Abstract
Introduction:
Polyamines – putrescine, spermidine and spermine are polycationic compounds ubiquitous for all living organisms. They are essential for the cell growth and differentiation, the control of cell cycle progress, apoptosis, and cancerogenesis. Accumulated scientific evidence suggests the central role of polyamines in the process of keratinocytic proliferation, differentiation, and regulation.
Objective:
To elucidate the polyamine metabolic changes that occur in benign keratinocytic proliferation. Fifty eight patients were enrolled in the study, 31 with plaque-form of psoriasis vulgaris, which had been referred to as a model of benign keratinocytic proliferation, and 27-healthy controls.
Materials and Methods:
An original, innovative chromatographic method was used to detect the levels of putrescine, spermidine, and spermine in all skin samples.
Results:
Were significantly proven (P < 0.05). No difference was found between the polyamines levels of non-lesional psoriatic skin and healthy controls. Psoriatic lesions showed a two-time higher concentration of all polyamines in lesional, compared to non-lesional skin. Spermine had the highest concentration and highest proliferation trend, which demonstrated the importance of propylamine synthesis in the pathogenesis of psoriasis. Spermine highest concentrations suggested the leading role of adenosine methionine decarboxylase (AMDC) in the pathogenesis of benign keratinocytic proliferations.
Conclusions:
Non-lesional skin in psoriatic patients did not show latent changes in polyamine metabolism. Psoriatic lesions demontrated two-time higher levels of the most essential biogenic polyamines compared to healthy controls. The highest level of spermine proved the crucial role of AMDC in the polyamine metabolism changes in psoriasis. Future therapeutic approaches should be focused on reduction of exogenic spermine intake, utilizing new spermine blockers, and synthesis of AMDC inhibitors.
Keywords: Psoriasis, putrescine, spermine, spermidine
Introduction
What was known?
Few data exists on polyamines concentration in lesional skin of psoriatic patients. Insignificant cohorts of psoriatic models have been investigated in the late70ties with no conclusive results on the epidermal distribution and levels of the main polyamine substances. The growing data indicating the importance of polyamine metabolism in neoplastic keratinocytic proliferations raises the question of their significance in the pathogenesis of epidermal cell synthesis.
The polyamines putrescine, spermidine and spermine are some of the major cations in eukaryotic cells. The majority of polyamine molecules are bound to polyanionic macromolecules such as desoxyribonucleic acids (DNA), ribonucleic acids (RNA), and phospholipids,[1] resulting in far-reaching effects upon cellular processes including DNA replication, transcription, and translation. It is not surprising that numerous studies using specific inhibitors of polyamine biosynthesis have documented that these small ubiquitous molecules are absolutely required for cell growth and differentiation.[2,3,4] The cellular functions of polyamines and their interaction with cellular components that play a key role in promoting the process of controlled proliferation and uncontrolled hyperproliferation - tumorogenesis - remain largely unknown.[2]
Various skin inflammations express a proliferation nature. Psoriasis is the most common example of hyperproliferation keratinocytic process. Histologically it is defined by parakeratosis, acanthosis and papillomatosis. Psoriasis is considered as a model of benign keratinocytic proliferation with higher synthetic rate and shortened turn-over of epidermal cells.
Objective
Comparative analysis of the polyamine metabolic changes that occur in lesions of psoriasis, compared to non-lesional skin of psoriatic patients, and healthy controls may elucidate specific pathogenetic biochemical mechanisms and open new therapeutic horizons.
Materials and Methods
Materials
Fifty eight patients were enrolled in the study after the appropriate approval for performing research with human skin had been obtained by the local ethical committee. An informed consent was obtained by each subject.
Thirty-one patients had mild to moderate plaque-form of psoriasis vulgaris. Inclusion criteria for psoriatic patients were: Age 18-64 yrs (mean 42.13 years), onset of the disease from six months to 30 years (mean 8.61 years), PASI ranging from 2 to 16 (mean 7.58). No patients with psoriatic arthritis, pustular psoriasis, and psoriatic erythroderma were enrolled in the study. All subjects were generally healthy. Obesitas, hypertension, metabolic syndrome, thrombophlebitis, and other dermatological diseases were specifically excluded. Psoriatic lesions were not triggered or exacerbated by drug intake or infectious agents. The patients were washed-out of any systemic and topical treatment one month prior the investigation.
Epidermal shave biopses, taken according to the standardized technique described by Lowe et al[16] and based on the method of Arthur and Shelley,[5] was used. The non-lesional skin samples were taken from the back of the patients, at minimum 5 cm from a psoriatic lesion. The lesions were free zed at −196°C and stored at −80°C.
Twenty seven volunteers, age range 30-68 years, were used as healthy controls. The skin samples were taken from the back of the patients via the described epidermal shave technique and processed in cryogenic nitrogen.
Methods
HPLC assay of polyamines
Sample preparation
Extraction
The tissue samples were lyophilized and weighted into eppendorf tubes. 300 μl perchloric acid (1M) were added and the samples were homogenized using homogenizer (Miccra D-1, Germany). The obtained mixture was centrifuged at 13000 g for 10 min. An aliquot of 50 μl of the supernatant was used for derivatization and HPLC analysis.
Derivatization
An aliquot of 50 μl of the supernatant was added to 80 μl of 0.5 M sodium hydrogen carbonate/sodium carbonate buffer with pH 10.2 in 0.6 ml well-capped reaction vessels. Then, 100 μl of the derivatizing reagent −2.5 mM Fmoc-Cl were added and allowed to react at 40o C for 25 min. 20 μl conc. HCl was used to quench the reaction.
HPLC analysis
Equipment
Analyses were carried out on HPLC system consisted of P2000 binary gradient pump (ThermoSystem, USA), fluorescent detector FL3000 (Thermo electron corporation, USA) and 20 μl manual injector model 7125NS (Rheodyne, USA).
The fluorescence of Fmoc derivatives was measured using 262 nm for excitation and 615 nm for emission wavelengths, respectively. Data acquisition and presiding was achieved with CW 1.7 chromatographic software (DataApex, Czech Republic).
Chromatographic conditions
Buffer A was a mixture of 20 mmol ammonium acetate and acetonitrile (9/55/%, v/v) containing 1 mmol dibutylamine. The pH of buffer was adjusted to 2.1 with 35% perchloric acid on pH mether (Schott, Germany), equipped with SenTix 41 electrode (WTW, Germany).
Buffer B was a mixture of 20 mmol ammonium acetate and acetonitrile (9/01/0%, v/v), containing 5 mmol dibutylamine. The resulting pH was 8.4.
Before use both buffers were filtered on nylon 0.45 μm membrane and degassed in sonic bath under vacuum.
The separation of Fmoc-derivatives of polyamines was achieved on Nucleodur 100-5 C18 ec (125 × 4) column (Masharey-Nagel, Germany) at flow rate of 1.5 ml/min using binary gradient elution. The gradient profile was as follows: 0’- 50% B, 5’- 75% B, 10’- 100% B, 14’-100% B, 16’- 50% B. The quantitative determination was carried out using external calibration method.
Results
Putrescine (PU), spermidine (SPD) and spermine (SM) were found significantly different in all 31 investigated specimen pairs (P < 0.000). The results are shown below [Table 1].
Table 1.
Statistic parameters of lesional and non-lesional skin of psoriatic patients specimen pairs
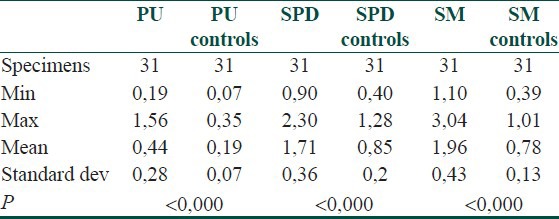
In lesional skin, SM had the highest concentration, followed by SPD and SM. Interestingly; all polyamines had two times greater concentrations in lesional compared to non-lesional skin in patients with psoriasis. The level of SM increased most – 2.5 times, followed by PU – 2.3 times and SPD – 2 times. The SPD/SM ratio was 1.09 in non-involved skin, and under 1 - in lesional skin. This result did not prove the SPD/SM ratio significance in evaluating the proliferation rate of keratinocytes in benign inflammatory proliferations.
More interesting results were obtained comparing the non-lesional skin of psoriatic patients (HLTH) with the healthy controls (aHLTH). The results showed no significant difference of the levels of PU, SPD, and SM in all 27 specimen pairs of non-involved skin in psoriatic patients and absolutely healthy controls – P > 0.05 [Table 2].
Table 2.
Statistic parameters of HLTH and aHLTH specimen pairs
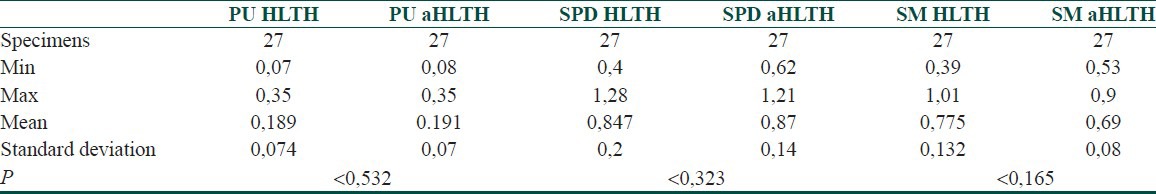
The absence of significant changes in the polyamine metabolism of healthy controls and uninvolved skin in psoriatic patients proved the local character of polyamine metabolic dysregulation in psoriasis. This finding repudiated the suggestion that the entire skin surface of the psoriatic patient demonstrated a latent morbid state with lower sensitivity threshold. In both categories SPD had the highest concentration. Moreover, the SPD/PU and SM/PU ratio was close to 4 in both groups, revealing that under normal conditions the healthy keratinocytes synthesized 4 times more SPD and SM that PU.
Discussion
The polyamines are differently distributed throughout epidermal and dermal compartments of normal skin.[2] Baze et al., indicated that polyamine concentrations varied significantly between the epidermis and dermis, both quantitatively and qualitatively: SPD and SM levels were much higher in the epidermis than in the dermis, and PU/SPD and SPD/SM ratios were much lower in the epidermis.[7] These differences characterized the very well-known specificity of cellularity, proliferative activity, and differentiation between the two cutaneous compartments. The higher SPD and SM concentrations in the epidermis suggested that both substances might play a special role in the epidermal metabolism.[3]
Polyamines concentration in proliferating tissues was found elevated. Moreover, blood samples of patients with psoriasis had twice higher SPD and two times higher SM levels compared to healthy controls.[11] Polyamine levels in blood, urine, and other biological fluid fluctuated in accordance with psoriasis activity.[12] SPD/SM ratio, considered as an indicator of proliferation activity, was found increased in psoriatic lesions, compared to non-lesional skin of patients affected by the disease.[13] Interestingly, patients with psoriasis receiving etretinate showed significant drop of all urinary polyamines, with the greatest prevalence of SM.[14] Lowe NJ et al., demonstrated that psoriatic lesions had increased ornithine decarboxylase/ODC/activity in both lesional and non-lesional skin, compared to healthy controls. Patients with eruptive forms of psoriasis had significantly higher levels of ODC than those with chronic-recalcitrant forms.[15] All these investigations, however, were carried out on small cohorts and were not significantly proven[16] as a consequence of the complicated laboratory conditions and technical difficulties.
Our chromatographic method is representative and innovative. It has been routinely used at an every-day basis to analyze various body substrates. As skin samples were investigated for the first time, the main technical pitfall was the proper adjustment of the binar time/pH gradient. The great sensitivity and reliability of the method provided quick technical utility and enrollment of a representative number of subjects.
Our results proved twice higher concentration of the basic polyamines in tissue samples of patients with psoriasis, compared to uninvolved psoriatic skin, and healthy subjects. There was no significant difference in polyamine metabolism of healthy subjects, compared to uninvolved skin of psoriatic patients. This denied the hypothesis of latent morbid state of non-lesional skin in psoriatic subjects.
The highest level of SM in psoriatic skin proved its essential role in epidermal metabolism and in particular, the process of keratinisation. SM demonstrated the highest proliferative trend, too. SPD/SM ratio showed negative results in all psoriatic patients. Thus, the hypothesis of positive correlation of SPD/SM ratio with the process of benign keratinocytic proliferation was not proven.
Another polyamine biosynthetic enzyme-S-adenosyl-L-methionine decarboxylase (AMDC), which is thought to regulate SPD and SM biosynthesis - probably plays a major role in the process of chronic benign keratinocytic proliferation. The suggestion is based on the positive correlation found between SPD and SM levels in psoriatic lesions. The utilization of a higher number of propylamine groups correlates with the lower levels of adenosine triphospate in psoriatic lesions explaining this well-known phenomenon. Moreover, Weinstein et al., proved the beneficial therapeutic role of a potent topical AMDC activity inhibitor - methylglyoxal-bis-guanylhydrazone, which reduced the induration and erythema of the psoriatic plaques.[17]
It is unlikely that polyamine abnormalities represent a primary defect in psoriatic skin because changes in polyamines have been noted in several other situations of epidermal hyperplasia. It is possible; however, that the polyamines are important as cell regulatory factors in psoriasis and that some treatments may partly act via modulation of polyamine metabolism.
Conclusions
Non-involved psoriatic skin did not show latent changes in polyamine metabolism.
Psoriatic lesions demontrated a twice-higher concentration of all essential biogenic polyamines.
The highest level of SM proved the crucial role of AMDC in the polyamine metabolic changes in psoriasis.
Manipulation of polyamine metabolism is a realistic target for therapeutic or preventive strategy in the treatment of psoriasis. Future therapeutic approaches should be focused on reduction of exogenic SM intake, utilizing new SM blockers, and synthesis of AMDC inhibitors.
Further scientific evidences are needed to elucidate the role of polyamines in the processes of cell proliferation and differentiation, as well as the regulatory mechanisms that cause the specific polyamine effects.
What is new?
1. Representative data on polyamines metabolism changes in psoriasis are provided. Non-lesional psoriatic skin shows no latent changes in polyamine metabolism.
2. Psoriatic lesions demontrated higher levels of the most essential biogenic polyamines compared to healthy controls.
3. The highest level of spermine proved the crucial role of AMDC in the polyamine metabolism changes in psoriasis.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Igarashi K, Sakamoto I, Goto N, Kashiwagi K, Honma R, Hirose S. Interaction between polyamines and nucleic acids or phospholipids. Arch Biochem Biophys. 1982;219:438–43. doi: 10.1016/0003-9861(82)90175-8. [DOI] [PubMed] [Google Scholar]
- 2.Praskova M, Mitev B. Regulation og gene expression in epidermal keratinocytes. Medicine. 2002;10:15–9. (in Bulgarian) [Google Scholar]
- 3.Nikolova E, Mitev V, Zhelev N, Deroanne C, Poumay Y. The suppression of Rac 1 expression alters cell proliferation in normal human skin fibroblast. BBRC. 2007;359:834–839. doi: 10.1016/j.bbrc.2007.05.214. [DOI] [PubMed] [Google Scholar]
- 4.Isaeva AR, Mitev VI. Inhibition of protein kinase CK2 induces E2F1 nuclear export, formation of p2/1/E2F1 complexes and suppression of DNA synthesis in normal human epidermal keratinocytes. J Dermatol Sci. 2009;55:134–6. doi: 10.1016/j.jdermsci.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Arthur R, Shelley W. The epidermal shave biopsy. Arch Dermatol. 1959;80:95–7. doi: 10.1001/archderm.1959.01560190097017. [DOI] [PubMed] [Google Scholar]
- 6.Seiler N. Catabolism of polyamines. Amino Acids. 2004;26:217–33. doi: 10.1007/s00726-004-0070-z. [DOI] [PubMed] [Google Scholar]
- 7.Baze PE, Milano G, Verrando P, Renee N, Ortonne JP. Distribution of polyamines in human epidermis. Br J Dermatol. 1985;112:393–6. doi: 10.1111/j.1365-2133.1985.tb02311.x. [DOI] [PubMed] [Google Scholar]
- 8.El Baze P, Milano G, Verrando P, Renée N, Ortonne JP. Polyamine levels in normal human skin: A comparative study of pure epidermis, pure dermis, and suction blister fluid. Arch Dermatol Res. 1983;275:218–21. doi: 10.1007/BF00416663. [DOI] [PubMed] [Google Scholar]
- 9.Peng HF, Jackson V. In vitro studies on the maintenance of transcription-induced stress by histones and polyamines. J Biol Chem. 2000;275:657–68. doi: 10.1074/jbc.275.1.657. [DOI] [PubMed] [Google Scholar]
- 10.Thomas T, Thomas TJ. Polyamine metabolism and cancer. J Cell Mol Med. 2003;7:113–26. doi: 10.1111/j.1582-4934.2003.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobbs CA, Paul BA, Gilmour SK. Deregulation of polyamine biosynthesis alters intrinsic histone acetyltransferase and deacetylase activities in murine skin and tumors. Cancer Res. 2002;62:67–74. [PubMed] [Google Scholar]
- 12.Proctor MS, Fletcher HV, Shukla JB, Rennert OM. Elevated spermidine and spermine levels in the blood of psoriatic patients. J Invest Dermatol. 1975;65:409–11. doi: 10.1111/1523-1747.ep12607659. [DOI] [PubMed] [Google Scholar]
- 13.Sakakibara S, Yoshikawa K. Urinary polyamine levels in patients with psoriasis. Arch Dermatol Res. 1979;265:133–7. doi: 10.1007/BF00407877. [DOI] [PubMed] [Google Scholar]
- 14.Lauharanta J, Kousa M, Kapyaho K, Linnamaa K, Mustakallio K. Reduction of increased polyamine levels in psoriatic lesions by retinoid and PUVA treatments. Br J Dermatol. 1981;105:267–72. doi: 10.1111/j.1365-2133.1981.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 15.Grekin RC, Ellis CN, Goldstein NG, Anderson TF, Duell EA, Voorhees JJ. Decreased urinary polyamines in patients with psoriasis treated with etretinate. J Invest Dermatol. 1983;80:181–4. doi: 10.1111/1523-1747.ep12533435. [DOI] [PubMed] [Google Scholar]
- 16.Lowe NJ, Breeding J, Russell D. Cutaneous polyamines in psoriasis. Br J Dermatol. 1982;107:21–5. doi: 10.1111/j.1365-2133.1982.tb00285.x. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein GD, McCullough JL, Eaglstein WH, Golub A, Cornell RC, Stoughton RB, et al. A clinical screening program for topical chemotherapeutic drugs in psoriasis. Arch Dermatol. 1981;117:388–93. [PubMed] [Google Scholar]


