Abstract
Background
Apamin sensitive potassium current (I KAS), carried by the type 2 small conductance Ca2+-activated potassium (SK2) channels, plays an important role in post-shock action potential duration (APD) shortening and recurrent spontaneous ventricular fibrillation (VF) in failing ventricles.
Objective
To test the hypothesis that amiodarone inhibits I KAS in human embryonic kidney 293 (HEK-293) cells.
Methods
We used the patch-clamp technique to study I KAS in HEK-293 cells transiently expressing human SK2 before and after amiodarone administration.
Results
Amiodarone inhibited IKAS in a dose-dependent manner (IC50, 2.67±0.25 µM with 1 µM intrapipette Ca2+). Maximal inhibition was observed with 50 µM amiodarone which inhibited 85.6±3.1% of IKAS induced with 1 µM intrapipette Ca2+ (n = 3). IKAS inhibition by amiodarone was not voltage-dependent, but was Ca2+-dependent: 30 µM amiodarone inhibited 81.5±1.9% of I KAS induced with 1 µM Ca2+ (n = 4), and 16.4±4.9% with 250 nM Ca2+ (n = 5). Desethylamiodarone, a major metabolite of amiodarone, also exerts voltage-independent but Ca2+ dependent inhibition of I KAS.
Conclusion
Both amiodarone and desethylamiodarone inhibit I KAS at therapeutic concentrations. The inhibition is independent of time and voltage, but is dependent on the intracellular Ca2+ concentration. SK2 current inhibition may in part underlie amiodarone's effects in preventing electrical storm in failing ventricles.
Introduction
Heart failure is a major public health problem with 300,000 directly attributable deaths annually, in the United States alone. It has a prevalence of 5.8 million in the U.S. and over 23 million worldwide [1]. Ventricular arrhythmias are a major cause of morbidity and mortality in heart failure [2]. Today, many patients with heart failure receive an implantable cardioverter defibrillator (ICD) for primary or secondary prevention of arrhythmic death. However, ICD itself does not reduce the incidence of arrhythmias. Electrical storm (ES) defined as recurrent ventricular arrhythmias in a short period of time, remains a frequent complication and a strong independent predictor of poor outcome even in patients with ICDs [3], [4]. Amiodarone is effective in the treatment of recurrent ventricular tachycardia or fibrillation [5] and is commonly used as the first line therapy for ES [6], [7]. However, the mechanism behind amiodarone's effectiveness in treating ES remains poorly understood.
Ca2+ activated K+ channels integrate intracellular calcium handling with membrane repolarization in various tissues including brain, peripheral nerve, endothelium, leukocytes, erythrocytes, heart, skeletal and smooth muscle [8]. They are classified into three types based on their conductance pattern: large (BK), intermediate (IK) and small (SK) conductance Ca2+ activated K+ channels. SK channels show weak voltage dependence, susceptibility to the bee venom toxin apamin, and they are highly Ca2+ sensitive [9]. However, the role of these channels in the heart is poorly understood.
Xu et al. identified three isoforms of SK channels (SK1, SK2 and SK3) in the mouse and human heart, and found that they play important roles in the maintenance of action potential duration (APD) in atrial myocytes and pacemaking tissues [10]. Subsequently, the same group demonstrated that mice engineered to lack SK2 have prolonged atrial APD and higher susceptibility to atrial fibrillation [11]. Interestingly, SK2 expression is strikingly higher in normal mouse, cat and human atria than their respective ventricles [10]. This preferential expression led researchers to propose SK2 as a target for treating atrial arrhythmias without ventricular proarrhythmic risk. However, in our recent study, we demonstrated that SK2 expression is significantly upregulated in failing ventricles compared to normal ones [12]. Moreover, upregulation of SK2 channels contributes to the development of ES in heart failure [13], [14] and apamin, a specific inhibitor of SK2 channels [15], [16], can prevent the post-shock APD shortening and ES in failing rabbit ventricles [13].
These findings led us to hypothesize that amiodarone, the most effective and commonly used antiarrhythmic agent for the treatment of ES, can inhibit SK2 channels. However, amiodarone also inhibits various ion currents (e.g., I Na, I Ca,L, I Ks, I Kr, and I to) as well as β-adrenergic receptors. One possible approach to test the effects of amiodarone on SK2 currents is to first inhibit other major repolarization currents (such as I Ks, and I Kr) before administering amiodarone in cardiac cells. However, we found that chromanol 293B and E-4031 (known inhibitors of I Ks and I Kr) are also inhibitors of SK2 channels. Using these two drugs in cardiac cells may prevent us to accurately study the effects of amiodarone on SK2 currents. Therefore, to test amiodarone's effects by itself on SK2 channels, we expressed the human SK2 in human embryonic kidney 293 (HEK-293) cells by transiently transfecting its coding gene KCNN2, variant 1. We then used the patch clamp method to demonstrate that both amiodarone and desethylamiodarone, a major metabolite of amiodarone, inhibit apamin-sensitive SK2 currents (I KAS) at therapeutic concentrations (10–20 µM). In addition, we confirmed that amiodarone prevents postshock APD shortening in failing rabbit ventricles. Our findings suggest that the antiarrhythmic action of amiodarone in the treatment of ES is, in part, due to I KAS inhibition.
Materials and Methods
All experimental protocols were approved by the Institutional Committee of Animal Use and Care, and the Institutional Committee of Human and DNA Research at Indiana University.
HEK-293 cell preparation and transfection
HEK-293 cells obtained from American Type Cell Culture were grown at 37°C and 5% CO2 in Dulbecco's Modified Eagle's Medium supplemented with 10% FBS. KCNN2, the gene encoding human small conductance Ca2+-activated K+ channel, subfamily N, member 2 (SK2), transcript variant 1 (Gene Bank Accession # NM_021614.2), was obtained from OriGene (Rockville, MD) and constructed in pCMV6-XL plasmids. Naïve HEK-293 cells in 35 mm dishes were co-transfected with 4 µg of the expression construct and 0.5 µg of pEGFP-C3 plasmid using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Transfected cells were incubated at 37°C and 5% CO2 for 48 hours prior to patch-clamp experiments. Cells showing green fluorescence were chosen to study the effects of amiodarone on SK2.
Expression of SK2 and IKAS
The HEK-293 cells transfected only with pEGFP-C3 were used as negative control for the patch clamp experiments. These did not generate significant I K with 1 µM intrapipette Ca2+ (Figure S1 in Data S1). Since apamin effectively suppressed almost all K+ currents in the transfected cells, nearly all currents in this study were apamin sensitive (I KAS). Apamin concentration used in our study (100 nM) was higher than the concentration shown to exert maximal inhibition on SK2 channels in other studies [15], [17]. DMSO as a vehicle at the concentration of 0.1% had no effects on I KAS (data not shown).
Patch-clamp Experiments
Effects of amiodarone on I KAS were studied using the whole-cell patch-clamp technique. Pipette resistances were 2–4 MΩ when filled with pipette solution. After whole-cell patch was obtained in Tyrode's solution, chamber bath was changed to a solution containing N-methylglucamine. Capacitance currents were monitored with repetitive 5 mV pulses for at least five minutes to measure the cell capacitance. Whole-cell compensation was not used for I KAS measurements. All experiments were performed at 36°C. Voltage pulse protocols were generated with an Axopatch 200B amplifier using pCLAMP-10 software (Molecular Devices/Axon, Sunnyvale, CA). The data were filtered with a built-in four-pole low-pass Bessel filter at 2 kHz and digitized at 5 kHz. Extracellular solution contained (in mM): NMG, 140; KCl, 4; MgCl2, 1; glucose, 5; and HEPES, 10 (pH 7.4 with HCl). Pipette solution contained (in mM): potassium gluconate, 144; MgCl2, 1.15; EGTA, 1; HEPES, 10; MgATP, 2; and CaCl2; 0.85 (pH 7.25 with KOH). This composition yields 1 µM free Ca2+ at 36°C based on the calculation method by Bers et al. [18]. For study of Ca2+-dependency, various combinations of EGTA and CaCl2 were used in the pipette solution to achieve different intracellular [Ca2+].
Since SK2 channels are activated by intracellular Ca2+, it usually took several minutes for the intracellular environment to reach equilibrium after whole-cell configuration was established. The change in I K amplitude was monitored using repetitive ramp pulses that were applied every 10 seconds (holding membrane potential −80 mV; test potentials from +20 mV to −120 mV for 400 ms). Once I K became steady, a step-pulse protocol was also used to record baseline I K (holding membrane potential −80 mV; test potentials in 10 mV steps from −140 mV to +40 mV for 300 ms). While I K were monitored with ramp-pulses, amiodarone in various concentrations was applied to the bath solution. After maximum inhibition of I K was achieved, above step pulse protocol was applied again to record the I K under drug effect. After recordings with amiodarone were obtained, the drug was washed out. Finally, apamin (100 nM) was applied to ensure the measured I K were apamin-sensitive. I K after 100 nM apamin application were subtracted from the I K under no drug application and the current difference was defined as I KAS that is carried by SK2 channels. Inhibition of IK under various concentrations of amiodarone was compared and normalized to the inhibition of IK with 100 nM apamin (i.e., I KAS).
Drugs and Reagents
Apamin was purchased from Tocris (catalog#1652), and was dissolved in water for a 500 µM stock solution. Amiodarone-HCl was also purchased from Tocris (catalog# 4095), and was dissolved in DMSO or ethanol for a 25 mM stock solution. All other chemicals were purchased from Sigma (St. Louis, MO).
Ethics Statement
The protocol for animal experiments was approved by the Indiana University Institutional Animal Care and Use Committee.
Data Analysis
Patch-clamp data were analyzed using Clampfit (Molecular Devices/Axon, Sunnyvale, CA). Inhibition of I K with amiodarone was compared and normalized to I KAS (I K with 100 nM apamin) and was plotted as a function of amiodarone concentration. The data were fitted with the Hill equation: y = 1/[1+(IC50/x)n], where y indicates normalized I KAS, x is concentration of amiodarone, IC50 is concentration of amiodarone at half-maximal inhibition of I KAS and n is the Hill coefficient. Curve fitting was performed by the method of least squares with data points weighted by the inverse of their variance with Igor Pro 6 (WaveMetrics, Lake Oswego, OR) and Prism 5 (GraphPad Software, La Jolla, CA).
Statistical Analysis
Comparison of continuous variables between two groups was performed using Mann-Whitney-Wilcoxon test. Continuous variables among three groups were compared with Kruskal-Wallis test with post-hoc Dunn's multiple comparison test to compare differences between any two groups. All comparisons were performed using two-tailed tests and a p value less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS PASW Statistics 17 software (IBM, Chicago, IL) and Prism 5 (GraphPad Software, La Jolla, CA). Data in text and figures are presented as median [25th percentile; 75th percentile] or mean ± S.D. unless otherwise stated.
Results
Amiodarone inhibits I KAS
We first tested whether the extracellularly applied amiodarone can inhibit I KAS. I K was activated with various intrapipette Ca2+ concentrations. Figure 1 shows representative traces of 1 µM Ca2+-activated IK obtained with a step-pulse protocol in the absence ( Figure 1A ) and in the presence of 10 µM amiodarone ( Figure 1B ) and 100 nM apamin ( Figure 1C ). Amiodarone significantly decreased I K in a time-independent manner. Figure 1D illustrates the current-voltage (I-V) relationship in the absence and presence of these drugs. Inhibition by amiodarone (10 µM) was observed at all membrane potentials.
Figure 1. Inhibitory effect of amiodarone on I KAS.
(A) Representative superimposed whole-cell I K traces obtained by the pulse protocol shown in the inset. The pipette solution contained 1 µM free Ca2+ to activate I KAS. (B) Superimposed IK traces in the presence of 10 µM amiodarone. (C) Superimposed I K traces after adding 100 nM apamin to the same preparation. (D) Current-voltage (I-V) relationships obtained in the absence and presence of 10 µM amiodarone, then 100 nM apamin. I K was measured between 280 ms and 290 ms of the test pulse, and plotted against membrane potentials. Similar results with 10 µM amiodarone were observed in 5 cells.
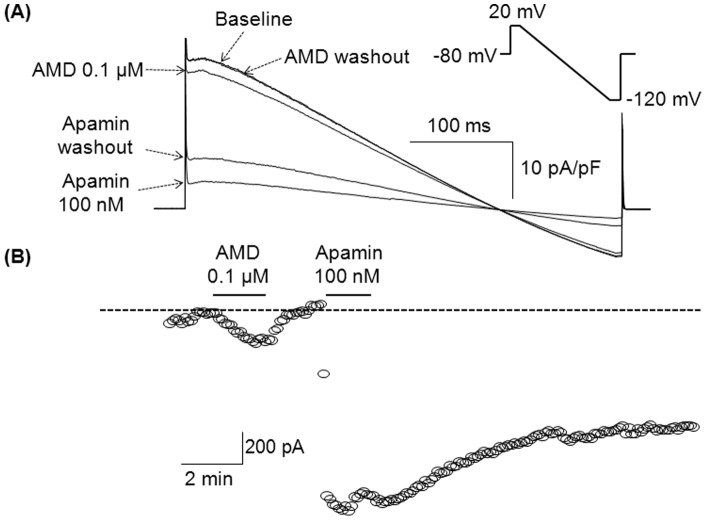
Inhibition of I KAS with amiodarone is reversible
We next tested whether the inhibitory effect of amiodarone was reversible. I K was recorded with a repetitive ramp pulse protocol. Figure 2A shows representative I K traces obtained in the absence (baseline) and in the presence of amiodarone (0.1 µM) and apamin (100 nM). While the inhibitory effect of amiodarone was completely reversed after washout, the inhibitory effect of apamin was only partially reversible. Figure 2B demonstrates the time course of IK measured at a membrane potential of 20 mV. We also performed experiments in which apamin (100 nM) was added first, followed by amiodarone administration. In these experiments, amiodarone did not further reduce the total I K.
Figure 2. Inhibition of I KAS with amiodarone is reversible.
(A) Superimposed I K traces in conditions as labeled. I K was obtained with the ramp pulse protocol shown in the inset, and normalized with cell-capacitance. Intrapipette Ca2+ was 1 µM. (B) Time course of I K measured at a membrane potential of 20 mV. The dotted line indicates the level of baseline I K. AMD indicates amiodarone.
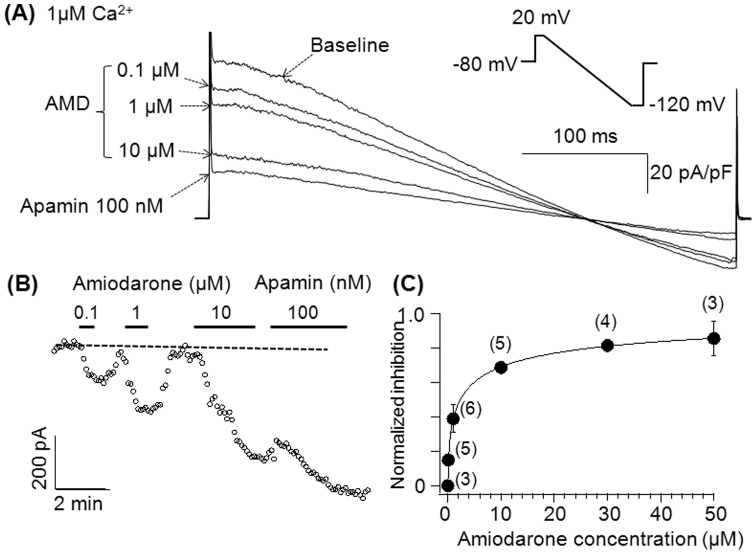
Inhibition of I KAS with amiodarone is dose-dependent
To study whether amiodarone's effects on I KAS were dose-dependent, various consecutive concentrations of amiodarone were applied to the chamber after achieving steady-state I KAS with repetitive ramp-pulse protocols. I KAS was induced with an intrapipette Ca2+ concentration of 1 µM. As indicated in Figure 3A , I KAS was inhibited by amiodarone in a dose-dependent manner. In Figure 3B , I KAS is shown in the presence of various concentrations of amiodarone obtained at a membrane potential of 20 mV and is plotted as a function of time. Figure 3C shows the dose-dependency of the inhibition of I KAS by amiodarone. The inhibition of I KAS with various amiodarone concentrations was normalized to the inhibition with 100 nM apamin (i.e. total I KAS), and plotted as a function of amiodarone concentration. Data were fit with the Hill equation, yielding an IC50 of 2.67±0.25 µM, and a Hill coefficient of 0.51±0.02.
Figure 3. Inhibition of I KAS with amiodarone is dose-dependent.
(A) I KAS was induced by the ramp pulse protocol used in Figure 2. (B) Time-course of I K measured at 0 mV in the absence and presence of various amiodarone concentrations and apamin. The dotted line indicates the level of baseline I K. (C) Dose-dependent inhibitory effects of amiodarone on I KAS. The numbers in parenthesis indicate number of patches. The error bars represent S.E.
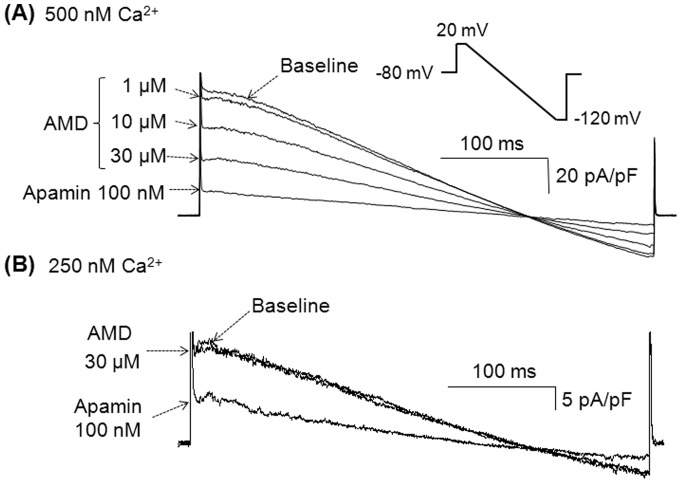
Inhibition of I KAS with amiodarone is affected by intracellular Ca2+ concentration
The open-probability of SK2 channels is dependent on intracellular concentration of Ca2+ which is suggestive of different conformational states for SK2 channels at different Ca2+ concentrations [19]. Therefore, we questioned whether the extent of inhibition might also be Ca2+-dependent. To test this hypothesis, we induced I KAS with various intrapipette Ca2+ concentrations and studied its inhibition by amiodarone. Figure 4A shows representative I K traces induced with an intrapipette Ca2+ concentration of 500 nM at baseline and in the presence of various concentrations of amiodarone. Amiodarone exerts less I KAS inhibition, when I KAS is induced with 500 nM intrapipette Ca2+ compared to its inhibition of the I KAS induced with 1 µM intrapipette Ca2+ ( Figure 3A ). Furthermore, the inhibitory effects of amiodarone (30 µM) on I KAS were even smaller when the currents were induced with 250 nM rather than with 500 nM intrapipette Ca2+ ( Figure 4B ).
Figure 4. Inhibition of I KAS with amiodarone is state-dependent.
(A) Superimposed I K traces in various conditions as labeled. The intrapipette Ca2+ was 500 nM. I K was obtained with the same ramp pulse protocol used in Figure 2 (also shown in the inset). (B) Superimposed I K traces induced with 250 nM intrapipette Ca2+. Similar results were observed in 5 cells. AMD indicates amiodarone.
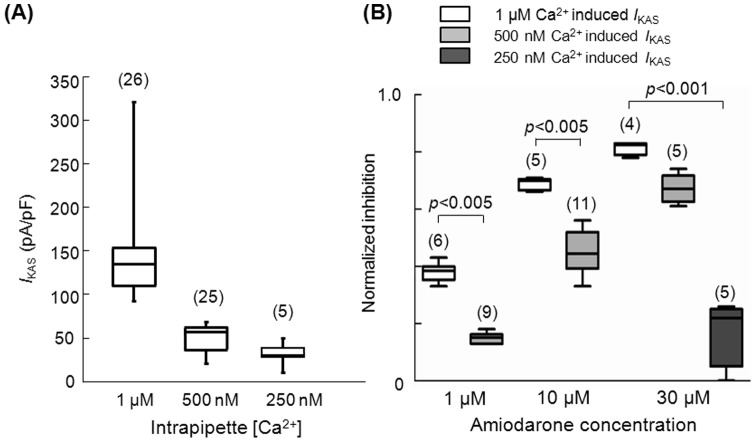
Figure 5A shows I KAS densities induced by various intrapipette Ca2+ concentrations. I KAS density was significantly larger when induced with 1 µM Ca2+ compared to 500 nM and 250 nM. Figure 5B shows that the extent of amiodarone's inhibitory effect on I KAS is dependent on whether the I KAS is induced with 1 µM, 500 nM or 250 nM intrapipette Ca2+ (i.e. different channel conformations respond differently to amiodarone). We used three different therapeutic amiodarone concentrations (1, 10 and 30 µM) to inhibit I KAS. The extent of inhibition in each condition was normalized to the extent of inhibition achieved by 100 nM apamin of the1 µM intracellular Ca2+ induced I KAS. With both 1 μM and 10 μM amiodarone, the extent of inhibition we observed was significantly smaller when the currents were activated with 500 nM intrapipette Ca2+ (light grey boxes) compared to1 μM intrapipette Ca2+ (white boxes). The inhibition of I KAS with 30 μM amiodarone was also highest when I KAS was induced with 1 μM intrapipette Ca2+. Amiodarone, at 30 μM, inhibited the I KAS induced by 500 nM Ca2+ less than by 1 μM Ca2+, and barely inhibited the I KAS induced by 250 nM intrapipette Ca2+ (dark grey box on the far right). These results indicate that the inhibition of I KAS by amiodarone is Ca2+ dependent.
Figure 5. Density of I KAS and its inhibition by amiodarone is dependent on the intracellular Ca2+ concentration.
(A) I KAS densities obtained with three different intrapipette Ca2+ concentrations. The box-plot indicates the median and 25 and 75 percentile values. The whiskers indicate the minimum and maximum values. (B) Inhibitory effects of various concentrations of amiodarone on I KAS induced with various intrapipette Ca2+ concentrations as shown in the top labels. In the left panel, inhibitory effects of 1 μM amiodarone were tested on I KAS induced with two different intrapipette Ca2+ concentrations (1 µM and 500 nM). In the mid panel, inhibitory effects of 10 μM amiodarone were tested on I KAS induced with two different intrapipette Ca2+ concentrations (1 µM and 500 nM). In the right panel, inhibitory effects of 30 μM amiodarone were tested on I KAS induced with three different Ca2+ concentrations (1 µM, 500 nM and 250 nM). Inhibition of 250 nM Ca2+ induced I KAS by 1 µM and 10 µM amiodarone is not shown since this was almost undetectable. The numbers in parentheses indicate the number of patches.
Desethylamiodarone, an amiodarone metabolite, also inhibits I KAS
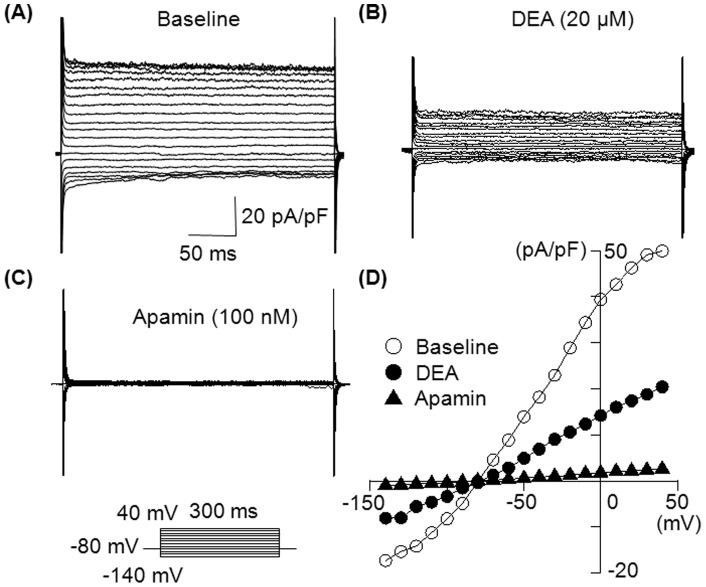
Once amiodarone is absorbed in the human body, it is extensively metabolized in the liver by cytochrome P450 3A4. The main metabolite is desethylamiodarone (DEA), which also has antiarrhythmic properties as the parent compound [20]. Therefore, we also studied the effects of DEA on I KAS. Figure 6A-C shows representative I K traces obtained with a step-pulse protocol in the absence of DEA (Panel A), in the presence of 20 µM DEA (Panel B) and after addition of 100 nM apamin (Panel C). Figure 6D illustrates the current-voltage (I-V) relationships of these currents. Similar to amiodarone, DEA inhibited I KAS in a time and voltage independent manner. The extent of inhibition with DEA (20 µM) was 79.0% [74.3; 86.8] of 1 µM intrapipette Ca2+ induced I KAS (n = 6).
Figure 6. Desethylamiodarone also inhibits I KAS.
(A) Superimposed whole-cell I K traces obtained by the same pulse protocol used in Figure 1. The intrapipette Ca2+ was 1 µM. (B) Current-voltage (I-V) relationships obtained in the absence and presence of 20 µM desethylamiodarone (DEA), and then 100 nM apamin. Similar results were observed in 6 cells.
Discussion
In this study, we examined the modulatory effects of amiodarone on human SK2 channels. Our novel findings are: (1) amiodarone reversibly inhibits the SK2 channels in a dose-dependent manner; (2) the inhibitory effect of amiodarone is time-independent and voltage independent; (3) the degree of IKAS inhibition by amiodarone is dependent on the intracellular calcium concentration; and (4) desethylamiodarone, the main metabolite of amiodarone, also inhibits SK2 channels.
Comparison with previous studies
Since the role of SK2 channels in ventricular arrhythmias of the failing heart had not been fully elucidated, effects of antiarrhythmic agents on SK2 channels were not studied systematically. Most studies about SK2 channels come from the neuroscience literature. Dreixler et al. [21] studied the effects of anesthetic agents on these channels and found that lidocaine inhibits SK2 channels expressed in HEK-293 cells. However, the IC50 they reported was 4.44 mM, which is much higher than the antiarrhythmic therapeutic concentration range (in µM) [22]. Although the underlying molecular mechanisms of lidocaine's effect on SK2 channels were not elucidated in the study, it was speculated that lidocaine interfered with the regulation of intracellular Ca2+, resulting in a change in SK2 activation. In our study, the intracellular Ca2+ concentration was clamped through the pipette solution and the extracellular buffer did not contain Ca2+. Therefore, it is unlikely that amiodarone inhibited SK2 channels by affecting intracellular Ca2+ in HEK-293 cells.
Very recently, Diness et al. [23] showed that three SK channel inhibitors (UCL1684, N-(pyridin-2-yl)-4-(pyridin-2-yl) thiazol-2-amine (ICA), and NS8593) successfully prevented the initiation of AF in various animal models. Since amiodarone (10 µM) also effectively prevented AF initiation in their models, they tested effects of 10 µM amiodarone on whole-cell SK2 currents expressed in HEK-293 cells. Surprisingly in their study, amiodarone did not show any significant effect on SK2 currents. Although detailed experimental conditions were not described in their paper, their previous study [24] indicated that the authors used 400 nM intrapipette Ca2+ to induce SK2 currents. Our study demonstrated that the inhibition of I KAS with 30 µM amiodarone was significantly smaller when I KAS was induced with 250 nM intracellular Ca2+ compared to 500 nM and 1 µM Ca2+, and inhibition with 10 µM amiodarone was also significantly smaller when I KAS was induced with 500 nM Ca2+ compared to 1 µM Ca2+ . Since open probability and state of SK2 channels are drastically different at various intracellular calcium concentrations below 1 µM [25], amiodarone's inhibitory effects may also be different. In other words, 400 nM intrapipette Ca2+ may not induce sufficient SK2 currents to test the inhibitory effects of amiodarone at 10 µM concentration. In addition, it appears that Diness et al. may have used nonphysiological K+ concentrations as their study showed a reversal potential of 0 mV. If the authors had used physiological K+ concentrations (e.g., [K+]o/ ([K+]i = 4/154 mM) in whole-cell experiments as they referenced [24], the outward SK2 currents would have been induced at the holding potential of 0 mV and the reversal potential would have been around −90 mV.
Antiarrhythmic effects of amiodarone
Since amiodarone affects various ion currents (e.g., I Na, I Ca,L, I Ks, I Kr, and I to) as well as β-adrenergic receptors, it is not straightforward to determine its most clinically important target for its anti-arrhythmic activity. Our study adds a new target ion channel to this list.
One mechanism for amiodarone's effectiveness in the treatment of electrical storm can be through prevention of excessive APD shortening after successful defibrillation. Persistence of Ca2+ elevation after the conclusion of repolarization results in depolarizing Na+ currents through the Na+/Ca2+ exchanger, which in turn results in late phase 3 EADs and triggered activity [13], [26]–[28]. Targeting the mechanism behind post-shock APD shortening would act as a specific therapeutic maneuver to prevent ES. In a recent study, we were able to effectively prevent spontaneous VF after defibrillation by the selective SK channel inhibitor apamin [13]. Using the same rabbit heart failure model, we demonstrated that amiodarone can decrease post-shock APD shortening, which underlie its effectiveness in treatment of ES (Figure S2 in Data S2).
Another mechanism behind amiodarone's effectiveness for ES could be through its effects on APD restitution. Previously, Omichi et al. reported that amiodarone flattened the APD restitution slope which in turn resulted in termination of VF in isolated swine ventricles [29]. Since VF induces Ca2+ accumulation due to high frequency depolarizations [30]–[33], it is reasonable to speculate that amiodarone's inhibitory action on SK2 channels are responsible for flattening of the APD restitution slope. This hypothesis is supported by a recent study that showed apamin flattens APD restitution curve at fast pacing rates [34].
We found that the inhibitory effects of amiodarone on SK2 channels were state-dependent (i.e., more inhibition for more current). Cytoplasmic domains of SK2 channels bind to calmodulin, and binding of Ca2+ to calmodulin may affect the conformation of SK2 channels and its gating kinetics [35]. This Ca2+-dependent conformational change may contribute to the state-dependent inhibition of SK2 by amiodarone. The state-dependent inhibition may be clinically important, since amiodarone may affect the channel only when the intracellular Ca2+ is elevated. This makes it particularly important in patients with heart failure or electrical storm where the basal intracellular Ca2+ is elevated.
In our study, an IC50 of amiodarone on whole-cell I KAS induced with 1 µM Ca2+ was 2.67 µM. Since acute and chronic amiodarone administration results in plasma levels of approximately 0.16 to 10 µM [36], inhibitory effects of amiodarone on SK2 channels can be seen with doses used in the clinical setting. Le Bouter et al. [37] demonstrated that mice fed with clinically used doses of amiodarone for 6 weeks showed significant increase of SK2 mRNA levels in their total heart tissue. Although I KAS were not determined in that study, it is reasonable to hypothesize that the transcripts could have increased as a compensatory mechanism to the inhibition of SK2 channels with chronic amiodarone therapy.
Study Limitations
Our study has several limitations: (1) we studied only one variant of SK2 channel (KCNN2 transcript variant 1). Several different SK2 variants are known, although cardiac expression of these variants remains unclear. In addition, other subtypes of SK channels (e.g., SK1 and SK3) exist in cardiomyocytes, and they may form heteromers with SK2 channel resulting in different sensitivity to amiodarone [38] in human hearts; (2) Clearly cytoplasmic environment of HEK-293 cells are very different from human cardiomyocytes. Since SK2 channels are regulated by various signal transduction pathways such as calmodulin, protein kinase CK2, and protein phosphatase 2A [35], effects of amiodarone on SK2 currents may differ between HEK-293 cells and human cardiomyocytes. To overcome this problem, testing amiodarone's effects on SK2 currents in cardiomyocytes or whole heart would have been helpful. However, since amiodarone inhibits various ion channels including I Kr, I Ks and I to in cardiomyocytes, specific inhibitors of these channels need to be used to isolate amiodarone's effects on SK2 currents. After attempting to study amiodarone effects in cardiomyocytes, we found that specific inhibitors of delayed rectifier K+ channels (IK s and IK r), chromanol 293B and E4031 [39], [40] also inhibit SK2 currents complicating the interpretation of data we would obtain using cardiomyocytes (Figure S3 in Data S3, Figure S4 in Data S4). To avoid the non-specific effects of various “specific” ion channel inhibitors, genetically manipulated models are needed. However, it is beyond a scope of this study; (3) Currently we do not know whether apamin can prevent electrical storm in human since it is a neurotoxin and cannot be used in the clinical setting. However, our recent study using rabbit heart failure models demonstrated that I KAS were upregulated and resulted in shortening of the APD after VF termination, predisposing the failing heart to spontaneous recurrence of VF. Apamin, through inhibition of SK2 channels, prevented the APD shortening, recurrence of VF and electrical storm. Similarly, we have shown that SK2 currents were upregulated in failing human ventricular myocytes compared with the non-failing myocytes, and Apamin prolonged the APD in failing human myocytes but not in the non-failing ones [12]. These facts, taken together, allow us to speculate that the inhibition of SK2 currents with amiodarone may, at least in part, underlie amiodarone's effects in termination and prevention of ES in human; (4) We assumed that the SK2 expression level is similar among different experiments, and thus I KAS density can reflect the open probability of the channel. Our assumption was based on our consistent cell culture and transfection protocols. However, we do not have single-channel recording data. Therefore, we do not know the detailed molecular mechanisms behind this inhibition. In spite of these limitations, the results of this study showed for the first time that amiodarone is a potent inhibitor of I KAS. It is possible that I KAS inhibition may partially account for the antiarrhythmic efficacy of amiodarone.
Supporting Information
Control experiments. Figure S1. Superimposed I K traces obtained from an HEK-293 cell with mock transfection using the pEGFP-C3 vector. The I K were recorded with an intra-pipette Ca2+ concentration of 1 µM. The voltage was clamped by the ramp-pulse protocol shown in the inset. Eighty repetitive ramp-pulses were applied every 10 seconds for approximately 5 minutes after the formation of whole-cell configuration.
(TIF)
Effects of amiodarone on the post-shock action potential durations. We sought to investigate whether amiodaorone can antagonize the post-shock action potential duration (APD) shortening. For this, we used pacing-induced rabbit heart failure models since induction of ventricular fibrillation (VF) was difficult in human ventricle wedge preparations. The protocol was approved by the Indiana University Institutional Animal Care and Use Committee. Pacing-induced rabbit heart failure model was created and optical mapping studies in the Langendorff-perfused hearts were performed as previously described [1]. Failing hearts were stained with RH237 for measurement of the membrane potential (V m). Rapid ventricular pacing and 3 to 5 ventricular fibrillation-defibrillation episodes were mapped in each heart. Amiodarone (10 μM) was added to the perfusate for 30 minutes and then the same protocol was repeated. Figure S2A shows a representative action potential (AP) recording at a pacing cycle length (PCL) of 300 ms obtained from the anterobasal left ventricle without amiodarone. The average APD80 obtained was 176 ms. VF was induced by programmed electrical stimulation, and the fibrillating heart was defibrillated with DC current at 130–230 V. First post-shock APD was 141 ms with a preceding diastolic interval of 478 ms (Figure S2B in Data S2). Next, same protocol was repeated in the presence of amiodarone (10 µM). The average APD80 was 182 ms (Figure S2C in Data S2). Figure S2D shows a representative AP recording after defibrillation. First post-shock APD was 162 ms with a preceding diastolic interval of 496 ms. VF was induced several times, and post-shock APDs were measured. Figure S2E shows a plot of post-shock APDs as a function of preceding diastolic intervals. The data were analyzed with simple linear regression. The slope was not significantly affected by amiodarone (baseline: 0.04±0.01, n = 12 beats from two animals; amiodarone, 0.02±0.02, n = 13 beats, p = 0.158). On the contrary, y-intercept was longer in the presence of amiodarone compared to the baseline (baseline: 116.3±3.8, n = 12 beats; amiodarone: 145.3±5.0, n = 13 beats, p<0.0001). Percent shortening of APD at a PCL of 300 ms was 26.5% at baseline and 15.8% in the presence of amiodarone. Figure S2. Amiodarone partially antagonizes post-shock APD shortening in failing hearts. (A) Baseline AP recordings in the absence of amiodarone. (B) Post-shock AP recordings in the absence of amiodarone (10 µM). Three beats of VF was shown. DC depicts direct current shock. (C) Baseline AP recordings in the presence of amiodarone (10 µM). (D) Post-shock AP recordings in the presence of amiodarone. (E) Plot of post-shock APD80 as a function of preceding diastolic intervals.
(TIF)
Inhibitory effects of chromanol 293B on SK2 currents. Amiodarone can inhibit various types of ion channels including slow and rapid components of the delayed rectifier K+ channels (I Ks and I Kr), and the inward rectifier K+ channel IK1. In order to study the specific effects of amiodarone on SK2 currents as well as on the action potential (AP) in cardiomyocytes, the effects of amiodarone on other K+ channels need to be eliminated. To achieve this, we planned to use chromanol 293B and E-4031 to specifically block I Ks and I Kr [2]. However, the effects of these drugs on SK2 currents were unknown. Therefore, we studied the effects of 293B and E-4031 on SK2 currents expressed in HEK-293 cells. Unexpectedly, the most specific I Ks blocker, chromanol 293B, blocked the I KAS induced with 1 µM intra-pipette Ca2+ and repetitive ramp-pulse protocols (Figure S3A in Data S3). Figure S3B shows the dose dependent inhibition of I KAS by chromanol 293B and its reversibility. Percent-inhibition of I KAS with 100 µM chromanol 293B was 38.2±15.0% (n = 5). Since the IC50 of chromanol 293B on I Ks is 10−30 µM, and 100 µM is necessary to fully block I Ks [3], [4], we conclude that it is not feasible to use chromanol 293B to differentiate I KAS from I Ks in cardiomyocytes in order to study the effects of amiodarone on I KAS and AP changes mediated by I KAS. Figure S3. Effect of chromanol 293B on SK2 currents. (A) Representative IK traces obtained with two different 293B concentrations. The ramp-pulse protocol used is shown in the inset. Note that apamin (100 nM) almost completely inhibited the I K meaning that the I K inhibited by 293B is I KAS. (B) The time course of the I K measured at +20 mV. The IK reached steady state (baseline) within a few minutes of whole-cell configuration. Chromanol 293B inhibited the I K in a dose-dependent manner. Subsequent application of apamin (100 nM) inhibited almost all of the I K.
(TIF)
Inhibitory effects of E4031 on SK2 currents. Next, we tested the effect of E4031 (a specific I Kr blocker) on I KAS. The IC50 of E4031 on tail I Kr is 397 nM, and 3−5 µM of E4031 is necessary to block all I Kr [2], [5]. Similar to 293B, E4031 also inhibited I KAS induced with 1 µM intra-pipette Ca2+ and repetitive ramp-pulse protocols (Figure S4A in Data S4). Figure S4B demonstrates that the inhibition of I KAS with E4031 is reversible. Percent-inhibition of I KAS with 500 nM E4031 was 37.6±14.9% (n = 5). Figure S4. Effect of E4031 on SK2 currents. (A) Representative I K traces in various conditions. The ramp-pulse protocol used is shown in the inset. (B) Time course of the I K measured at +20 mV. The I K reached steady state (dotted line) within a few minutes after the formation of whole-cell configuration. E4031 (0.5 µM) reversibly blocked the I K. Subsequent application of apamin (100 nM) inhibited most of the I K.
(TIF)
(DOCX)
Funding Statement
This study was supported in part by NIH Grants P01HL78931, R01HL78932, R01HL71140, a Medtronic-Zipes Endowment (P.-S.C.) and an Indiana University Health-Indiana University School of Medicine Strategic Research Initiative. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bui AL, Horwich TB, Fonarow GC (2011) Epidemiology and risk profile of heart failure. Nat Rev Cardiol 8: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, et al. (2005) Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 352: 225–237. [DOI] [PubMed] [Google Scholar]
- 3. Israel CW, Barold SS (2007) Electrical storm in patients with an implanted defibrillator: a matter of definition. AnnNoninvasiveElectrocardiol 12: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gatzoulis KA, Andrikopoulos GK, Apostolopoulos T, Sotiropoulos E, Zervopoulos G, et al. (2005) Electrical storm is an independent predictor of adverse long-term outcome in the era of implantable defibrillator therapy. Europace 7: 184–192. [DOI] [PubMed] [Google Scholar]
- 5. Kowey PR, Levine JH, Herre JM, Pacifico A, Lindsay BD, et al. (1995) Randomized, double-blind comparison of intravenous amiodarone and bretylium in the treatment of patients with recurrent, hemodynamically destabilizing ventricular tachycardia or fibrillation. Circulation 92: 3255–3263. [DOI] [PubMed] [Google Scholar]
- 6. Credner SC, Klingenheben T, Mauss O, Sticherling C, Hohnloser SH (1998) Electrical storm in patients with transvenous implantable cardioverter-defibrillators: incidence, management and prognostic implications. J Am Coll Cardiol 32: 1909–1915. [DOI] [PubMed] [Google Scholar]
- 7. Vassallo P, Trohman RG (2007) Prescribing amiodarone: an evidence-based review of clinical indications. JAMA 298: 1312–1322. [DOI] [PubMed] [Google Scholar]
- 8. Vergara C, Latorre R, Marrion NV, Adelman JP (1998) Calcium-activated potassium channels. Curr Opin Neurobiol 8: 321–329. [DOI] [PubMed] [Google Scholar]
- 9. Weatherall KL, Goodchild SJ, Jane DE, Marrion NV (2010) Small conductance calcium-activated potassium channels: from structure to function. Prog Neurobiol 91: 242–255. [DOI] [PubMed] [Google Scholar]
- 10. Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, et al. (2003) Molecular identification and functional roles of a Ca(2+)-activated K+ channel in human and mouse hearts. J Biol Chem 278: 49085–49094. [DOI] [PubMed] [Google Scholar]
- 11. Li N, Timofeyev V, Tuteja D, Xu D, Lu L, et al. (2009) Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. JPhysiol 587: 1087–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang PC, Turker I, Lopshire JC, Masroor S, Nguyen BL, et al. (2013) Heterogeneous Upregulation of Apamin-Sensitive Potassium Currents in Failing Human Ventricles. Journal of the American Heart Association 2. [DOI] [PMC free article] [PubMed]
- 13. Chua SK, Chang PC, Maruyama M, Turker I, Shinohara T, et al. (2011) Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res 108: 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen PS, Turker I (2012) Epicardial adipose tissue and neural mechanisms of atrial fibrillation. Circ Arrhythm Electrophysiol 5: 618–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, et al. (1996) Small-conductance, calcium-activated potassium channels from mammalian brain. Science 273: 1709–1714. [DOI] [PubMed] [Google Scholar]
- 16.Adelman JP, Maylie J, Sah P (2011) Small-Conductance Ca(2+)-Activated K(+) Channels: Form and Function. Annu Rev Physiol. [DOI] [PubMed]
- 17. Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, et al. (2005) Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol 289: H2714–2723. [DOI] [PubMed] [Google Scholar]
- 18. Bers DM, Patton CW, Nuccitelli R (2010) A practical guide to the preparation of Ca(2+) buffers. Methods Cell Biol 99: 1–26. [DOI] [PubMed] [Google Scholar]
- 19. Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, et al. (1998) Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395: 503–507. [DOI] [PubMed] [Google Scholar]
- 20. Zhang YH, Cheng H, Alexeenko VA, Dempsey CE, Hancox JC (2010) Characterization of recombinant hERG K(+) channel inhibition by the active metabolite of amiodarone desethyl-amiodarone. J Electrocardiol 43: 440–448. [DOI] [PubMed] [Google Scholar]
- 21. Dreixler JC, Jenkins A, Cao YJ, Roizen JD, Houamed KM (2000) Patch-clamp analysis of anesthetic interactions with recombinant SK2 subtype neuronal calcium-activated potassium channels. Anesth Analg 90: 727–732. [DOI] [PubMed] [Google Scholar]
- 22. Sbarbaro JA, Rawling DA, Fozzard HA (1979) Suppression of ventricular arrhythmias with intravenous disopyramide and lidocaine: efficacy comparison in a randomized trial. Am J Cardiol 44: 513–520. [DOI] [PubMed] [Google Scholar]
- 23. Diness JG, Sorensen US, Nissen JD, Al-Shahib B, Jespersen T, et al. (2010) Inhibition of small-conductance Ca2+-activated K+ channels terminates and protects against atrial fibrillation. Circ Arrhythm Electrophysiol 3: 380–390. [DOI] [PubMed] [Google Scholar]
- 24. Strobaek D, Hougaard C, Johansen TH, Sorensen US, Nielsen EO, et al. (2006) Inhibitory gating modulation of small conductance Ca2+-activated K+ channels by the synthetic compound (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphtylamine (NS8593) reduces afterhyperpolarizing current in hippocampal CA1 neurons. Mol Pharmacol 70: 1771–1782. [DOI] [PubMed] [Google Scholar]
- 25. Hirschberg B, Maylie J, Adelman JP, Marrion NV (1998) Gating of recombinant small-conductance Ca-activated K+ channels by calcium. J Gen Physiol 111: 565–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burashnikov A, Antzelevitch C (2003) Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation 107: 2355–2360. [DOI] [PubMed] [Google Scholar]
- 27. Patterson E, Lazzara R, Szabo B, Liu H, Tang D, et al. (2006) Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am CollCardiol 47: 1196–1206. [DOI] [PubMed] [Google Scholar]
- 28. Ogawa M, Morita N, Tang L, Karagueuzian HS, Weiss JN, et al. (2009) Mechanisms of recurrent ventricular fibrillation in a rabbit model of pacing-induced heart failure. Heart Rhythm 6: 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Omichi C, Zhou S, Lee MH, Naik A, Chang CM, et al. (2002) Effects of amiodarone on wave front dynamics during ventricular fibrillation in isolated swine right ventricle. Am J Physiol Heart Circ Physiol 282: H1063–1070. [DOI] [PubMed] [Google Scholar]
- 30. Koretsune Y, Marban E (1989) Cell calcium in the pathophysiology of ventricular fibrillation and in the pathogenesis of postarrhythmic contractile dysfunction. Circulation 80: 369–379. [DOI] [PubMed] [Google Scholar]
- 31. Zaugg CE, Ziegler A, Lee RJ, Barbosa V, Buser PT (2002) Postresuscitation stunning: postfibrillatory myocardial dysfunction caused by reduced myofilament Ca2+ responsiveness after ventricular fibrillation-induced myocyte Ca2+ overload. J Cardiovasc Electrophysiol 13: 1017–1024. [DOI] [PubMed] [Google Scholar]
- 32. Zaugg CE, Wu ST, Barbosa V, Buser PT, Wikman-Coffelt J, et al. (1998) Ventricular fibrillation-induced intracellular Ca2+ overload causes failed electrical defibrillation and post-shock reinitiation of fibrillation. J Moll Cell Cardiol 30: 2183–2192. [DOI] [PubMed] [Google Scholar]
- 33. Kojima S, Wikman-Coffelt J, Wu ST, Parmley WW (1994) Nature of [Ca2+]i transients during ventricular fibrillation and quinidine treatment in perfused rat hearts. Am J Physiol 266: H1473–1484. [DOI] [PubMed] [Google Scholar]
- 34. Hsieh YC, Chang PC, Hsueh CH, Lee YS, Shen C, et al. (2013) Apamin-sensitive potassium current modulates action potential duration restitution and arrhythmogenesis of failing rabbit ventricles. Circ Arrhythm Electrophysiol 6: 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stocker M (2004) Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5: 758–770. [DOI] [PubMed] [Google Scholar]
- 36. Ikeda N, Nademanee K, Kannan R, Singh BN (1984) Electrophysiologic effects of amiodarone: experimental and clinical observation relative to serum and tissue drug concentrations. Am Heart J 108: 890–898. [DOI] [PubMed] [Google Scholar]
- 37. Le Bouter S, El Harchi A, Marionneau C, Bellocq C, Chambellan A, et al. (2004) Long-term amiodarone administration remodels expression of ion channel transcripts in the mouse heart. Circulation 110: 3028–3035. [DOI] [PubMed] [Google Scholar]
- 38. Benton DC, Monaghan AS, Hosseini R, Bahia PK, Haylett DG, et al. (2003) Small conductance Ca2+-activated K+ channels formed by the expression of rat SK1 and SK2 genes in HEK 293 cells. J Physiol 553: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bosch RF, Gaspo R, Busch AE, Lang HJ, Li GR, et al. (1998) Effects of the chromanol 293B, a selective blocker of the slow, component of the delayed rectifier K+ current, on repolarization in human and guinea pig ventricular myocytes. Cardiovasc Res 38: 441–450. [DOI] [PubMed] [Google Scholar]
- 40. Zhou Z, Gong Q, Ye B, Fan Z, Makielski JC, et al. (1998) Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J 74: 230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Control experiments. Figure S1. Superimposed I K traces obtained from an HEK-293 cell with mock transfection using the pEGFP-C3 vector. The I K were recorded with an intra-pipette Ca2+ concentration of 1 µM. The voltage was clamped by the ramp-pulse protocol shown in the inset. Eighty repetitive ramp-pulses were applied every 10 seconds for approximately 5 minutes after the formation of whole-cell configuration.
(TIF)
Effects of amiodarone on the post-shock action potential durations. We sought to investigate whether amiodaorone can antagonize the post-shock action potential duration (APD) shortening. For this, we used pacing-induced rabbit heart failure models since induction of ventricular fibrillation (VF) was difficult in human ventricle wedge preparations. The protocol was approved by the Indiana University Institutional Animal Care and Use Committee. Pacing-induced rabbit heart failure model was created and optical mapping studies in the Langendorff-perfused hearts were performed as previously described [1]. Failing hearts were stained with RH237 for measurement of the membrane potential (V m). Rapid ventricular pacing and 3 to 5 ventricular fibrillation-defibrillation episodes were mapped in each heart. Amiodarone (10 μM) was added to the perfusate for 30 minutes and then the same protocol was repeated. Figure S2A shows a representative action potential (AP) recording at a pacing cycle length (PCL) of 300 ms obtained from the anterobasal left ventricle without amiodarone. The average APD80 obtained was 176 ms. VF was induced by programmed electrical stimulation, and the fibrillating heart was defibrillated with DC current at 130–230 V. First post-shock APD was 141 ms with a preceding diastolic interval of 478 ms (Figure S2B in Data S2). Next, same protocol was repeated in the presence of amiodarone (10 µM). The average APD80 was 182 ms (Figure S2C in Data S2). Figure S2D shows a representative AP recording after defibrillation. First post-shock APD was 162 ms with a preceding diastolic interval of 496 ms. VF was induced several times, and post-shock APDs were measured. Figure S2E shows a plot of post-shock APDs as a function of preceding diastolic intervals. The data were analyzed with simple linear regression. The slope was not significantly affected by amiodarone (baseline: 0.04±0.01, n = 12 beats from two animals; amiodarone, 0.02±0.02, n = 13 beats, p = 0.158). On the contrary, y-intercept was longer in the presence of amiodarone compared to the baseline (baseline: 116.3±3.8, n = 12 beats; amiodarone: 145.3±5.0, n = 13 beats, p<0.0001). Percent shortening of APD at a PCL of 300 ms was 26.5% at baseline and 15.8% in the presence of amiodarone. Figure S2. Amiodarone partially antagonizes post-shock APD shortening in failing hearts. (A) Baseline AP recordings in the absence of amiodarone. (B) Post-shock AP recordings in the absence of amiodarone (10 µM). Three beats of VF was shown. DC depicts direct current shock. (C) Baseline AP recordings in the presence of amiodarone (10 µM). (D) Post-shock AP recordings in the presence of amiodarone. (E) Plot of post-shock APD80 as a function of preceding diastolic intervals.
(TIF)
Inhibitory effects of chromanol 293B on SK2 currents. Amiodarone can inhibit various types of ion channels including slow and rapid components of the delayed rectifier K+ channels (I Ks and I Kr), and the inward rectifier K+ channel IK1. In order to study the specific effects of amiodarone on SK2 currents as well as on the action potential (AP) in cardiomyocytes, the effects of amiodarone on other K+ channels need to be eliminated. To achieve this, we planned to use chromanol 293B and E-4031 to specifically block I Ks and I Kr [2]. However, the effects of these drugs on SK2 currents were unknown. Therefore, we studied the effects of 293B and E-4031 on SK2 currents expressed in HEK-293 cells. Unexpectedly, the most specific I Ks blocker, chromanol 293B, blocked the I KAS induced with 1 µM intra-pipette Ca2+ and repetitive ramp-pulse protocols (Figure S3A in Data S3). Figure S3B shows the dose dependent inhibition of I KAS by chromanol 293B and its reversibility. Percent-inhibition of I KAS with 100 µM chromanol 293B was 38.2±15.0% (n = 5). Since the IC50 of chromanol 293B on I Ks is 10−30 µM, and 100 µM is necessary to fully block I Ks [3], [4], we conclude that it is not feasible to use chromanol 293B to differentiate I KAS from I Ks in cardiomyocytes in order to study the effects of amiodarone on I KAS and AP changes mediated by I KAS. Figure S3. Effect of chromanol 293B on SK2 currents. (A) Representative IK traces obtained with two different 293B concentrations. The ramp-pulse protocol used is shown in the inset. Note that apamin (100 nM) almost completely inhibited the I K meaning that the I K inhibited by 293B is I KAS. (B) The time course of the I K measured at +20 mV. The IK reached steady state (baseline) within a few minutes of whole-cell configuration. Chromanol 293B inhibited the I K in a dose-dependent manner. Subsequent application of apamin (100 nM) inhibited almost all of the I K.
(TIF)
Inhibitory effects of E4031 on SK2 currents. Next, we tested the effect of E4031 (a specific I Kr blocker) on I KAS. The IC50 of E4031 on tail I Kr is 397 nM, and 3−5 µM of E4031 is necessary to block all I Kr [2], [5]. Similar to 293B, E4031 also inhibited I KAS induced with 1 µM intra-pipette Ca2+ and repetitive ramp-pulse protocols (Figure S4A in Data S4). Figure S4B demonstrates that the inhibition of I KAS with E4031 is reversible. Percent-inhibition of I KAS with 500 nM E4031 was 37.6±14.9% (n = 5). Figure S4. Effect of E4031 on SK2 currents. (A) Representative I K traces in various conditions. The ramp-pulse protocol used is shown in the inset. (B) Time course of the I K measured at +20 mV. The I K reached steady state (dotted line) within a few minutes after the formation of whole-cell configuration. E4031 (0.5 µM) reversibly blocked the I K. Subsequent application of apamin (100 nM) inhibited most of the I K.
(TIF)
(DOCX)