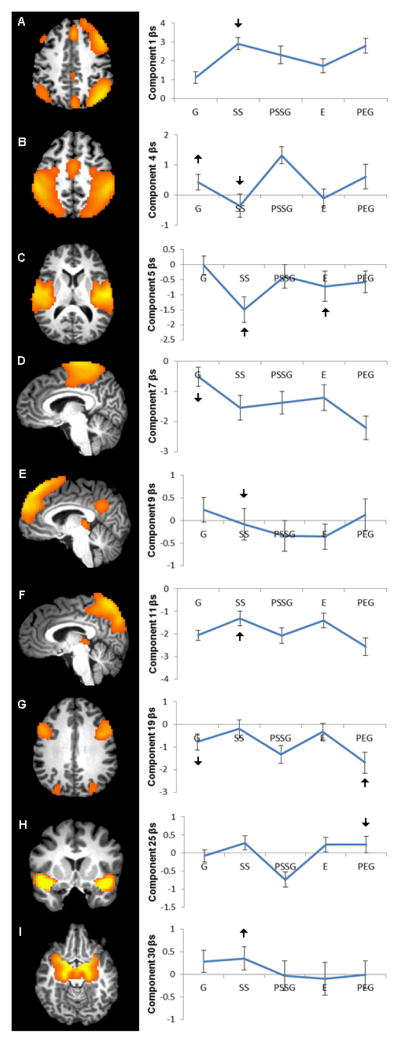
Of these 75 variables, the step-wise variable selection procedure conducted with the training set identified 12 variables derived from 9 different components with potential to discriminate cocaine-dependent and control subjects (Table 3). Six variables were associated with the successful stop condition, one variable with the error condition, three variables with the go condition (not following a stop trial), and two variables with post-error go trials. Mean beta estimates for control subjects for each of the nine components across all five stop signal task conditions are depicted in Figure 1.
Table 3.
Linear Discriminant Function for Cocaine Dependence
| IC # | Network Label | Task Condition | Controls | Cocaine | t |
|---|---|---|---|---|---|
| Constant | −8.49 | −1.87 | |||
| 11 | precuneus network | SS | −3.55 | −0.09 | 6.37 |
| 1 | right frontal-parietal network | SS | 2.66 | −0.12 | −5.68 |
| 4 | lateral motor cortex | G | −2.86 | 1.22 | 4.99 |
| 4 | lateral motor cortex | SS | 3.35 | −0.86 | −4.85 |
| 9 | dorsomedial prefrontal cortex | SS | 1.83 | −0.39 | −2.96 |
| 7 | supplementary motor area | G | 1.65 | −0.50 | −2.91 |
| 19 | inferior frontal junction, intraparietal sulcus | PEG | −2.50 | −0.65 | 2.59 |
| 5 | posterior insula / superior temporal gyrus | SS | −1.78 | −0.12 | 2.51 |
| 25 | orbital inferior frontal cortex / insula | PEG | 1.48 | −0.29 | −2.50 |
| 5 | posterior insula / superior termporal gyrus | E | −0.82 | 0.27 | 2.34 |
| 30 | amygdala, striatum | SS | −1.22 | 0.05 | 1.85 |
| 19 | inferior frontal junction, intraparietal sulcus | G | 1.06 | −0.19 | −1.58 |
Constant and classification function coefficients for each variable of the linear discriminant function to determine classification into the control group or the cocaine group. T values represent the statistical strength of each variable for differentiating between the groups, covarying for the other variables in the function.
IC, independent component; G, go trials; SS, successful stops, PSSG, post-successful stop go trials; E, errors of commission on stop trials; PEG, post-error go trials.
Figure 1. Independent components discriminating between cocaine-dependent men and healthy control men.
Nine components (A–I) contributed to the classifier. Mean beta coefficients for the control group for go trials (G), successful stops (SS), post-successful stop go trials (PSSG), errors of commission on stop trials (E), post-error go trials (PEG) are displayed for each component. Arrows designate those task conditions for which the component discriminated between the groups, where β denotes cocaine-dependent < control men and β denotes cocaine-dependent > control men. A. component 1, right frontal-parietal network; B. component 4, lateral motor cortex; C. component 5, posterior insula/superior temporal gyrus; D. component 7, supplementary motor area; E. component 9, dorsomedial prefrontal cortex; F. component 11, precuneus network; G. component 19, inferior frontal junction and intraparietal sulcus; H. component 25, orbital inferior frontal cortex/insula; I. component 30, amygdala and striatum.
The linear discriminant function (classification function coefficients) for cocaine dependence produced in the training set is presented in Table 3. Group allocation was determined by the larger of the control or cocaine-dependent classification scores, obtained by adding the intercept to the sum of the products of the coefficients and the corresponding independent variables. Leave-one-out cross validation (LOOCV) correctly classified 89.5% (39/44) of subjects with 84.6% (22/26) sensitivity and 94.4% (17/18) specificity (Table 4). The LOOCV estimate of classifier sensitivity exceeded that attributed to chance accuracy (59.1%). To assess an effect of site on classifier performance, cross validation of the linear discriminant function defined in the 30 subject training set subsample from UAMS correctly classified 91.7% (13/14) of the training set subsample from Emory University with 83.3% (5/6) sensitivity and 100% (8/8) specificity.
Table 4.
Classification Accuracy
| Classified Control | Classified Cocaine | Correctly classified | |
|---|---|---|---|
| Training set | 89.5% | ||
| Controls | 17 | 1 | 94.4% |
| Cocaine | 4 | 22 | 84.6% |
|
| |||
| Test set | 81.9% | ||
| Controls | 8 | 1 | 88.9% |
| Cocaine | 3 | 9 | 75.0% |
Total accuracy, specificity and sensitivity for cocaine dependence from leave-one-out cross validation of the training set and classification of the test set.
The classification accuracy of the linear discriminant function in the independent test set was 81.9% (17/21) with 75% (9/12) sensitivity and 88.9% (8/9) specificity (Table 4). The independent estimate of classifier sensitivity exceeded that attributed to chance accuracy (57.1%).
The classification score for controls was positively correlated with the BIS motor impulsiveness score for control subjects (r=0.48, p=0.02), but not for cocaine-dependent subjects (r=0.09, NS). Conversely, the classification score for cocaine dependence was correlated with the motor impulsiveness score for individuals in the cocaine-dependent group (r=0.43, p=0.01), but not control group (r=0.06, NS). The cocaine dependence classification score also correlated with the lifetime number of years of cocaine use in the cocaine-dependent sample (r=0.52 p=0.004). However, years of cocaine use was not itself associated with BIS motor impulsiveness (r=0.18, NS), and a regression model including both variables as predictors indicated that they independently predicted the cocaine addiction classification score (R2 adjusted=0.50; BIS motor: t=3.28, p=0.003; years of use: t=3.57, p=0.002). BIS nonplanning and attentional impulsiveness factor scores, stop-signal reaction time, and post error slowing were not correlated with either classification score for cocaine dependent or control men.
DISCUSSION
This study provides a novel, comprehensive analysis of cocaine addiction-related neural network alterations associated with multiple sub-processes involved in inhibitory control of motor behavior. We used ICA as a dimensionality reduction tool to address the complex feature space of fMRI datasets and identified spatially-independent networks of neural activation associated with performance of a stop-signal task. The statistical association of components of brain activation, rather than individual voxels, was defined for each task condition. We incorporated component beta coefficients for stop-signal task conditions into a multivariate group-level analysis using stepwise variable selection, linear discriminant analysis and binary classification approaches. The result was a pattern classifier for cocaine addiction with high sensitivity and specificity in both cross-validation and for an independent sample. The twelve variables contributing to the classifier were derived from nine independent components and from four different stop-signal task conditions.
Successful Stop trials
The variable with the greatest discriminant power was for activation during successful stop trials of a component of activation (#11) centered in the precuneus and involving activations in the bilateral middle occipital gyri, bilateral supramarginal gyri, bilateral dorsal superior frontal gyri, and anterior cingulate cortex. This network was less deactivated during successful stop trials for cocaine-dependent compared with control men. Hypoactivity related to cocaine dependence during response inhibition trials for either go/no-go or stop-signal tasks has previously been reported for some of the regions of this network (bilateral supramarginal gyrus, left superior frontal gyrus, and anterior cingulate cortex) (Hester and Garavan, 2004; Kaufman et al., 2003; Li et al., 2007). Decreased activation of the precuneus has been associated with practice effects (Goldstein et al., 2007; Sakai et al., 1998), and cocaine addiction was associated with diminished task habituation-related deactivation of the precuneus in a go/no-go task with monetary incentives (Goldstein et al., 2007). These results suggest that cocaine dependence is associated with the impaired ability to transition to more efficient and automated task performance processes.
A frontal-parietal component (#1) associated with predominantly right hemisphere activity in the dorsolateral and ventrolateral prefrontal cortex, anterior insula, middle temporal gyrus, dorsomedial prefrontal cortex, precuneus, and right inferior parietal cortex also significantly differentiated the groups. For this component, activation during successful stop trials was greater in control than cocaine-dependent men, perhaps reflecting the association of cocaine dependence with deficits in the cognitive regulation of motor, motivational, interoceptive, or attentional responses (Kober et al., 2010; Li et al., 2006b; Zhang and Li, 2012). Cocaine-dependent men also demonstrated relatively diminished activity during successful stop trials for a dorsomedial prefrontal cortex component (#9) that included activation of the bilateral inferior frontal gyri, angular gyri, middle temporal gyri, and posterior cingulate cortex. Activity in both of these networks during successful stop trials was significantly negatively correlated with SSRTs (component 1: r =−0.33, p=0.007; component 9: r =−0.36, p=0.003), corroborating the importance of these networks for inhibitory control and the inference of deficits in their functional engagement associated with cocaine dependence.
A limbic-striatal component (#30) of activation of the amygdala, hippocampus, caudate, and putamen was more engaged during successful stopping for cocaine-dependent compared with control men. Additionally, a component (#5) of activation of the bilateral posterior insula and superior temporal gyrus was also more active in cocaine dependent men during successful response inhibition. These regions have also been implicated in craving responses to drug cues (Kilts et al., 2001; Potenza et al., 2012; Volkow et al., 2010) and may represent a general marker of impaired control of emotional and motivational responses in addiction.
Go trials
During go trials not following a stop trial, motor regulation network activity most differentiated the groups, a finding consistent with the role of motor pathways in go trials (Congdon et al., 2010). A motor network of activation (#4) involving the lateral primary motor cortex and pre-supplementary motor area (pre-SMA), likely associated with executing motor plans, exhibited greater activation in cocaine-dependent men versus controls during go trials. For the same task condition, a supplementary motor area (SMA) network of activation (#7) of areas involved in motor planning was more deactivated in cocaine-dependent individuals. These group differences suggest that less inhibitory control of prepotent behaviors in cocaine-dependent men during go trials drives the binary classification. However, the discriminant function also indicated greater suppression of primary motor-pre-SMA network (#4) activity in cocaine-dependent versus control men during successful stop trials, an enhancement which may enable the overcoming of functional deficits in other networks and preserve inhibitory control ability.
Errors of commission
As with the successful stop condition, cocaine-dependent men also demonstrated greater activity in the posterior insula network (#5) relative to controls during errors on stop trials. The increased activity of this network across both errors and successful stop trials may reflect an enhanced sensory salience of the stop signal.
Post-error go trials
Cocaine-dependent participants demonstrated less deactivation compared to controls of a spatial attention network (#19) involving the bilateral intraparietal sulci, posterior inferior/middle frontal gyri, superior frontal gyri, and posterior cingulate cortex for go trials following an unsuccessful stop trial. In addition, a network of activation involving bilateral orbital/inferior frontal gyri (#25) was less active in cocaine-dependent men for post-error go trials. These group differences corroborate evidence for reduced response to errors of commission in cocaine dependence, perhaps reflecting a lesser awareness of errors (Hester et al., 2007).
In contrast to prior response inhibition studies (Ersche et al., 2012; Fillmore and Rush, 2002; Li et al., 2006b) the sample of cocaine-dependent men demonstrated motor inhibition ability similar to controls, although cocaine dependence was associated with the utilization of different neural processing strategies to achieve this outcome. Neural processing differences in the absence of stop-signal task performance deficits have previously been reported for cocaine-dependent individuals (Li et al., 2007), corroborating the improved sensitivity of functional neuroimaging over behavioral measures of underlying processes (Costafreda et al., 2011; Rose and Donohoe, 2012). Furthermore, the neural processing differences identified by this study may translate to practical individual or group differences not readily detected by task performance, such as treatment outcome or compulsive drug use behavior. The correlation of the BIS motor impulsiveness factor score with the classification score for cocaine dependence supports this contention.
The motor impulsiveness factor of the BIS encompasses items related to acting without thinking or living in the moment (Patton et al., 1995). Thus, the selective association of classification scores with this measure indicates that the classifier reflects the impact of cocaine dependence on these specific decision processes and that deficits in neural processing networks underlying impulsive behavior represent biomarkers of cocaine addiction and addiction-related behavioral impairments. For example, follow-up and treatment studies have demonstrated that greater impulsivity predicts poorer treatment retention for cocaine-dependent individuals (Moeller et al., 2001; Patkar et al., 2004). High impulsivity has also been associated with a genetic predisposition for drug addiction (Ersche et al., 2010).
The extent to which the derived classifier reflects patterns of altered functional brain organization that preceded drug use – and therefore could identify individuals at risk for cocaine dependence – cannot be addressed by this cross-sectional study. However, previous work has identified adolescent inhibitory control-related behavioral (Nigg et al., 2006; Tarter et al., 2004) and neural processing (Norman et al., 2011) deficits as predictors of future drug use problems, as well as response inhibition-related deficits in brain structure as familial risk factors for stimulant addiction (Ersche et al., 2012). On the other hand, there is also evidence that a greater extent of cocaine exposure is associated with worse inhibitory control ability (Colzato et al., 2007). This latter association is consistent with the finding that, for drug-dependent subjects, the cocaine dependence classification score was positively correlated with years of cocaine use. An integrated theory proposes that chronic drug use exacerbates preexisting deficits in inhibitory control and impulsivity (Ersche et al., 2010; Garavan and Stout, 2005; Verdejo-García et al., 2008), and thus these cognitive impairments both increase susceptibility to drug use problems and serve to maintain drug addiction.
The high classification accuracy in the sub optimally matched independent test set demonstrates the robustness of the classifier. Furthermore, the ability of the subsample of the training set from UAMS to classify the subsample from Emory University suggests that the classifier is reliable across sites as well as across diverse demographics. In addition, this cross-site validation indicates that the classifier is not influenced by treatment or treatment motivation, as these were significant differences in the cocaine-dependent samples across sites. Therefore, the classifier seems to represent consistent differences between cocaine-dependent men and non-drug using men that supersede the effects of variables such as treatment or recent drug use.
Limitations
Although we successfully used task-related fMRI data to train a binary classifier with good sensitivity and specificity for cocaine addiction, the study had several limitations. The subjects in the test set were included in the group ICA which was used to calculate the variables (beta coefficients) that were entered into the classifier. Even though the actual beta coefficients calculated from subjects in the test set were not included in the variable selection process that built the classifier, the inclusion of these subjects’ data in the independent component analysis could have indirectly biased the subsequent independent analyses of binary classification accuracy. In addition, the current dataset only contained males; therefore, these linear functions may not accurately classify cocaine dependence in females. More importantly, the interpretation of neural processing alterations identified in the current sample of cocaine-dependent men may not apply to cocaine-dependent women. Future studies should therefore examine response inhibition-related neural network alterations associated with cocaine dependence in women. Likewise, the generalizability of these findings to other drugs of abuse is unknown. Although many of the cocaine-dependent men abused marijuana and alcohol, cocaine was the primary drug in every case. While there are similarities in inhibitory control deficits across drug addictions (Fillmore and Rush, 2002; Hester et al., 2009; Hester et al., 2007; Kaufman et al., 2003), differences have also been identified (Verdejo-Garcia et al., 2007). Also, group differences other than cocaine dependence status may contribute to the pattern classification outcome. In this study, the samples of cocaine-dependent and control men comprising the training set differed in education, depression symptoms, and impulsiveness. However, these variables tend to be concomitant with – if not secondary to – drug addiction (Ersche et al., 2010; Lundqvist, 2010; Swendsen et al., 2009; Verdejo-García et al., 2008). Finally, varying degrees of orbitofrontal signal dropout and inconsistent coverage of the cerebellum limited the contribution of these brain regions to the discriminant analysis.
Conclusions
Cocaine addiction is associated with altered recruitment of multiple neural networks that support inhibitory motor control processes, including response execution, response inhibition, error processing, and post-error adaptation. Comparative pattern classification of neural responses during a stop-signal task yielded a binary classifier for cocaine addiction based upon these collective neural processing differences that exhibited reliable classification accuracy and was associated with both trait impulsiveness and duration of cocaine use. These results support the use of pattern classification of brain inhibitory control processes as a biomarker for cocaine addiction.
Supplementary Material
Acknowledgments
This study was funded by the National Institute on Drug Abuse grant RO1DA019999. Portions of this work were supported by grants from the National Center for Research Resources (UL1RR029884 and KL2RR029883), National Institute on Drug Abuse (T32DA022981) and support from the Glidden Family Foundation. The authors thank Tim Ely for fMRI stop-signal task design and Kristina Davidson for her assistance with data entry.
Footnotes
AUTHOR CONTRIBUTIONS
CK was responsible for the study concept and design. JY, SS, REG, TM screened study participants, administered assessments, and collected data. AE performed data analyses. AE drafted the manuscript. CK provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
None of the authors report any biomedical financial interests or potential conflicts of interest.
References
- Aron AR, Poldrack RA. Cortical and Subcortical Contributions to Stop Signal Response Inhibition: Role of the Subthalamic Nucleus. The Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender Differences in Substance Use Disorders. Psychiatric Clinics of North America. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson TA, Schrater P, He S. Patterns of Activity in the Categorical Representations of Objects. Journal of Cognitive Neuroscience. 2003;15:704–717. doi: 10.1162/089892903322307429. [DOI] [PubMed] [Google Scholar]
- Colzato LS, van den Wildenberg WPM, Hommel B. Impaired Inhibitory Control in Recreational Cocaine Users. PLoS ONE. 2007;2:e1143. doi: 10.1371/journal.pone.0001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa N, Adal T, Calhoun VD. Performance of blind source separation algorithms for fMRI analysis using a group ICA method. Magnetic Resonance Imaging. 2007;25:684–694. doi: 10.1016/j.mri.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda S, Fu C, Picchioni M, Toulopoulou T, McDonald C, Kravariti E, Walshe M, Prata D, Murray R, McGuire P. Pattern of neural responses to verbal fluency shows diagnostic specificity for schizophrenia and bipolar disorder. BMC Psychiatry. 2011;11:18. doi: 10.1186/1471-244X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanza MC, Afifi AA. Comparison of Stopping Rules in Forward Stepwise Discriminant Analysis. Journal of the American Statistical Association. 1979;74:777–785. [Google Scholar]
- Cotto JH, Davis E, Dowling GJ, Elcano JC, Staton AB, Weiss SRB. Gender effects on drug use, abuse, and dependence: A special analysis of results from the national survey on drug use and health. Gender Medicine. 2010;7:402–413. doi: 10.1016/j.genm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dai Z, Yan C, Wang Z, Wang J, Xia M, Li K, He Y. Discriminative analysis of early Alzheimer’s disease using multi-modal imaging and multi-level characterization with multi-classifier (M3) NeuroImage. 2012;59:2187–2195. doi: 10.1016/j.neuroimage.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal Brain Structure Implicated in Stimulant Drug Addiction. Science. 2012;335:601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry. 2010;68:770–773. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Impaired inhibitory control of behavior in chronic cocaine users. Drug and Alcohol Dependence. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York, New York, USA: 2007. Structured Clinical Interview for DSM-IV-TR Axis I Disorders–Non-patient Edition (SCID-I/NP, 1/2007 revision. [Google Scholar]
- Ford J, Farid H, Makedon F, Flashman L, McAllister T, Megalooikonomou V, Saykin A. Patient Classification of fMRI Activation Maps. In: Ellis R, Peters T, editors. Medical Image Computing and Computer-Assisted Intervention - MICCAI 2003. Springer; Berlin / Heidelberg: 2003. pp. 58–65. [Google Scholar]
- Fu CHY, Mourao-Miranda J, Costafreda SG, Khanna A, Marquand AF, Williams SCR, Brammer MJ. Pattern Classification of Sad Facial Processing: Toward the Development of Neurobiological Markers in Depression. Biological Psychiatry. 2008;63:656–662. doi: 10.1016/j.biopsych.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends in Cognitive Sciences. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Alia-Klein N, Zhang L, Telang F, Volkow ND. The effect of practice on a sustained attention task in cocaine abusers. NeuroImage. 2007;35:194–206. doi: 10.1016/j.neuroimage.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive Dysfunction in Cocaine Addiction: Evidence for Discordant Frontal, Cingulate, and Cerebellar Activity. The Journal of Neuroscience. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired Error Awareness and Anterior Cingulate Cortex Hypoactivity in Chronic Cannabis Users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Simoes-Franklin C, Garavan H. Post-Error Behavior in Active Cocaine Users: Poor Awareness of Errors in the Presence of Intact Performance Adjustments. Neuropsychopharmacology. 2007;32:1974–1984. doi: 10.1038/sj.npp.1301326. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate Hypoactivity in Cocaine Users During a GO-NOGO Task as Revealed by Event-Related Functional Magnetic Resonance Imaging. The Journal of Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KPG. Neural Activity Related to Drug Craving in Cocaine Addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences. 2010;107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaConte S, Strother S, Cherkassky V, Anderson J, Hu X. Support vector machines for temporal classification of block design fMRI data. NeuroImage. 2005;26:317–329. doi: 10.1016/j.neuroimage.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Li C-s, Zhang S, Duann J-R, Yan P, Sinha R, Mazure C. Gender Differences in Cognitive Control: an Extended Investigation of the Stop Signal Task. Brain Imaging and Behavior. 2009;3:262–276. doi: 10.1007/s11682-009-9068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-sR, Huang C, Constable RT, Sinha R. Gender differences in the neural correlates of response inhibition during a stop signal task. NeuroImage. 2006a;32:1918–1929. doi: 10.1016/j.neuroimage.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Li C-sR, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R. Neural Correlates of Impulse Control During Stop Signal Inhibition in Cocaine-Dependent Men. Neuropsychopharmacology. 2007;33:1798–1806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-sR, Milivojevic V, Kemp K, Hong K, Sinha R. Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug and Alcohol Dependence. 2006b;85:205–212. doi: 10.1016/j.drugalcdep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Logan GD, editor. On the ability to inhibit thought and action: A user’s guide to the stop signal paradigm. Academic Press; San Diego: 1994. [Google Scholar]
- Lundqvist T. Imaging cognitive deficits in drug abuse. Curr Top Behav Neurosci. 2010;3:247–275. doi: 10.1007/7854_2009_26. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Mourão-Miranda J, Bokde ALW, Born C, Hampel H, Stetter M. Classifying brain states and determining the discriminating activation patterns: Support Vector Machine on functional MRI data. NeuroImage. 2005;28:980–995. doi: 10.1016/j.neuroimage.2005.06.070. [DOI] [PubMed] [Google Scholar]
- Najavits LM, Lester KM. Gender differences in cocaine dependence. Drug and Alcohol Dependence. 2008;97:190–194. doi: 10.1016/j.drugalcdep.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor Response Inhibition as a Predictor of Problem Drinking and Illicit Drug Use in Adolescents at Risk for Alcoholism and Other Substance Use Disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole AJ, Jiang F, Abdi H, Pénard N, Dunlop JP, Parent MA. Theoretical, Statistical, and Practical Perspectives on Pattern-based Classification Approaches to the Analysis of Functional Neuroimaging Data. Journal of Cognitive Neuroscience. 2007;19:1735–1752. doi: 10.1162/jocn.2007.19.11.1735. [DOI] [PubMed] [Google Scholar]
- Office of Applied Studies. Results from the 2003 National Survey on Drug Use and Health: National findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2004. [Google Scholar]
- Patkar AA, Murray HW, Mannelli P, Gottheil E, Weinstein SP, Vergare MJ. Pre-treatment measures of impulsivity, aggression and sensation seeking are associated with treatment outcome for African-American cocaine-dependent patients. J Addict Dis. 2004;23:109–122. doi: 10.1300/J069v23n02_08. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Hong K-iA, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural Correlates of Stress-Induced and Cue-Induced Drug Craving: Influences of Sex and Cocaine Dependence. American Journal of Psychiatry. 2012;169:406–414. doi: 10.1176/appi.ajp.2011.11020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose EJ, Donohoe G. Brain vs Behavior: An Effect Size Comparison of Neuroimaging and Cognitive Studies of Genetic Risk for Schizophrenia. Schizophrenia Bulletin. 2012 doi: 10.1093/schbul/sbs056. Advance Access published April 12 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DB, Hoffmann RG. Multivariate statistical analysis in fMRI. IEEE Eng Med Biol Mag. 2006;25:60–64. doi: 10.1109/memb.2006.1607670. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Takino R, Sasaki Y, Pütz B. Transition of Brain Activation from Frontal to Parietal Areas in Visuomotor Sequence Learning. The Journal of Neuroscience. 1998;18:1827–1840. doi: 10.1523/JNEUROSCI.18-05-01827.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen J, Conway KP, Degenhardt L, Dierker L, Glantz M, Jin R, Merikangas KR, Sampson N, Kessler RC. Socio-demographic risk factors for alcohol and drug dependence: the 10-year follow-up of the national comorbidity survey. Addiction. 2009;104:1346–1355. doi: 10.1111/j.1360-0443.2009.02622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Habeych M, Reynolds M, Vanyukov M. Neurobehavior disinhibition in childhood predisposes boys to substance use disorder by young adulthood: direct and mediated etiologic pathways. Drug and Alcohol Dependence. 2004;73:121–132. doi: 10.1016/j.drugalcdep.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience & Biobehavioral Reviews. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. NeuroImage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li C-sR. Functional networks for cognitive control in a stop signal task: Independent component analysis. Human Brain Mapping. 2012;33:89–104. doi: 10.1002/hbm.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.