Abstract
Large-scale interspecific studies of mammals ranging between 0.04–280 kg have shown that larger animals walk with more extended limb joints. Within a taxon or clade, however, the relationship between body size and joint posture is less straightforward. Factors that may affect the lack of congruence between broad and narrow phylogenetic analyses of limb kinematics include limited sampling of (1) ranges of body size, and/or (2) numbers of individuals. Unfortunately, both issues are inherent in laboratory-based or zoo locomotion research. In this study, we examined the relationship between body mass and elbow and knee joint angles (our proxies of fore- and hind limb posture, respectively) in a cross-sectional ontogenetic sample of wild chacma baboons (Papio hamadryas ursinus) habituated in the De Hoop Nature Reserve, South Africa. Videos were obtained from 33 individuals of known age (12 to ≥108 months) and body mass (2–29.5 kg) during walking trials. Results show that older, heavier baboons walk with significantly more extended knee joints but not elbow joints. This pattern is consistent when examining only males, but not within the female sample. Heavier, older baboons also display significantly less variation in their hind limb posture compared to lighter, young animals. Thus, within this ontogenetic sample of a single primate species spanning an order of magnitude in body mass, hind limb posture exhibited a postural scaling phenomenon while the forelimbs did not. These findings may further help explain 1) why younger mammals (including baboons) tend to have relatively stronger bones than adults, and 2) why humeri appear relatively weaker than femora (in at least baboons). Finally, this study demonstrates how field-acquired kinematics can help answer fundamental biomechanical questions usually addressed only in animal gait laboratories.
Introduction
For centuries, biologists have observed that small mammals tend to have crouched limb postures with flexed joints, whereas larger animals move with erect limbs and extended joints (e.g. [1]–[4]). Biomechanical models suggest that crouched postures require greater muscle force to counteract torques generated by substrate reaction forces (typically at midstance when forces are highest), contributing in turn to high bending strains in long bones [4]. In contrast, by increasing the effective mechanical advantage (EMA; ratio of muscle moment arm to substrate reaction force moment arm) of the anti-gravity muscles, erect postures help attenuate the magnitude of joint moments [4], thereby reducing compensatory muscles forces and potentially moderating bone strain. Additional attenuation of bone strain can be obtained by aligning limb segments more closely with the resultant of the substrate reaction force vector during weight support, which also minimizes shaft bending moments [4]–[6]. By using erect limb postures, larger animals are able to maintain tissue mechanical safety factors [bone: between 2 and 4] similar to those of smaller animals without ‘over-building’ the skeletal system [6]–[8].
The best evidence supporting this relationship between limb posture and body size comes from a large-scale interspecific study of kinematic data from mammals spanning 0.04 to 280 kg in size, in which limb EMA correlated positively with body size [4]. Analyses of the scaling of limb posture within narrower phylogenetic ranges (i.e., clade specific), however, have yielded mixed results. Some studies, such as one on rodents [7] and one on cercopithecoid primates [9], found similar trends of increasing joint angle with body size but with weaker statistical support. There is, however, more convincing support from morphological indicators of limb posture in a broad sample of primates (e.g., mid-shaft cross-sectional geometry of the femur [10] and subchondral bone radiodensity patterns in the distal femur [11]). Conversely, a kinematic study on felids only found a correlation between body size and one of the 12 measured limb posture characteristics (elbow angle at mid-stance), prompting the authors to suggest that there is no scaling phenomenon between body size and limb posture in this group [12].
Several factors likely contribute to the lack of congruence among these analyses. A primary and acknowledged concern among all of the previously cited studies is sampling bias, both within and across species. For example, nine of the 14 species used in Biewener's [4] original study were rodents, and the largest rodents (capybaras) are a semi-aquatic species [13], and thus may deviate from the generalized rodent pattern. Similarly, Polk [9] was only able to include two each (one male and one female) for three species of cercopithecoid primates yielding a total sample size of six individuals. Although Day and Jayne [12] were able to examine nine species of felids that ranged in mass from ∼3.7 kg to 200 kg, the number of individuals per species was small (N<6) and consequently data from both sexes were pooled. This could be problematic as felids are sexually size dimorphic, with male tending to be much larger than females [13]. Furthermore, previous studies have shown that pooling of sexes can confound kinematic studies since males and females often display sex specific biases in body size, differ in skeletal shape, and often differ in locomotor and positional behavior (see examples in [14], [15]). Finally, these data were collected in laboratory settings or unnatural outdoor enclosures (e.g., zoos), environment that may affect the postural and locomotor kinematics of animals (e.g. [16], [17]).
An alternative approach that avoids the confounding influence of different phylogenetic histories is to focus on limb postural change as a function of ontogeny within a taxon (e.g. [18], [19]). The range of body sizes a single species experiences during growth can span several orders of magnitude, particularly in altricial mammals such as primates [20]. For example, in Vilensky and Gankiewicz's [21] ontogenetic study spanning a three-fold mass range in vervet monkeys, some individuals were observed to have more extended knee joints when they were heavier and older compared to when they were younger and lighter. However, Young [19], [22] did not observe statistically significant differences in knee joint angles in older, heavier squirrel monkeys compared to younger, lighter individuals (0.2–0.5 kg size range) even though the larger-bodied individuals did experience greater hind limb forces relative to forelimb forces. Potential sources of error in these studies again include small sample size (five individuals were used in each study), as well as locomotion behavior altered by treadmill use (in the case of the vervet monkeys), and general kinematic differences due to the laboratory setting [17]. It also may be the case that the animal models used were not large enough to elicit change in limb posture. Small animals (typically less than <1–2 kg) are able to limit bone stresses while in crouched postures (e.g. [5]) and limb posture of small species is likely determined by behavioral influences (e.g., crypsis, maneuverability) rather than by biomechanical constraints (e.g. [5], [11]).
In an effort to both take advantage of the insights offered by body size change over ontogeny and overcome the sample size limitations of lab based studies, we examine scaling of limb posture in a cross-sectional ontogenetic sample using a field-based approach. We selected a wild troop of chacma baboons (Papio hamadryas ursinus) that are well-suited for this line of inquiry because they (1) live in large multi-male/multi-female groups with typically more than 35 individuals [23], (2) undergo >10 fold increase in body size between birth and adulthood (see Table 1), and exhibit large size differences between sexes (where adult males can outweigh adult females by ∼15 kgs [24]), and (3) are (semi-) terrestrial and can easily be viewed where they occupy and utilize open habitats [23]. Moreover, infant baboons are able to locomote independently at an early age (2–5 months [25]), and previous ontogenetic studies of a closely related baboon subspecies (Papio hamadryus cyncocephalus) in captivity demonstrated both morphological (i.e., posterior center of mass shift) and kinematic (e.g., stride and step length) changes between infants and young juveniles [15]. Similar morphological, behavioral and biomechanical changes likely take place in wild baboons. Finally, Polk [26] reported that of the three cercopithecoid species in his study sample, the baboons showed the strongest support for Biewener's [4] biomechanical model. Thus, if body size is a primary determinant of limb posture, then we predict that older, larger individuals will walk with more extended fore- and hind limbs compared to younger, smaller individuals. Additionally, we predict that smaller juveniles will walk with more variation in limb posture than large adults.
Table 1. Tables 1. Sample and descriptive statistics.
| Individual | Sex | Age (months) | Mass (kg) | Knee Joint Angle (degrees) | Elbow Joint Angle (degrees) | ||||||
| N | Mean | St. Dev. | CV | N | Mean | St. Dev. | CV | ||||
| Sylvestor | Male | 12.0 | 2.0 | 2 | 127 | 0.69 | – | 2 | 158 | 7.63 | – |
| Oscar | Male | 13.0 | 2.5 | 7 | 131 | 12.14 | 9.241 | 9 | 151 | 8.52 | 5.637 |
| Chester | Male | 13.0 | 3.3 | 1 | 127 | – | – | 1 | 157 | – | – |
| Elissa | Female | 20.0 | 4.0 | 3 | 136 | 4.06 | – | 3 | 161 | 4.15 | – |
| Luke | Male | 19.0 | 4.9 | 7 | 139 | 7.70 | 5.544 | 7 | 152 | 4.67 | 3.069 |
| Emilio | Male | 20.0 | 7.0 | 9 | 140 | 7.52 | 5.374 | 9 | 152 | 3.55 | 2.329 |
| Bono | Male | 36.0 | 7.0 | 4 | 147 | 1.43 | – | 6 | 158 | 4.01 | 2.533 |
| Quincy | Male | 46.0 | 9.0 | 14 | 134 | 4.80 | 3.592 | 17 | 156 | 4.22 | 2.710 |
| Doug | Male | 49.0 | 9.0 | 5 | 138 | 4.74 | 3.425 | 5 | 148 | 3.14 | 2.127 |
| Turtle | Male | 44.0 | 9.3 | 15 | 139 | 4.98 | 3.584 | 19 | 158 | 4.67 | 2.955 |
| Kyle | Male | 55.0 | 11.5 | 1 | 131 | – | – | 1 | 158 | – | – |
| Cartman | Male | 56.0 | 12.0 | 4 | 135 | 7.82 | – | 4 | 154 | 3.82 | – |
| Vicky | Female | 61.0 | 13.0 | 5 | 141 | 4.34 | 3.066 | 5 | 156 | 4.05 | 2.595 |
| Ulrike | Female | 62.0 | 13.0 | 4 | 136 | 7.19 | – | 5 | 152 | 2.41 | 1.590 |
| Kevin | Male | 62.0 | 13.3 | 6 | 135 | 3.52 | 2.612 | 7 | 147 | 4.46 | 3.026 |
| Catherine | Female | – | 16.2 | 0 | – | – | – | 5 | 159 | 4.18 | 2.624 |
| Lynn | Female | – | 17.0 | 7 | 132 | 4.16 | 3.145 | 6 | 157 | 1.30 | 0.829 |
| Rushenka | Female | 87.0 | 17.5 | 3 | 132 | 7.98 | – | 4 | 156 | 2.78 | – |
| Jane | Female | – | 17.5 | 8 | 140 | 3.89 | 2.777 | 6 | 155 | 5.01 | 3.237 |
| Alice | Female | – | 17.8 | 9 | 139 | 4.14 | 2.990 | 8 | 154 | 5.40 | 3.508 |
| Olga | Female | – | 17.8 | 9 | 134 | 4.72 | 3.510 | 9 | 157 | 4.27 | 2.725 |
| Alison | Female | – | 18.0 | 3 | 142 | 1.44 | – | 3 | 157 | 3.74 | – |
| Emma | Female | – | 18.0 | 1 | 134 | – | – | 1 | 151 | – | – |
| Sarah | Female | – | 18.5 | 8 | 140 | 5.18 | 3.704 | 9 | 161 | 4.09 | 2.538 |
| Christina | Female | – | 19.0 | 5 | 141 | 1.30 | 0.920 | 5 | 160 | 8.03 | 5.019 |
| Watson | Male | 108.0 | 24.0 | 22 | 145 | 4.36 | 3.012 | 26 | 160 | 4.34 | 2.713 |
| Guy | Male | – | 26.0 | 3 | 143 | 1.35 | – | 3 | 162 | 4.27 | – |
| Pinker | Male | – | 26.0 | 6 | 144 | 1.47 | 1.017 | 7 | 154 | 5.38 | 3.490 |
| Schwartze | Male | – | 27.0 | 12 | 140 | 3.10 | 2.211 | 10 | 161 | 4.43 | 2.751 |
| Caliban | Male | – | 28.0 | 4 | 137 | 5.59 | – | 4 | 155 | 4.64 | – |
| Prof Higgins | Male | – | 28.0 | 10 | 144 | 5.23 | 3.627 | 11 | 158 | 4.13 | 2.621 |
| Redfur | Male | – | 29.0 | 16 | 139 | 4.82 | 3.476 | 12 | 156 | 4.93 | 3.150 |
| Seth | Male | – | 29.5 | 8 | 149 | 2.15 | 1.440 | 9 | 159 | 2.65 | 1.667 |
Materials and Methods
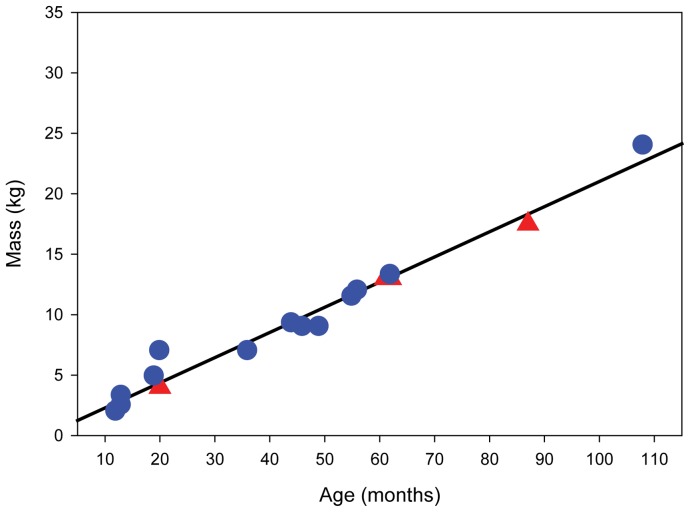
The study site was the De Hoop Nature Preserve (34°27′00′′S, 20°24′00”E) in the Western Cape Province in South Africa. De Hoop is a winter rainfall habitat characterized by endemic fynbos habitat that is home to a large population of chacma baboons. The chacma baboons are not a protected species in De Hoop Nature Preserve, and no permits were necessary for this study. Data come from a single, habituated troop (VT) that has been studied since 1997 [27]. Videos of all animals used in this study were recorded in July 2004 when the troop was making extensive use of the area adjacent to a large inland lake. The open, flat terrain facilitated collection of locomotion data from a large number of individually identifiable animals of all age-sex classes. The focal animals (total N = 33) included both males (N = 20) and females (N = 13) with an age range of 12 to >108 months and a body mass range of 2 kg to 29.5 kg (Table 1). A custom built, portable electronic digital scale placed opportunistically at sleeping sites was used to obtain body mass data. We found that age (in months) and body mass (in kg) were significantly correlated (r2 = 0.974; p<0.001; Fig. 1). A Shapiro-Wilk test for normality revealed that the body mass data in our sample follow a normal distribution (p = 0.103) and therefore we did not log transform this variable in our statistical analyses (see below). It is necessary to point out that although we had a diverse sampling of males across all body size ranges, most of our female sample comes from older, larger individuals. All juveniles and sub-adults were already weaned and were independent of their mothers for locomotor behaviors.
Figure 1. Age vs. mass.
Correlation between known age (in months) and body mass (in kg) for a subset of the comparative sample. Red triangles are for females. Blue circles are for males. Statistics: r2 = 0.974; p<0.001.
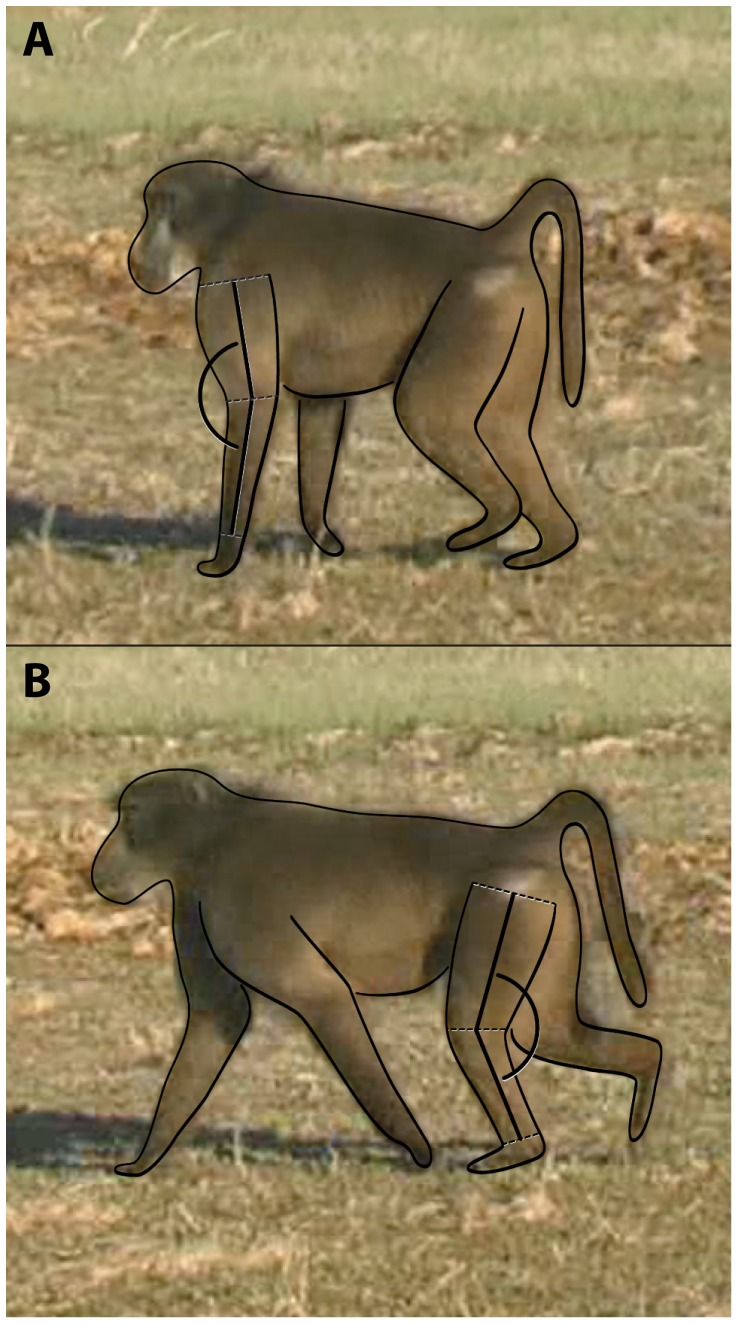
Video was recorded (60 frames/s) with a commercial handheld digital video camera placed on a tripod. The camera was typically positioned in close proximity to the focal animal (∼2–3 meters). Trials were included in the analysis only if the animal appeared to be moving in a straight line and perpendicular (<20 degrees relative) to the camera for two consecutive strides while on the ground [28]. Ultimately, sample sizes ranged between 1–26 trials per individual (Table 1). Angle measurements were obtained from still-frame images of each video sequence during fore- and hind limb mid-support, similar to previous published studies of primate kinematics [29]–[31]. Mid-support was defined as the kinematic event when the wrist joint was below the shoulder joint and the ankle joint was below the hip joint. Mid-support is typically the time during the step cycle when the vertical component of the GRF vector is at its peak in baboons [32], thereby making it the most relevant kinematic event to address our hypotheses about limb loading. The low, scrubby vegetation of this field site facilitated viewing of the animal's hands and feet. Frame-by-frame analysis of video trials was performed using Virtual Dub software (http://www.virtualdub.org). Angle values (in degrees) from each trial were measured either using ImageJ software (http://rsbweb.nih.gov/ij/) or Didge software (http://biology.creighton.edu/faculty/cullum/Didge/index.html). Specifically, two-dimensional knee and elbow angles were measured between the thigh/leg and arm/forearm segments, respectively (Fig. 2). Each trial was measured at least three times and the average value was used in all analyses. Trials for each joint were measured by only one author (knee: AMH; elbow: NET). Joint angle values were considered more extended if they converged on 180 degrees. For subsequent statistical analyses, we calculated the mean joint angle (for all individuals) and the coefficient of variation (CV) about the mean value (for individuals that had five or more trials).
Figure 2. Angle measurements.
Illustration showing how joint angles (in degrees) were measured. (A) Elbow joint angle was measured as the angle between the arm segment and the forearm segment. A line determined the arm segment with its proximal end approximating the midpoint between the anterior and posterior contours of the arm at the shoulder joint and the distal end approximating the midpoint between the anterior and posterior contours of the elbow joint. A line determined the forearm segment with its proximal end approximating the midpoint between anterior and posterior contours of the elbow joint and the distal end approximating the midpoint between the anterior and posterior contours of the wrist joint. (B) Knee joint angle was measured as the angle between the thigh segment and the leg segment. A line determined the thigh segment with its proximal end approximating the midpoint between the anterior and posterior contours of the thigh at the hip joint and the distal end approximating the midpoint between the anterior and posterior contours of the knee joint. A line determined the leg segment with its proximal end approximating the midpoint between anterior and posterior contours of the knee joint and the distal end approximating the midpoint between the anterior and posterior contours of the ankle joint.
Because speed and gait can affect limb joint kinematics in baboons [26], [33], we chose trials only when duty factor was greater than 0.50 (i.e., kinematic symmetrical walks [34]). Duty factor was determined by taking the percentage of time the hind limb was in contact with the ground (i.e., step duration) relative to total hind limb stride duration. Ultimately, we found that duty factor was not significantly correlated with joint angles in any of the individuals (p>0.05) and thus all statistical analyses were performed without duty factor as a covariate.
Our analyses consisted of a series of Pearson's product moment correlations and least squares (LS) regressions. We regressed mean knee and elbow angles against body mass for each individual in the entire sample, and then again for each sex independently. Second, we regressed the CVs of knee and elbow angles against body mass in the entire sample and within each sex. Following Day and Jayne [12], we focused our attention on the correlation coefficient and the sign of the calculated slope (positive or negative). We were also interested in evaluating whether males adopt more extended fore- and hind limbs compared to females across all size ranges. Therefore, we performed a pair of analyses of covariance (ANCOVA) with body mass as the covariate. In the ANCOVA, least-square (LS) means for elbow and knee joint angle for each sex were calculated and then compared using a Tukey's HSD tests. All statistical analyses were performed in JMP v.9.0 (SAS Institute Inc.) or SPSS v.16 (SPSS Inc.) software packages.
Results
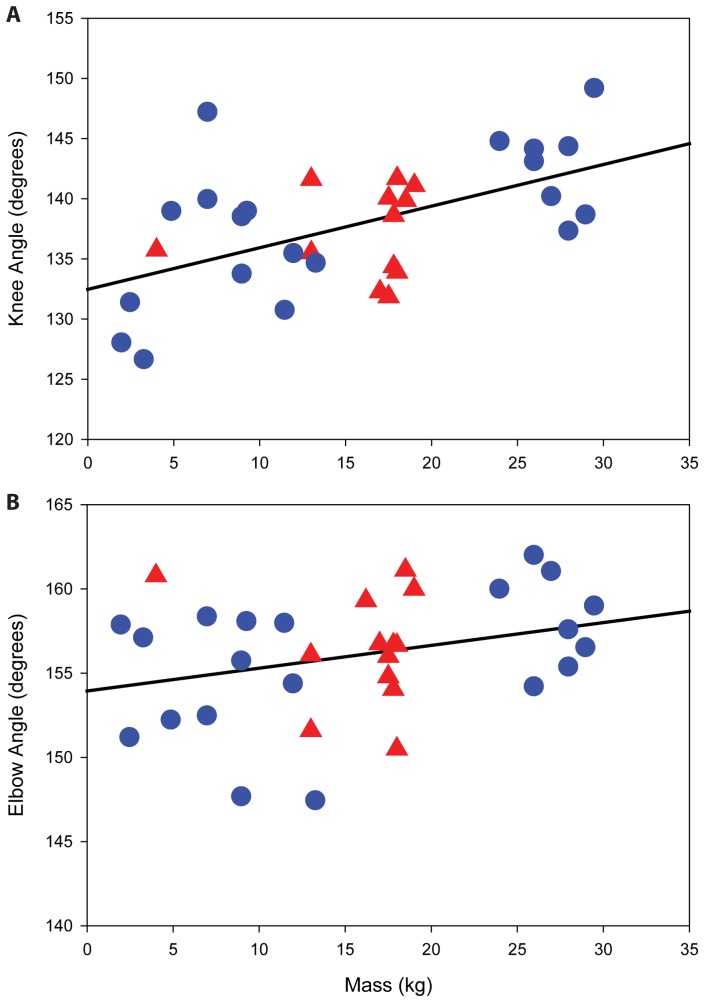
Descriptive statistics for knee and elbow angle variables are shown in Table 1. Results of regression analyses are presented in Table 2 and illustrated in Figure 3. For the entire sample, there was a positive significant relationship between body mass and knee angle (r2 = 0.301, p = 0.001). Although body mass had a small positive effect on elbow angle, this relationship was not statistically significant (r2 = 0.093, p = 0.084). Thus, as individuals mature and increase mass, only hind limbs become significantly more extended (Fig. 3).
Table 2. Results of least squares regressions against body mass*.
| Variable | Sample | N | Slope | Intercept | r2 | p |
| Knee Joint Angle | All individuals | 32 | 0.346 | 132.47 | 0.301 | 0.001 |
| Males | 20 | 0.372 | 132.51 | 0.386 | 0.004 | |
| Females | 12 | 0.096 | 135.68 | 0.012 | 0.732 | |
| Elbow Joint Angle | All individuals | 33 | 0.135 | 153.94 | 0.093 | 0.084 |
| Males | 20 | 0.163 | 153.27 | 0.176 | 0.066 | |
| Females | 13 | −0.161 | 159.05 | 0.039 | 0.516 | |
| Knee Joint CV | All individuals | 20 | −0.144 | 5.85 | 0.448 | 0.001 |
| Males | 13 | −0.143 | 6.11 | 0.502 | 0.007 | |
| Females | 7 | −0.123 | 4.99 | 0.070 | 0.566 | |
| Elbow Joint CV | All individuals | 23 | −0.019 | 3.17 | 0.026 | 0.465 |
| Males | 14 | −0.027 | 3.35 | 0.098 | 0.275 | |
| Females | 9 | 0.264 | −1.66 | 0.248 | 0.173 |
Significant at p<0.05.
Figure 3. Body mass vs. mean joint angle.
Relationship between mean knee joint angle (A), and mean elbow joint angle (B) and individual body mass. Data fit with a least squares regression line. Red triangles are for females. Blue circles are for males. See Table 2 for relevant statistics.
When regressions between body mass and joint angle were performed within each sex separately, we found that the significant positive relationship between body mass and knee angle was upheld in the male-only sample (r2 = 0.386, p = 0.004), and that the slope and intercept of this relationship were similar to that of the regression equation for the combined sample (slope = 0.372 males only; slope = 0.346 both sexes). The female-only regression demonstrated no relationship between knee angle and body mass (r2 = 0.012, p = 0.732). Neither males nor females displayed a significant relationship between elbow joint angle and body mass, though males in the sample had a positive trend of body mass on elbow angle (r2 = 0.176, p = 0.066).
Although some of the relationships discussed above are statistically significant, their correlation coefficients can be considered rather low. After an outlier-analysis was performed on the spread of values in Figure 3 and Table 2, it became apparent that Bono, who is one of the smallest individuals at 7 kg, adopted a highly extended knee joint similar to Seth, who is the largest animal at 29.5 kg (147 vs. 149 degrees, respectively). When removing Bono from the analyses, r2 values increase and the p values decrease for the knee joint (combined sample: r2 = 0.424, p<0.001; male-only sample: r2 = 0.551, p = 0.0003).
For the combined sample, and in the male-only subset of the data, there was a significant negative relationship between body mass and knee joint CV (combined sample: r2 = 0.448, p = 0.001; male sample: r2 = 0.502, p = 0.007). Thus smaller individuals had more variation in hind limb posture. This relationship was not significant for the elbow joint in the combined sample and in the male-only sample (combined sample: r2 = 0.026, p = 0.465; male-only sample: r2 = 0.098, p = 0.275). For both the knee and elbow joints, body mass had no affect on limb posture variation in females (p>0.05; see Table 2).
Table 3 presents the distribution of LS means of knee and elbow angles for each sex that were calculated in the ANCOVA (with body mass as the covariate). Even though the LS means for knee angle appear larger in males compared to females, and the LS means for elbow angle appear larger in females compared to males, the Tukey's HSD tests demonstrate that males and females do not significantly differ from each other in both elbow and knee joint angles (knee joint: p = 0.473; elbow joint: p = 0.626).
Table 3. Least squares (LS) means and results of ANCOVAs between males and females*.
| Variable | Sex | Mean (degrees) | LS Mean | Std. Error | p |
| Knee Joint Angle | Female | 137 | 137 | 1.323 | 0.473 |
| Male | 138 | 138 | 1.025 | ||
| Elbow Joint Angle | Female | 156 | 156 | 1.006 | 0.626 |
| Male | 155 | 155 | 0.811 |
Significant at p<0.05.
Discussion
Large-scale interspecific analyses have previously demonstrated that larger species tend to walk with more extended limb postures. Such kinematic modifications can help decrease the magnitude of muscle force needed to counteract gravity, which in turn attenuates musculoskeletal stresses arising from both muscle and substrate reaction forces [4]–[6]. Some studies using more narrow phylogenetic samples have found support for this hypothesis (e.g., primates [9], [11]; rodents [7]), while others have demonstrated little to no relationship between body mass and limb posture (i.e., felids [12]). The goal of the present study was to test if large-scale interspecific patterns of limb orientation hold true in a more phylogenetically restricted but ontogenetically expanded sample, specifically in a population of wild chacma baboons (Papio hamadryas ursinus). By filming naturalistic locomotion in the wild, we were able to include a greater number of individuals spanning a large range of body size, as well as avoid many of the potential problems of studying posture and behavior inherent in laboratory studies. Furthermore, we were able to analyze males and females separately, which to our knowledge has not been rigorously investigated in previous studies. Using this approach, our findings for hind limb posture support Biewener's [4] biomechanical model in that older, heavier baboons tend to walk with more extended knee joints. In contrast to the model, however, elbow joint angles did not become more extended with an increase in body size.
The fact that knee angle, but not elbow angle, was positively correlated with body mass in our primate sample indicate that there may be different biomechanical demands imposed on hind limbs versus forelimbs over ontogeny in baboons. In general, primates tend to support a greater proportion of their mass on their hind limbs than on their forelimbs [32], [35]–[37] and thus a more extended hind limb could be a way to mitigate potentially higher musculoskeletal stresses acting on the thigh and leg bones. Baboons, however, are among the few primate quadrupeds that have an approximately equal distribution of mass on their fore- and hind limbs, particularly during ground locomotion [26], [32], [35], [38] making this an unlikely explanation for the different scaling relationships for elbow angle and knee angles in our wild chacma baboon sample.
In addition to not changing with increasing body size, the elbow joints of chacma baboons are more extended than the knee joint across ontogeny (as also seen in many other cercopithecoid monkeys in general; see [31]), which likely serves to create a longer effective forelimb length. Longer effective limb length can help to lower energetic costs associated with long distance travel [39], [40]. This could be important for terrestrial primates like baboons who spend a significant portion of their locomotor time walking long distances within their home range ([41], see also [42], [43]). This follows other postural adaptations of effective forelimb elongation in baboons, such as adopting digitigrade hand postures when walking [33], [44]–[46]. In fact, baboons start to habitually use a digitigrade hand posture early in their development, possibly as soon as two months of age [47]. Thus, by adopting extended elbow joints very early in ontogeny, baboons may already be approaching the limits to joint ranges of motion in the forelimb and thus cannot extend their forelimbs any more during maturation.
Why then are the hind limbs also not as extended early in ontogeny in baboons, and possibly other cercopithecoid monkeys? Increasing effective limb length should be just as beneficial in the hind limb as in the forelimb. One possibility may be that younger baboons need to maintain crouched hind limbs to increase maneuverability (e.g. [48], [49]); the hind limbs are used more in propulsion than are the forelimbs [32]. Greater maneuverability and agility is important for younger animals for several reasons. For example, younger baboons may need to be more agile to be able to follow their mothers closely during group dispersal events (i.e., being able to catch up to group leaders), to better avoid terrestrial predators [50], or because of social dynamics (i.e., submissive displays to older individuals, play behavior). However, as noted above, crouched limbs come with the tradeoff of an increase in musculoskeletal stresses. This may be a reason why younger animals tend to have relatively stronger long bones than older animals. In both precocial (rodents, lagomorphs, bovids) and altricial (primates) mammals, bending strength and geometric safety factors scale with negative allometry as animals grow in body size and develop with age [51]–[54]. In baboons specifically, Ruff [55] found that younger individuals of a closely related baboon subspecies (Papio hamadryas cyncocephalus) have relatively stronger femora than adults. Ruff [55], citing published bone biology literature, originally suggested that the overall age-related decline in relative femoral strength could be compensated by an increase in bone mineral strength. But, it is also likely that habitually adopting a more extended hind limb at older ages and when masses are greater could also attenuate musculoskeletal stresses in their relatively weaker femora. Interestingly, while the baboon femur decreases in bone strength during ontogeny, Ruff [55] also documented that the strength of the humerus actually appears to decrease at a faster rate than femoral strength, especially after 2.5 years in age. This discrepancy in humerus to femur bone strength later in ontogeny may further explain why baboons always have forelimbs that are more extended than their hind limbs (Table 1; see also [30]) and why the elbow joint remains extended over ontogeny (see above).
Another possibility, although not mutually exclusive with those proposed above, may be related to different allometries of distal limb-segment growth. In building upon Biewener's biomechanical model, Polk [9] proposed that among animals of similar body size, those with longer distal limb segments will adopt more extended postures in order to compensate for longer moment arms at the knee or elbow joint (see Figure 1 in [9]). In baboons (Papio hamadryas cyncocephalus), Raichlen [56] documented that the relative length of the leg segment (tibia/fibula) significantly increases over ontogeny whereas the relative length of the forearm segment (ulna/radius) does not; thus, older individuals have relatively longer distal hind-limb versus distal forelimb segments. Older baboons may then attenuate relatively larger knee joint moments by adopting an extended posture, but may not be necessary in the forelimb since the forearm does not increase in relative length, thus accounting for the fore- vs. hind limb scaling difference observed in this study.
The observed relationship between knee angle and body mass is similar when all animals are included in the analysis, and when males are examined independently. In contrast, we found no significant relationship between any of the limb posture variables and mass in the females. Behavioral differences between males and females may contribute to the observed differences in scaling relationships. Adopting more erect limb postures may come with the cost of decreasing agility, acceleration and overall speed [48], [49]. In general, these costs may be detrimental for habitually terrestrial primates like baboons when trying to escape from predators. For females this loss would additionally hamper their ability to compete with other females of the same group for resources [57], as well as to avoid aggression from dominant males in the group. Their smaller body sizes (on average of 15 kg) relative to males also reduce their ability to resist attack during bouts of male-male competition [58]. While these are all possibilities, results of the ANCOVAs comparing males and females demonstrated no statistically significant sex differences in knee angle after controlling for body mass (Table 3). One possible explanation for this result could be related to the fact that our sample of females is smaller than males (13 vs. 20 individuals, respectively), and the females encompassed a smaller range of sizes and ages (Table 1). Thus, the data from females in this study does not span a full ontogenetic spectrum, especially at younger ages. The potential for sex-based differences in joint angles remain intriguing, however, as such effects may be indicative of potential tradeoffs between locomotor biomechanics and social dynamics. Larger data sets with known dominance ranks, for example, would be necessary to fully investigate this hypothesis (e.g. [59]).
Biewener's [4], [5] biomechanical model hypothesizes that postural accommodations by anti-gravity muscles are most constrained in larger animals because small taxa (0.01–1.0 kg) can operate at higher bone safety factors relative to their body size. Recently, Polk and colleagues [10], investigated whether smaller primate taxa are indeed less constrained in adopting a specific hind limb joint orientation by examining variability in knee angles. Their results indicate that smaller taxa display more variation in knee joint angles when compared to larger species; and the latter consistently had more extended knees. In our intraspecific, ontogenetic sample of chacma baboons, we observed significantly higher levels of variation (as identified by the CV around mean values) in knee joint angles in smaller, younger individuals. Again, no relationship was found for the elbow joint. Thus, the results reported here further support Biewener's [4], [5] biomechanical model and support Polk et al. 's [10] conclusions of larger primates being more biomechanically constrained in their choice of hind limb posture.
Conclusions
Though large, interspecific data are conducive for investigating emergent, large-scale properties among species [4], investigation of these patterns within clades [12], or within species (this study; see also [19], [22]), often deviate from the larger scale patterns in biologically meaningful ways. In chacma baboons, we found that Biewener 's [4] biomechanical model of size-dependent limb posture was supported across a broad range of body sizes for the knee joint but not the elbow joint. Furthermore, we found additional evidence that limb posture variation is size dependent for the knee joint, but again, not for the elbow. While the lack of congruence between the fore- and hind limbs may be specific to chacma baboons, it is also likely that other large-bodied terrestrial primates may exhibit similar patterns. This possibility, however, can only be fully tested with additional data. Like others (e.g. [60]), we also propose that using field data from wild animals holds promise for future studies of comparative biomechanics, as it permits data collection from a larger, more diverse population of naturally behaving individuals. Primates are an especially amenable group for field-collected data as they can be acclimated to human contact and observation, and large populations of semi-free ranging animals are also available at primate-centered biomedical facilities [61], [62]. Our results underscore how field data can provide a wealth of opportunity to address large-scale biomechanical questions not possible in animal gait laboratories. By collecting these data in the field, future studies will be able to tackle previously unaddressable questions related to sexual dimorphism, ontogeny patterns, and effects of social structure (e.g., dominance and rank) on posture and locomotion.
Acknowledgments
We are grateful to Cape Nature for permission to work at De Hoop Nature Reserve. We thank Susan Larson, Brigitte Demes, John Polk and an anonymous reviewer who provided valuable feedback on an earlier draft of this manuscript.
Funding Statement
This study was funded by a National Research Foundation of South Africa grant to S.P. Henzi (http://www.nrf.ac.za/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Galilei G (1638) Dialogues concerning two new species [Crew H, de Salvio A, translators]. New York: Dover. 300 p.
- 2. Osborn H (1900) The angulation of the limbs of proboscidia, dinocerata, and other quadrupeds, in adaptation to weight. Am Nat 34: 89–94. [Google Scholar]
- 3.Gray J (1968) Animal locomotion. New York: Norton. 479 p.
- 4. Biewener AA (1989) Scaling body support in mammals: limb posture and muscle mechanics. Science 245: 45–48. [DOI] [PubMed] [Google Scholar]
- 5. Biewener AA (1990) Biomechanics of mammalian terrestrial locomotion. Science 250: 1097–1103. [DOI] [PubMed] [Google Scholar]
- 6. Biewener AA (1991) Musculoskeletal design in relation to body size. J Biomech 24 Suppl 119–29. [DOI] [PubMed] [Google Scholar]
- 7. Biewener AA (2005) Biomechanical consequences of scaling. J Exp Biol 208: 1665–1676 doi:10.1242/jeb.01520. [DOI] [PubMed] [Google Scholar]
- 8. Brassey CA, Kitchener AC, Withers PJ, Manning PL, Sellers WI (2013) The role of cross-sectional geometry, curvature, and limb posture in maintaining equal safety factors: a computed tomography study. Anat Rec 296: 395–413. [DOI] [PubMed] [Google Scholar]
- 9. Polk JD (2002) Adaptive and phylogenetic influences on musculoskeletal design in cercopithecine primates. J Exp Biol 205: 3399–3412. [DOI] [PubMed] [Google Scholar]
- 10. Polk JD, Demes B, Jungers WL, Biknevicius AR, Heinrich RE, et al. (2000) A comparison of primate, carnivoran and rodent limb bone cross-sectional properties: are primates really unique? J Hum Evol 39: 297–325. [DOI] [PubMed] [Google Scholar]
- 11. Polk JD, Williams SA, Peterson JV (2009) Body size and joint posture in primates. Am J Phys Anthropol 140: 359–367 doi:10.1002/ajpa.21083. [DOI] [PubMed] [Google Scholar]
- 12. Day LM, Jayne BC (2007) Interspecific scaling of the morphology and posture of the limbs during the locomotion of cats (Felidae). J Exp Biol 210: 642–654 doi:10.1242/jeb.02703. [DOI] [PubMed] [Google Scholar]
- 13.Nowak RM (1999) Walker's mammals of the world, 6th edition. Baltimore: The Johns Hopkins University Press. 2015 p.
- 14.Plavcan JM (2001) Sexual dimorphism in primate evolution. Am J Phys Anthropol, Suppl 33: 25–53. doi:10.1002/ajpa.10011. [DOI] [PubMed]
- 15. Carlson KJ (2005) Investigating the form-function interface in African apes: Relationships between principal moments of area and positional behaviors in femoral and humeral diaphyses. Am J Phys Anthropol 127: 312–334 doi:10.1002/ajpa.20124. [DOI] [PubMed] [Google Scholar]
- 16. Nekaris KAI, Stevens NJ (2007) Not all lorises are slow: rapid arboreal locomotion in Loris tardigradus of southwestern Sri Lanka. Am J Primatol 69: 112–120. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro LJ, Young JW, Souther A (2011) Quadrupedal locomotion of Saimiri boliviensis: a comparison of field and laboratory-based kinematic data: In: D'Août K, Vereecke EE, editors. Primate locomotion: linking field and laboratory research. New York: Springer. 335–356.
- 18. Raichlen DA (2005) Effects of limb mass distribution on the ontogeny of quadrupedalism in infant baboons (Papio cynocephalus) and implications for the evolution of primate quadrupedalism. J Hum Evol 49: 415–431 doi:10.1016/j.jhevol.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19. Young JW (2012) Ontogeny of limb force distribution in squirrel monkeys (Saimiri boliviensis): Insights into the mechanical bases of primate hind limb dominance. J Hum Evol 62: 473–485 doi:10.1016/j.jhevol.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 20. Altmann J, Alberts SC (2005) Growth rates in a wild primate population: ecological influences and maternal effects. Behav Ecol Sociobiol 57: 490–501. [Google Scholar]
- 21. Vilensky JA, Gankiewicz E (1990) Effects of growth and speed on hindlimb joint angular displacement patterns in vervet monkeys (Cercopithecus aethiops). Am J Phys Anthropol 81: 441–449 doi:10.1002/ajpa.1330810313. [DOI] [PubMed] [Google Scholar]
- 22. Young JW (2009) Ontogeny of joint mechanics in squirrel monkeys (Saimiri boliviensis): functional implications for mammalian limb growth and locomotor development. J Exp Biol 212: 1576–1591 doi:10.1242/jeb.025460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henzi SP, Barrett L (2005) The historical socioecology of savanna baboons (Papio hamadryas). J Zool 265: 215–226 doi:10.1017/S0952836904006399. [Google Scholar]
- 24. Smith RJ, Jungers WL (1997) Body mass in comparative primatology. J Hum Evol 32: 523–559 doi:10.1006/jhev.1996.0122. [DOI] [PubMed] [Google Scholar]
- 25.Altmann J (2001) Baboon mothers and infants. Cambridge: Harvard University Press. 272 p.
- 26.Polk JD (2001) The influence of body size and body proportions on primate quadrupedal locomotion. PhD Dissertation. SUNY Stony Brook.
- 27. Barrett L, Gaynor D, Rendall D, Mitchell D, Henzi SP (2004) Habitual cave use and thermoregulation in chacma baboons (Papio hamadryas ursinus). J Hum Evol 46: 215–222 doi:10.1016/j.jhevol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 28. Stevens NJ, Schmitt DO, Cole TM, Chan LK (2006) Technical note: out-of-plane angular correction based on a trigonometric function for use in two-dimensional kinematic studies. Am J Phys Anthropol 129: 399–402 doi:10.1002/ajpa.20359. [DOI] [PubMed] [Google Scholar]
- 29. Larson SG, Schmitt D, Lemelin P, Hamrick M (2000) Uniqueness of primate forelimb posture during quadrupedal locomotion. Am J Phys Anthropol 112: 87–101. [DOI] [PubMed] [Google Scholar]
- 30. Larson S, Schmitt D, Lemelin P, Hamrick M (2001) Limb excursion during quadrupedal walking: how do primates compare to other mammals? J Zool 255: 353–365. [Google Scholar]
- 31. Larney E, Larson SG (2004) Compliant walking in primates: elbow and knee yield in primates compared to other mammals. Am J Phys Anthropol 125: 42–50 doi:10.1002/ajpa.10366. [DOI] [PubMed] [Google Scholar]
- 32. Demes B, Larson SG, Stern JT, Jungers WL, Biknevicius AR, et al. (1994) The kinetics of primate quadrupedalism: “hindlimb drive” reconsidered. J Hum Evol 26: 353–374. [Google Scholar]
- 33. Patel BA (2009) Not so fast: speed effects on forelimb kinematics in cercopithecine monkeys and implications for digitigrade postures in primates. Am J Phys Anthropol 140: 92–112 doi:10.1002/ajpa.21039. [DOI] [PubMed] [Google Scholar]
- 34. Hildebrand M (1967) Symmetrical gaits of primates. Am J Phys Anthropol 26: 119–130. [Google Scholar]
- 35.Kimura T, Okada M, Ishida H (1979) Kinesiological characteristics of primate walking: its significance in human walking. In: Morbeck ME, Preuschoft H, Gomberg N, editors. Environment, behavior, and morphology: dynamic interactions in primates. New York: G. Fischer. 297–311.
- 36. Franz TM, Demes B, Carlson KJ (2005) Gait mechanics of lemurid primates on terrestrial and arboreal substrates. J Hum Evol 48: 199–217 doi:10.1016/j.jhevol.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 37. Wallace IJ, Demes B (2008) Symmetrical gaits of Cebus apella: implications for the functional significance of diagonal sequence gait in primates. J Hum Evol 54: 783–794 doi:10.1016/j.jhevol.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 38. Schmitt D, Hanna JB (2004) Substrate alters forelimb to hindlimb peak force ratios in primates. J Hum Evol 46: 239–254 doi:10.1016/j.jhevol.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 39. Altmann SA (1987) The impact of locomotor energetics on mammalian foraging. J Zool 211: 215–225. [Google Scholar]
- 40. Pontzer H (2007) Effective limb length and the scaling of locomotor cost in terrestrial animals. J Exp Biol 210: 1752–1761 doi:10.1242/jeb.002246. [DOI] [PubMed] [Google Scholar]
- 41. Henzi SP, Byrne RW, Whiten A (1992) Patterns of movement by baboons in the Drakensberg mountains: primary responses to the environment. Int J Primatol 13: 601–629. [Google Scholar]
- 42. Rose M (1977) Positional behaviour of olive baboons (Papio anubis) and its relationship to maintenance and social activities. Primates 18: 59–116. [Google Scholar]
- 43. Isbell LA, Pruetz JD, Lewis M, Young TP (1998) Locomotor activity differences between sympatric patas monkeys (Erythrocebus patas) and vervet monkeys (Cercopithecus aethiops): implications for the evolution of long hindlimb length in Homo . Am J Phys Anthropol 105: 199–207. [DOI] [PubMed] [Google Scholar]
- 44. Patel BA (2010) The interplay between speed, kinetics, and hand postures during primate terrestrial locomotion. Am J Phys Anthropol 141: 222–234 doi:10.1002/ajpa.21138. [DOI] [PubMed] [Google Scholar]
- 45. Patel BA, Polk JD (2010) Distal forelimb kinematics in Erythrocebus patas and Papio anubis during walking and galloping. Int J Primatol 31: 191–207 doi:10.1007/s10764-010-9394-6. [Google Scholar]
- 46. Patel BA, Wunderlich RE (2010) Dynamic pressure patterns in the hands of olive baboons (Papio anubis) during terrestrial locomotion: implications for cercopithecoid primate hand morphology. Anat Rec 293: 710–718 doi:10.1002/ar.21128. [DOI] [PubMed] [Google Scholar]
- 47.Zeininger A, Shapiro LJ, Raichlen DA (2009) The effect of digitigrade cheiridial postures on speed and gait in infant baboons. Am J Phys Anthropol, Suppl. 48: 279.
- 48. Rubin CT, Lanyon LE (1982) Limb mechanics as a function of speed and gait: a study of functional strains in the radius and tibia of horse and dog. J Exp Biol 101: 187–211. [DOI] [PubMed] [Google Scholar]
- 49. Rubin CT, Lanyon LE (1984) Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J Theor Biol 107: 321–327. [DOI] [PubMed] [Google Scholar]
- 50.Busse C (1980) Leopard and lion predation upon chacma baboons living in the Moremi Wildlife Reserve. Botswana Notes and Records: 15–21.
- 51. Carrier DR (1983) Postnatal ontogeny of the musculoskeletal system in the black-tailed jack rabbit (Lepus californicus). J Zool 201: 27–55. [Google Scholar]
- 52. Heinrich RE, Ruff CB, Adamczewski JZ (1999) Ontogenetic changes in mineralization and bone geometry in the femur of muskoxen (Ovibos moschatus). J Zool 247: 215–223. [Google Scholar]
- 53. Lammers AR, German RZ (2002) Ontogenetic allometry in the locomotor skeleton of specialized half-bounding mammals. J Zool 258: 485–495 doi:10.1017/S0952836902001644. [Google Scholar]
- 54. Young JW, Fernandez D, Fleagle JG (2010) Ontogeny of long bone geometry in capuchin monkeys (Cebus albifrons and Cebus apella): implications for locomotor development and life history. Biol Lett 6: 197–200 doi:10.1098/rsbl.2009.0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ruff C (2003) Ontogenetic adaptation to bipedalism: age changes in femoral to humeral length and strength proportions in humans, with a comparison to baboons. J Hum Evol 45: 317–349 doi:10.1016/j.jhevol.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Raichlen DA (2004) The relationship between limb muscle mass distribution and the mechanics and energetics of quadrupedalism in infant baboons (Papio cyncephalus). PhD Dissertation. University of Texas at Austin.
- 57. Ron T, Henzi SP, Motro U (1996) Do female chacma baboons compete for a safe spatial position in a southern woodland habitat? Behaviour 133: 475–490. [Google Scholar]
- 58. Henzi SP, Lycett JE, Weingrill T (1998) Mate guarding and risk assessment by male mountain baboons during inter-troop encounters. Anim Behav 55: 1421–1428. [DOI] [PubMed] [Google Scholar]
- 59. Hausfater G (1977) Tail carriage in baboons (Papio cynocephalus): relationship to dominance rank and age. Folia Primatol 27: 41–59. [DOI] [PubMed] [Google Scholar]
- 60.Schmitt D (2011) Translating primate locomotor biomechanical variables from the laboratory to the field. In: D'Août K, Vereecke EE, editors. Primate locomotion: linking field and laboratory research. New York: Springer. 7–27.
- 61. Wells JP, Turnquist JE (2001) Ontogeny of locomotion in rhesus macaques (Macaca mulatta): II. Postural and locomotor behavior and habitat use in a free-ranging colony. Am J Phys Anthropol 115: 80–94 doi:10.1002/ajpa.1059. [DOI] [PubMed] [Google Scholar]
- 62.Wang Q (2012) Bones, genetics, and behavior of Rhesus macaques: Macaca mulatta of Cayo Santiago and beyond. New York: Springer. 308 p.