Abstract
Oroxylin A, a natural flavonoid, is one of the main bioactive compounds that underlie the anti-inflammatory effect of the medicinal herb Scutellaria baicalensis Georgi widely used in southeastern Asia; however, the molecular mechanisms for the therapeutic benefits remain largely unclear. In this study, we found that Oroxylin A induces estrogen-responsive gene expression and promoter activity. In macrophages, Oroxylin A treatment significantly attenuates lipopolysaccharide (LPS)-induced but not basal inflammatory response, including nitric oxide (NO) production and the expression of inflammatory mediators (i.e., iNOS and COX-2) and cytokines (i.e., TNF-α, IL-1β, and IL-6), in an estrogen receptor (ER)-dependent manner. Oroxylin A treatment also dramatically decreases LPS-induced secretion of pro-inflammatory cytokines. Furthermore, the downregulation of all these inflammatory parameters by Oroxylin A was abolished when cells were pretreated with specific ER antagonist. Thus, Oroxylin A is a novel phytoestrogen and exhibits anti-inflammatory effects that are mediated by ER activity.
Introduction
Oroxylin A (C16H12O5, Figure S1) is one of the main bioactive compounds purified from the root of the medicinal herb Scutellaria baicalensis Georgi that has been widely used in China, Japan, and Korea for treating inflammation and infections in the respiratory and gastrointestinal system [1]. Although many biological activities of Oroxylin A, including anti-tumor [2,3], anti-bacterial [4], and cognitive enhancement [5], have been reported, the underlying molecular mechanisms are largely unknown.
Oroxylin A is a natural flavonoid, and flavonoids are a class of polyphenolic compounds. Studies from our group and others have showed that some flavonoids have estrogenic activity [6–10] and thus, are named phytoestrogens. Whether Oroxylin A is also a phytoestrogen remains to be determined.
The effects of estrogen and phytoestrogens are mediated through two well-characterized intracellular receptors, estrogen receptor (ER) α and β [11,12]. ERs are members of the nuclear receptor superfamily and act as a ligand-activated transcription factors to regulate the expression of target genes. They are expressed in various immune cells, including macrophages [13,14] that play a critical role in many inflammatory diseases by expressing pro-inflammatory mediators, including tumor necrosis factor (TNF)-α, Interleukin (IL)-1, interleukin (IL)-6, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS) [15,16]. Among various inflammatory stimuli, lipopolysaccharide (LPS) is most frequently used to study macrophage biology [17].
In this study, we have evaluated the estrogenic activity of Oroxylin A and investigated its anti-inflammatory properties by using an in vitro model system where the inflammatory response is induced in RAW 264.7 macrophages by LPS treatment [18]. Our results suggest that Oroxylin A is a new phytoestrogen, and it activates the expression of estrogen target genes and potently attenuates LPS-induced expression of a panel of pro-inflammatory mediators through the activity of ERs.
Materials and Methods
Reagents
Oroxylin A was purchased from the Chinese Institute for Drug and Biological Product Control (Beijing, China). Charcoal dextran–stripped FBS (CD–FBS) was purchased from Biological Industries (Kibbutz Beit Haeme of Israel). Specific ER antagonist ICI 182,780 was purchased from Tocris Bioscience (Ellisville, MO, USA). IL-1β, IL-6, and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were obtained from R&D Systems (Minneapolis, MN, USA). Griess Reagent System was purchased from Beyotime (Nanjin, Jiangsu, China). TaqMan Reverse Transcription Reagents and SYBR Green PCR Master Mix reagent kit were obtained from Applied Biosystems (Foster City, CA, USA). Nuclear Extraction kit was purchased from Millipore (Billerica, MA, USA). 17β-estradiol (E2), ICI 182,780, and Oroxylin A were dissolved in DMSO and further diluted in cell culture media so that the final DMSO concentration did not exceed 0.1% v/v. All other reagents used in cell culture and transfection were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell Culture
HeLa, MCF-7, and RAW264.7 cells were obtained from American Type Culture Collection (ATCC). Cells were maintained in high glucose Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS and penicillin and streptomycin in a 37°C humidified incubator containing 5% CO2.
Transient transfection and reporter assay
Mammalian expression vectors ERα and ERβ were gifts from Dr. R.H. Karas (Tufts Medical Center, Boston, USA). The luciferase reporter plasmid carrying 3× vitellogenin ERE was kindly provided by Dr. J. Zhang (Nankai University, Tianjin, China). Cells were plated in triplicate in 24-well plates at a density of 2×105 cells/well in 10% CD–FBS. After attachment and growth for 24 h, the cells were co-transfected with the reporter plasmid ERE-TK-Luc, ERα/βexpression plasmids, and pRL-TK control plasmid, which contains a Renilla luciferase gene to allow for normalizing transfection efficiency. Transfection was performed in serum-free, antibiotic-free DMEM media using Lipofectamine 2000 (Invitrogen/Life Technologies, Carlsbad, CA) according to manufacturer’s instructions. Then, the cells were treated with individual test compounds for 24 h and lysed. Aliquots from each well were divided into two 96-well plates for luciferase and renillia activity determination using a luminescence counter (Flexstation 3 Molecular Devices Company, CA, USA). Experiments were performed at least three times and the data were assessed as units of firefly luciferase activities normalized to the renilla luciferase control activities from individual wells.
RNA isolation and quantitative real time RT-PCR (qRT-PCR)
For assessments of pS2 mRNA expression, MCF-7 cells were seeded into 6-well plates at a density of 5×105 cells per well and incubated with phenol red-free DMEM supplemented with 3% CD–FBS and maintained in 5% CO2 in air at 37°C for overnight. Then, test compounds were added to the medium and 10 h later, the medium was removed and the cells were scraped from the dish for RNA extraction. The total RNA was purified with TRIzol reagent (GIBCO) according to the manufacturer’s protocol. The RNA samples were treated with DNase I, and cDNA was made from each sample. cDNAs of the pS2 and an internal reference gene (GAPDH) were quantified using a fluorescence-based real time detection method (SYBR Green PCR Master Mix reagent kits, Applied Biosystems, Foster City, USA), which was performed with the ABI PRISM 7300 Sequence Detection System. The sequences of primers for amplification of human pS2 are reported in Table S1. Data were analyzed by using the comparative threshold cycle (Ct) method. Ct values from the genes of interest were normalized with the values from corresponding GAPDH reactions.
For assessments of the mRNA expression of pro-inflammatory mediators, the RAW264.7 cells were grown in 6-well plates in phenol red-free DMEM with 10% CD–FBS. After approximately 80% confluence, cells were pretreated with Oroxylin A for 2 h and then stimulated with LPS (1µg/ml) for an additional 8 h. Total RNA extracted from RAW264.7 cells by using the Trizol reagent (Invitrogen) was reverse-transcribed into cDNA with TaqMan Reverse Transcription Reagents. Real time PCR was performed using SYBR Green PCR Master Mix reagent kits (Applied Biosystems) and the specific primers (Table S1).
Measurement of nitric oxide (NO) in the cell culture medium
The RAW264.7 cells were plated in 48-well plates at a density of 2x106 cells per well in phenol red-free DMEM with 10% CD–FBS for 24 h. After pretreatment with Oroxylin A for 2 h the cells were stimulated with LPS (1µg/ml) in serum-free DMEM for an additional 18 h. The supernatant from the cultured cells was collected and centrifuged to remove cell debris and transferred to 96-well plates, and then reacted using a NO detection kit (Beyotime, China). Values were calculated by measuring the absorbance at 540 nm using a plate reader.
Enzyme-linked immunosorbent assay (ELISA)
One day after seeding in 48-well plates in phenol red-free DMEM, the RAW264.7 cells were treated with Oroxylin A and 2 h later, stimulated with LPS (1µg/ml) for additional 18 h. Then the medium was collected from each well and centrifuged and the supernatant was analyzed by using special ELISA kits for IL-1β, IL-6 and TNFα(R&D systems Inc, Minneapolis, MN) as directed by the manufacturer’s instructions.
Statistical analysis
The results are expressed as mean ± S.D. ANOVA followed by a post hoc multiple comparison was performed. Dunnett’s test was used to compare increasing doses of the test compounds with the respective control. A P-value less than 0.05 is considered significant.
Results
Oroxylin A induces estrogen-responsive gene expression and promoter activity through estrogen receptor (ER) activity
We initiated our investigation by assessing whether Oroxylin A displays estrogenic activity. ERE-dependent promoter-luciferase reporter assays were performed in HeLa cells that had been transfected with plasmids coding for ERα or ERβ and then treated with estradiol (E2) or Oroxylin A (at concentrations from 10-7 ~10-5 M). Oroxylin A treatment increased the activity of both ERα and ERβ in a concentration-dependent manner (Figure 1).
Figure 1. Oroxylin A activates the transcriptional activity of ERα and ERβ.
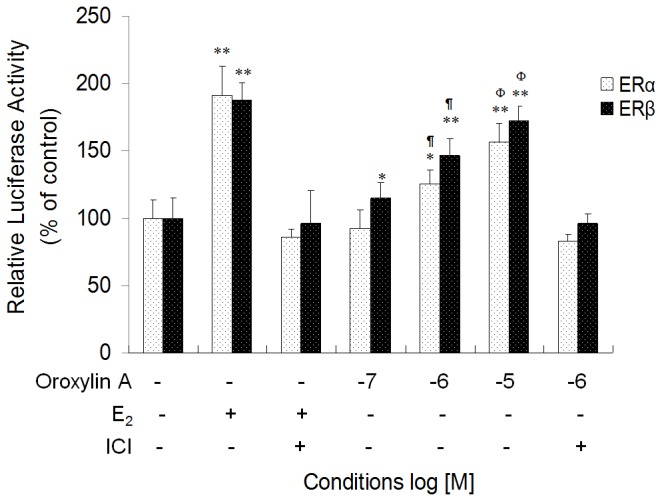
HeLa cells were co-transfected with pERE-luc, pRL-TK, and a plasmid coding for ERα or ERβ and 18 h later, treated with Vehicle (-), Estrogen (E2; 10-8 M), Oroxylin A (10-7–10-5M), and/or ICI 182,780 (10-7 M) for an additional 24 h. Then, ER activities (i.e., Luciferase activities) of different treatment groups were measured and presented as fold increase relative to the levels in cells treated with Vehicle only. n=3 per group; *p<0.05, **p<0.01 vs. Vehicle controls. ¶P < 0.05 vs. cells treated with Oroxylin A (10-7 M); Φ P < 0.05 vs. cells treated with Oroxylin A (10-6 M). Shown is representative of three independent experiments with similar results.
Next, we evaluated whether Oroxylin A activates the expression of endogenous estrogen target genes. MCF-7 cells were treated with E2 and serial concentrations of Oroxylin A for 10 h and then, analyzed for the mRNA expression of pS2 gene by qRT-PCR. Oroxylin A induced a marked increase in pS2 mRNA expression, which peaked 2.2 folds-of-induction (Figure 2), while the cell viability was unaffected (Figure S2 ). Notably, the induction of both pS2 mRNA expression and ERE promoter activity were abolished when the cells were co-treated with specific ER antagonist ICI 182,780 (Figures 1 and 2). These results suggest that Oroxylin A can induce transcription of estrogen target genes through the action of ER. Thus, Oroxylin A is an estrogen mimetic or a “phytoestrogen”.
Figure 2. Oroxylin A induces mRNA expression of endogenous estrogen target gene pS2.
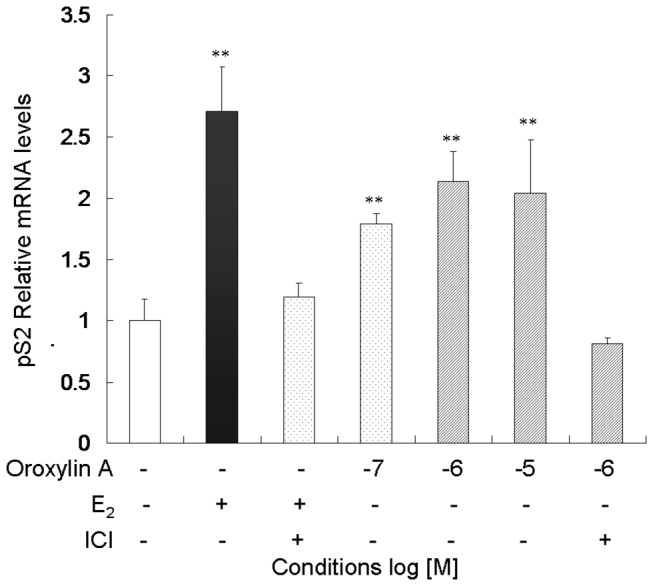
MCF-7 cells were incubated for 10 h in the presence of Vehicle (-), E2 (10-8 M), ICI 182,780 (10-7 M), and/or Oroxylin A at indicated concentrations and then subjected to qRT-PCR analyses. N=3 per treatment; **p<0.01 vs. Vehicle (-). Shown is representative of three independent experiments with similar results.
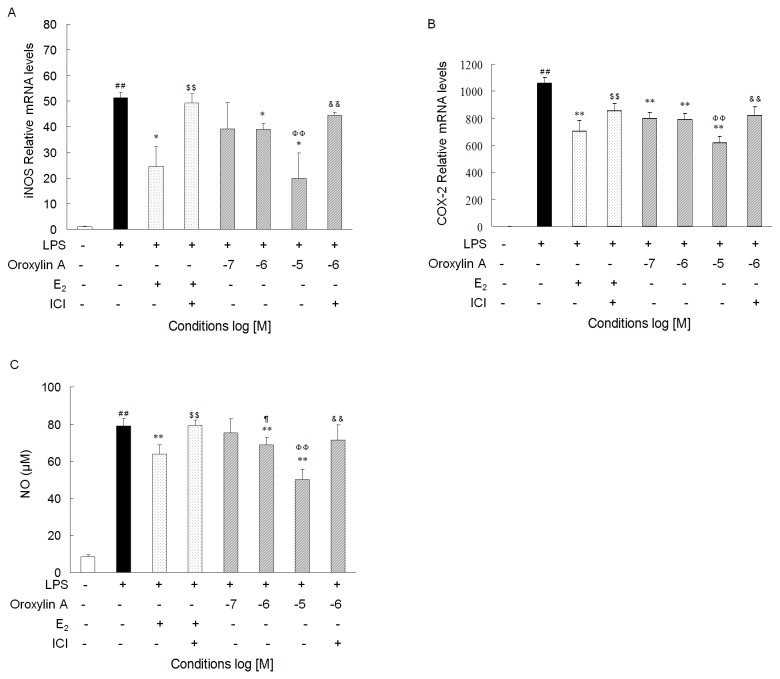
Oroxylin A treatment attenuates LPS-induced iNOS and COX-2 expression and the production of nitric oxide (NO) in macrophages in an ER-dependent manner
Because estrogen plays an important role in the regulation of inflammation, we investigated whether Oroxylin A modulates LPS-induced inflammatory response in macrophages. RAW264.7 cells were incubated with Oroxylin A, E2, or vehicle for 2 h; then LPS was added, and the cells were culture for additional 8 h before assessments of iNOS and COX-2 expression. LPS treatment led to a marked increase in the levels of iNOS and COX-2 mRNAs (Figure 3A-B). Pre-treatment with Oroxylin A and E2, but not with vehicle, significantly attenuated LPS induction of iNOS and COX-2 expression. However, the repressor effects of Oroxylin A and E2 was largely abrogated when the cells were co-treated with ICI 182,780 (Figure 3A-B).
Figure 3. Oroxylin A suppresses LPS-induced iNOS and COX-2 mRNA expression and the production of NO in ER-dependent manner.
(A–B) RAW 264.7 cells were pre-incubated with Oroxylin A at indicated concentrations, E2 (10-8 M), and/or ICI 182,780 (10-7 M) for 2 h, then LPS was added (1µg/ml), and the cells were cultured for an additional 8 h. The iNOS and COX-2 gene expression was analyzed by qRT-PCR. N=3; *P < 0.05, **P < 0.01 vs. cells treated with LPS only; # # P < 0.01 vs. Vehicle; $ $ P < 0.01 vs. cells treated with LPS plus E2 (10-8 M); & & P < 0.01 vs. cells treated with LPS plus Oroxylin A in the same concentration. (C) RAW 264.7 cells were treated with LPS (1 µg/ml), Oroxylin A, E2 (10-8 M), and/or ICI 182,780 (10-7 M) for 18 h, and the amount of nitrite in the supernatant of each treatment group was quantified using Griess reagent. N=5; **P < 0.01 vs. cells treated with LPS only; # # P < 0.01 vs. Vehicle; $ $ P < 0.01 vs. cells treated with LPS plus E2 (10-8 M); & & P < 0.01 vs. cells treated with LPS plus Oroxylin A in the same concentration. ¶P < 0.05 vs. cells treated with LPS plus Oroxylin A (10-7 M); ΦΦ P < 0.01 vs. cells treated with LPS plus Oroxylin A (10-6 M). Shown are representatives of three independent experiments with similar results.
Because iNOS catalyzes the generation of NO, we measured NO in the culture media of cells that had been treated with LPS for 18 h with or without pre-treatment with Oroxylin A or E2. Consistently, we found that NO was markedly induced by LPS treatment, that the LPS induction of NO is repressed by E2 and Oroxylin A in a concentration-dependent manner, and that the repressor effects of E2 and Oroxylin A on LPS induction of NO were abolished by co-treatment of cells with ICI 182,780 (Figure 3C). Collectively, these results suggest that Oroxylin A represses LPS-induced expression of inflammatory mediators, and this repressor effect of Oroxylin A is mediated by ER activity. Of note, neither a cytotoxic effect (CCK assay) nor an alteration of cell viability (MTT assay) was observed in RAW264.7 cells treated with Oroxylin A at the experimental concentrations (i.e., 10-7M, 10-6M, and 10-5M) (data not shown).
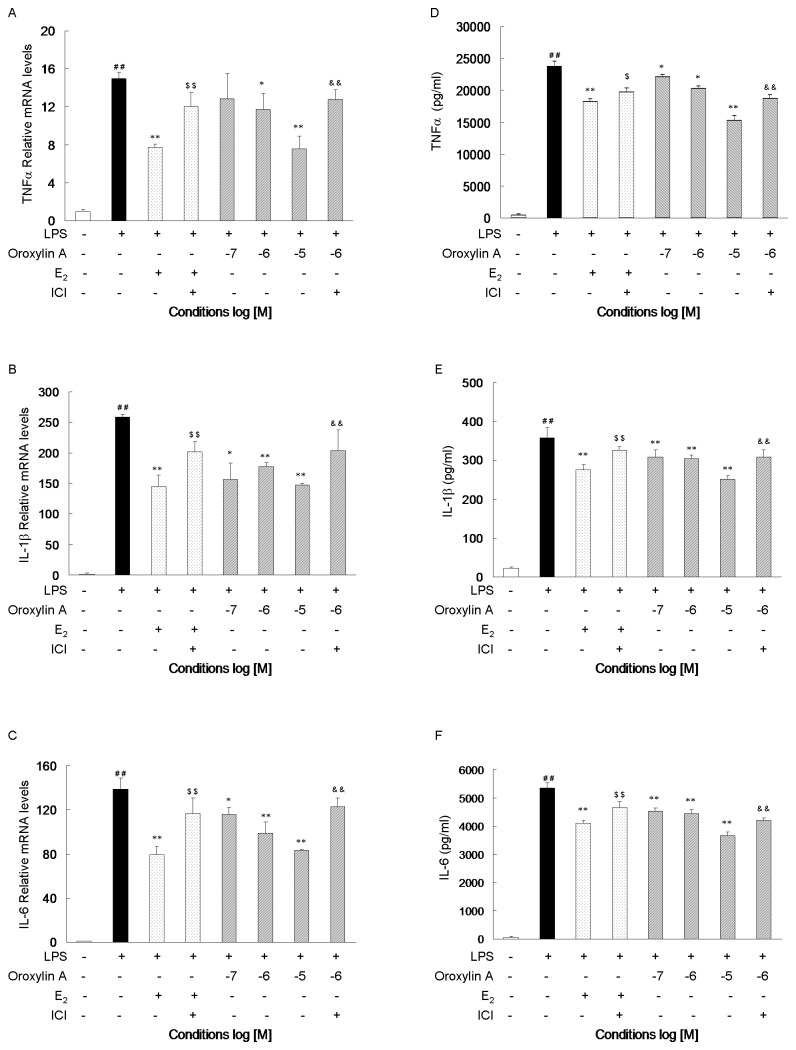
Oroxylin A treatment suppresses LPS-induced secretion of pro-inflammatory Cytokines in an ER-dependent manner
Because TNF-α, IL-1β and IL-6 play a critical role in the inflammatory process, we investigated the effect of Oroxylin A on their expression. RAW264.7 cells were treated for 6 h and 18 h with or without co-treatment with Oroxylin A, E2, or ICI, and then analyzed for mRNA expression (qRT-PCR) and protein secretion (ELISA), respectively (Figure 4). Consistent with our observations with iNOS, COX-2, and NO, treatment of cells with Oroxylin A suppressed the LPS-induced mRNA expression and secretion of TNF-α, IL-1β, and IL-6 in a concentration-dependent manner (Figure 4). Furthermore, the Oroxylin A-mediated repression of mRNA expression and protein secretion were largely abolished by ICI co-treatment.
Figure 4. Oroxylin A suppresses LPS-induced mRNA expression and secretion of TNF-α, IL-1β, and IL-6 in ER-dependent manner.
(A–C) RAW 264.7 cells were incubated with Oroxylin A, E2 (10-8 M), and/or ICI 182,780 (10-7 M) for 2 h, then LPS was added (1µg/ml), and the cells were cultured for an additional 6 h before qRT-PCR analyses. (D–F) RAW 264.7 cells were cultured with LPS (1µg/ml), Oroxylin A, E2 (10-8 M), and/or ICI 182,780 (10-7 M) for 18 h and then, the amount of cytokines in the supernatant were quantified by ELISA. N=3; *P < 0.05**P < 0.01 vs. cells treated with LPS only; # # P < 0.01 vs. Vehicle; $ P < 0.05, $ $ P < 0.01 vs. cells treated with LPS plus E2 (10-8 M); & & P < 0.01 vs. cells treated with LPS plus Oroxylin A in the same concentration. Shown are representatives of three independent experiments with similar results.
Discussion
In this study, we found that Oroxylin A is a new phytoestrogen that activates estrogen-responsive promoter activity and the expression of endogenous estrogen target genes. Our results also indicate that Oroxylin A displays a potent anti-inflammatory activity, attenuating LPS-induced expression of pro-inflammatory mediators and cytokines in macrophages. The anti-inflammatory effects and ER transcriptional activity of Oroxylin A occur at similar concentrations, and importantly all these Oroxylin A functions appear to be mediated by ER activity.
Macrophages are recognized as an important source of inflammatory factors including NO, iNOS, COX-2, TNF-α, IL-1β and IL-6, and therefore, represent a cellular target for the prevention, control, and cure of inflammatory damages [17,19–21]. We observed that the anti-inflammatory effects of Oroxylin in macrophages are abolished by co-administration of a specific ER antagonist, suggesting that activation of ER is a necessary step in mediating the inflammatory proteins synthesis. These findings are in agreement with previous observations made by us and other groups, which demonstrated that estrogen decreases production of inflammatory cytokines and inhibits macrophage infiltration in damaged tissues [22–27]. Interestingly, some other flavonoids have also been shown to inhibit NO production in response to inflammatory stimuli [28–31]. Collectively, our data and the results from other labs suggest that ERs in macrophage could serve a molecular target for reducing inflammation.
A main finding of our study is that Oroxylin A activates ERs. Such a function is also supported by its polyphenolic structures. Nevertheless, the domains mediating the interaction between Oroxylin A and ERs and other potential associating proteins are yet to be identified. An outstanding question is whether Oroxylin A-induced ER activation directly down-regulates the transcription of those pro-inflammatory genes. This is intriguing particularly for iNOS and COX-2 since the promoters of both genes do not appear to contain a recognizable ERE. One explanation is that iNOS and COX-2 may be regulated indirectly by other Oroxylin A-induced genes. Alternatively, the activated ERs may interact and form complex with other transcription factors, such as NF-κB, AP-1, and STATs and prevent them from binding to their cognate response elements [32,33]. Clearly, these hypotheses need to be tested in future studies.
In conclusion, Oroxylin A possesses estrogenic activity and inhibits LPS-elicited expression of pro-inflammatory mediators and cytokines through ER activity. In the last decade, there has been an increasing interest in the use of natural products to modulate inflammatory disorders because of their lesser side effects and cytotoxicity. The observations made in this study suggest the mode of actions of phytoestrogenic compounds in preventing inflammation, which may be helpful in developing new therapeutics for the treatment of inflammatory disease, especially in the population of aging women with low level of estrogen.
Supporting Information
(TIF)
MCF-7 cells were incubated in the presence of Vehicle (-) and Oroxylin at concentrations from 10-7-10-5 M for 24 h and then, cell viability was assessed with MTT assay. N=3; NS, not significant.
(TIF)
(DOC)
Acknowledgments
We thank Dr. R.H. Karas (Tufts Medical Center, Boston, USA) for providing Mammalian expression vectors ERα and ERβ Dr. J. Zhang (Nankai University, Tianjin, China) for luciferase reporter plasmid.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81173592, 81125024), Tianjin Applied Basic Research and Frontier Technological Program (11JCZDJC21100), and the Program for Changjiang Scholars and Innovative Research Team in University, PCSIRT (IRT0973,IRT1276). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hu (1996) National regulatory authority for Chinese medicinal drugs (Chinese). Shanghai: Shanghai Science and Technology.
- 2. Hu Y, Yang Y, You QD, Liu W, Gu HY et al. (2006) Oroxylin A induced apoptosis of human hepatocellular carcinoma cell line HepG2 was involved in its antitumor activity. Biochem Biophys Res Commun 351: 521-527. doi:10.1016/j.bbrc.2006.10.064. PubMed: 17069758. [DOI] [PubMed] [Google Scholar]
- 3. Mu R, Qi Q, Gu H, Wang J, Yang Y et al. (2009) Involvement of p53 in oroxylin A-induced apoptosis in cancer cells. Mol Carcinog 48: 1159-1169. doi:10.1002/mc.20570. PubMed: 19626645. [DOI] [PubMed] [Google Scholar]
- 4. Suresh Babu K, Hari Babu T, Srinivas PV, Sastry BS, Hara Kishore K et al. (2005) Synthesis and in vitro study of novel 7-O-acyl derivatives of Oroxylin A as antibacterial agents. Bioorg Med Chem Lett 15: 3953-3956. doi:10.1016/j.bmcl.2005.05.045. PubMed: 16046127. [DOI] [PubMed] [Google Scholar]
- 5. Lee S, Kim DH, Lee DH, Jeon SJ, Lee CH et al. (2010) Oroxylin A, a flavonoid, stimulates adult neurogenesis in the hippocampal dentate gyrus region of mice. Neurochem Res 35: 1725-1732. doi:10.1007/s11064-010-0235-y. PubMed: 20680459. [DOI] [PubMed] [Google Scholar]
- 6. Wanda GJ, Njamen D, Yankep E, Fotsing MT, Fomum ZT et al. (2006) Estrogenic properties of isoflavones derived from Millettia griffoniana. Phytomedicine 13: 139-145. doi:10.1016/j.phymed.2005.06.003. PubMed: 16428019. [DOI] [PubMed] [Google Scholar]
- 7. Zhang G, Qin L, Shi Y (2007) Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: a 24-month randomized, double-blind and placebo-controlled trial. J Bone Miner Res 22: 1072-1079. doi:10.1359/jbmr.070405. PubMed: 17419678. [DOI] [PubMed] [Google Scholar]
- 8. Wilkinson AP, Wähälä K, Williamson G (2002) Identification and quantification of polyphenol phytoestrogens in foods and human biological fluids. J Chromatogr B Anal Technol Biomed Life Sci 777: 93-109. doi:10.1016/S1570-0232(02)00095-8. PubMed: 12270203. [DOI] [PubMed] [Google Scholar]
- 9. Xin D, Wang H, Yang J, Su YF, Fan GW et al. (2010) Phytoestrogens from Psoralea corylifolia reveal estrogen receptor-subtype selectivity. Phytomedicine 17: 126-131. doi:10.1016/j.phymed.2009.05.015. PubMed: 19577453. [DOI] [PubMed] [Google Scholar]
- 10. Wang Hong, Li Ming-Cong, Yang Jing, Yang Dan, Yan-Fang Su et al. (2011) Estrogenic properties of six compounds derived from Eucommia ulmoides Oliv. and their differing biological activity through estrogen receptors a and b. Food Chemistry 129: 408-416 [DOI] [PubMed]
- 11. Kuiper GG, Gustafsson JA (1997) The novel estrogen receptor-beta subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett 410: 87-90. doi:10.1016/S0014-5793(97)00413-4. PubMed: 9247129. [DOI] [PubMed] [Google Scholar]
- 12. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J et al. (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138: 863-870. doi:10.1210/en.138.3.863. PubMed: 9048584. [DOI] [PubMed] [Google Scholar]
- 13. Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT (1995) Estrogens, the immune response and autoimmunity. Clin Exp Rheumatol 13: 217-226. PubMed: 7656468. [PubMed] [Google Scholar]
- 14. Vegeto E, Pollio G, Pellicciari C, Maggi A (1999) Estrogen and progesterone induction of survival of monoblastoid cells undergoing TNF-alpha-induced apoptosis. FASEB J 13: 793-803. PubMed: 10224223. [DOI] [PubMed] [Google Scholar]
- 15. Grip O, Janciauskiene S, Lindgren S (2003) Macrophages in inflammatory bowel disease. Curr Drug Targets Inflamm Allergy 2: 155-160. doi:10.2174/1568010033484179. PubMed: 14561168. [DOI] [PubMed] [Google Scholar]
- 16. Kim SS, Oh OJ, Min HY, Park EJ, Kim Y et al. (2003) Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci 73: 337-348. doi:10.1016/S0024-3205(03)00288-1. PubMed: 12757841. [DOI] [PubMed] [Google Scholar]
- 17. Barrios-Rodiles M, Tiraloche G, Chadee K (1999) Lipopolysaccharide modulates cyclooxygenase-2 transcriptionally and posttranscriptionally in human macrophages independently from endogenous IL-1 beta and TNF-alpha. J Immunol 163: 963-969. PubMed: 10395693. [PubMed] [Google Scholar]
- 18. Han HS, Qiao Y, Karabiyikoglu M, Giffard RG, Yenari MA (2002) Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J Neurosci 22: 3921-3928. PubMed: 12019311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duval DL, Miller DR, Collier J, Billings RE (1996) Characterization of hepatic nitric oxide synthase: identification as the cytokine-inducible form primarily regulated by oxidants. Mol Pharmacol 50: 277-284. PubMed: 8700134. [PubMed] [Google Scholar]
- 20. Willoughby DA, Moore AR, Colville-Nash PR (2000) COX-1, COX-2, and COX-3 and the future treatment of chronic inflammatory disease. Lancet 355: 646-648. doi:10.1016/S0140-6736(99)12031-2. PubMed: 10696997. [DOI] [PubMed] [Google Scholar]
- 21. Krakauer T (2004) Molecular therapeutic targets in inflammation: cyclooxygenase and NF-kappaB. Curr Drug Targets Inflamm Allergy 3: 317-324. doi:10.2174/1568010043343714. PubMed: 15379601. [DOI] [PubMed] [Google Scholar]
- 22. Hayashi T, Yamada K, Esaki T, Muto E, Chaudhuri G et al. (1998) Physiological concentrations of 17beta-estradiol inhibit the synthesis of nitric oxide synthase in macrophages via a receptor-mediated system. J Cardiovasc Pharmacol 31: 292-298. doi:10.1097/00005344-199802000-00016. PubMed: 9475272. [DOI] [PubMed] [Google Scholar]
- 23. Zancan V, Santagati S, Bolego C, Vegeto E, Maggi A et al. (1999) 17beta-estradiol decreases nitric oxide synthase II synthesis in vascular smooth muscle cells. Endocrinology 140: 2004-2009. doi:10.1210/en.140.5.2004. PubMed: 10218948. [DOI] [PubMed] [Google Scholar]
- 24. Cuzzocrea S, Santagati S, Sautebin L, Mazzon E, Calabrò G et al. (2000) 17beta-estradiol antiinflammatory activity in carrageenan-induced pleurisy. Endocrinology 141: 1455-1463. doi:10.1210/en.141.4.1455. PubMed: 10746650. [DOI] [PubMed] [Google Scholar]
- 25. Saito S, Aras RS, Lou H, Ramwell PW, Foegh ML (1999) Effects of estrogen on nitric oxide synthase expression in rat aorta allograft and smooth muscle cells. J Heart Lung Transplant 18: 937-945. doi:10.1016/S1053-2498(99)00060-1. PubMed: 10561103. [DOI] [PubMed] [Google Scholar]
- 26. Cuzzocrea S, Mazzon E, Sautebin L, Serraino I, Dugo L et al. (2001) The protective role of endogenous estrogens in carrageenan-induced lung injury in the rat. Mol Med 7: 478-487. PubMed: 11683373. [PMC free article] [PubMed] [Google Scholar]
- 27. Mendelsohn ME, Karas RH (1999) The protective effects of estrogen on the cardiovascular system. N Engl J Med 340: 1801-1811. doi:10.1056/NEJM199906103402306. PubMed: 10362825. [DOI] [PubMed] [Google Scholar]
- 28. Wang J, Mazza G (2002) Inhibitory effects of anthocyanins and other phenolic compounds on nitric oxide production in LPS/IFN-gamma-activated RAW. p. 264.7 macrophages. J Agric Food Chem 50: 850-857. [DOI] [PubMed]
- 29. Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP (1999) Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem Pharmacol 58: 759-765. doi:10.1016/S0006-2952(99)00160-4. PubMed: 10449184. [DOI] [PubMed] [Google Scholar]
- 30. Kim AR, Cho JY, Zou Y, Choi JS, Chung HY (2005) Flavonoids differentially modulate nitric oxide production pathways in lipopolysaccharide-activated RAW264.7 cells. Arch Pharm Res 28: 297-304. doi:10.1007/BF02977796. PubMed: 15832817. [DOI] [PubMed] [Google Scholar]
- 31. Raso GM, Meli R, Di Carlo G, Pacilio M, Di Carlo R (2001) Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci 68: 921-931. doi:10.1016/S0024-3205(00)00999-1. PubMed: 11213362. [DOI] [PubMed] [Google Scholar]
- 32. Xie QW, Kashiwabara Y, Nathan C (1994) Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem 269: 4705-4708. PubMed: 7508926. [PubMed] [Google Scholar]
- 33. Faulds MH, Pettersson K, Gustafsson JA, Haldosén LA (2001) Cross-talk between ERs and signal transducer and activator of transcription 5 is E2 dependent and involves two functionally separate mechanisms. Mol Endocrinol 15: 1929-1940. doi:10.1210/me.15.11.1929. PubMed: 11682624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
MCF-7 cells were incubated in the presence of Vehicle (-) and Oroxylin at concentrations from 10-7-10-5 M for 24 h and then, cell viability was assessed with MTT assay. N=3; NS, not significant.
(TIF)
(DOC)