Abstract
In Hodgkin's disease a possible mechanism for impaired cellular immunity is cell-mediated suppression, defined as the inhibitory interaction between suppressor cells and effector lymphocytes. To test for the presence of suppressor cells in peripheral blood, we have modified the standard, one-way mixed lymphocyte culture by adding mitomycin C-treated mononuclear cells from the responder. Suppression, expressed as a percent of the base-line mixed lymphocyte culture in which these extra cells are not present, results in a reduction of thymidine incorporated in the modified culture (i.e., 100% suppression = no net thymidine incorporation; 0% suppression = identical thymidine incorporation in both the modified and baseline culture).
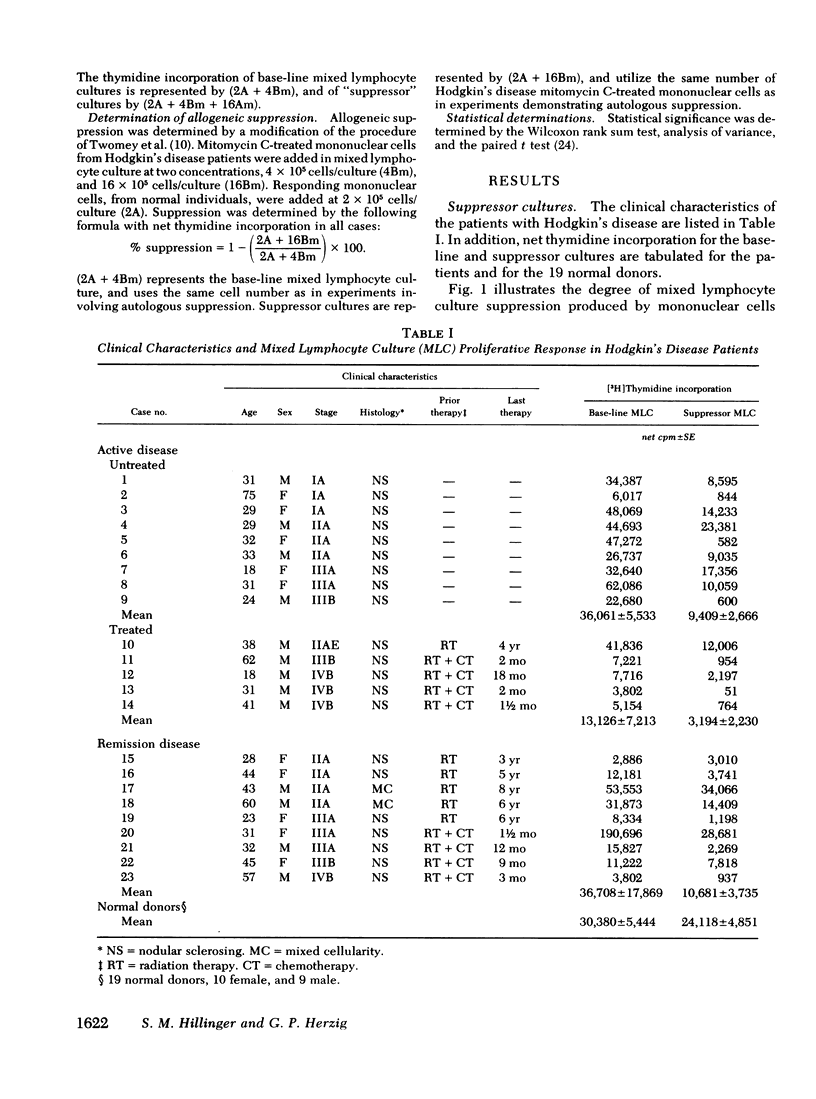
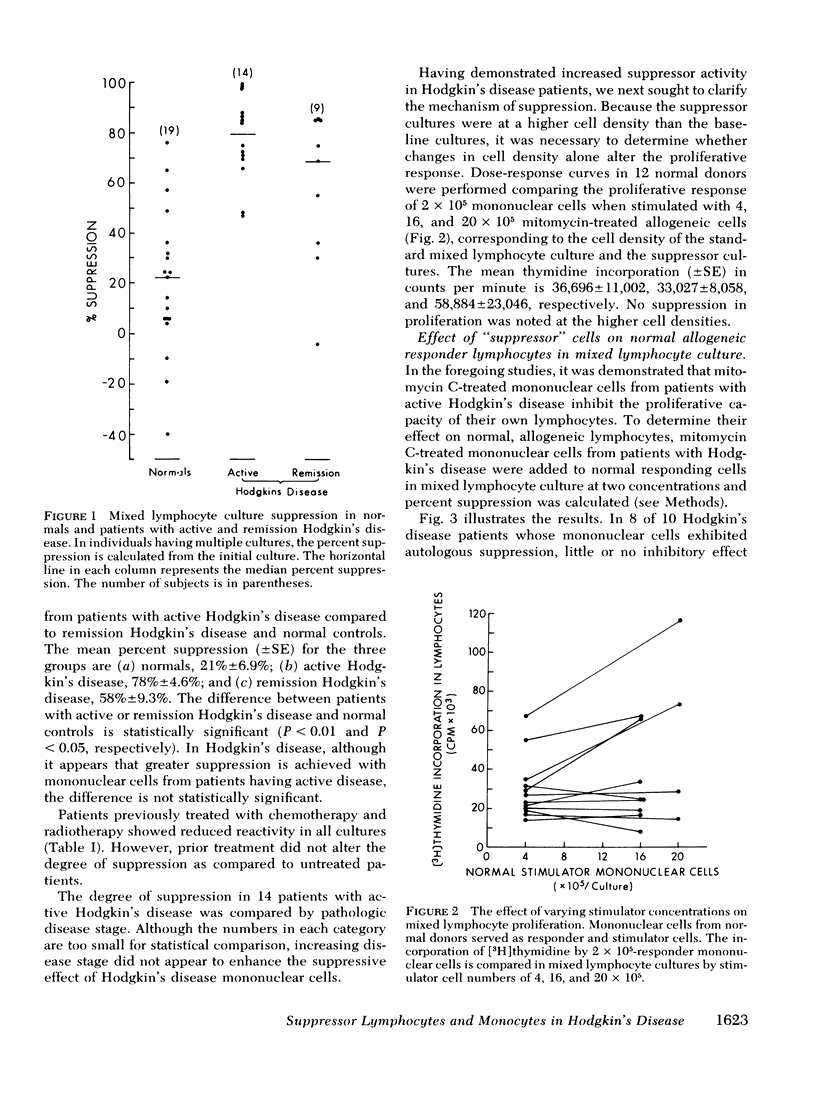
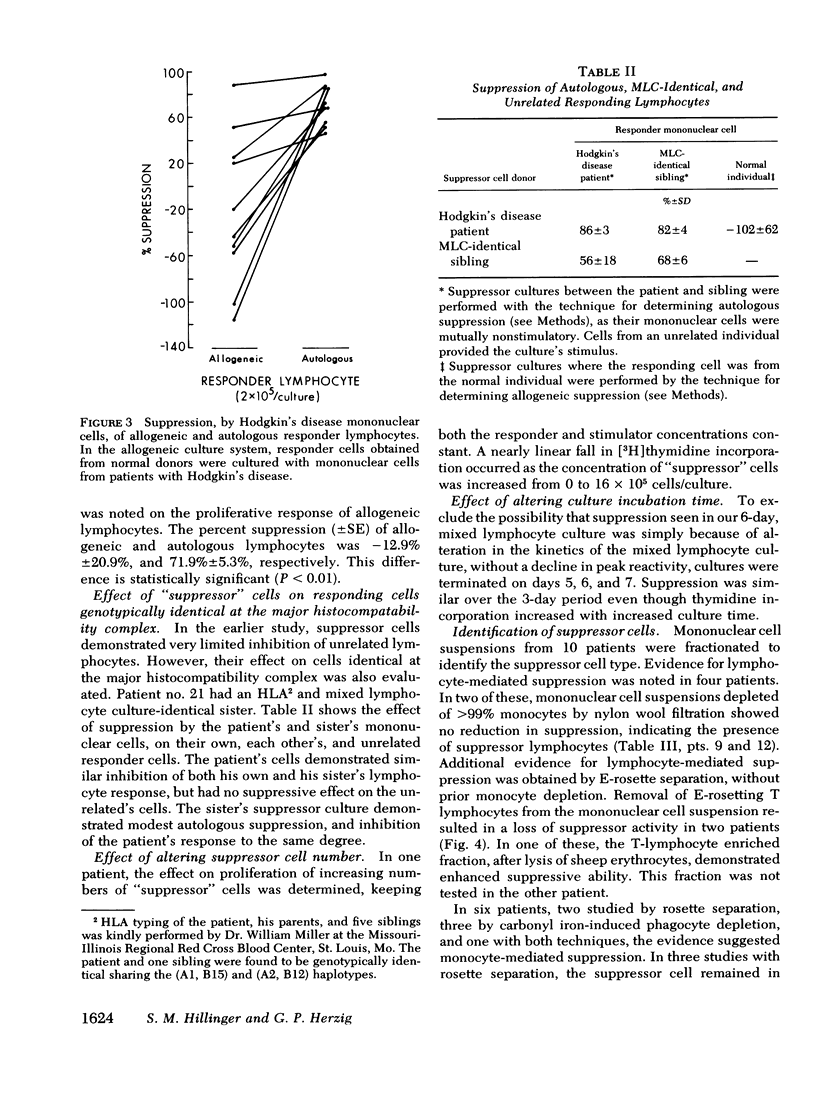
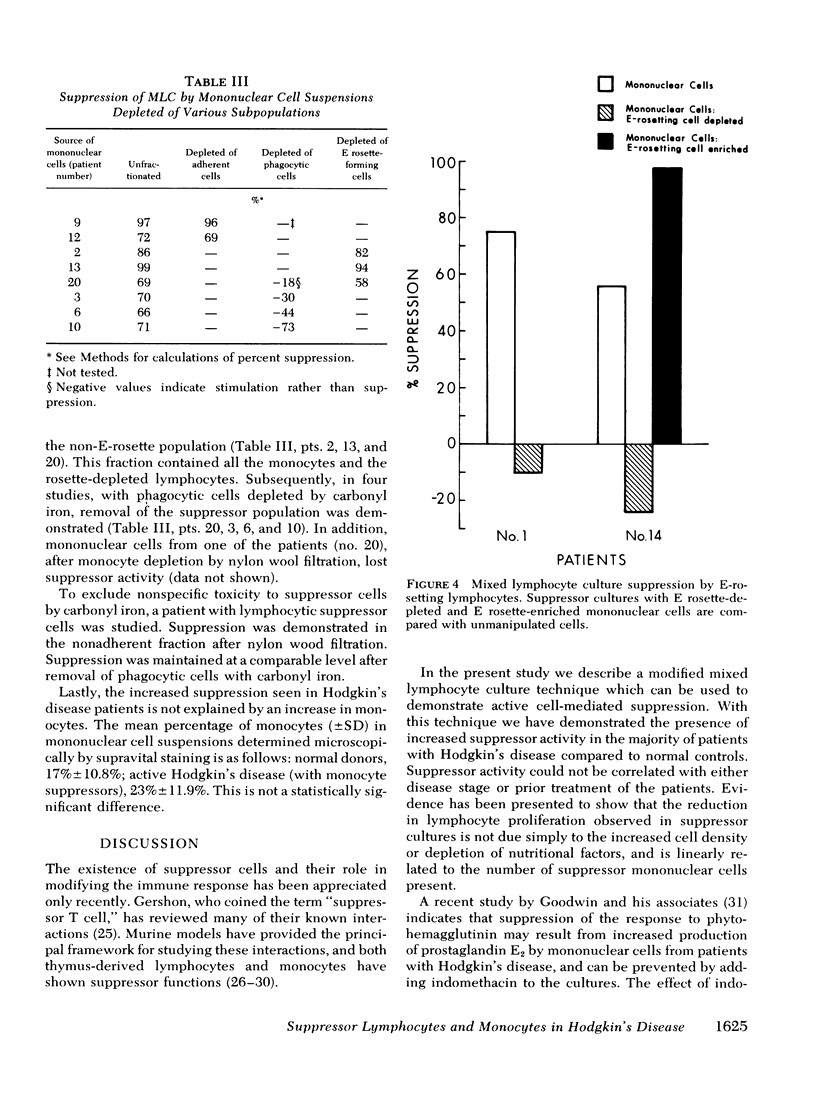
Suppression was found to be significantly increased in patients with both active Hodgkin's disease (78±4.6%) and remission Hodgkin's disease (58±9.3%) compared to normal individuals (21±6.9%) (mean±SE). The degree and frequency of suppression were not influenced by disease stage or prior therapy. Cell purification techniques revealed (in 10 patients studied) the suppressor cell to be a monocyte in 6, and a thymus-derived lymphocyte in 4. Possible genetic restriction of the suppressor cell interaction was indicated by a failure of suppressor cells to alter the response of lymphocytes from unrelated individuals, but suppression was obtained with lymphocytes from a histocompatible sibling. Although mononuclear cells from normal individuals suppress less frequently than cells from patients with Hodgkin's disease, normals may demonstrate suppression comparable to that observed in Hodgkin's patients. This finding suggests that suppression is a normal immunoregulatory mechanism which is altered in Hodgkin's disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaum G., Tsairis P. Suppressor lymphocytes in myasthenia gravis and effect of adult thymectomy. Ann N Y Acad Sci. 1976;274:527–535. doi: 10.1111/j.1749-6632.1976.tb47713.x. [DOI] [PubMed] [Google Scholar]
- Björkholm M., Holm G., Mellstedt H., Pettersson D. Immunological capacity of lymphocytes from untreated patients with Hodgkin's disease evaluated in mixed lymphocyte culture. Clin Exp Immunol. 1975 Dec;22(3):373–377. [PMC free article] [PubMed] [Google Scholar]
- Bobrove A. M., Fuks Z., Strober S., Kaplan H. S. Quantitation of T and B lymphocytes and cellular immune function in Hodgkin's disease. Cancer. 1975 Jul;36(1):169–179. doi: 10.1002/1097-0142(197507)36:1<169::aid-cncr2820360115>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carbone P. P., Kaplan H. S., Musshoff K., Smithers D. W., Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971 Nov;31(11):1860–1861. [PubMed] [Google Scholar]
- Case D. C., Hansen J. A., Corrales E., Young C. W., Dupont B., Pinsky C. M., Good R. A. Comparison of multiple in vivo and in vitro parameters in untreated patients with Hodgkin's disease. Cancer. 1976 Oct;38(4):1807–1815. doi: 10.1002/1097-0142(197610)38:4<1807::aid-cncr2820380458>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Cohnen G., Augener W., Brittinger G., Douglas S. D. Rosette-forming lymphocytes in Hodgkin's disease. N Engl J Med. 1973 Oct 18;289(16):863–863. doi: 10.1056/NEJM197310182891616. [DOI] [PubMed] [Google Scholar]
- Eggers A. E., Wunderlich J. R. Suppressor cells in tumor-bearing mice capable of nonspecific blocking of in vitro immunization against transplant antigens. J Immunol. 1975 May;114(5):1554–1556. [PubMed] [Google Scholar]
- Eltringham J. R., Kaplan H. S. Impaired delayed-hypersensitivity responses in 154 patients with untreated Hodgkin's disease. Natl Cancer Inst Monogr. 1973 May;36:107–115. [PubMed] [Google Scholar]
- Fuks Z., Strober S., Kaplan H. S. Interaction between serum factors and T lymphocytes in Hodgkin's disease. Use as a diagnostic test. N Engl J Med. 1976 Dec 2;295(23):1273–1278. doi: 10.1056/NEJM197612022952301. [DOI] [PubMed] [Google Scholar]
- GREEN I., CORSO P. F. A study of skin homografting in patients with lymphomas. Blood. 1959 Mar;14(3):235–245. [PubMed] [Google Scholar]
- Gast G. C., Halie M. R., Nieweg H. O. Immunological responsiveness against two primary antigens in untreated patients with Hodgkin's disease. Eur J Cancer. 1975 Apr;11(4):217–224. doi: 10.1016/0014-2964(75)90001-8. [DOI] [PubMed] [Google Scholar]
- Gershon R. K. A disquisition on suppressor T cells. Transplant Rev. 1975;26:170–185. doi: 10.1111/j.1600-065x.1975.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Graze P. R., Perlin E., Royston I. In vitro lymphocyte dysfunction in Hodgkin's disease. J Natl Cancer Inst. 1976 Feb;56(2):239–243. doi: 10.1093/jnci/56.2.239. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Muchmore A. V., Chused T. M., Holden H. T., Herberman R. B. Inhibition of proliferation of lymphoma cells and T lymphocytes by suppressor cells from spleens of tumor-bearing mice. J Immunol. 1975 Jan;114(1 Pt 1):206–210. [PubMed] [Google Scholar]
- Levy R., Kaplan H. S. Impaired lymphocyte function in untreated Hodgkin's disease. N Engl J Med. 1974 Jan 24;290(4):181–186. doi: 10.1056/NEJM197401242900402. [DOI] [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Cellular interactions in the proliferative response of human T and B lymphocytes to phytomitogens and allogeneic lymphocytes. J Exp Med. 1974 Jun 1;139(6):1553–1567. doi: 10.1084/jem.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER D. G., LIZARDO J. G., SNYDERMAN R. K. Homologous and heterologous skin transplantation in patients with lymphomatous disease. J Natl Cancer Inst. 1961 Mar;26:569–583. [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich S. S., Rich R. R. Regulatory mechanisms in cell-mediated immune responses. I. Regulation of mixed lymphocyte reactions by alloantigen-activated thymus-derived lymphocytes. J Exp Med. 1974 Dec 1;140(6):1588–1603. doi: 10.1084/jem.140.6.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich S. S., Rich R. R. Regulatory mechanisms in cell-mediated immune responses. II. A genetically restricted suppressor of mixed lymphocyte reactions released by alloantigen-activated spleen cells. J Exp Med. 1975 Dec 1;142(6):1391–1402. doi: 10.1084/jem.142.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich S. S., Rich R. R. Regulatory mechanisms in cell-mediated immune responses. IV. Expression of a receptor for mixed lymphocyte reaction suppressor factor on activated T lymphocytes. J Exp Med. 1976 Nov 2;144(5):1214–1226. doi: 10.1084/jem.144.5.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson D., Grotelueschen C., Kauffman H. M., Jr The human splenic suppressor cell. Transplantation. 1975 Nov;20(5):362–367. doi: 10.1097/00007890-197511000-00002. [DOI] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M., Good R. A. Suppression of B-cell differentiation by leukocytes from hypogammaglobulinemic patients. J Clin Invest. 1976 Jul;58(1):109–122. doi: 10.1172/JCI108439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey J. J., Laughter A. H., Farrow S., Douglass C. C. Hodgkin's disease. An immunodepleting and immunosuppressive disorder. J Clin Invest. 1975 Aug;56(2):467–475. doi: 10.1172/JCI108113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]


