Abstract
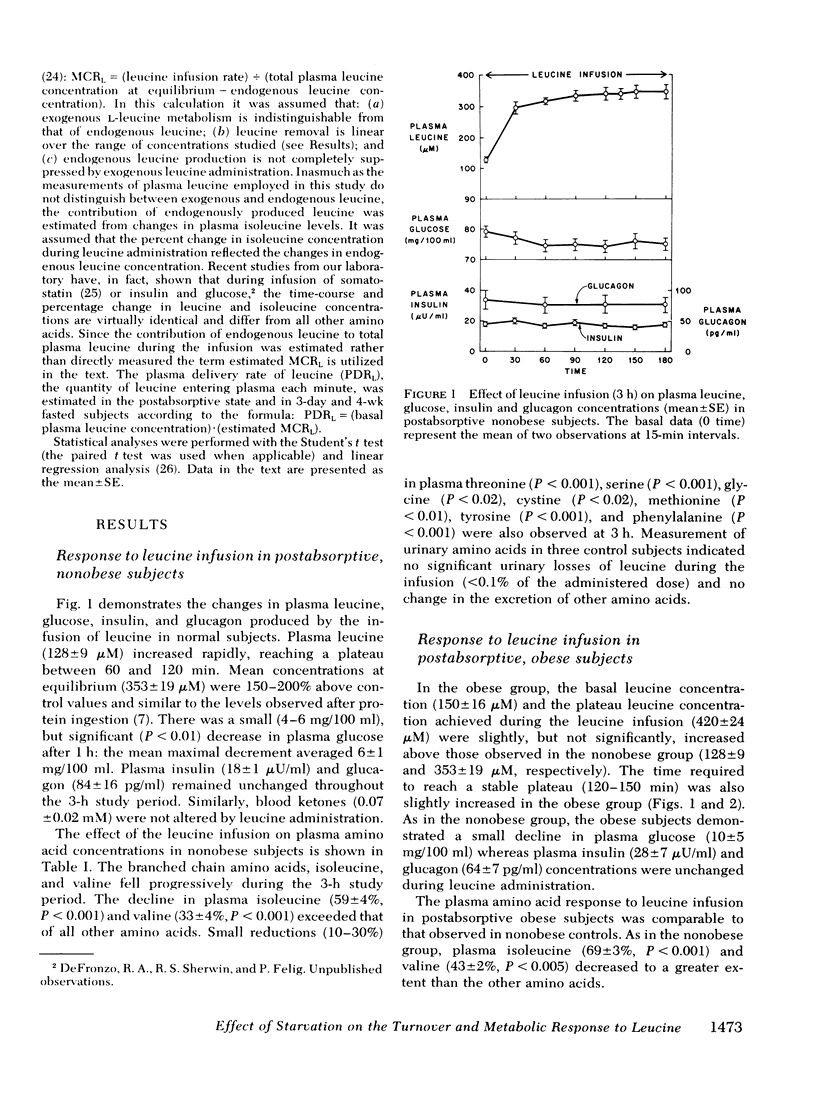
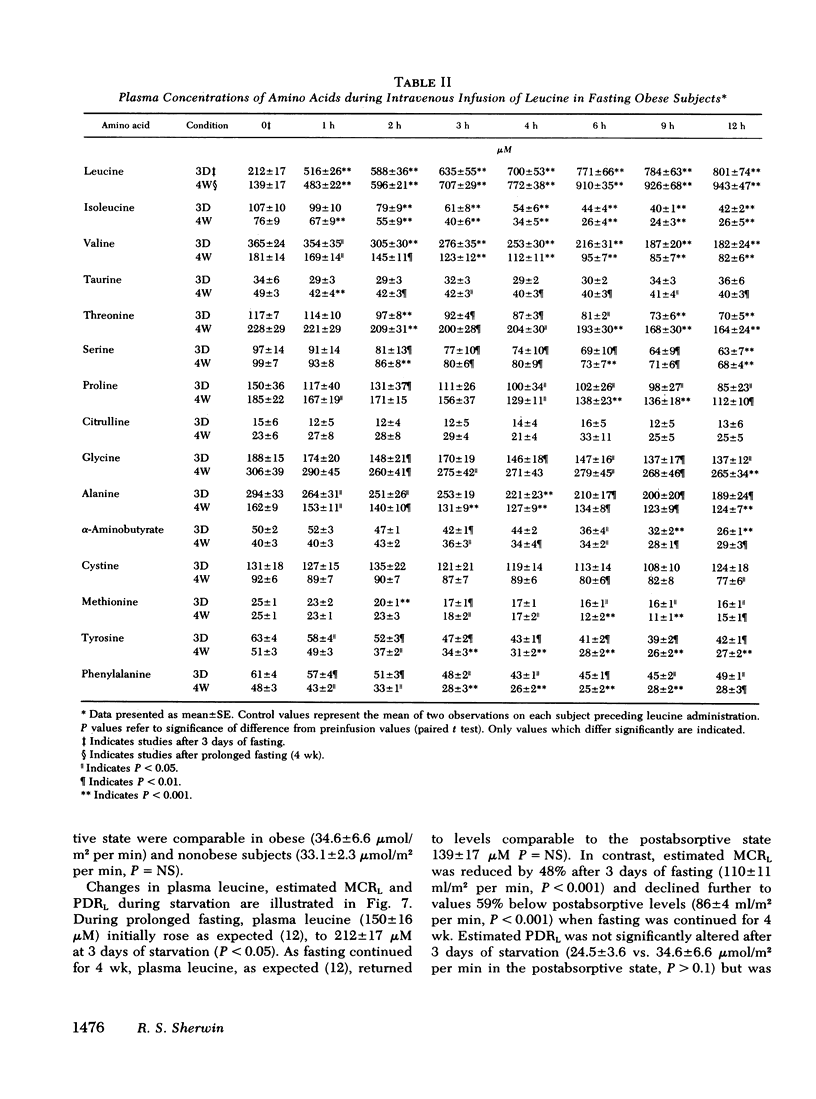
l-Leucine was administered as a primed continuous 3-4-h infusion in nonobese and obese subjects in the postabsorptive state and for 12 h in obese subjects after a 3-day and 4-wk fast. In nonobese and obese subjects studied in the post-absorptive state, the leucine infusion resulted in a 150-200% rise in plasma leucine above preinfusion levels, a small decrease in plasma glucose, and unchanged levels of plasma insulin and glucagon and blood ketones. Plasma isoleucine (60-70%) and valine (35-40%) declined to a greater extent than other amino acids (P < 0.001).
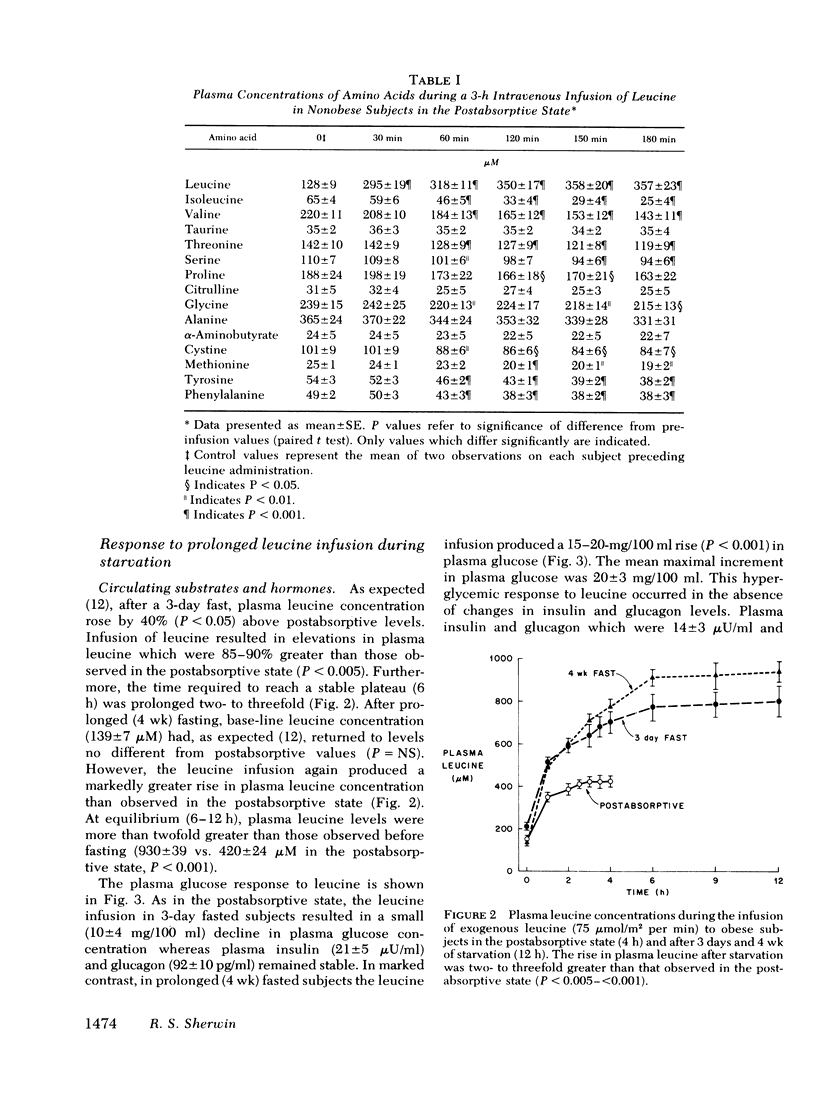
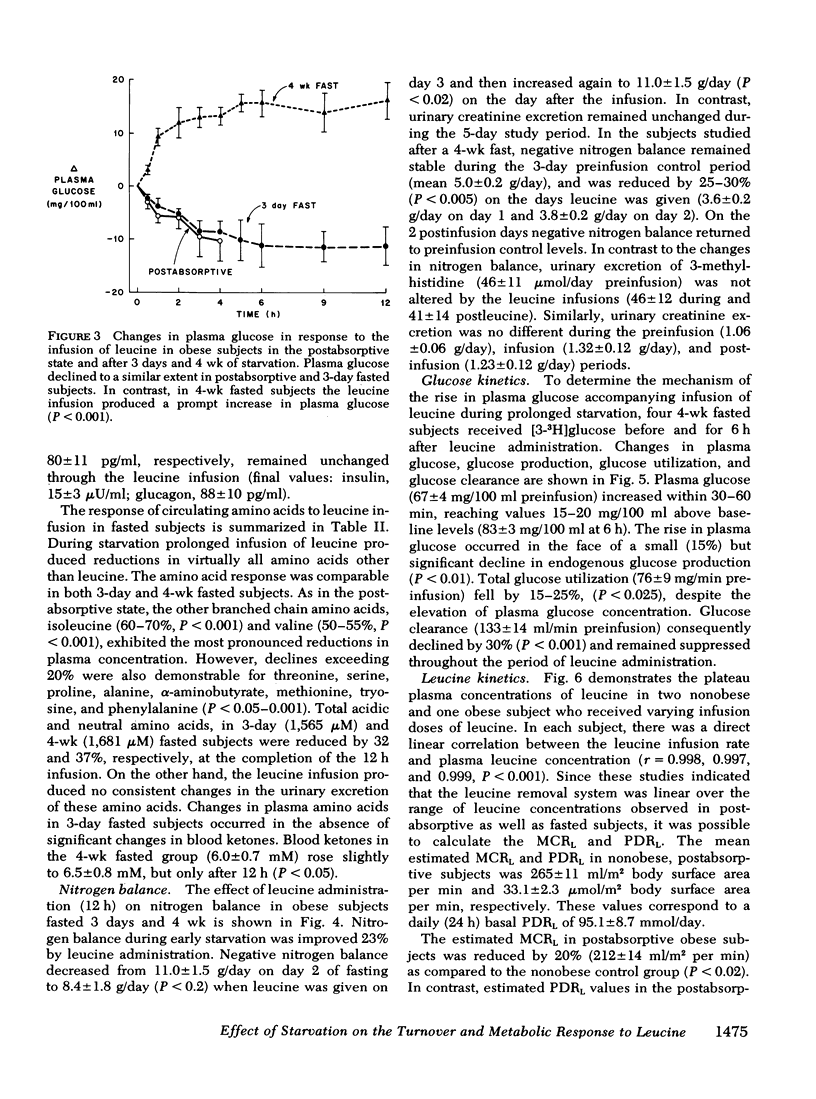
After 3 days and 4 wk of fasting, equimolar infusions of leucine resulted in two- to threefold greater increments in plasma leucine as compared to post-absorptive subjects, a 30-40% decline in other plasma amino acids, and a 25-30% decrease in negative nitrogen balance. Urinary excretion of 3-methylhistidine was however, unchanged. Plasma glucose which declined in 3-day fasted subjects after leucine administration, surprisingly rose by 20 mg/100 ml after 4 wk of fasting. The rise in blood glucose occurred in the absence of changes in plasma glucagon and insulin and in the face of a 15% decline in endogenous glucose production (as measured by infusion of [3-3H]glucose). On the other hand, fractional glucose utilization fell by 30% (P < 0.001), thereby accounting for hyperglycemia.
The estimated metabolic clearance rate of leucine fell by 48% after 3 days of fasting whereas the plasma delivery rate of leucine was unchanged, thereby accounting for a 40% rise in plasma leucine during early starvation. After a 4-wk fast, the estimated metabolic clearance rate of leucine declined further to 59% below base line. Plasma leucine nevertheless fell to postabsorptive levels as the plasma delivery rate of leucine decreased 65% below postabsorptive values.
Conclusions: (a) Infusion of exogenous leucine in prolonged fasting results in a decline in plasma levels of other amino acids, improvement in nitrogen balance and unchanged excretion of 3-methylhistidine, thus suggesting stimulation of muscle protein synthesis, (b) leucine infusion also reduces glucose production and to an even greater extent, glucose consumption, thereby raising blood glucose concentration; and (c) the rise in plasma leucine in early starvation results primarily from a decrease in leucine clearance which drops progressively during starvation.
Full text
PDF

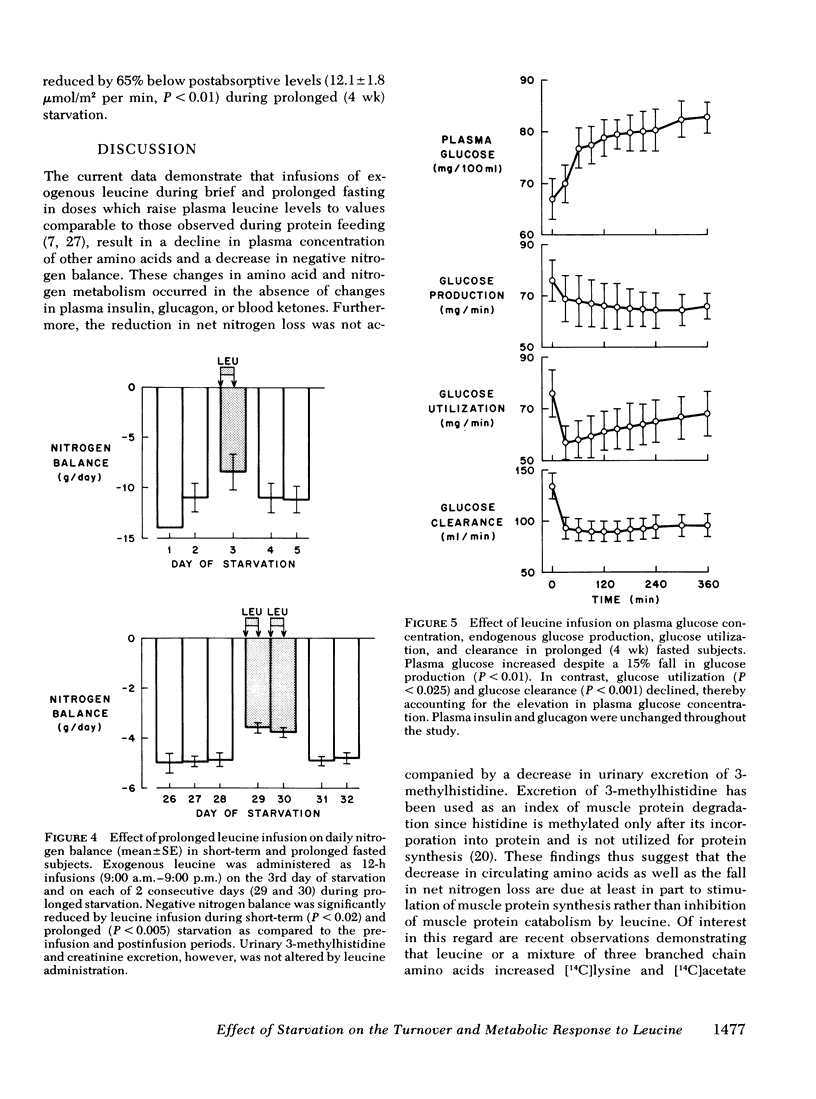
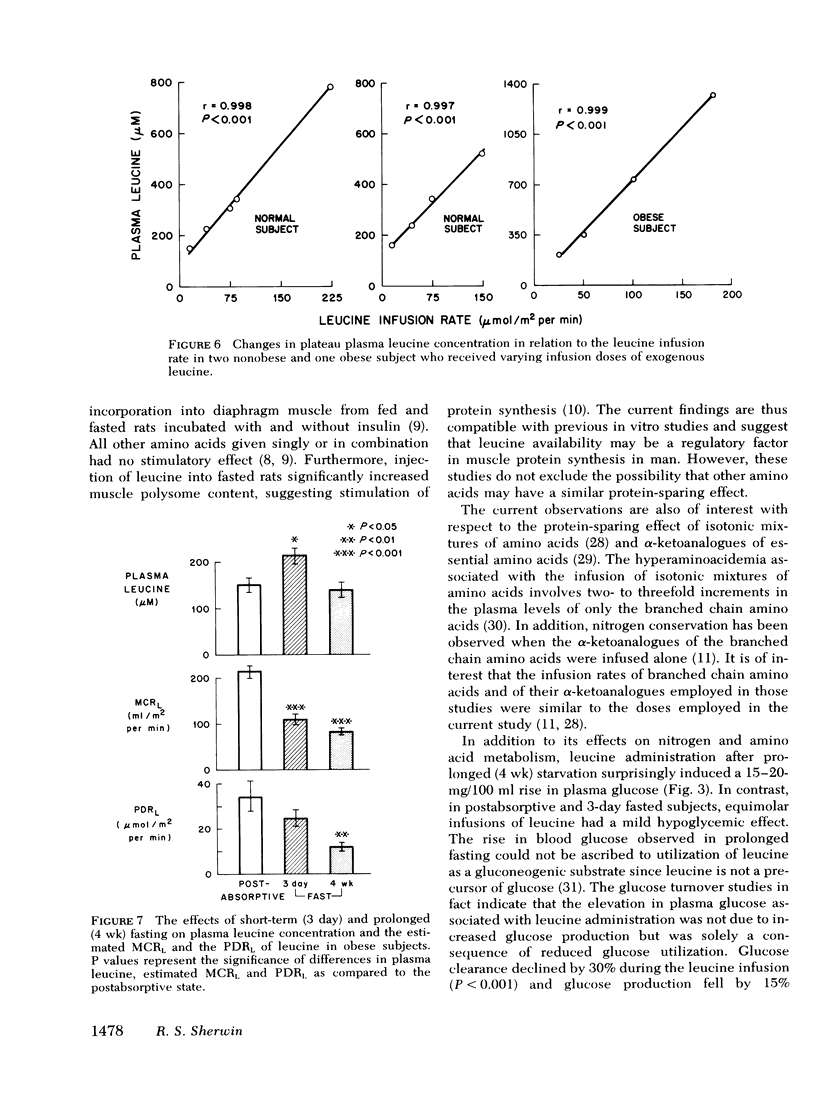



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adibi S. A. Metabolism of branched-chain amino acids in altered nutrition. Metabolism. 1976 Nov;25(11):1287–1302. doi: 10.1016/s0026-0495(76)80012-1. [DOI] [PubMed] [Google Scholar]
- Adibi S. A., Peterson J. A., Krzysik B. A. Modulation of leucine transaminase activity by dietary means. Am J Physiol. 1975 Feb;228(2):432–435. doi: 10.1152/ajplegacy.1975.228.2.432. [DOI] [PubMed] [Google Scholar]
- Altszuler N., Barkai A., Bjerknes C., Gottlieb B., Steele R. Glucose turnover values in the dog obtained with various species of labeled glucose. Am J Physiol. 1975 Dec;229(6):1662–1667. doi: 10.1152/ajplegacy.1975.229.6.1662. [DOI] [PubMed] [Google Scholar]
- Blackburn G. L., Flatt J. P., Clowes G. H., O'Donnell T. E. Peripheral intravenous feeding with isotonic amino acid solutions. Am J Surg. 1973 Apr;125(4):447–454. doi: 10.1016/0002-9610(73)90080-9. [DOI] [PubMed] [Google Scholar]
- Buse M. G., Biggers J. F., Friderici K. H., Buse J. F. Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat. The effect of fatty acids, glucose, and pyruvate respiration. J Biol Chem. 1972 Dec 25;247(24):8085–8096. [PubMed] [Google Scholar]
- Buse M. G., Reid S. S. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975 Nov;56(5):1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- DARE J. G., MOGEY G. A. Rabbit responses to human threshold doses of a bacterial pyrogen. J Pharm Pharmacol. 1954 May;6(5):325–332. doi: 10.1111/j.2042-7158.1954.tb10954.x. [DOI] [PubMed] [Google Scholar]
- FLOYD J. C., Jr, FAJANS S. S., KNOPF R. F., CONN J. W. EVIDENCE THAT INSULIN RELEASE IS THE MECHANISM FOR EXPERIMENTALLY INDUCED LEUCINE HYPOGLYCEMIA IN MAN. J Clin Invest. 1963 Nov;42:1714–1719. doi: 10.1172/JCI104857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajans S. S., Floyd J. C., Jr, Knopf R. F., Conn F. W. Effect of amino acids and proteins on insulin secretion in man. Recent Prog Horm Res. 1967;23:617–662. doi: 10.1016/b978-1-4831-9826-2.50017-9. [DOI] [PubMed] [Google Scholar]
- Fajans S. S., Knopf R. F., Floyd J. C., Power L., Conn J. W. THE EXPERIMENTAL INDUCTION IN MAN OF SENSITIVITY TO LEUCINE HYPOGLYCEMIA. J Clin Invest. 1963 Feb;42(2):216–229. doi: 10.1172/JCI104708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P. Amino acid metabolism in man. Annu Rev Biochem. 1975;44:933–955. doi: 10.1146/annurev.bi.44.070175.004441. [DOI] [PubMed] [Google Scholar]
- Felig P., Marliss E. B., Cahill G. F., Jr Metabolic response to human growth hormone during prolonged starvation. J Clin Invest. 1971 Feb;50(2):411–421. doi: 10.1172/JCI106508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Owen O. E., Wahren J., Cahill G. F., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Goldberg A. L., Odessey R. Oxidation of amino acids by diaphragms from fed and fasted rats. Am J Physiol. 1972 Dec;223(6):1384–1391. doi: 10.1152/ajplegacy.1972.223.6.1384. [DOI] [PubMed] [Google Scholar]
- Goldstein L., Newsholme E. A. The formation of alanine from amino acids in diaphragm muscle of the rat. Biochem J. 1976 Feb 15;154(2):555–558. doi: 10.1042/bj1540555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg G. R., Marliss E. B., Anderson G. H., Langer B., Spence W., Tovee E. B., Jeejeebhoy K. N. Protein-sparing therapy in postoperative patients. Effects of added hypocaloric glucose or lipid. N Engl J Med. 1976 Jun 24;294(26):1411–1416. doi: 10.1056/NEJM197606242942601. [DOI] [PubMed] [Google Scholar]
- Greenberg R., Reaven G. The effect of L-leucine on hepatic glucose formation. Pediatrics. 1966 Jun;37(6):934–941. [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Meikle A. W., Klain G. J. Effect of fasting and fasting-refeeding on conversion of leucine into CO 2 and lipids in rats. Am J Physiol. 1972 May;222(5):1246–1250. doi: 10.1152/ajplegacy.1972.222.5.1246. [DOI] [PubMed] [Google Scholar]
- Nallathambi S. A., Goorin A. M., Adibi S. A. Hepatic and skeletal muscle transport of cycloleucine during starvation. Am J Physiol. 1972 Jul;223(1):13–19. doi: 10.1152/ajplegacy.1972.223.1.13. [DOI] [PubMed] [Google Scholar]
- Odessey R., Goldberg A. L. Oxidation of leucine by rat skeletal muscle. Am J Physiol. 1972 Dec;223(6):1376–1383. doi: 10.1152/ajplegacy.1972.223.6.1376. [DOI] [PubMed] [Google Scholar]
- Odessey R., Khairallah E. A., Goldberg A. L. Origin and possible significance of alanine production by skeletal muscle. J Biol Chem. 1974 Dec 10;249(23):7623–7629. [PubMed] [Google Scholar]
- Owen O. E., Cahill G. F., Jr Metabolic effects of exogenous glucocorticoids in fasted man. J Clin Invest. 1973 Oct;52(10):2596–2605. doi: 10.1172/JCI107452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul H. S., Adibi S. A. Assessment of effect of starvation, glucose, fatty acids and hormones on alpha-decarboxylation of leucine in skeletal muscle of rat. J Nutr. 1976 Aug;106(8):1079–1088. doi: 10.1093/jn/106.8.1079. [DOI] [PubMed] [Google Scholar]
- Pozefsky T., Tancredi R. G., Moxley R. T., Dupre J., Tobin J. D. Effects of brief starvation on muscle amino acid metabolism in nonobese man. J Clin Invest. 1976 Feb;57(2):444–449. doi: 10.1172/JCI108295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Measurement and validation of nonsteady turnover rates with applications to the inulin and glucose systems. Fed Proc. 1974 Jul;33(7):1855–1864. [PubMed] [Google Scholar]
- Rocha D. M., Faloona G. R., Unger R. H. Glucagon-stimulating activity of 20 amino acids in dogs. J Clin Invest. 1972 Sep;51(9):2346–2351. doi: 10.1172/JCI107046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. D., Hems R., Krebs H. A. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem J. 1967 Mar;102(3):942–951. doi: 10.1042/bj1020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEELE R., WALL J. S., DE BODO R. C., ALTSZULER N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956 Sep;187(1):15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- Sapir D. G., Owen O. E., Pozefsky T., Walser M. Nitrogen sparing induced by a mixture of essential amino acids given chiefly as their keto-analogues during prolonged starvation in obese subjects. J Clin Invest. 1974 Oct;54(4):974–980. doi: 10.1172/JCI107838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir D. G., Walser M. Nitrogen sparing induced early in starvation by infusion of branched-chain ketoacids. Metabolism. 1977 Mar;26(3):301–308. doi: 10.1016/0026-0495(77)90077-4. [DOI] [PubMed] [Google Scholar]
- Sketcher R. D., Fern E. B., James W. P. The adaptation in muscle oxidation of leucine to dietary protein and energy intake. Br J Nutr. 1974 May;31(3):333–342. doi: 10.1079/bjn19740041. [DOI] [PubMed] [Google Scholar]
- TAIT J. F. REVIEW: THE USE OF ISOTOPIC STEROIDS FOR THE MEASUREMENT OF PRODUCTION RATES IN VIVO. J Clin Endocrinol Metab. 1963 Dec;23:1285–1297. doi: 10.1210/jcem-23-12-1285. [DOI] [PubMed] [Google Scholar]
- Tamborlane W. V., Sherwin R. S., Hendler R., Felig P. Metabolic effects of somatostatin in maturity-onset diabetes. N Engl J Med. 1977 Jul 28;297(4):181–183. doi: 10.1056/NEJM197707282970403. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P., Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976 Apr;57(4):987–999. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J. K., Hendler R., Felig P. Influence of glucocorticoids on glucagon secretion and plasma amino acid concentrations in man. J Clin Invest. 1973 Nov;52(11):2774–2782. doi: 10.1172/JCI107473. [DOI] [PMC free article] [PubMed] [Google Scholar]



