Abstract
Klotho, a transmembrane protein, which can be cleaved off as β-glucuronidase and hormone, is released in both, kidney and choroid plexus and encountered in blood and cerebrospinal fluid. Klotho deficiency leads to early appearance of age-related disorders and premature death. Klotho may modify transport by inhibiting 1,25(OH)2D3 formation or by directly affecting channel and carrier proteins. The present study explored whether Klotho influences the activity of the Na+-coupled excitatory amino acid transporters EAAT3 and EAAT4, which are expressed in kidney (EAAT3), intestine (EAAT3) and brain (EAAT3 and EAAT4). To this end, cRNA encoding EAAT3 or EAAT4 was injected into Xenopus oocytes with and without additional injection of cRNA encoding Klotho. EAAT expressing Xenopus oocytes were further treated with recombinant human β-Klotho protein with or without β-glucuronidase inhibitor D-saccharic acid 1,4-lactone monohydrate (DSAL). Electrogenic excitatory amino acid transport was determined as L-glutamate-induced current (Iglu) in two electrode voltage clamp experiments. EAAT3 and EAAT4 protein abundance in the Xenopus oocyte cell membrane was visualized by confocal microscopy and quantified utilizing chemiluminescence. As a result, coexpression of Klotho cRNA significantly increased Iglu in both, EAAT3 or EAAT4-expressing Xenopus oocytes. Klotho cRNA coexpression significantly increased the maximal current and cell membrane protein abundance of both EAAT3 and EAAT4. The effect of Klotho coexpression on EAAT3 and EAAT4 activity was mimicked by treating EAAT3 or EAAT4-expressing Xenopus oocytes with recombinant human β-Klotho protein. The effects of Klotho coexpression and of treatment with recombinant human β-Klotho protein were both abrogated in the presence of DSAL (10 µM). In conclusion, Klotho is a novel, powerful regulator of the excitatory amino acid transporters EAAT3 and EAAT4.
Introduction
Klotho is expressed in several tissues with particularly high expression in kidney and choroid plexus of the brain [1], [2]. The extracellular domain of the Klotho protein may be cleaved off and released into blood or cerebrospinal fluid and affect neighbouring cells as β-glucuronidase or hormone [3], [4]. Klotho-deficient mice suffer from severe growth retardation and premature appearance of a variety of age-related disorders resulting in death within less than 5 months [5], [6]. Conversely, the life span of mice is substantially extended by Klotho overexpression [5], [6].
Klotho is required for the inhibitory effect of FGF23 on 1α-hydroxylase and thus on 1,25(OH)2D3 formation [2], [6]–[8]. Functions of 1,25(OH)2D3 include up-regulation of renal Ca2+ and phosphate transport [9], [10]. Largely due to excessive 1,25(OH)2D3 formation, plasma Ca2+ [11] and phosphate [10] concentrations are increased in Klotho-deficient mice [2], [7], [8], leading to vascular calcification [12], [13] and growth deficit [2]. Beyond its impact on 1,25(OH)2D3 formation, Klotho may more directly influence transport processes, including Na+, phosphate cotransport [4], [14], Na+/K+ ATPase [15], Ca2+ channels [16] and renal outer medullary K+ channels [17].
Transport systems expressed in intestine, kidney and brain, include the excitatory amino acid transporter EAAT3, which is required for dicarboxylic amino acid absorption in intestine and reabsorption in renal proximal tubules [18], [19] as well as for cellular excitatory amino acid uptake at the blood-brain barrier [20], into neurons [21]–[28], into retinal ganglion cells [29] and into glial cells [30]–[33]. Excitatory amino acid uptake into cerebellar Purkinje cells is accomplished by the excitatory amino acid transporter EAAT4 [23], [25], [34].
Compromised excitatory amino acid uptake in the brain may result in excitotoxicity [35]. Deranged function of EAAT3 may further contribute to the pathophysiology of schizophrenia [28], [36]–[41], epilepsy [42]–[46] and hepatic encephalopathy [47]. Impaired function of EAAT4 has similarly been implicated in schizophrenia [36], [39].
The excitatory amino acid transporters EAAT3 and EAAT4 are regulated by phosphatidylinositide (PI)- 3-kinase signaling [29], [48]–[50], which is in turn sensitive to klotho [51].
To explore, whether Klotho participates in the regulation of the excitatory amino acid transporters EAAT3 and EAAT4, cRNA encoding EAAT3 or EAAT4 was injected into Xenopus oocytes either without or with additional injection of cRNA encoding Klotho. Moreover, EAAT3 or EAAT4-expressing oocytes were treated with recombinant human β-Klotho protein. To elucidate glutamate transport, glutamate-induced current was determined utilizing the two electrode voltage clamp and EAAT3 and EAAT4 protein abundance by confocal microscopy and chemiluminescence.
Methods
Animal Experiments
Xenopus Oocytes were explanted from adult Xenopus Laevis (NASCO, Fort Atkinson, USA). Xenopus Laevis frogs were anaesthesized by a 0.1% Tricain solution. After confirmation of anaesthesia and disinfection of the skin, a small abdominal incision was made and oocytes were removed, followed by closure of the skin with sutures. All animal experiments were conducted according to the German law for the welfare of animals and the surgical procedures on the adult Xenous laevis were reviewed and approved by the respective government authority of the state Baden-Württemberg (Regierungspräsidium) prior to the start of the study (Anzeige für Organentnahme nach §6).
Constructs
For generation of cRNA constructs were used encoding Klotho [14], EAAT3 [52], [53] and EAAT4 [54]. The constructs were used for the generation of cRNA as described previously [55].
Voltage Clamp in Xenopus Oocytes
Xenopus oocytes were prepared as previously described [56]. cRNA encoding EAAT3 or EAAT4 (10 ng) with or without additional 7 ng of cRNA encoding Klotho was injected on the first day after preparation of the Xenopus oocytes [57]. All experiments were performed at room temperature (about 22°C) 3 days after the injection. Two electrode voltage clamp recordings were performed at a holding potential of -60 mV [58]. Pipettes were filled with 3 M KCl and had resistances of 0.3–3.0 MΩ. The data were filtered at 10 Hz and recorded with a GeneClamp 500 amplifier, a DigiData 1300 A/D-D/A converter and the pClamp 9.2 software packages for data acquisition and analysis (Axon Instruments, Foster City, CA, USA) [55]. The oocytes were maintained at 17°C in ND96 solution containing 88.5 mM NaCl, 2 mM KCl, 1 mM MgC12, 1.8 mM CaC12, 5 mM HEPES, 0.11 mM tretracycline (Sigma, Schnelldorf, Germany), 4 µM ciprofloxacin (Sigma, Schnelldorf, Germany), 0.2 mM gentamycin (Refobacin©), 0.5 mM theophylline (Euphylong©) and 5 mM sodium pyruvate (Sigma, Schnelldorf, Germany), pH was adjusted to 7.5 by addition of NaOH [59]. The control superfusate ND96 contained 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2 and 5 mM HEPES, pH 7.4. The flow rate of the superfusion was 20 ml/min, and a complete exchange of the bath solution was reached within about 10 s. L-glutamate was added to the solutions at a concentration of 2 mM unless otherwise stated. Where indicated, recombinant human β-Klotho protein (10, 30 or 50 ng/ ml, R&D Systems) and D-saccharic acid 1,4-lactone monohydrate (DSAL, 10µ M, Sigma, Schnelldorf, Germany) were added.
Detection of EAAT Cell Surface Expression by Chemiluminescence
Oocytes were incubated with primary mouse monoclonal anti-EAAC1/EAAT3 antibody (diluted 1∶200, Invitrogen, USA) or with monoclonal anti-HA antibody conjugated to Horseradish Peroxidase (diluted 1∶500, Miltenyi Biotec, Germany) in order to determine HA-tagged EAAT4. Next, oocytes were incubated with secondary, HRP-conjugated sheep anti-mouse IgG antibody (for EAAT3; diluted 1∶1000, GE Healthcare, München, Germany). The individual oocytes were placed in 96 well plates with 20 µl of SuperSignal ELISA Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL, USA) and chemiluminescence of single oocytes was quantified in a luminometer (Walter Wallac 2 plate reader, Perkin Elmer, Juegesheim, Germany) by integrating the signal over a period of 1 s. The results display normalized relative light units [60].
Immunocytochemistry
The oocytes were fixed in 4% paraformaldehyde for at least 4 h at room temperature. After washing with PBS, the oocytes were cryoprotected in 30% sucrose, frozen in mounting medium and placed on a cryostat. Sections were collected at a thickness of 8 µm on coated slides and stored at −20°C. For immunostaining, the slides were dried at room temperature, fixed in aceton/methanol (1∶1), washed in PBS and blocked for 1h in 5% bovine serum albumin in PBS. The primary antibodies used were goat anti-EAAT3 antibody (for detection of EAAT3, diluted 1∶2500, Millipore Corporation, USA) or rat anti-HA antibody (for detection of EAAT4, diluted 1∶100, clone 3 F10, Roche, Switzerland). Incubation was performed in a moist chamber overnight at 4°C. In the case of EAAT3, binding of primary antibodies was visualised with a swine anti-goat conjugated Alexa488 antibody (diluted 1∶1000, Invitrogen, Molecular Probes, Eugene, OR, USA). For detection of EAAT4, a goat anti-rat conjugated Alexa488 antibody (diluted 1∶200, Invitrogen, Carlsbad, California, USA) was used. The oocytes were analyzed by a fluorescence laser scanning microscope (LSM 510, Carl Zeiss MicroImaging GmbH, Germany) with A-Plan 40×/0.25. Brightness and contrast settings were kept constant during imaging of all oocytes in each injection series [61].
Statistical Analysis
Data are provided as means ± SEM, n represents the number of oocytes investigated. To avoid any bias from differences between oocyte batches, statistical comparisons were always made within batches of oocytes. Data were tested for significance using analysis of variance (ANOVA) or student’s unpaired t-test, as appropriate. Results with p<0.05 were considered statistically significant.
Results
The present study explored whether Klotho influences the excitatory amino acid transporters EAAT3 and EAAT4. To this end, cRNA encoding EAAT3 or EAAT4 was injected into Xenopus oocytes with or without additional injection of cRNA encoding Klotho and the glutamate-induced current was taken as a measure of the electrogenic glutamate transport.
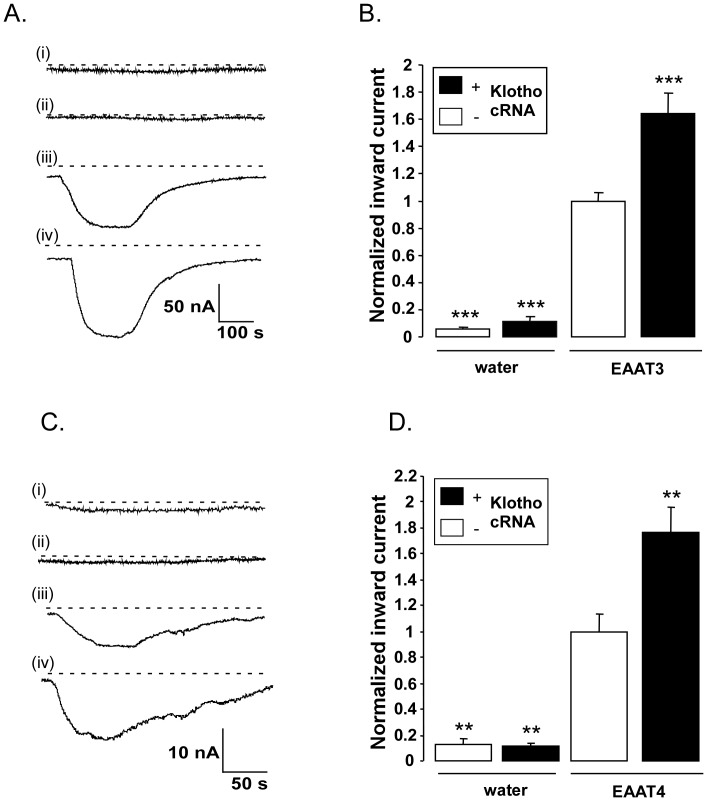
As illustrated in Fig. 1, negligible glutamate-induced current was observed in water-injected Xenopus oocytes or in oocytes injected with cRNA encoding Klotho alone. In contrast, the injection of cRNA encoding EAAT3 (Fig. 1 A,B) or EAAT4 (Fig. 1 C,D) was followed by the appearance of a marked inward current in the presence of glutamate. Additional injection of cRNA encoding Klotho led to a significant increase of the glutamate-induced current through EAAT3 (Fig. 1 A,B) and EAAT4 (Fig. 1 C,D).
Figure 1. Effect of Klotho coexpression on electrogenic glutamate transport in EAAT3 or EAAT4 expressing Xenopus oocytes.
A: Representative original tracings of glutamate (2 mM)-induced current (Iglu) at −60 mV in Xenopus oocytes injected with water (i), or with cRNA encoding Klotho alone (ii), EAAT3 alone (iii) or both, EAAT3 and Klotho (iv). B: Means ± SEM (n = 7–36) of glutamate (2 mM)-induced current (Iglu) in Xenopus oocytes injected without (left bars) or with (right bars) cRNA encoding EAAT3 and injected without (white bars) or with (black bars) cRNA encoding Klotho.***(p<0.001) indicates statistically significant difference from Xenopus oocytes injected with cRNA encoding EAAT3 alone (ANOVA). C: Representative original tracings of glutamate (2 mM)-induced current (Iglu) measured at a holding potential of −60 mV in Xenopus oocytes injected with water (i), or with cRNA encoding Klotho alone (ii), EAAT4 alone (iii) or both EAAT4 and Klotho (iv). D: Means ± SEM (n = 5–8) of glutamate (2 mM)-induced current (Iglu) in Xenopus oocytes injected without (left bars) or with (right bars) cRNA encoding EAAT4, and injected without (white bars) or with (black bars) cRNA encoding Klotho.**(p<0.01) indicate statistically significant difference from Xenopus oocytes injected with cRNA encoding EAAT3 or EAAT4 alone (ANOVA).
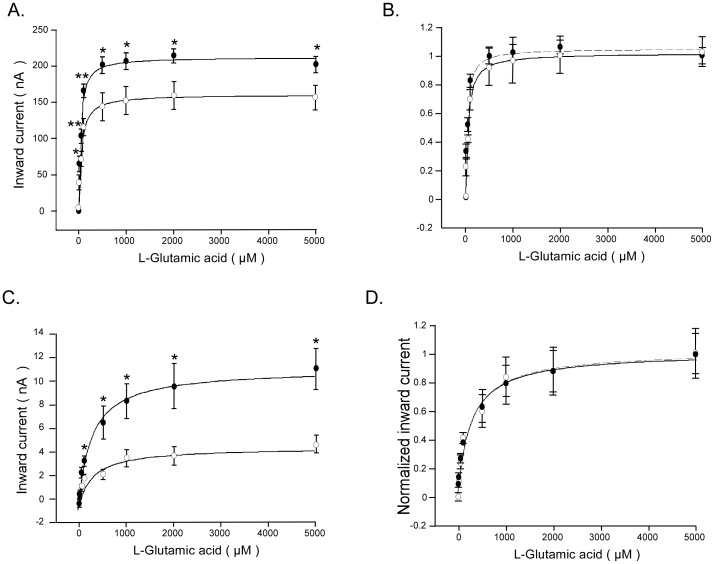
Kinetic analysis of glutamate induced currents was performed to elucidate whether Klotho coexpression modifies the affinity of the carriers. As illustrated in Fig. 2, the glutamate-induced current increased as a function of the substrate concentration. The maximal glutamate-induced current was significantly (p<0.05) higher in Xenopus oocytes injected with cRNA encoding both EAAT3 and Klotho (212.8±7.1 nA, n = 9) than in Xenopus oocytes injected with cRNA encoding EAAT3 alone (161.3±4.3 nA, n = 9) (Fig. 2 A). Similarly, the maximal glutamate induced current was significantly (p<0.05) higher in Xenopus oocytes injected with cRNA encoding both EAAT4 and Klotho (11.2±0.4 nA, n = 6) than in Xenopus oocytes injected with cRNA encoding EAAT4 alone (5.0±0.3 nA, n = 6) (Fig. 2C). The glutamate concentration required for half maximal glutamate-induced current was not significantly different (p = 0.1815) between Xenopus oocytes injected with cRNA encoding both EAAT3 and Klotho (34.5±6.9 µM, n = 9) and in Xenopus oocytes injected with cRNA encoding EAAT3 alone (48.7±7.4 µM, n = 9). Similarly, the glutamate concentration required for halfmaximal glutamate induced current was not significantly (p = 0.9236) different between Xenopus oocytes injected with cRNA encoding both EAAT4 and Klotho cRNA (274.3±48 µM, n = 6) and in Xenopus oocytes injected with cRNA encoding EAAT4 alone (283.3±78.2 µM, n = 6). It should be pointed out, however, that the scatter of the data precludes safe conclusions regarding effects of klotho on affinity of the glutamate carriers.
Figure 2. Glutamate-induced currents as a function of glutamate concentration in EAAT3/EAAT4-expressing Xenopus oocytes wihout or with Klotho coexpression.
A, C: Means ± SEM of glutamate-induced current (Iglu) as a function of glutamate concentration in Xenopus oocytes injected with cRNA encoding EAAT3 (A, n = 9) or EAAT4 (C, n = 6) without (open circles) or with (closed circles) additional coexpression of Klotho.*,**(p<0.05, p<0.01) indicate statistically significant difference from Xenopus oocytes injected with cRNA encoding EAAT3 (A) or EAAT4 (C) alone (two-tailed unpaired t-test). B, D: Means ± SEM of glutamate induced current (Iglu) normalized to Iglu at 5 mM glutamate as a function of glutamate concentration in Xenopus oocytes injected with cRNA encoding EAAT3 (B, n = 9) or EAAT4 (D, n = 6) without (open circles) and with (closed circles) additional coexpression of Klotho. The values were fitted to a hyperbola function.
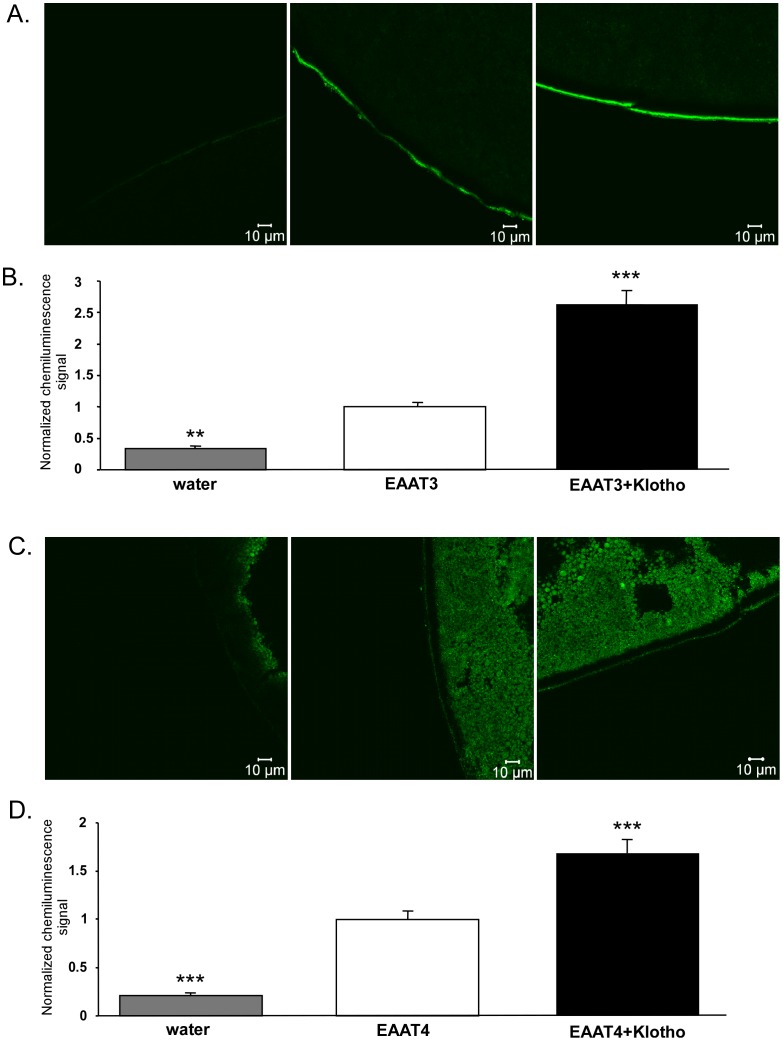
The increased maximal transport rate upon Klotho coexpression could have been due to an increase of EAAT3/EAAT4 protein abundance in the cell membrane. Confocal microscopy and chemiluminescence were thus employed in order to determine the EAAT3/EAAT4 protein abundance in the cell membrane of Xenopus oocytes. As illustrated in Fig. 3, injection of cRNA encoding Klotho significantly enhanced the EAAT3 (Fig. 3 A,B) and EAAT4 (Fig. 3 C,D) protein abundance in the cell membrane of oocytes injected with cRNA encoding EAAT3 or EAAT4.
Figure 3. Effect of Klotho coexpression on protein abundance of both EAAT3 and EAAT4 in the Xenopus oocyte cell membrane.
A, C: Confocal images of EAAT3 (A) and EAAT4 (C) protein abundance in the plasma membrane of Xenopus oocytes injected with water (1st panel), injected with cRNA encoding EAAT3 (A) or EAAT4 (C) without (2nd panel) or with additional coexpression of Klotho (3rd panel). B, D: Means ± SEM of EAAT3 (B, n = 75–80) and EAAT4 (D, n = 82–87) protein abundance as determined by chemiluminescence in the plasma membrane of Xenopus oocytes injected with cRNA encoding EAAT3 (B) or EAAT4 (D) without (white bars) or with (black bars) coexpression of Klotho. For comparison, water injected oocytes (grey bars).**,***(p<0.01, p<0.001) indicate statistically significant difference from Xenopus oocytes injected with cRNA encoding EAAT3/EAAT4 alone (ANOVA).
Further experiments explored whether the effect of Klotho coexpression was mimicked by the pretreatment of EAAT3-expressing Xenopus oocytes with recombinant human β-Klotho protein. As shown in Fig. 4A, pretreatment of Xenopus oocytes injected with cRNA encoding EAAT3 with recombinant human β-Klotho protein (10, 30 and 50 ng/ ml) for 24 hours was followed by a gradual increase in the glutamate-induced inward current, an effect reaching statistical significance at the concentration of 30 ng/ ml. The effect of recombinant human β-Klotho protein (30 ng/ml) on the glutamate induced current of oocytes injected with cRNA encoding EAAT3 was time-dependent and reached statistical significance after 24 hours of treatment (Fig. 4B). Accordingly, in the next series of experiments β-Klotho protein was used at a concentration of 30 ng/ ml and an incubation time of 24 hours.
Figure 4. Effect of recombinant human β-Klotho protein on electrogenic glutamate transport in EAAT3 -expressing Xenopus oocytes.
A: Means ± SEM (n = 7–16) of glutamate (2 mM)-induced current (Iglu) in Xenopus oocytes injected with water (grey bar) or injected with cRNA encoding EAAT3 and pretreated prior to measurements for 24 hours without (white bar) or with 10, 30 and 50 ng/ml recombinant human β-Klotho protein (1st, 2nd and 3rd black bar respectively).*,**(p<0.05, p<0.01) indicate statistically significant difference from untreated Xenopus oocytes (ANOVA). B: Means ± SEM (n = 12–17) of glutamate (2 mM)-induced current (Iglu) in Xenopus oocytes injected with water (grey bar) or injected with cRNA encoding EAAT3 pretreated prior to measurements with 30 ng/ml recombinant human β-Klotho protein for 0 hr (white bar) or 1, 6, 12 or 24hr (black bars respectively).**(p<0.01) indicates statistically significant difference from untreated Xenopus oocytes (ANOVA).
An additional series of experiments explored whether the effect of Klotho was related to its β-glucuronidase activity. To this end, Xenopus oocytes, which were injected with cRNA encoding both, EAAT3 and Klotho (Fig. 5A) or both, EAAT4 and Klotho (Fig. 5C), were treated with the β-glucuronidase inhibitor DSAL (10 µM) for 24 hours prior the measurement. As illustrated in Fig. 5A, C, pretreatment of Xenopus oocytes with DSAL (10µM) abrogated the effect of Klotho encoding cRNA injection on glutamate-induced inward current of oocytes injected with cRNA encoding EAAT3 (Fig. 5A) and EAAT4 (Fig. 5C). Similarly, parallel pretreatment with β-glucuronidase inhibitor DSAL (10 µM) for 24 hours abrogated the effect of recombinant human β-Klotho protein (30 ng/ ml) on glutamate induced inward current of oocytes injected with cRNA encoding EAAT3 (Fig. 5B) and EAAT4 (Fig. 5D).
Figure 5. Reversal of the effect of Klotho on electrogenic glutamate transport in EAAT3 or EAAT4 expressing Xenopus oocytes by β-glucuronidase inhibitor DSAL.
A: Means ± SEM (n = 9–21) of glutamate (2 mM)-induced current (Iglu) in Xenopus oocytes injected with water (grey bar) or injected with cRNA encoding EAAT3 alone (white bar) or both EAAT3 and Klotho (black bars). Where indicated, the oocytes were treated with β-glucuronidase inhibitor DSAL (10 µM).**(p<0.01) indicates statistically significant difference from oocytes injected with cRNA encoding EAAT3 alone (ANOVA). ### (p<0.001) indicates statistically significant difference from oocytes injected with cRNA encoding both EAAT3 and Klotho (ANOVA).B: Means ± SEM (n = 11–23) of glutamate (2 mM)-induced current (Iglu) in Xenopus oocytes injected with water (grey bar) or injected with cRNA encoding EAAT3 alone (white bar) or pretreated for 24 hours with 30ng/ ml recombinant human β-Klotho protein without (first black bar) or with the presence of β-glucuronidase inhibitor DSAL (10µM ) (second black bar).***(p<0.001) indicates statistically significant difference from non-treated oocytes injected with cRNA encoding EAAT3 alone (ANOVA). ##(p<0.01). indicates statistically significant difference from oocytes injected with cRNA encoding EAAT3 and treated for 24 hours with 30ng/ ml recombinant human β-Klotho protein (ANOVA).C: Means ± SEM (n = 5–23) of glutamate (2 mM)-induced current (Iglu) in Xenopus oocytes injected with water (grey bar) or injected with cRNA encoding EAAT4 alone (white bar) or both EAAT4 and Klotho (black bars). Where indicated, the oocytes were treated with β-glucuronidase inhibitor DSAL (10 µM).***(p<0.001) indicates statistically significant difference from oocytes injected with cRNA encoding EAAT4 alone (ANOVA). ###(p<0.001) indicates statistically significant difference from oocytes injected with cRNA encoding both EAAT4 and Klotho (ANOVA).D: Means ± SEM (n = 9–21) of glutamate (2 mM)-induced current (Iglu) in Xenopus oocytes injected with water (grey bar) or injected with cRNA encoding EAAT4 alone (white bar) and pretreated for 24 hours with 30 ng/ ml recombinant human β-Klotho protein without (first black bar) or with the presence of β-glucuronidase inhibitor DSAL (10µM, second black bar).***(p<0.001) indicates statistically significant difference from non-treated oocytes injected with cRNA encoding EAAT4 alone (ANOVA). ##(p<0.01). indicates statistically significant difference from oocytes injected with cRNA encoding EAAT4 and treated for 24 hours with 30 ng/ml recombinant human Klotho protein (ANOVA).
Discussion
The present observations uncover a completely novel function of Klotho, i.e. the up-regulation of the excitatory amino acid transporters EAAT3 and EAAT4. Klotho increased the carrier protein abundance in the cell membrane and thus enhanced the maximal transport rate of the carriers. The effect apparently required the hydrolysis of β-D-glucuronic acid by Klotho, as it was reversed by the β-glucuronidase inhibitor. The effect of klotho on EAAT3 and EAAT4 contrasts the effect of klotho on Na+ coupled phosphate transporter NaPiIIa and NaPiIIb, which are both donwregulated by klotho [14].
Klotho further up-regulates the Na+/K+ATPase [15], [62], which is required to maintain the chemical gradient for Na+ coupled transport [63]. Thus, Klotho modifies excitatory anino acid transport not only by up-regulating the carrier protein, but at least in theory by additional maintaining the electrochemical gradient for Na+.
The effect of Klotho on EAAT3 may contribute to the regulation of renal tubular amino acid transport. In the kidneys, the excitatory amino acid transporter EAAT3 accomplishes dicarboxylic amino acid reabsorption in renal proximal tubules [19] and defective EAAT3 leads to dicarboxylic aminoaciduria [18]. Whether Klotho deficient mice suffer from amino aciduria is – to the best of our knowledge – not known. Dicarboxylic amino aciduria is expected only, if the lack of Klotho decreases the maximal transport rate of EAAT3 below the filtered load.
In the brain, EAAT3 contributes to excitatory amino acid transport at the blood-brain barrier [20], and to the clearance of excitatory amino acids from synaptic clefts by cellular uptake into neurons [21]–[28], retinal ganglion cells [29] and glial cells [30]–[33]. EAAT4 accomplishes excitatory amino acid transport into cerebellar Purkinje cells [23], [25], [34]. Decreased cerebral or cerebellar excitatory amino acid uptake in the brain is expected to cause excitotoxicity [35], [64]. Acccordingly, impaired function of EAAT3 may lead to epilepsy [42]–[46] and hepatic encephalopathy [65]. Moreover, deranged cellular excitatory amino acid uptake by EAAT3 [28], [36]–[41] or EAAT4 [36], [39] may foster the development of schizophrenia. Evidence for a role of glutamatergic neurotransmission in the pathophysiology of psychiatric disorders comes from studies using magnetic resonance spectroscopy, a technique that non-invasively measures in vivo concentrations of glutamate and other amino acids under different experimental conditions [66]. Morover, recent clinical studies have demonstrated that a single subpsychotomimetic dose of ketamine, an ionotropic glutamatergic N-methyl-D-aspartate (NMDA) receptor antagonist, produces a rapid antidepressant response in patients with major depressive disorder, with effects lasting up to 2 weeks [67]. Along those lines, altered EAAT expression has been found in schizophrenic and bipolar patients in frontal and temporal brain regions [40], [68]–[70]. Furthermore, administration of riluzole, a drug that enhances glutamate uptake through EAATs, reverses stress-induced motivational deficits and restores prefrontal BDNF expression after corticosterone [71]. Because riluzole has antidepressant effects in both, animal models and human subjects, it may represent the prototype for a novel class of antidepressants with the modulation of glial physiology as a primary mechanism of action [72].
Klotho deficiency has been shown to foster the degeneration of mesencephalic dopaminergic neurons leading to decreased levels of striatal dopamine [73]. The effect was, however, reversed by vitamin D restriction [73] and is thus presumably not the result from direct regulation of excitatory amino acid transport. Lack of Klotho expression further leads to cognitive deficits [65]. Klotho induces maturation of rat primary oligodendrocytic progenitor cells, an effect attributed in part to stimulation of Akt and ERK [47]. Klotho deficiency leads to a decrease of major myelin protein expression due to a decreased number of total and mature oligodendrocytes [65]. Klotho is downregulated in the aged brain, which is paralleled by decrease of white matter and myelin abnormalities [38], [74]. Whether or not oligodendrocyte maturation and survival is modified by the abundance of extracellular excitatory amino acids and thus by EAAT3 and EAAT4 activities, remains to be shown. Klotho abundance is downregulated by TNFα and thus, deranged expression of Klotho may participate in the pathophysiology of neuroinflammation [75]. It is tempting to speculate that Klotho sensitivity of EAAT3 and EAAT4 contributes to neurodegeneration during neuroinflammation. Clearly, additional studies will be required, however, to define the in vivo relevance of Klotho-sensitive excitatory amino acid transport.
In conclusion, the anti-aging protein Klotho up-regulates the excitatory amino acid transporters EAAT3 and EAAT4, an effect which may participate in the regulation of renal tubular transport of dicarboxylic amino acids and the clearance of excitatory amino acids from synaptic clefts in the brain. Mechanisms regulating glutamate cycling and metabolism including Klotho may be viable drug targets for depression and schizophrenia.
Acknowledgments
The authors acknowledge the technical assistance of E. Faber. The manuscript was meticulously prepared by Ali Soleimanpour.
Funding Statement
This study was supported by the Deutsche Forschungsgemeinschaft (GK 1302, FO 695/1-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Takeshita K, Fujimori T, Kurotaki Y, Honjo H, Tsujikawa H, et al. (2004) Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation 109: 1776–1782. [DOI] [PubMed] [Google Scholar]
- 2. Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y (2003) Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol 17: 2393–2403. [DOI] [PubMed] [Google Scholar]
- 3. Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, et al. (2004) Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 565: 143–147. [DOI] [PubMed] [Google Scholar]
- 4. Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, et al. (2010) Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24: 3438–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, et al. (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51. [DOI] [PubMed] [Google Scholar]
- 6. Kuro-o M (2010) Klotho. Pflugers Arch 459: 333–343. [DOI] [PubMed] [Google Scholar]
- 7. Razzaque MS, Sitara D, Taguchi T, St Arnaud R, Lanske B (2006) Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J 20: 720–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoshida T, Fujimori T, Nabeshima Y (2002) Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocrinology 143: 683–689. [DOI] [PubMed] [Google Scholar]
- 9. Ramasamy I (2006) Recent advances in physiological calcium homeostasis. ClinChemLab Med 44: 237–273. [DOI] [PubMed] [Google Scholar]
- 10. Segawa H, Yamanaka S, Ohno Y, Onitsuka A, Shiozawa K, et al. (2007) Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Renal Physiol 292: F769–F779. [DOI] [PubMed] [Google Scholar]
- 11. Kuro-o M (2006) Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. CurrOpinNephrolHypertens 15: 437–441. [DOI] [PubMed] [Google Scholar]
- 12. Ohnishi M, Nakatani T, Lanske B, Razzaque MS (2009) Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int 75: 1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voelkl J, Alesutan I, Leibrock CB, Quintanilla-Martinez L, Kuhn V, et al.. (2013) Spironolactone ameliorates PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice. J Clin Invest. [DOI] [PMC free article] [PubMed]
- 14. Dermaku-Sopjani M, Sopjani M, Saxena A, Shojaiefard M, Bogatikov E, et al. (2011) Downregulation of NaPi-IIa and NaPi-IIb Na-coupled phosphate transporters by coexpression of Klotho. Cell Physiol Biochem 28: 251–258. [DOI] [PubMed] [Google Scholar]
- 15. Sopjani M, Alesutan I, Dermaku-Sopjani M, Gu S, Zelenak C, et al. (2011) Regulation of the Na+/K+ ATPase by Klotho. FEBS Lett 585: 1759–1764. [DOI] [PubMed] [Google Scholar]
- 16. Boros S, Bindels RJ, Hoenderop JG (2009) Active Ca(2+) reabsorption in the connecting tubule. Pflugers Arch 458: 99–109. [DOI] [PubMed] [Google Scholar]
- 17. Cha SK, Hu MC, Kurosu H, M K-o, Moe O, et al. (2009) Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol 76: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bailey CG, Ryan RM, Thoeng AD, Ng C, King K, et al. (2011) Loss-of-function mutations in the glutamate transporter SLC1A1 cause human dicarboxylic aminoaciduria. J Clin Invest 121: 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peghini P, Janzen J, Stoffel W (1997) Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J 16: 3822–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Kane RL, Martinez-Lopez I, DeJoseph MR, Vina JR, Hawkins RA (1999) Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier. A mechanism for glutamate removal. J BiolChem 274: 31891–31895. [DOI] [PubMed] [Google Scholar]
- 21. Amara SG, Fontana AC (2002) Excitatory amino acid transporters: keeping up with glutamate. NeurochemInt 41: 313–318. [DOI] [PubMed] [Google Scholar]
- 22. Collin M, Backberg M, Ovesjo ML, Fisone G, Edwards RH, et al. (2003) Plasma membrane and vesicular glutamate transporter mRNAs/proteins in hypothalamic neurons that regulate body weight. EurJ Neurosci 18: 1265–1278. [DOI] [PubMed] [Google Scholar]
- 23. Furuta A, Rothstein JD, Martin LJ (1997) Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci 17: 8363–8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Furuta A, Takashima S, Yokoo H, Rothstein JD, Wada K, et al. (2005) Expression of glutamate transporter subtypes during normal human corticogenesis and type II lissencephaly. Brain ResDevBrain Res 155: 155–164. [DOI] [PubMed] [Google Scholar]
- 25. Huang YH, Dykes-Hoberg M, Tanaka K, Rothstein JD, Bergles DE (2004) Climbing fiber activation of EAAT4 transporters and kainate receptors in cerebellar Purkinje cells. J Neurosci 24: 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nieoullon A, Canolle B, Masmejean F, Guillet B, Pisano P, et al. (2006) The neuronal excitatory amino acid transporter EAAC1/EAAT3: does it represent a major actor at the brain excitatory synapse? J Neurochem 98: 1007–1018. [DOI] [PubMed] [Google Scholar]
- 27. Shashidharan P, Huntley GW, Murray JM, Buku A, Moran T, et al. (1997) Immunohistochemical localization of the neuron-specific glutamate transporter EAAC1 (EAAT3) in rat brain and spinal cord revealed by a novel monoclonal antibody. Brain Res 773: 139–148. [DOI] [PubMed] [Google Scholar]
- 28. Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH (2001) Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. AmJ Psychiatry 158: 1393–1399. [DOI] [PubMed] [Google Scholar]
- 29. Schniepp R, Kohler K, Ladewig T, Guenther E, Henke G, et al. (2004) Retinal colocalization and in vitro interaction of the glutamate transporter EAAT3 and the serum- and glucocorticoid-inducible kinase SGK1 [correction]. Invest OphthalmolVisSci 45: 1442–1449. [DOI] [PubMed] [Google Scholar]
- 30. Maragakis NJ, Dietrich J, Wong V, Xue H, Mayer-Proschel M, et al. (2004) Glutamate transporter expression and function in human glial progenitors. Glia 45: 133–143. [DOI] [PubMed] [Google Scholar]
- 31. Miralles VJ, Martinez-Lopez I, Zaragoza R, Borras E, Garcia C, et al. (2001) Na+ dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) in primary astrocyte cultures: effect of oxidative stress. Brain Res 922: 21–29. [DOI] [PubMed] [Google Scholar]
- 32. Schmitt A, Zink M, Petroianu G, May B, Braus DF, et al. (2003) Decreased gene expression of glial and neuronal glutamate transporters after chronic antipsychotic treatment in rat brain. NeurosciLett 347: 81–84. [DOI] [PubMed] [Google Scholar]
- 33. van Landeghem FK, Weiss T, von Deimling A (2007) Expression of PACAP and glutamate transporter proteins in satellite oligodendrocytes of the human CNS. RegulPept 142: 52–59. [DOI] [PubMed] [Google Scholar]
- 34. Huang YH, Sinha SR, Tanaka K, Rothstein JD, Bergles DE (2004) Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons. J Neurosci 24: 4551–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grewer C, Gameiro A, Zhang Z, Tao Z, Braams S, et al. (2008) Glutamate forward and reverse transport: from molecular mechanism to transporter-mediated release after ischemia. IUBMBLife 60: 609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deng X, Shibata H, Takeuchi N, Rachi S, Sakai M, et al. (2007) Association study of polymorphisms in the glutamate transporter genes SLC1A1, SLC1A3, and SLC1A6 with schizophrenia. AmJ MedGenetB NeuropsychiatrGenet 144B: 271–278. [DOI] [PubMed] [Google Scholar]
- 37. Huerta I, McCullumsmith RE, Haroutunian V, Gimenez-Amaya JM, Meador-Woodruff JH (2006) Expression of excitatory amino acid transporter interacting protein transcripts in the thalamus in schizophrenia. Synapse 59: 394–402. [DOI] [PubMed] [Google Scholar]
- 38. Kim JH, Do SH, Kim YL, Zuo Z (2005) Effects of chronic exposure to ethanol on glutamate transporter EAAT3 expressed in Xenopus oocytes: evidence for protein kinase C involvement. Alcohol Clin ExpRes 29: 2046–2052. [DOI] [PubMed] [Google Scholar]
- 39. Lang UE, Puls I, Muller DJ, Strutz-Seebohm N, Gallinat J (2007) Molecular mechanisms of schizophrenia. Cell Physiol Biochem 20: 687–702. [DOI] [PubMed] [Google Scholar]
- 40. McCullumsmith RE, Meador-Woodruff JH (2002) Striatal excitatory amino acid transporter transcript expression in schizophrenia, bipolar disorder, and major depressive disorder. Neuropsychopharmacology 26: 368–375. [DOI] [PubMed] [Google Scholar]
- 41.Nudmamud-Thanoi S, Piyabhan P, Harte MK, Cahir M, Reynolds GP (2007) Deficits of neuronal glutamatergic markers in the caudate nucleus in schizophrenia. J Neural TransmSuppl: 281–285. [DOI] [PubMed]
- 42. Crino PB, Jin H, Shumate MD, Robinson MB, Coulter DA, et al. (2002) Increased expression of the neuronal glutamate transporter (EAAT3/EAAC1) in hippocampal and neocortical epilepsy. Epilepsia 43: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mathern GW, Mendoza D, Lozada A, Pretorius JK, Dehnes Y, et al. (1999) Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology 52: 453–472. [DOI] [PubMed] [Google Scholar]
- 44. Proper EA, Hoogland G, Kappen SM, Jansen GH, Rensen MG, et al. (2002) Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain 125: 32–43. [DOI] [PubMed] [Google Scholar]
- 45. Rakhade SN, Loeb JA (2008) Focal reduction of neuronal glutamate transporters in human neocortical epilepsy. Epilepsia 49: 226–236. [DOI] [PubMed] [Google Scholar]
- 46. Simantov R, Crispino M, Hoe W, Broutman G, Tocco G, et al. (1999) Changes in expression of neuronal and glial glutamate transporters in rat hippocampus following kainate-induced seizure activity. Brain ResMolBrain Res 65: 112–123. [DOI] [PubMed] [Google Scholar]
- 47. Chan H, Zwingmann C, Pannunzio M, Butterworth RF (2003) Effects of ammonia on high affinity glutamate uptake and glutamate transporter EAAT3 expression in cultured rat cerebellar granule cells. NeurochemInt 43: 137–146. [DOI] [PubMed] [Google Scholar]
- 48. Alesutan IS, Ureche ON, Laufer J, Klaus F, Zurn A, et al. (2010) Regulation of the glutamate transporter EAAT4 by PIKfyve. Cell Physiol Biochem 25: 187–194. [DOI] [PubMed] [Google Scholar]
- 49. Klaus F, Laufer J, Czarkowski K, Strutz-Seebohm N, Seebohm G, et al. (2009) PIKfyve-dependent regulation of the Cl- channel ClC-2. Biochem BiophysResCommun 381: 407–411. [DOI] [PubMed] [Google Scholar]
- 50. Rajamanickam J, Palmada M, Lang F, Boehmer C (2007) EAAT4 phosphorylation at the SGK1 consensus site is required for transport modulation by the kinase. J Neurochem 102: 858–866. [DOI] [PubMed] [Google Scholar]
- 51. Wang Y, Chen L, Huang G, He D, He J, et al. (2013) Klotho sensitizes human lung cancer cell line to cisplatin via PI3k/Akt pathway. PLoS One 8: e57391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dowd LA, Robinson MB (1996) Rapid stimulation of EAAC1-mediated Na+-dependent L-glutamate transport activity in C6 glioma cells by phorbol ester. J Neurochem 67: 508–516. [DOI] [PubMed] [Google Scholar]
- 53. Dowd LA, Coyle AJ, Rothstein JD, Pritchett DB, Robinson MB (1996) Comparison of Na+-dependent glutamate transport activity in synaptosomes, C6 glioma, and Xenopus oocytes expressing excitatory amino acid carrier 1 (EAAC1). MolPharmacol 49: 465–473. [PubMed] [Google Scholar]
- 54. Bohmer C, Philippin M, Rajamanickam J, Mack A, Broer S, et al. (2004) Stimulation of the EAAT4 glutamate transporter by SGK protein kinase isoforms and PKB. Biochem BiophysResCommun 324: 1242–1248. [DOI] [PubMed] [Google Scholar]
- 55. Strutz-Seebohm N, Pusch M, Wolf S, Stoll R, Tapken D, et al. (2011) Structural basis of slow activation gating in the cardiac I Ks channel complex. Cell Physiol Biochem 27: 443–452. [DOI] [PubMed] [Google Scholar]
- 56. Bogatikov E, Munoz C, Pakladok T, Alesutan I, Shojaiefard M, et al. (2012) Up-regulation of amino acid transporter SLC6A19 activity and surface protein abundance by PKB/Akt and PIKfyve. Cell Physiol Biochem 30: 1538–1546. [DOI] [PubMed] [Google Scholar]
- 57. Alesutan I, Sopjani M, Dermaku-Sopjani M, Munoz C, Voelkl J, et al. (2012) Upregulation of Na-coupled glucose transporter SGLT1 by Tau tubulin kinase 2. Cell Physiol Biochem 30: 458–465. [DOI] [PubMed] [Google Scholar]
- 58. Pathare G, Foller M, Daryadel A, Mutig K, Bogatikov E, et al. (2012) OSR1-Sensitive Renal Tubular Phosphate Reabsorption. Kidney Blood Press Res 36: 149–161. [DOI] [PubMed] [Google Scholar]
- 59. Henrion U, Zumhagen S, Steinke K, Strutz-Seebohm N, Stallmeyer B, et al. (2012) Overlapping cardiac phenotype associated with a familial mutation in the voltage sensor of the KCNQ1 channel. Cell Physiol Biochem 29: 809–818. [DOI] [PubMed] [Google Scholar]
- 60. Hosseinzadeh Z, Bhavsar SK, Lang F (2012) Downregulation of ClC-2 by JAK2. Cell Physiol Biochem 29: 737–742. [DOI] [PubMed] [Google Scholar]
- 61. Mia S, Munoz C, Pakladok T, Siraskar G, Voelkl J, et al. (2012) Downregulation of Kv1.5 K Channels by the AMP-Activated Protein Kinase. Cell Physiol Biochem 30: 1039–1050. [DOI] [PubMed] [Google Scholar]
- 62. Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, et al. (2007) alpha-Klotho as a regulator of calcium homeostasis. Science 316: 1615–1618. [DOI] [PubMed] [Google Scholar]
- 63. Lang F, Rehwald W (1992) Potassium channels in renal epithelial transport regulation. Physiol Rev 72: 1–32. [DOI] [PubMed] [Google Scholar]
- 64. Hertz L (2008) Bioenergetics of cerebral ischemia: a cellular perspective. Neuropharmacology 55: 289–309. [DOI] [PubMed] [Google Scholar]
- 65. Chen CD, Sloane JA, Li H, Aytan N, Giannaris EL, et al. (2013) The Antiaging Protein Klotho Enhances Oligodendrocyte Maturation and Myelination of the CNS. J Neurosci 33: 1927–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maddock RJ, Buonocore MH (2012) MR Spectroscopic Studies of the Brain in Psychiatric Disorders. CurrTopBehavNeurosci. [DOI] [PubMed]
- 67. Kavalali ET, Monteggia LM (2012) Synaptic mechanisms underlying rapid antidepressant action of ketamine. AmJ Psychiatry 169: 1150–1156. [DOI] [PubMed] [Google Scholar]
- 68. Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE (2010) Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. SchizophrRes 117: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rao JS, Kellom M, Reese EA, Rapoport SI, Kim HW (2012) Dysregulated glutamate and dopamine transporters in postmortem frontal cortex from bipolar and schizophrenic patients. J AffectDisord 136: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70. Shan D, Lucas EK, Drummond JB, Haroutunian V, Meador-Woodruff JH, et al. (2013) Abnormal expression of glutamate transporters in temporal lobe areas in elderly patients with schizophrenia. SchizophrRes 144: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gourley SL, Swanson AM, Jacobs AM, Howell JL, Mo M, et al. (2012) Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. ProcNatlAcadSciUSA 109: 20714–20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, et al. (2007) Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. BiolPsychiatry 61: 822–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kosakai A, Ito D, Nihei Y, Yamashita S, Okada Y, et al. (2011) Degeneration of mesencephalic dopaminergic neurons in klotho mouse related to vitamin D exposure. Brain Res 1382: 109–117. [DOI] [PubMed] [Google Scholar]
- 74. Abraham CR, Chen C, Cuny GD, Glicksman MA, Zeldich E (2012) Small-molecule Klotho enhancers as novel treatment of neurodegeneration. FutureMedChem 4: 1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Teocchi MA, Ferreira AE, da Luz de Oliveira EP, Tedeschi H, D’Souza-Li L (2013) Hippocampal gene expression dysregulation of Klotho, nuclear factor kappa B and tumor necrosis factor in temporal lobe epilepsy patients. J Neuroinflammation 10: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]