Abstract
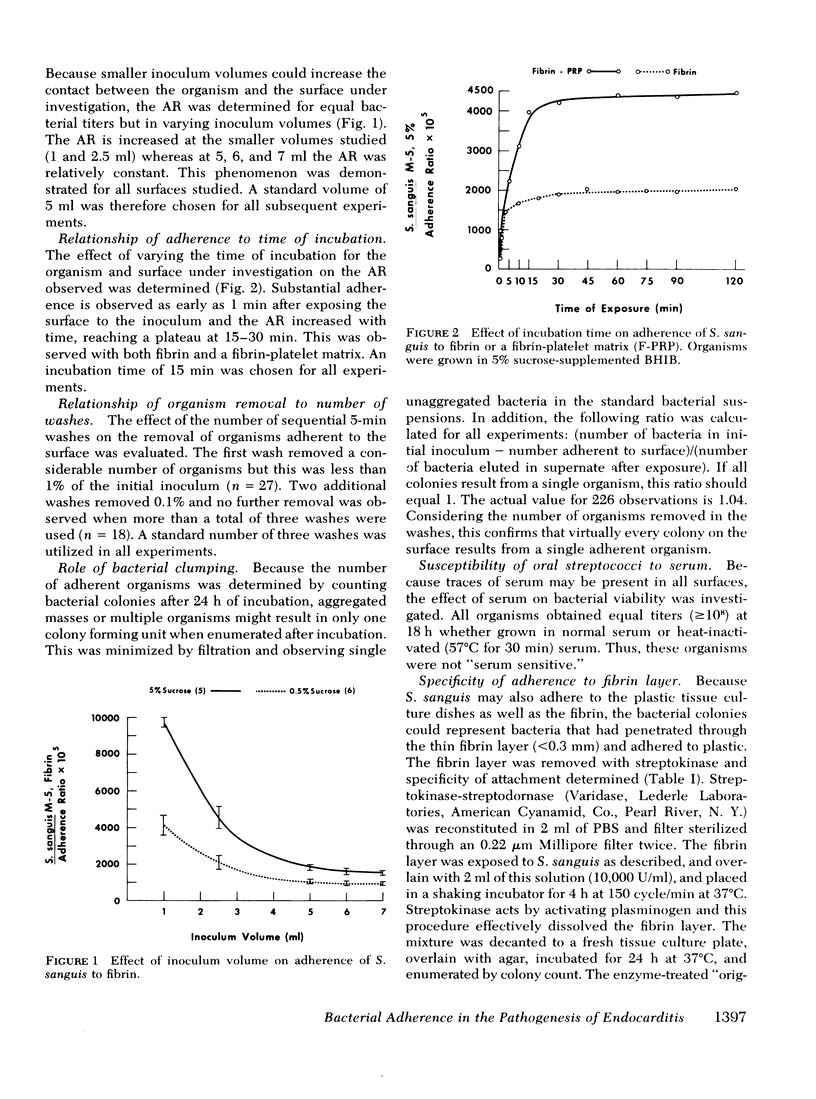
The role of dextran in the pathogenesis of bacterial endocarditis was investigated by studying the adherence of dextran producing oral streptococci to the constituents of nonbacterial thrombotic endocarditis (NBTE) in vitro and in vivo. The adherence of Streptococcus sanguis to fibrin and platelets was determined in an in vitro assay system simulating nonbacterial thrombotic endocarditis. Adherence was increased when the organisms were grown in sucrose-supplemented media (adherence ratio X 10(4), 177 +/- 6 in 5% sucrose vs. 140 +/- 7 in 0.5% sucrose, P less than 0.001), and decreased by incubating the organisms in dextranase (adherence ratio X 10(4), 117 +/- 16, P less than 0.001), an effect which was nullified by heat inactivating this enzyme (adherence ratio X 10(4), 192 +/- 7, P less than 0.001). The amount of dextran produced in broth by three different oral streptococci correlated directly with the adherence observed to fibrin and a fibrin-platelet matrix in vitro (P less than 0.001). These organisms adhered more readily to a fibrin-platelet matrix than to fibrin alone (adherence ratio X 10(4), 455 +/- 30 vs. 177 +/- 6, respectively, P less than 0.001). The role of dextran formation was also examined in vivo in rabbits with preexisting NBTE. After injection of 10(7) S. sanguis, 12 of 17 animals developed endocarditis. In contrast, when the organisms were pretreated with dextranase (an enzyme that removes dextran from the bacterial cell surface), the same inoculum resulted in endocarditis in only 5 of 19 animals (P less than 0.05). In addition, a fresh strain of S. sanguis that produced high levels of dextran (1,220 +/- 50 microgram/ml) and adhered avidly to fibrin (adherence ratio X 10(4), 220 +/- 11) produced endocarditis in 12 of 18 rabbits after injection of 10(7) organisms. Another isolate of the same strain that had been passed repeatedly in the laboratory produced less dextran (400 +/- 30 microgram/ml), adhered poorly to fibrin (adherence ratio X 10(4), 140 +/- 7), and produced endocarditis in only 3 of 14 rabbits under identical conditions (P less than 0.05). This study demonstrates that dextran production is important in the adherence of oral streptococci to the constituents of NBTE and may play a role in the pathogenesis of bacterial endocarditis by oral streptococci.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANGRIST A. A., OKA M. Pathogenesis of bacterial endocarditis. JAMA. 1963 Jan 26;183:249–252. doi: 10.1001/jama.1963.63700040009010b. [DOI] [PubMed] [Google Scholar]
- ASTRUP T., MULLERTZ S. The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys. 1952 Oct;40(2):346–351. doi: 10.1016/0003-9861(52)90121-5. [DOI] [PubMed] [Google Scholar]
- Clawson C. C., Rao G. H., White J. G. Platelet interaction with bacteria. IV. Stimulation of the release reaction. Am J Pathol. 1975 Nov;81(2):411–420. [PMC free article] [PubMed] [Google Scholar]
- Clawson C. C., White J. G. Platelet interaction with bacteria. I. Reaction phases and effects of inhibitors. Am J Pathol. 1971 Nov;65(2):367–380. [PMC free article] [PubMed] [Google Scholar]
- Daneo-Moore L., Terleckyj B., Shockman G. D. Analysis of growth rate in sucrose-supplemented cultures of Streptococcus mutans. Infect Immun. 1975 Nov;12(5):1195–1205. doi: 10.1128/iai.12.5.1195-1205.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972 Feb;53(1):44–49. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B., Petersdorf R. G. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. Br J Exp Pathol. 1973 Apr;54(2):142–151. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J Pathol. 1975 Feb;115(2):81–89. doi: 10.1002/path.1711150204. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. Parameters affecting the adherence and tissue tropisms of Streptococcus pyogenes. Infect Immun. 1974 Jan;9(1):85–91. doi: 10.1128/iai.9.1.85-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald R. J., Spinell D. M., Stoudt T. H. Enzymatic removal of artificial plaques. Arch Oral Biol. 1968 Jan;13(1):125–128. doi: 10.1016/0003-9969(68)90042-3. [DOI] [PubMed] [Google Scholar]
- Garrison P. K., Freedman L. R. Experimental endocarditis I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J Biol Med. 1970 Jun;42(6):394–410. [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. Induction of dental caries in gnotobiotic rats with a levan-forming streptococcus and a streptococcus isolated from subacute bacterial endocarditis. Arch Oral Biol. 1968 Mar;13(3):297–308. doi: 10.1016/0003-9969(68)90128-3. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Spinell D. M., Skobe Z. Selective adherence as a determinant of the host tropisms of certain indigenous and pathogenic bacteria. Infect Immun. 1976 Jan;13(1):238–246. doi: 10.1128/iai.13.1.238-246.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K., Ramirez-Ronda C. H., Holmes R. K., Sanford J. P. Adherence of bacteria to heart valves in vitro. J Clin Invest. 1975 Dec;56(6):1364–1370. doi: 10.1172/JCI108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheim B. Streptococci of dental plaques. Caries Res. 1968;2(2):147–163. doi: 10.1159/000259553. [DOI] [PubMed] [Google Scholar]
- Hamada S., Mizuno J., Murayama Y., Ooshima Y., Masuda N. Effect of dextranase on the extracellular polysaccharide synthesis of Streptococcus mutans; chemical and scanning electron microscopy studies. Infect Immun. 1975 Dec;12(6):1415–1425. doi: 10.1128/iai.12.6.1415-1425.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkate F., Brakman P. Letter: The fibrin plate assay -- some crucial conditions. Thromb Diath Haemorrh. 1975 Sep 30;34(1):312–313. [PubMed] [Google Scholar]
- Hohmann A., Wilson M. R. Adherence of enteropathogenic Escherichia coli to intestinal epithelium in vivo. Infect Immun. 1975 Oct;12(4):866–880. doi: 10.1128/iai.12.4.866-880.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook E. W., 3rd, Sande M. A. Role of the vegetation in experimental Streptococcus viridans endocarditis. Infect Immun. 1974 Dec;10(6):1433–1438. doi: 10.1128/iai.10.6.1433-1438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K., Ingersoll L. Differential inhibition of Streptococcus mutans in vitro adherence by anti-glucosyltransferase antibodies. Infect Immun. 1976 Jun;13(6):1775–1777. doi: 10.1128/iai.13.6.1775-1777.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner P. I., Weinstein L. Infective endocarditis in the antibiotic era. N Engl J Med. 1966 Jan 27;274(4):199–contd. doi: 10.1056/NEJM196601272740407. [DOI] [PubMed] [Google Scholar]
- Levison M. E., Carrizosa J., Tanphaichitra D., Schick P. K., Rubin W. Effect of aspirin on thrombogenesis and on production of experimental aortic valvular Streptococcus viridans endocarditis in rabbits. Blood. 1977 Apr;49(4):645–650. [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers L. L., Guinée P. A. Occurrence and characteristics of enterotoxigenic Escherichia coli isolated from calves with diarrhea. Infect Immun. 1976 Apr;13(4):1117–1119. doi: 10.1128/iai.13.4.1117-1119.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian J., Freedman M. L., Tanzer J. M., Lovelace S. M. Ultrastructure of Mutants of Streptococcus mutans with Reference to Agglutination, Adhesion, and Extracellular Polysaccharide. Infect Immun. 1974 Nov;10(5):1170–1179. doi: 10.1128/iai.10.5.1170-1179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven C. F., Jr, White J. C. A Study of Streptococci Associated with Subacute Bacterial Endocarditis. J Bacteriol. 1946 Jun;51(6):790–790. doi: 10.1128/jb.51.6.790-790.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka M., Shirota A., Angrist A. Experimental endocarditis. Endocrine factors in valve lesions on AV shunt rats. Arch Pathol. 1966 Jul;82(1):85–92. [PubMed] [Google Scholar]
- Olson G. A., Bleiweis A. S., Small P. A., Jr Adherence inhibition of Streptococcus mutans: an assay reflecting a possible role of antibody in dental caries prophylaxis. Infect Immun. 1972 Apr;5(4):419–427. doi: 10.1128/iai.5.4.419-427.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. T., Ball L. C. Streptococci and aerococci associated with systemic infection in man. J Med Microbiol. 1976 Aug;9(3):275–302. doi: 10.1099/00222615-9-3-275. [DOI] [PubMed] [Google Scholar]
- Pedersen K. B., Froholm L. O., Bovre K. Fimbriation and colony type of Moraxella bovis in relation to conjunctival colonization and development of keratoconjunctivitis in cattle. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):911–918. doi: 10.1111/j.0365-5563.1973.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Perlman B. B., Freedman L. R. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J Biol Med. 1971 Oct;44(2):206–213. [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D. E. Studies on bacteriemia. I. Mechanisms relating to the persistence of bacteriemia in rabbits following the intravenous injection of staphylococci. J Exp Med. 1956 Jun 1;103(6):713–742. doi: 10.1084/jem.103.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sande M. A., Irvin R. G. Penicillin-aminoglycoside synergy in experimental Streptococcus viridans endocarditis. J Infect Dis. 1974 May;129(5):572–576. doi: 10.1093/infdis/129.5.572. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Staat R. H., Harlander S. K. Dextranases from oral bacteria: inhibition of water-insoluble glucan production and adherence to smooth surfaces by Streptococcus mutans. Infect Immun. 1975 Aug;12(2):309–317. doi: 10.1128/iai.12.2.309-317.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. R., Hohmann A. W. Immunity to Escherichia coli in pigs: adhesion of enteropathogenic Escherichia coli to isolated intestinal epithelial cells. Infect Immun. 1974 Oct;10(4):776–782. doi: 10.1128/iai.10.4.776-782.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]




