Abstract
Background:
Actinic keratosis (AK) is a cutaneous neoplasm caused by prolonged sun exposure, and may progress into squamous cell carcinoma (SCC). The p53 gene plays a central role in the development of SCC, and mutations in this gene are found in 90% of SCC and up to 100% of AK cases.
Objective:
To identify AK cases that are highly susceptible to developing SCC.
Materials and Methods:
Fifty-six AK cases were classified into two groups: AK adjacent to “normal” skin and AK adjacent to SCC. The groups were compared based on epithelial atypia, inflammation, solar elastosis, histopathological AK classification and p53 protein expression.
Results:
Of the 56 AK cases analyzed, 23% were associated with SCC. The types of AK observed were classified as follows: common, hypertrophic, atrophic, acantholytic, pigmented and bowenoid. SCC was associated with common and hypertrophic AK, and p53 staining was observed in 78% of AK cases. The mean difference in p53 immunopositivity between common AK cases associated with SCC (17%) and not associated with SCC (45.4%) was significant (p=0.011).
Conclusions:
Hypertrophic and common AK are associated with SCC, and the low percentage of p53 immunopositivity in the common type indicates a greater probability of developing into SCC.
Keywords: Actinic keratosis, histopathology, immunohistochemical, p53, squamous cell carcinoma
Introduction
What was known?
The abnormal expression of p53 is considered a marker of pre-malignant lesions and plays a central role in the development of SCC.
Actinic keratosis (AK) is a cutaneous neoplasm caused by prolonged sun exposure and may progress into invasive squamous cell carcinoma (SCC). SCC is the second most frequent type of skin cancer, and its incidence has increased over the last several years in all regions of the world.[1,2] Epidemiological data suggest that the risk factors for AK and cutaneous SCC are the same, where the strongest supporting evidence for this link is the presence of histopathological alterations in tissues with AK in epithelium adjacent to SCC.[3]
In accordance with the field cancerization hypothesis, the presence of multiple AK lesions in the same patient indicates severe actinic damage to all the skin. In these cases, clinically normal skin surrounding AK lesions shows genetic alterations associated with carcinoma.[4,5]
The p53, a tumor suppressor protein, interrupts the cell cycle to allow the repair of damaged DNA. Mutations in the p53 gene are among the most common genetic abnormalities associated with diverse types of cancer.[6] Aberrant p53 protein expression is generally considered to be an indirect indication of mutation of this gene.[7,8] The abnormal expression of p53 is considered a marker of pre-malignant lesions and plays a central role in the development of SCC. Chromosomal mutations in this gene are found in 90% of SCC cases and in 16.6% to 100% of AK cases.[9,10,11]
However, to date, no study has compared the histopathology and p53 protein expression in AK tissue, SCC tissue and “normal” skin adjacent to AK with SCC and “normal” skin adjacent to AK without a tumor. Such a study could help determine the importance of the activity of the p53 protein in the progression of AK to SCC. Thus, we aimed to verify the differences in AK between these two groups, with the goal of identifying cases of AK with greater susceptibility to developing invasive SCC.
Materials and Methods
This retrospective study was conducted between 2004 and 2006 after approval from the Research Ethics Committee of the Federal University of São Paulo, Brazil. AK and SCC cases arising in photoexposed skin sites were selected from the archives of the Department of Pathology. AK cases that presented “normal” adjacent skin and AK cases adjacent to SCC with “normal” skin on the surgical margins were included. “Normal skin,” photodamaged skin with no epithelial atypia, was used as the control tissue. These requirements were met in 56 cases, which were then separated into two groups: AK adjacent to SCC and AK without SCC.
Clinical study
The following data were obtained from patient registers: Age, gender, phototype, and lesion location. Patients with other risk factors for skin cancer were excluded.
Histopathology
The following aspects of the tissue sample were evaluated through the study of histology slides stained with hematoxylin–eosin:
For AK: (1) the intensity of epithelial atypia: Light, moderate or intense; (2) the location of atypia on the epidermis: Lower 1/3, lower 2/3 or the entire epidermis; (3) the intensity of solar elastosis: Light, moderate or intense; (4) the type of AK, according to histopathological classification[12]: Atrophic, hypertrophic, acantholytic, pigmented, bowenoid, lichenoid, or common (the term “common” was adopted to designate a case of AK with epidermal thickness close to normal and without any distinguishing histopathological characteristics that did not fit into any cited histopathological type); and (5) the intensity of dermal inflammatory infiltrate: Light, moderate, or intense.
For SCC: The degree of differentiation, according to Broders’ classification:[12] I, II or III, (well-, moderately or poorly differentiated, respectively). Tumors of degree IV were excluded.
Immunohistochemical study of the p53 protein: The immunoperoxidase technique and the biotin avidin-peroxidase method were used to study the p53 protein, utilizing a mouse monoclonal anti-human p53 antibody, clone D0-7 (Dako, code no. M7001, CA, USA) at a 1/30 dilution. Histopathological slides were digitized at a resolution of 0.50 μm/pixel using the ScanScope GL System™ (Aperio, Vista, CA, USA) with a Plan Apo 20x/0.75 objective lens.[13,14] Selected fields were analyzed using Image Lab software (Diracom Bioinformática Ltd, SP, Brazil).[15,16] The p53 protein expression in the sample was considered positive when the cell nucleus stained brown.[17] Colon adenocarcinoma tissue was used as a positive control. Ten consecutive fields of AK, SCC and adjacent “normal” skin were selected, and a minimum of 500 cells was counted at × 200 magnification. The images obtained with the ScanScope System GL slide scanning system provided excellent staining visibility for the observer who determined the cell counts. The following parameters were evaluated in each field: (1) percentage of immunoreactive cells, according to the following semi-quantitative scale:[15,16] 0 = negative (<1%), 1 = positive (1% to 25%), 2 = positive (25% to 50%) and 3 = positive (>50%); (2) intensity of staining: Weak, moderate or intense; and (3) epidermal location of positive cells: In the basal or suprabasal layer.
The following associations between AK cases were analyzed statistically: (1) the degree of epithelial atypia was compared to the atypia location, degree of solar elastosis, intensity of dermal inflammatory infiltrate, and histopathological type; (2) clinical data and histopathological criteria were compared to the presence or absence of SCC; and (3) p53 protein expression was compared to (a) the histopathological AK type, according to the presence or absence of SCC; (b) p53 levels in adjacent “normal” skin; and c) p53 levels in SCC.
The Chi-squared test, Student's t-test, Fisher's exact test, and ANOVA were employed for data analysis using Minitab 15.1 statistical software. Some categories were condensed during analysis. Values of P < 0.05 were considered statistically significant. Quantitative data are represented by mean ± SE.
Results
Of the 56 cases of AK, 13 (23.21%) were associated with SCC. All the patients were Caucasian, with an average age of 78.3 years; 34 (60.71%) of the patients were women. The majority of lesions were located on the extremities (45%) or the head (41%). The following histopathological types were encountered: Acantholytic (4/56), atrophic (4/56), bowenoid (2/56), hypertrophic (21/56), pigmented (3/56), and common (22/56). Only the hypertrophic (5/21) and common (8/22) types presented with associated tumors. None of the clinical or histopathological criteria was correlated with the presence or absence of tumors [Table 1]. p53 immunoreactivity was observed in 78.57% of AK samples and in 38.46% of SCC samples [Table 2]. Figures 1 and 2 illustrate examples of immunoreactivity to p53.
Table 1.
Summary of the associations between clinical, histopathological and immunohistochemical criteria
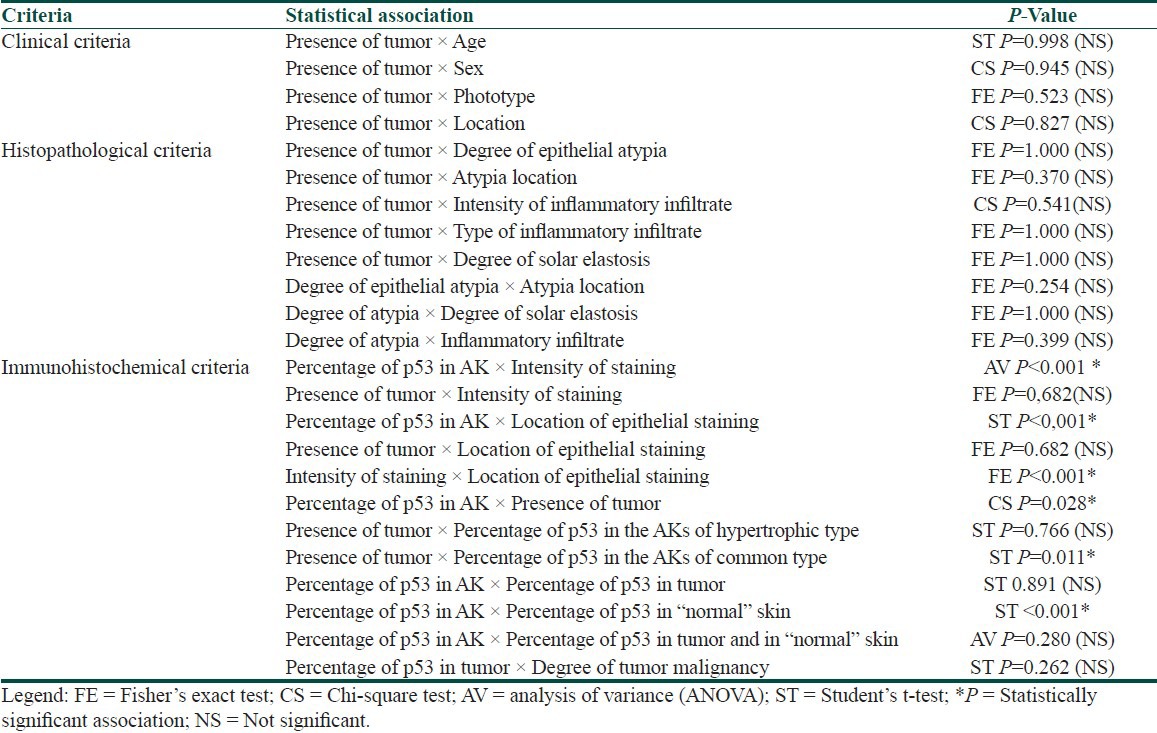
Table 2.
Immunoexpression of the p53 protein in AK, tumor and “normal” skin
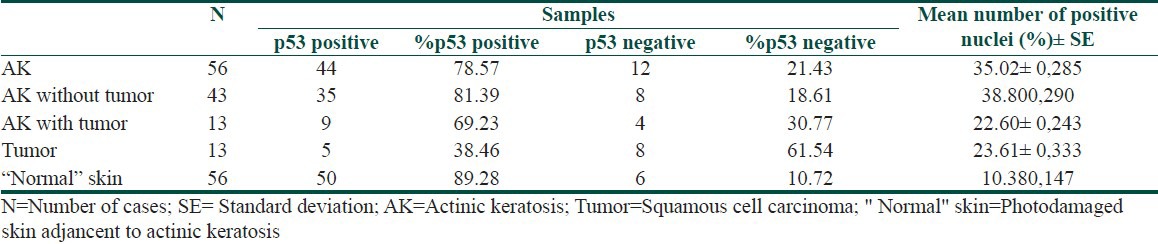
Figure 1.
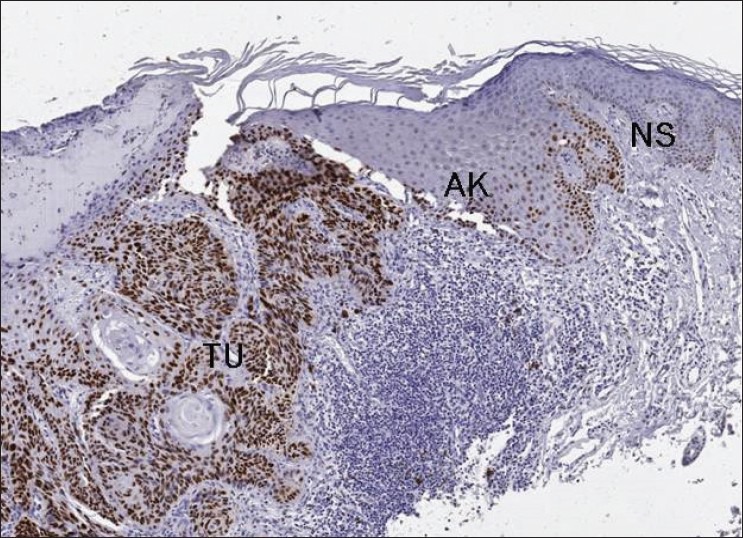
p53 protein expression - Strongly positive degree II squamous cell carcinoma (TU) with actinic keratosis (AK) and “normal” skin (NS) with low positivity (×50)
Figure 2.
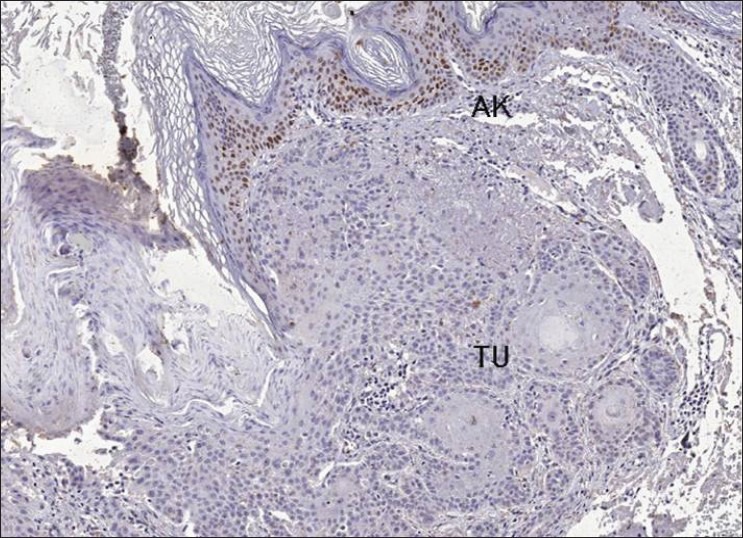
p53 protein expression - Histological slide sho wing absence of p53 immunereactivity in squamous cell carcinoma (TU) adjacent to actinic keratosis (AK) area with moderate immunereactivity (×50)
A significant relationship was found between the presence and absence of SCC and the quantity of p53-positive cells in AK tissue. The results of a Chi-squared test showed that the prevalence of SCC was significantly greater (P = 0.028) in cases where the proportion of p53-positive cells in AK was less than 25% [Tables 1 and 3 and Figure 3].
Table 3.
Percentage of p53 positive nuclei in AK associated or not with SCC
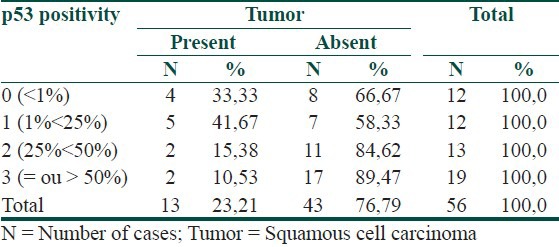
Figure 3.
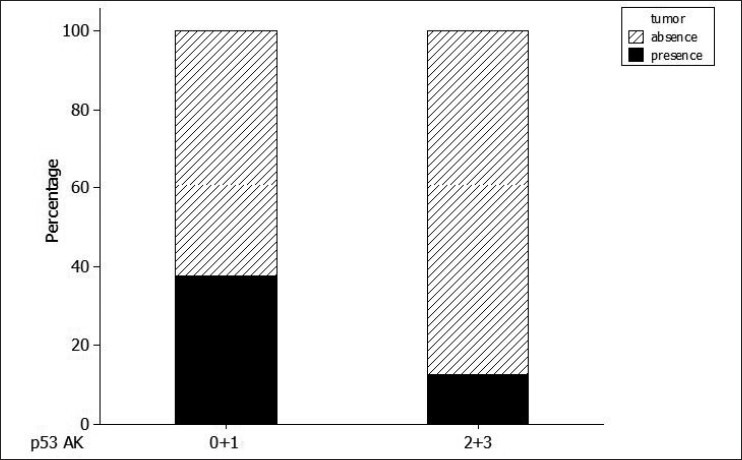
Percentage of p53 in actinic keratosis (AK), grouped according to the semi-quantitative classification
We used Student's t-test to separately analyze hypertrophic and common AK associated with tumors. There was a significant (P = 0.011) decrease in p53 protein immunopositivity in cases of common AK not associated with tumors (45.41 ± 23.80; n = 14) in comparison with AK associated with tumors (17.03 ± 20.62; n = 8). However, there was no difference in the mean percentage of p53-positive cells for the hypertrophic AK group (P = 0.766) [Table 1].
Of the 13 SCCs, 7 were degree I, and 6 were degree II. p53 protein expression was detected in seven cases, with averages of 13.65% and 35.20% of cells staining positive for degree I and degree II cases, respectively. Staining was negative in eight tumors [Figures 1 and 2].
Discussion
AK produces lesions that shelter the molecular alterations that lead to SCC in the skin.[18] The area affected by AK, the adjacent skin, and SCCs present typical histopathological alterations that are characteristic of sun damage. Of the 56 cases of AK studied here, 13 (23.2%) were associated with SCC. This finding demonstrates the necessity for studying suspected lesions,[19,20] with the goal of acquiring criteria that can facilitate the early diagnosis of invasive cancer. To achieve this goal, cases of AK that were associated with SCCs were compared to those not associated with tumors.
In terms of histopathological AK type, a predominance of common AK was noticed in 39.28% (22/56) of cases in this study, followed by hypertrophic AK in 37.50% (21/56) of cases. Only these two types of AK presented with associated SCC, in accordance with the findings of Sim et al.,[21] who found five tumors among eight cases of hypertrophic AK. The results of this study are also in accordance with the findings of Pimentel et al.[16] That study found a greater proportion of the same histopathological type, followed by the hypertrophic type, along with a greater frequency of SCC of the lip in cases of simple actinic cheilitis (AK of the lip would fall under the “common” type in this study).
AK with a nearly normal epithelial thickness and without histopathological characteristics allowing its classification as one specific variant was called “common.”[12,22]
The majority of AK cases showed p53 immunoreactivity (78.57%) and presented intense staining (66.7%); the greater the intensity, the greater the percentage of immunoreactive nuclei (P < 0.01) [Table 1]. Generally, these areas of intense staining are where p53 mutations are found.[23]
However, there was no significant difference in the intensity of the staining based on the presence or absence of tumors. All AK histopathological types presented p53 protein immunoreactivity, with similar average percentages of stained nuclei, in agreement with the findings of Sim et al.,[21] who found positivity in all AK types.
When comparing p53 protein immunoreactivity between cases of AK according to the presence or absence of a tumor, SCC prevalence was found to be greater in cases in which the proportion of p53-positive cells in the AK tissue was up to 25% (P = 0.028). In light of this unexpected finding, the histopathological types that presented with associated tumors were analyzed separately. For the hypertrophic type, there was no significant difference with respect to the percentage of p53-positive cells according to the presence or the absence of a tumor. However, in common AK, there was a significant difference (P = 0.011) between the percentages of p53-positive nuclei in lesions associated with SCC (8/22) and not associated with SCC (14/22), with averages of 17.0% and 45.4%, respectively. This surprising observation suggests that the low percentage of positive cells in “common” AK indicates a greater chance of progression to invasive carcinoma.
To explain how AK with no or low p53 immunoreactivity would be more likely to develop into skin SCC, the following data should be considered.
Studies have reported cases of adjacent AK and SCC, with both being either negative or positive for p53 expression. Nagano et al.[24] showed that not only p53-positive AK can progress to SCC. Several facts cited in the literature can justify the occurrence of AK with low immunopositivity for p53 expression.
Mutations in the p53 gene in cases of AK may either inhibit the normal function of the protein or provide it with a new function, including leading it to associate with the products of oncogenes.[25,26] If there has been the loss of an allele[2,27,28] or the deletion of both alleles, no p53 would be produced, and the gene would therefore lose its tumor suppression function.[2,8,29,30] However, not all mutant p53 genes are biologically identifiable, and there is a strong selective advantage for some mutation hotspots.[31]
One interesting finding is the higher percentage of specific mutations caused by UV radiation in AK and exposed skin, as compared to SCC.[32] However, not all UV-induced mutations are predictors of the stability of p53. On the other hand, UV radiation of non-melanoma skin cancer, particularly aggressive variants of SCC, induces mutations in the p16 gene,[33] which leads to the assumption that the p16 and p53 genes are involved in two independent cutaneous carcinogenesis pathways.[34] It is noteworthy that the common polymorphism at codon 72 of the p53 gene preferentially predisposes the arginine-containing allele to mutations along with the selective loss of the proline-containing allele, which could be a facilitating factor in the development of SCC in skin types I and II.[35]
Another explanation for the poor expression of p53 in AK is the ability of the protein to form complexes with other proteins, such as the oncoprotein MDM2[6,36,37] and E6, present in the high-risk human papillomavirus (HPV) subtypes HPV 16 and HPV 18, which can be detected in AK because the virus is involved in the etiology of AK.[24,38,39] Such associations can inactivate the normal function of p53.
The studies cited above may explain the low p53 protein expression in AK that has developed into invasive SCC, demonstrating that this progression could have occurred due to an increased number of mutations and/or deletions. Consequently, these mutations would have had such large impacts that the ability to inhibit cellular proliferation and initiate apoptosis would be lost in the mutant cells. Progression may also occur through the participation of other genes, such as p16 and MDM2, or even due to HPV. Another surprising finding in this study was that cases of common AK with the highest percentages of p53 immunopositivity were not associated with SCC. Possible explanations for this finding are provided by several observations from previous studies, cited below.
These cases of AK may possess mutations capable of stabilizing p53 but unable to modify its function. Einspahr et al.[32] found a higher percentage of mutations in cases of AK than in cases of SCC, which led them to conclude that not all mutations detected in AK and photodamaged skin are predisposed to malignancy.[4,40,41] Instead, certain mutations in limited cases of AK would have selective advantages in progressing to SCC. In this study, the high percentage of immunoreactive cells in the tumors without AK may have reflected a combination of normal and mutant proteins, as reported by Kastan et al.[42] and Shaw.[43] The antibody used here (DO-7) recognizes both the normal and mutant forms of the protein.
It has been shown that normal p53 decreases the expression of the nuclear proliferation antigen Ki-67.[44] However, a normal increase in the amount of p53 may be concomitant with increased cell proliferation in some tumors because p53 fails to exercise its function due to other mechanisms involved in carcinogenesis affecting p53-dependent pathways.[45] Einspahr et al.[32] suggested that p53-independent Bax apoptosis could be a means of preventing tumor development in p53 mutant cells, by inducing apoptosis even in the absence of normal p53. Consistent with the hypothesis that p53 acts as the “guardian of the genome,” this immunoreactivity may be due to the induction of synthesis of normal p53 in response to the high frequency of DNA errors in proliferating tissue.[42]
Jiang et al.[46] observed mutations induced by UV radiation, but not loss of alleles, in p53 heterozygous rats. The smaller rates of SCC and AK compared to rats homozygous for p53 loss show that p53 still has a protective function after UV irradiation.[2] The expression of a normal p53 gene can suppress the neoplastic phenotype; that is, the normal p53 gene is dominant over the mutated gene.
The mutations observed in AK may be different from those seen in SCC,[47,48] and different mutant alleles of the p53 gene may not have the same biological and biochemical properties. Therefore, the presence of some mutant genes in AK contributes to its progression into SCC in contrast with others. Therefore, it is presumed that gains in mutations may lead to transformation into SCC. This transition was probably prevented by the normal action of p53 in the studied cases of AK with high immunoreactivity for p53 and no associated SCC.
Assuming that the immunoreactivity observed in common AK in this study was a false positive, the normal protein could have accumulated due to a defect in degradation.[29,30] However, this interpretation is unlikely to be true because the adjacent photodamaged skin from the same patient was used as a control in all of the cases studied here.
In summary, the high percentage of p53-positive cells can be explained by the small and numerous mutations in AK that lead to greater synthesis of normal and/or mutant p53 protein with preserved function. This production would impede invasive neoplastic transformation by inhibiting proliferation and inducing apoptosis via p53-dependent or p53-independent pathways.
Another unexpected result was the observation of p53 immunopositivity in only 38.46% of tumors, with a corresponding negative rate of 61.54%. This ratio is similar to the findings of Batinac et al.,[44] who found a p53 tumor positivity rate of 35.7%.
Similar average p53 positivity values have been noted in cases of SCC and their associated incidences of AK.[24,32,49] Out of 13 tumors, both AK and SCC were negative for p53 in t cases, and both were positive in 4 cases. This finding demonstrates that it is unlikely that only p53-positive AK will develop into SCC.[21,24] The possibility remains that other genes, such as p16 and MDM2, may be involved in tumors that are negative for p53 protein expression.[33,34,36] In 5 of 13 tumors, the AK tissue was positive, and the tumors were negative, suggesting the occurrence of additional genetic alterations, such as mutant allele loss during tumor development.[24] In cases in which both the AK and the tumor were positive or negative, it is possible that the mutations necessary for tumor development had occurred before tumor invasion.[24]
Considering only the 13 cases that presented tumors, p53 protein immunoreactivity in “normal” skin (9.21%), adjacent AK (22.57%) and SCC (23.61%) progressively increased from “normal” skin to cancer; however, the difference between AK and SCC was small.[24,32,49]
In this study, it was possible to conclude the following: (1) only hypertrophic and common AK histopathological types appear to be associated with SCC; (2) there was no association between clinical and histopathological aspects in relation to the presence or the absence of SCC; (3) frequent p53 protein immunoreactivity in AK suggests that these lesions involve changes in the p53 gene; and (4) the small percentage of p53 immunopositivity in common AK suggests that this histopathological type has a high probability of developing into SCC, as compared to AK with stronger positive signals.
To evaluate the potential of AK to develop into SSC, and thereby answer the questions raised by this preliminary study, we suggest an increase in research on the topic. In addition, more information could be gathered with the quantification of p53, sequencing of the p53 gene and other oncogenes and detection of HPV in normal and tumor tissues microdissected from AK lesions. Thus, AK, p53 expression and the level and type of the gene mutation may be correlated
What is new?
The small percentage of p53 immunopositivity in common AK suggests that this histopathological type has a high probability of developing into SCC, as compared to AK with stronger positive signals.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: Incidence. J Am Acad Dermatol. 1994;30:774–8. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–6. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz RM, Monger LE. Solar keratosis: An evolving squamous cell carcinoma. Benign or malignant? Dermatol Surg. 1995;21:184. doi: 10.1111/j.1524-4725.1995.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 4.Roewert-Huber J, Stockfleth E, Kerl H. Pathology and pathobiology of actinic (solar) keratosis-an update. Br J Dermatol. 2007;157(Suppl 2):18–20. doi: 10.1111/j.1365-2133.2007.08267.x. [DOI] [PubMed] [Google Scholar]
- 5.Vatve M, Ortonne JP, Birch-Machin MA, Gupta G. Management of field change in actinic keratosis. Br J Dermatol. 2007;157(Suppl 2):21–4. doi: 10.1111/j.1365-2133.2007.08268.x. [DOI] [PubMed] [Google Scholar]
- 6.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 7.Berhane T, Halliday GM, Cooke B, Barnetson RS. Inflammation is associated with progression of actinic keratoses to squamous cell carcinomas in humans. Br J Dermatol. 2002;146:810–15. doi: 10.1046/j.1365-2133.2002.04720.x. [DOI] [PubMed] [Google Scholar]
- 8.Queille S, Luron L, Spatz A, Avril MF, Ribrag V, Duvillard P, et al. Analysis of skin cancer risk factors in immunosuppressed renal transplant patients shows high levels of UV-specific tandem CC to TT mutations of the p53 gene. Carcinogenesis. 2007;28:724–31. doi: 10.1093/carcin/bgl191. [DOI] [PubMed] [Google Scholar]
- 9.Leffell DJ. The scientific basis of skin cancer. J Am Acad Dermatol. 2000;42:18–22. doi: 10.1067/mjd.2000.103340. [DOI] [PubMed] [Google Scholar]
- 10.McGregor JM, Yu CC, Dublin EA, Levison DA, MacDonald DM. Aberrant expression of p53 tumour-suppressor protein in non-melanoma skin cancer. Br J Dermatol. 1992;127:463–9. doi: 10.1111/j.1365-2133.1992.tb14841.x. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler A, Leffell DJ, Kunala S, Sharma HW, Gailani M, Simon JA, et al. Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc Natl Acad Sci U S A. 1993;90:4216–20. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeBoit PE, Burg G, Weedon D. Pathology and genetics of skin tumors. Lyon: IARC Press; 2006. World health organization classification tumours. [Google Scholar]
- 13.Stewart J, 3rd, Bevans-Wilkins K, Bhattacharya A, Ye C, Miyazaki K, Kurtycz DF. Virtual microscopy: An educator's tool for the enhancement of cytotechnology students’ locator skills. Diagn Cytopathol. 2008;36:363–8. doi: 10.1002/dc.20821. [DOI] [PubMed] [Google Scholar]
- 14.Yagi Y, Gilbertson JR. A relationship between slide quality and image quality in whole slide imaging (WSI) Diagn Pathol. 2008;3(Suppl 1):S12. doi: 10.1186/1746-1596-3-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Araujo VC, Loyola AM, Pinto DD, Junior, Borra RC, De Araujo NS. p53 In biopsies of oral squamous cell carcinoma. A comparative study with a malignancy grading system. Oral Oncol. 1997;33:5–9. doi: 10.1016/s0964-1955(96)00055-3. [DOI] [PubMed] [Google Scholar]
- 16.Neto Pimentel DR, Michalany N, Alchorne M, Abreu M, Borra RC, Weckx L. Actinic cheilitis: Histopathology and p53. J Cutan Pathol. 2006;33:539–44. doi: 10.1111/j.1600-0560.2006.00440.x. [DOI] [PubMed] [Google Scholar]
- 17.Hall PA, Lane DP. p53 in tumour pathology: Can we trust immunohistochemistry? Revisited! J Pathol. 1994;172:1–4. doi: 10.1002/path.1711720103. [DOI] [PubMed] [Google Scholar]
- 18.Ackerman AB, Mones J. Squamous-cell carcinoma in situ is a fiction! J Cutan Pathol. 2009;36:74–6. doi: 10.1111/j.1600-0560.2008.00984.x. [DOI] [PubMed] [Google Scholar]
- 19.Callen JP, Bickers DR, Moy RL. Actinic keratoses. J Am Acad Dermatol. 1997;36:650–3. doi: 10.1016/s0190-9622(97)70265-2. [DOI] [PubMed] [Google Scholar]
- 20.Moy RL. Clinical presentation of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42:8–10. doi: 10.1067/mjd.2000.103343. [DOI] [PubMed] [Google Scholar]
- 21.Sim CS, Slater S, McKee PH. Mutant p53 expression in solar keratosis: An immunohistochemical study. J Cutan Pathol. 1992;19:302–8. doi: 10.1111/j.1600-0560.1992.tb01366.x. [DOI] [PubMed] [Google Scholar]
- 22.Rossi R, Mori M, Lotti T. Actinic keratosis. Int J Dermatol. 2007;46:895–904. doi: 10.1111/j.1365-4632.2007.03166.x. [DOI] [PubMed] [Google Scholar]
- 23.Girod SC, Pfeiffer P, Ries J, Pape HD. Proliferative activity and loss of function of tumour suppressor genes as ‘biomarkers’ in diagnosis and prognosis of benign and preneoplastic oral lesions and oral squamous cell carcinoma. Br J Oral Maxillofac Surg. 1998;36:252–60. doi: 10.1016/s0266-4356(98)90708-2. [DOI] [PubMed] [Google Scholar]
- 24.Nagano T, Ueda M, Ichihashi M. Expression of p53 protein is an early event in ultraviolet light-induced cutaneous squamous cell carcinogenesis. Arch Dermatol. 1993;129:1157–61. [PubMed] [Google Scholar]
- 25.Finlay CA, Hinds PW, Levine AJ. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989;57:1083–93. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- 26.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–6. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 27.Rehman I, Quinn AG, Healy E, Rees JL. High frequency of loss of heterozygosity in actinic keratoses, a usually benign disease. Lancet. 1994;344:788–9. doi: 10.1016/s0140-6736(94)92343-4. [DOI] [PubMed] [Google Scholar]
- 28.Rehman I, Takata M, Wu YY, Rees JL. Genetic change in actinic keratoses. Oncogene. 1996;12:2483–90. [PubMed] [Google Scholar]
- 29.McNutt NS, Saenz-Santamaria C, Volkenandt M, Shea CR, Albino AP. Abnormalities of p53 protein expression in cutaneous disorders. Arch Dermatol. 1994;130:225–32. [PubMed] [Google Scholar]
- 30.Ro YS, Cooper PN, Lee JA, Quinn AG, Harrison D, Lane D, et al. p53 protein expression in benign and malignant skin tumours. Br J Dermatol. 1993;128:237–41. doi: 10.1111/j.1365-2133.1993.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 31.Giglia-Mari G, Sarasin A. TP53 mutations in human skin cancers. Hum Mutat. 2003;21:217–28. doi: 10.1002/humu.10179. [DOI] [PubMed] [Google Scholar]
- 32.Einspahr JG, Alberts DS, Warneke JA, Bozzo P, Basye J, Grogan TM, et al. Relationship of p53 mutations to epidermal cell proliferation and apoptosis in human UV-induced skin carcinogenesis. Neoplasia. 1999;1:468–75. doi: 10.1038/sj.neo.7900061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soufir N, Moles JP, Vilmer C, Moch C, Verola O, Rivet J, et al. P16 UV mutations in human skin epithelial tumors. Oncogene. 1999;18:5477–81. doi: 10.1038/sj.onc.1202915. [DOI] [PubMed] [Google Scholar]
- 34.Nindl I, Gottschling M, Krawtchenko N, Lehmann MD, Rowert-Huber J, Eberle J, et al. Low prevalence of p53, p16(INK4a) and Ha-ras tumour-specific mutations in low-graded actinic keratosis. Br J Dermatol. 2007;156(Suppl 3):34–9. doi: 10.1111/j.1365-2133.2007.07857.x. [DOI] [PubMed] [Google Scholar]
- 35.McGregor JM, Harwood CA, Brooks L, Fisher SA, Kelly DA, O’Nions J, et al. Relationship between p53 codon 72 polymorphism and susceptibility to sunburn and skin cancer. J Invest Dermatol. 2002;119:84–90. doi: 10.1046/j.1523-1747.2002.01655.x. [DOI] [PubMed] [Google Scholar]
- 36.Ganguli G, Abecassis J, Wasylyk B. MDM2 induces hyperplasia and premalignant lesions when expressed in the basal layer of the epidermis. EMBO J. 2000;19:5135–47. doi: 10.1093/emboj/19.19.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu S, Tiekso J, Hietanen S, Syrjanen K, Havu VK, Syrjanen S. Expression of cell-cycle proteins p53, p21 (WAF-1), PCNA and Ki-67 in benign, premalignant and malignant skin lesions with implicated HPV involvement. Acta Derm Venereol. 1999;79:268–73. doi: 10.1080/000155599750010634. [DOI] [PubMed] [Google Scholar]
- 38.Kvlividze O, Gogiashvili L, Burkadze G. The characteristics of human papillomavirus expression and cell proliferation in actinic keratosis and Bowen's disease of the skin. Georgian Med News. 2006;136:108–12. [PubMed] [Google Scholar]
- 39.Mitsuishi T, Kawana S, Kato T, Kawashima M. Human papillomavirus infection in actinic keratosis and bowen's disease: Comparative study with expression of cell-cycle regulatory proteins p21(Waf1/Cip1), p53, PCNA, Ki-67, and Bcl-2 in positive and negative lesions. Hum Pathol. 2003;34:886–92. doi: 10.1016/s0046-8177(03)00352-6. [DOI] [PubMed] [Google Scholar]
- 40.Brash DE. Sunlight and the onset of skin cancer. Trends Genet. 1997;13:410–14. doi: 10.1016/s0168-9525(97)01246-8. [DOI] [PubMed] [Google Scholar]
- 41.Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991;88:10124–8. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer res. 1991;51:6304–11. [PubMed] [Google Scholar]
- 43.Shaw PH. The role of p53 in cell cycle regulation. Pathol Res Pract. 1996;192:669–75. doi: 10.1016/S0344-0338(96)80088-4. [DOI] [PubMed] [Google Scholar]
- 44.Batinac T, Zamolo G, Jonjic N, Gruber F, Petrovecki M. p53 protein expression and cell proliferation in non-neoplastic and neoplastic proliferative skin diseases. Tumori. 2004;90:120–7. doi: 10.1177/030089160409000124. [DOI] [PubMed] [Google Scholar]
- 45.Kumar MG, Spandau DF. p53-binding proteins in squamous cell carcinoma and normal human keratinocytes. Cell Growth Differ. 1995;6:1601–8. [PubMed] [Google Scholar]
- 46.Jiang W, Ananthaswamy HN, Muller HK, Kripke ML. p53 protects against skin cancer induction by UV-B radiation. Oncogene. 1999;18:4247–53. doi: 10.1038/sj.onc.1202789. [DOI] [PubMed] [Google Scholar]
- 47.Ren ZP, Ahmadian A, Ponten F, Nister M, Berg C, Lundeberg J, et al. Benign clonal keratinocyte patches with p53 mutations show no genetic link to synchronous squamous cell precancer or cancer in human skin. Am J Pathol. 1997;150:1791–803. [PMC free article] [PubMed] [Google Scholar]
- 48.Ren ZP, Ponten F, Nister M, Ponten J. Two distinct p53 immunohistochemical patterns in human squamous-cell skin cancer, precursors and normal epidermis. Int J Cancer. 1996;69:174–9. doi: 10.1002/(SICI)1097-0215(19960621)69:3<174::AID-IJC4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 49.Barzilai A, Lyakhovitsky A, Trau H, Fogel M, Huszar M. Expression of p53 in the evolution of squamous cell carcinoma: Correlation with the histology of the lesion. J Am Acad Dermatol. 2007;57:669–76. doi: 10.1016/j.jaad.2007.04.025. [DOI] [PubMed] [Google Scholar]


