Abstract
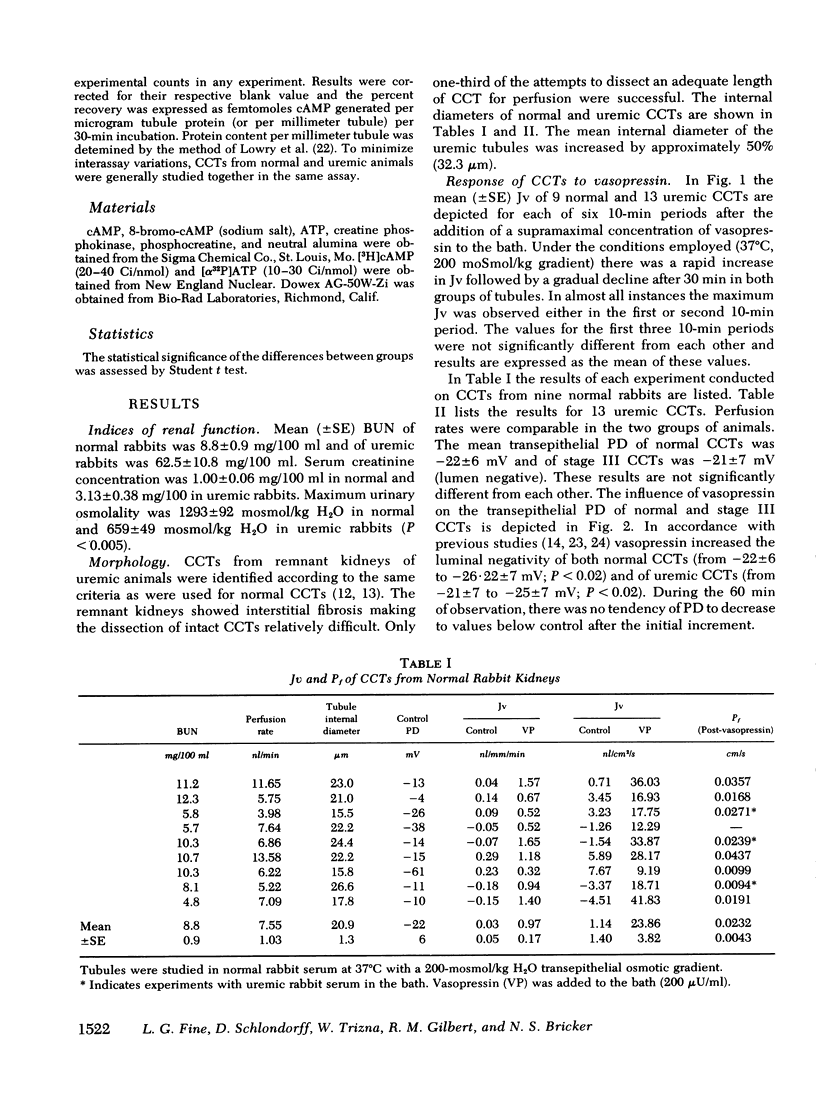
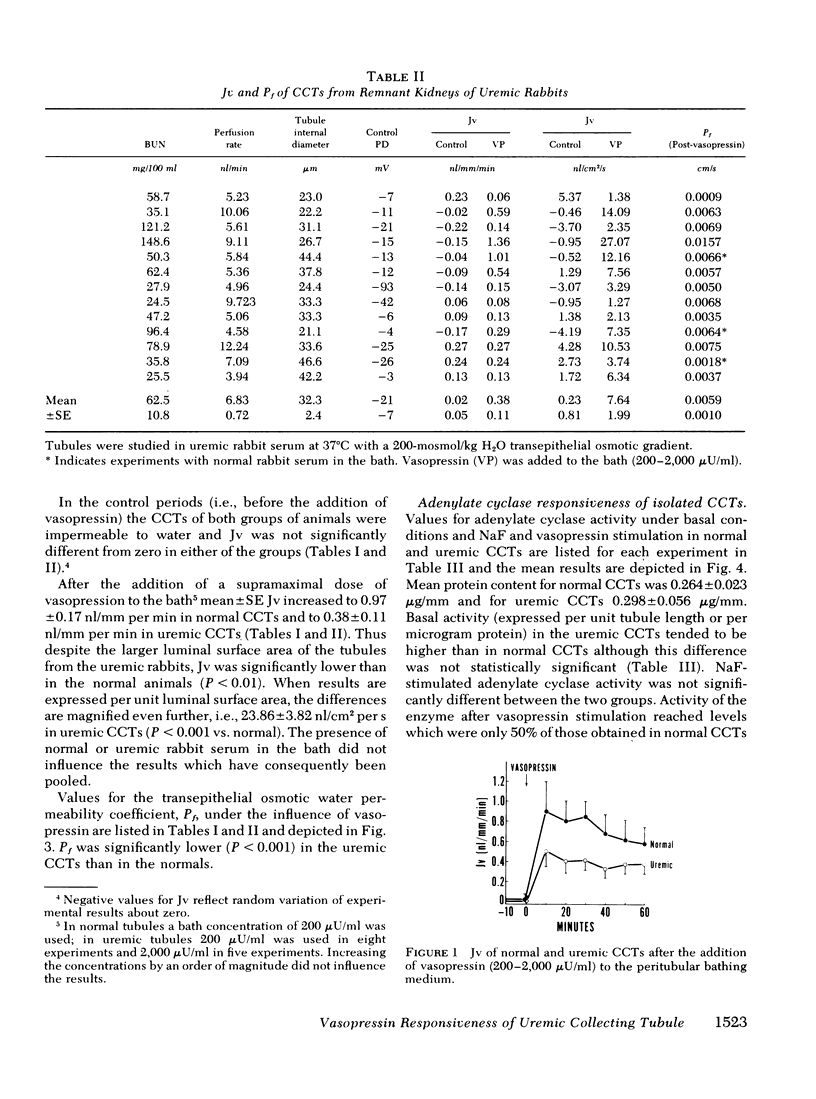
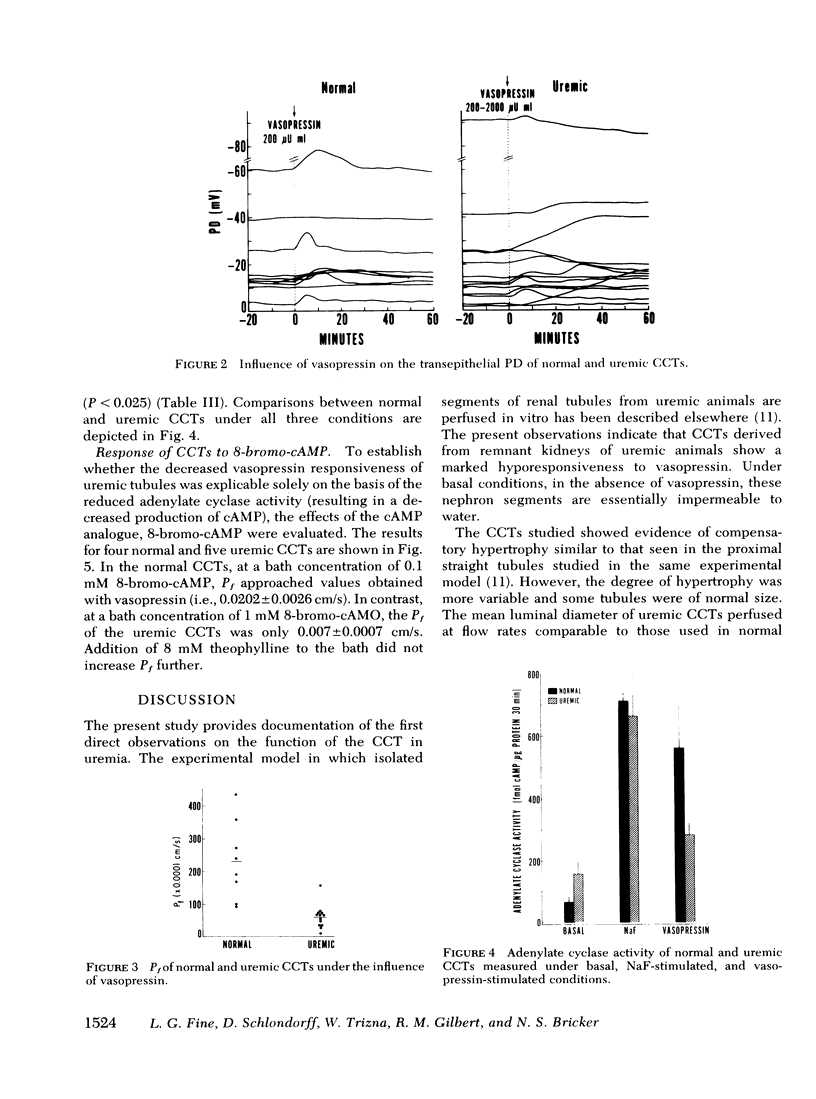
Resistance of the chronically diseased kidney to vasopressin has been proposed as a possible explanation for the urinary concentrating defect of uremia. The present studies examined the water permeability and adenylate cyclase responsiveness of isolated cortical collecting tubules (CCT) from remnant kidneys of uremic rabbits to vasopressin. In the absence of vasopressin the CCTs of both normal and uremic rabbits were impermeable to water. At the same osmotic gradient, addition of a supramaximal concentration of vasopressin to the peritubular bathing medium led to a significantly lower net water flux per unit length (and per unit luminal surface area) in uremic CCTs than in normal CCTs. Transepithelial osmotic water permeability coefficient, Pf, was 0.0232 ±0.0043 cm/s in normal CCTs and 0.0059±0.001 cm/s in uremic CCTs (P < 0.001). The impaired vasopressin responsiveness of the uremic CCTs was observed whether normal or uremic serum was present in the bath.
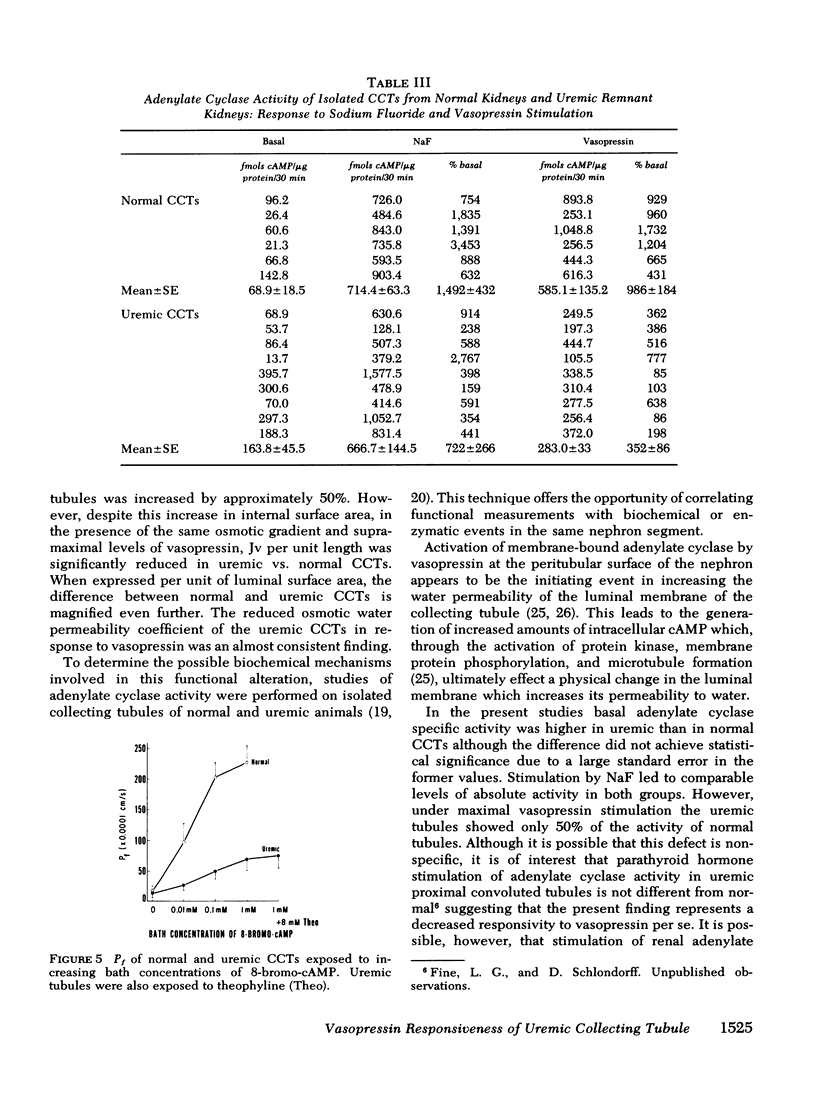
Basal adenylate cyclase activity per microgram protein was comparable in normal and uremic CCTs. Stimulation by NaF led to equivalent levels of activity in both, whereas vasopressin-stimulated activity was 50% lower in the uremic than in the normal CCTs (P < 0.025).
The cyclic AMP analogue, 8-bromo cyclic AMP, produced an increase in the Pf of normal CCTs closely comparable to that observed with vasopressin. In contrast, the Pf of uremic CCTs was only minimally increased by this analogue and was not further stimulated by theophylline.
These studies demonstrate an impaired responsiveness of the uremic CCT to vasopressin. This functional defect appears to be a result, at least in part, of a blunted responsiveness of adenylate cyclase to vasopressin. The data further suggest that an additional defect in the cellular response to vasopressin may exist, involving a step (or steps) subsequent to the formation of cyclic AMP.
A unifying concept of the urinary concentrating defect of uremia is proposed which incorporates a number of hitherto unexplained observations on the concentrating and diluting functions of the diseased kidney.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Zahid G., Schafer J. A., Troutman S. L., Andreoli T. E. Effect of antidiuretic hormone on water and solute permeation, and the activation energies for these processes, in mammalian cortical collecting tubules: evidence for parallel ADH-sensitive pathways for water and solute diffusion in luminal plasma membranes. J Membr Biol. 1977 Feb 24;31(1-2):103–129. doi: 10.1007/BF01869401. [DOI] [PubMed] [Google Scholar]
- Bank N., Aynedjian H. S. Individual nephron function in experimental bilateral pyelonephritis. II. Distal tubular sodium and water reabsorption and the concentrating defect. J Lab Clin Med. 1966 Nov;68(5):728–739. [PubMed] [Google Scholar]
- Carriere S., Wong N. L., Dirks J. H. Redistribution of renal blood flow in acute and chronic reduction of renal mass. Kidney Int. 1973 Jun;3(6):364–371. doi: 10.1038/ki.1973.58. [DOI] [PubMed] [Google Scholar]
- Chabardès D., Imbert M., Clique A., Montégut M., Morel F. PTH sensitive adenyl cyclase activity in different segments of the rabbit nephron. Pflugers Arch. 1975;354(3):229–239. doi: 10.1007/BF00584646. [DOI] [PubMed] [Google Scholar]
- DORHOUT MEES E. J. Role of osmotic diuresis in impairment of concentrating ability in renal disease. Br Med J. 1959 May 2;1(5130):1156–1158. doi: 10.1136/bmj.1.5130.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dousa T. P., Valtin H. Cellular actions of vasopressin in the mammalian kidney. Kidney Int. 1976 Jul;10(1):46–63. doi: 10.1038/ki.1976.78. [DOI] [PubMed] [Google Scholar]
- Fine L. G., Bourgoignie J. J., Hwang K. H., Bricker N. S. On the influence of the natriuretic factor from patients with chronic uremia on the bioelectric properties and sodium transport of the isolated mammalian collecting tubule. J Clin Invest. 1976 Sep;58(3):590–597. doi: 10.1172/JCI108505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine L. G., Trizna W., Bourgoignie J. J., Bricker N. S. Functional profile of the isolated uremic nephron. Role of compensatory hypertrophy in the control of fluid reabsorption by the proximal straight tubule. J Clin Invest. 1978 Jun;61(6):1508–1518. doi: 10.1172/JCI109071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine L. G., Trizna W. Influence of prostaglandins on sodium transport of isolated medullary nephron segments. Am J Physiol. 1977 Apr;232(4):F383–F390. doi: 10.1152/ajprenal.1977.232.4.F383. [DOI] [PubMed] [Google Scholar]
- Frindt G., Burg M. B. Effect of vasopressin on sodium transport in renal cortical collecting tubules. Kidney Int. 1972 Apr;1(4):224–231. doi: 10.1038/ki.1972.32. [DOI] [PubMed] [Google Scholar]
- Gilbert R. M., Weber H., Turchin L., Fine L. G., Bourgoignie J. J., Bricker N. S. A study of the intrarenal recycling of urea in the rat with chronic experimental pyelonephritis. J Clin Invest. 1976 Dec;58(6):1348–1357. doi: 10.1172/JCI108590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J. J., Burg M. B. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966 Jul;211(1):255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- Gross J. B., Imai M., Kokko J. P. A functional comparison of the cortical collecting tubule and the distal convoluted tubule. J Clin Invest. 1975 Jun;55(6):1284–1294. doi: 10.1172/JCI108048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. A., Barnes L. D., Dousa T. P. Cyclic AMP in action of antidiuretic hormone: effects of exogenous cyclic AMP and its new analogue. Am J Physiol. 1977 Apr;232(4):F368–F376. doi: 10.1152/ajprenal.1977.232.4.F368. [DOI] [PubMed] [Google Scholar]
- Hays R. M., Levine S. D. Vasopressin. Kidney Int. 1974 Nov;6(5):307–322. doi: 10.1038/ki.1974.116. [DOI] [PubMed] [Google Scholar]
- Helman S. I., Grantham J. J., Burg M. B. Effect of vasopressin on electrical resistance of renal cortical collecting tubules. Am J Physiol. 1971 Jun;220(6):1825–1832. doi: 10.1152/ajplegacy.1971.220.6.1825. [DOI] [PubMed] [Google Scholar]
- Holliday M. A., Egan T. J., Morris C. R., Jarrah A. S., Harrah J. L. Pitressin-resistant hyposthenuria in chronic renal disease. Am J Med. 1967 Mar;42(3):378–387. doi: 10.1016/0002-9343(67)90266-5. [DOI] [PubMed] [Google Scholar]
- Imbert M., Chabardès D., Montégut M., Clique A., Morel F. Adenylate cyclase activity along the rabbit nephron as measured in single isolated segments. Pflugers Arch. 1975;354(3):213–228. doi: 10.1007/BF00584645. [DOI] [PubMed] [Google Scholar]
- KLEEMAN C. R., ADAMS D. A., MAXWELL M. H. An evaluation of maximal water diuresis in chronic renal disease. I. Normal solute intake. J Lab Clin Med. 1961 Aug;58:169–184. [PubMed] [Google Scholar]
- Kokko J. P., Rector F. C., Jr Countercurrent multiplication system without active transport in inner medulla. Kidney Int. 1972 Oct;2(4):214–223. doi: 10.1038/ki.1972.97. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lubowitz H., Purkerson M. L., Bricker N. S. Investigation of single nephrons in the chronically diseased (Pyelonephritic) kidney of the rat using micropuncture techniques. Nephron. 1966;3(2):73–83. doi: 10.1159/000179449. [DOI] [PubMed] [Google Scholar]
- Meyer R. B., Jr, Miller J. P. Analogs of cyclic AMP and cyclic GMP: general methods of synthesis and the relationship of structure to enzymic activity. Life Sci. 1974 Mar 16;14(6):1019–1040. doi: 10.1016/0024-3205(74)90228-8. [DOI] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Permeability of medullary nephron segments to urea and water: Effect of vasopressin. Kidney Int. 1974 Dec;6(6):379–387. doi: 10.1038/ki.1974.123. [DOI] [PubMed] [Google Scholar]
- Schafer J. A., Andreoli T. E. The effect of antidiuretic hormone on solute flows in mammalian collecting tubules. J Clin Invest. 1972 May;51(5):1279–1286. doi: 10.1172/JCI106922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlondorff D., Weber H., Trizna W., Fine L. G. Vasopressin responsiveness of renal adenylate cyclase in newborn rats and rabbits. Am J Physiol. 1978 Jan;234(1):F16–F21. doi: 10.1152/ajprenal.1978.234.1.F16. [DOI] [PubMed] [Google Scholar]
- Schrier R. W., Regal E. M. Influence of aldosterone on sodium, water and potassium metabolism in chronic renal disease. Kidney Int. 1972 Mar;1(3):156–168. doi: 10.1038/ki.1972.23. [DOI] [PubMed] [Google Scholar]
- Tannen R. L., Regal E. M., Dunn M. J., Schrier R. W. Vasopressin-resistant hyposthenuria in advanced chronic renal disease. N Engl J Med. 1969 May 22;280(21):1135–1141. doi: 10.1056/NEJM196905222802101. [DOI] [PubMed] [Google Scholar]


