Abstract
Background:
Inflammatory cytokines are the key factor in the pathophysiology of acne. It is well known that keratinocytes synthesize many kinds of inflammatory cytokines. In addition, it is reported that inflammatory cytokines are also expressed from sebocytes, which originate from the same stem cells with keratinocytes.
Aim:
To clarify changes in the expression of inflammatory biomarkers from cultured sebocytes after treatment with vitamin D.
Materials and Methods:
Reverse transcription-polymerase chain reaction (RT-PCR) was done to measure changes in the expression of inflammatory biomarkers, including interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor-α (TNF-α), and several subtypes of matrix metalloproteinases (MMPs) after treatment of a group of cultured sebocytes with vitamin D. Vitamin D receptor (VDR) small interfering RNA (siRNA) was added in the other group of cultured sebocytes to assure the role of vitamin D on the expression of inflammatory biomarkers. Enzyme-linked immunosorbent assay (ELISA) was also performed in the vitamin D-treated sebocytes.
Results:
Cultured sebocytes showed non-significant changes in the gene expression of inflammatory biomarkers after treatment with vitamin D. In cultured sebocytes treated with a VDR siRNA, the expression of inflammatory biomarkers was not blocked after treatment with vitamin D. ELISA showed a significant decrease in the expression of IL-6, IL-8, and MMP-9, but a significant increase in the expression of MMP-1 and MMP-3, after treatment with vitamin D (10-6 M).
Conclusion:
Expression of inflammatory biomarkers is influenced by treatment with vitamin D in cultured sebocytes, but not through VDR.
Keywords: Acne, cultured sebocyte, inflammatory cytokines, vitamin D, vitamin D siRNA
Introduction
What was known?
1. Production of inflammatory cytokines in sebaceous glands can affect the development of acne.
2. Sebocytes have been identified as bioactive vitamin D-responsive target cells.
Acne is a multifactorial disease based on an alteration in the pattern of keratinization within the pilosebaseous follicles, resulting in comedo formation, increase in sebum production, which is influenced by androgens, proliferation of Propionibacterium (P.) acnes, and peri-follicular inflammation.[1] The sebaceous gland plays an important role in the pathophysiology of acne. Excessive sebum production and its abnormal lipid ingredients play an important role in contributing to the formation of the primary lesions associated with acne.[2]
Vitamin D regulates the growth and differentiation of keratinocytes and other cell types as its bioactive form, 1,25-dihydroxyvitamin D3 [1,25(OH2)D3]. Recently, sebocytes were identified as bioactive vitamin D-responsive target cells, indicating that vitamin D analog may be effective in the treatment of acne.[3]
Based on previous studies that substance P and dihydrotestosterone might be involved in the production of inflammatory cytokines in sebaceous glands in acne,[4,5] we conducted this study to define the effect of vitamin D on the expression of inflammatory biomarkers from sebocytes, such as interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor-α (TNF-α), and matrix metalloproteinase-1 (MMP-1), MMP-3, and MMP-9.
Materials and Methods
Sebocyte culture
Specimens for sebocyte culture were obtained from the non-balding occipital scalp region of patients with androgenic alopecia. The specimens were obtained under their consents.
Sebaceous glands were isolated from dissected hair follicles under a binocular microscope and transferred to a tissue culture dish. The cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Hyclone Laboratories, Logan, UT. USA) at 37°C in a humidified atmosphere of 5% CO2. Explants were left for 5 days then the medium was changed to Epilife (MEPI500CA; Gibco BRL, Grand Island, NY. USA). The medium was changed every 3 days. DMEM was supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and 20% heat-inactivated fetal calf serum (Hyclone Laboratories) and Epilife was supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and fungizone (250 μg/ml).
After cell outgrowth became sub-confluent, cells were harvested with 0.25% trypsin and 10 mM EDTA in Hank's balanced salt solution and sub-cultured at a split ratio of 1:3. Cells obtained after the second passage were used in this study after identifying the cultured sebocytes with hematoxylin and eosin, Oil red O, Nile red (Sigma, St Louis, MO. USA) staining, and immunocytofluorescence against cytokeratin 1 and 7 (Chemicon, MA. Billerica, USA).
Treatment of cultured sebocytes with vitamin D and vitamin D receptor siRNA
The cultured sebocytes were treated with 1,25-dihydroxyvitamin D3 (vitamin D) (10−10-10−6 M) for 5 days. The concentrations of vitamin D were decided after CCK-8 assay (Cell Counting Kit-8; Dojindo Laboratories, Kumamoto, Japan). In one experimental group, a vitamin D receptor (VDR) small interfering RNA (siRNA) was added into the cultured sebocytes to inhibit the expression of VDR. After then, vitamin D (10−10-10−6 M) was added to the VDR siRNA-treated cultured sebocytes for 5 days.
siRNA duplex oligonucleotides were purchased from Bioneer Corporation (Daejeon, Korea). Transfection was performed as indicated by the manufacturer. One day before transfection, sebocytes were seeded at a density of 3 × 105 per well in six-well plates with 2 ml of Epilife without antibiotics. After 1 day, the growth medium was removed from the cells, and 500 μl of fresh Epilife was added. For each well to be transfected, siRNA duplex-Lipofectamine™ RNAiMAX complexes were prepared. The siRNA duplex and Lipofectamine RNAiMAX (Invitrogen, Grand Island, NE. USA) were diluted in 500 μl growth medium, and they were mixed and incubated for 20 min at room temperature. Then the mixture was added to each well containing sebocytes. The cells were incubated 48 h at 37°C in a 5% CO2 incubator. For selecting efficient siRNA, we used three types of siRNAs at 100 nM concentration. The sequences were VDR1 (siRNA no. 1161711: 5′-CUAAGAUGAUACCAGGAUU, 5′-AAUCCUGGUAUCAUCUUAG), VDR2 (siRNA no. 1161712: 5′-GACCAGAUCGUACUGCUGA, 5′-UCA GCAGUACGAUCUGGUC), and VDR3 (siRNA no. 1161707: 5′-CCAACCCAUCAGAAGGAGA, 5′-UCUCCU UCUGAUGGGUUGG). Then we selected one and transfected with various concentrations, 10, 20, 50, and 100 nM, for optimal concentration. We performed reverse transcription-polymerase chain reaction (RT-PCR) to assay decrease in gene expression.
Reverse transcription-polymerase chain reaction
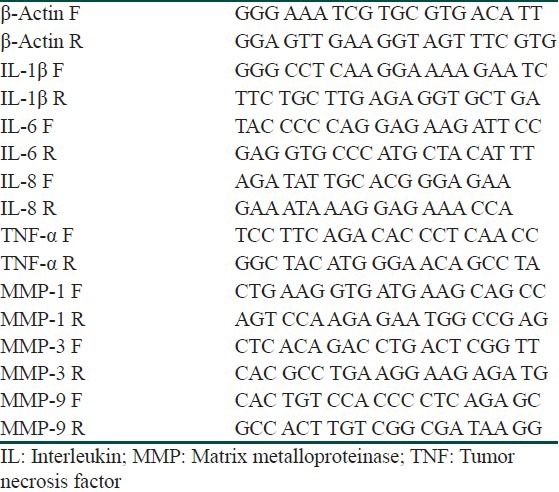
PCR amplification was repeated in quintuplicate using a first-strand cDNA synthesis kit (Promega, Madison, WI. USA) and oligonucleotide primers (Genotech, Daejeon, Korea) for IL-1β, IL-6, IL-8, TNF-α, MMP-1, MMP-3, and MMP-9 [Table 1]. Image J (NIH Image, Bethesda, MD, USA) was used for quantitative analysis. The mRNA levels were normalized to β-actin, which was presented as the control group, and represented as a relative ratio.
Table 1.
Primers of biomarkers
Total RNA extraction
Total RNA was isolated from the cells using the TRIzol reagent (Invitrogen) according to a modified acid phenol method.
Isolation of mRNA
mRNA was isolated from total RNA using the Dynabead mRNA purification kit (Dynal, Carlsbad, CA. USA).
RNA amplification
cDNA was synthesized from 5 μg total RNA using the cDNA synthesis kit containing ImProm-II™ reverse transcriptase and random primers (Promega). PCR amplification was conducted using GoTaq Flexi DNA Polymerase. All amplifications except IL-1β were performed for 35 cycles using the following conditions: 95°C for 1 min, 56°C for 1 min, and 72°C for 1 min. IL-1β amplifications were performed for 25 cycles using same parameters.
Statistical analysis
Statistical analysis was carried out on all data by analysis of variance (ANOVA). A probability value less than 0.05 was considered statistically significant.
ELISA
Analysis of IL-1β, IL-6, IL-8, TNF-α, MMP-1, MMP-3, and MMP-9 (R and D Systems, Shanghai, China) by enzyme-linked immunosorbent assay (ELISA) followed the manual provided by the manufacturer. Vitamin D (10−10, 10−8, and 10−6 M)-treated cultured sebocytes were evaluated. Briefly, we added 50 μl of the samples to each well in duplicate. Then, 50 μl of biotinylated antibody reagent, 100 μl of prepared streptavidin-horseradish peroxidase (HRP), and 100 μl of a premixed TMB substrate solution were added to each well in order. We developed the plate in the dark at room temperature for 30 min; stopped the reaction by adding 100 μl of stop solution to each well; and finally measured absorbance using a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA. USA).
Results
Expression of IL-6 and IL-8 in cultured sebocytes decreased after treatment with vitamin D
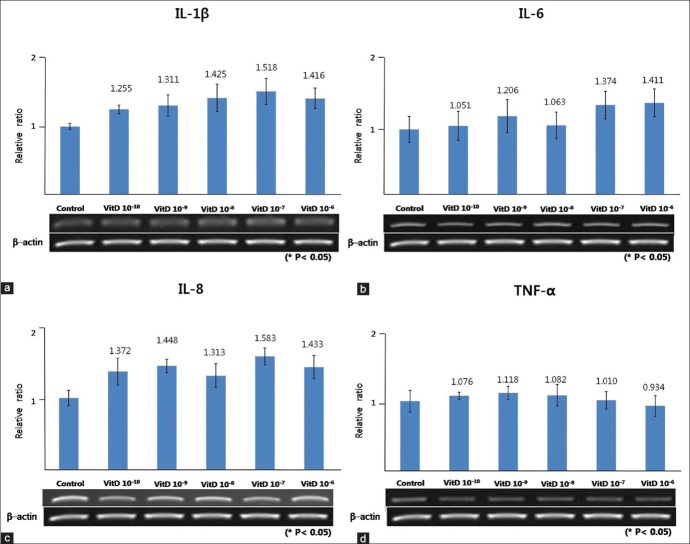
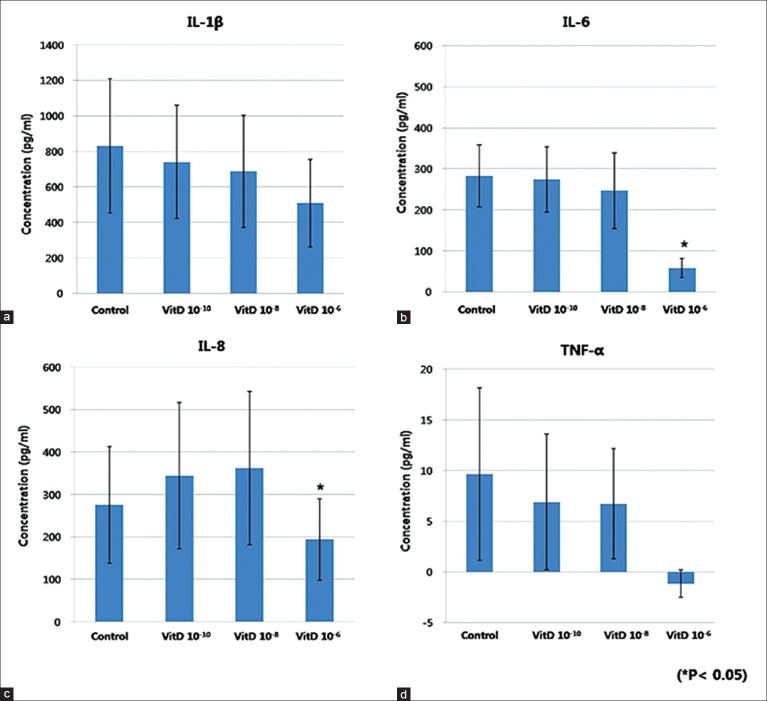
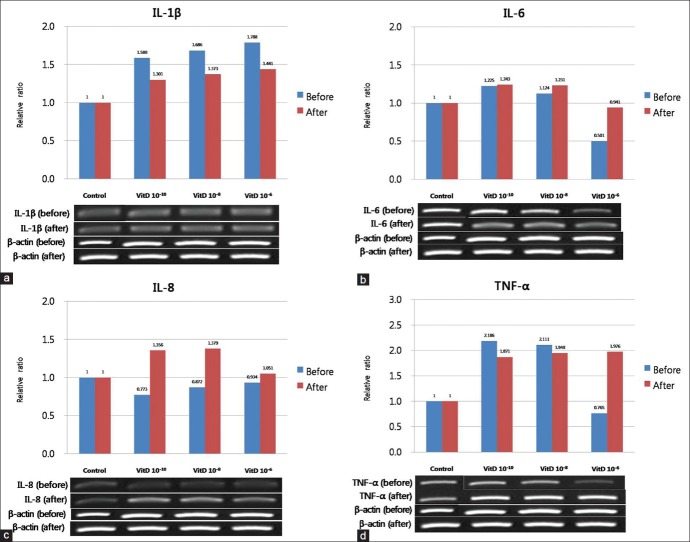
The gene expression of IL-1β, IL-6, IL-8, and TNF-α from cultured sebocytes did not show significant changes after treatment with vitamin D (10−10 -10−6 M) (P > 0.05) [Figure 1]. However, in ELISA, release of IL-6 and IL-8 from cultured sebocytes showed a significant decrease after treatment with vitamin D (10−6 M) (P < 0.05) [Figure 2]. The gene expression of IL-1β, IL-6, IL-8, and TNF-α in the VDR siRNA-treated sebocytes was not blocked after treatment with vitamin D (10−10, 10−8, and 10−6 M) [Figure 3].
Figure 1.
The effect of vitamin D on the gene expression of IL-1 b (a), IL - 6 (b), IL - 8 (c), and TNF -α (d) in cultured sebocytes
Figure 2.
ELISA of IL-1 b (a), IL - 6 (b), IL - 8 (c), and TNF -α (d) in cultured sebocytes
Figure 3.
Effect of VDR siRNA on the gene expression of IL-1 b (a), IL - 6 (b), IL - 8 (c), and TNF -α (d) in cultured sebocytes after treatment with vitamin D
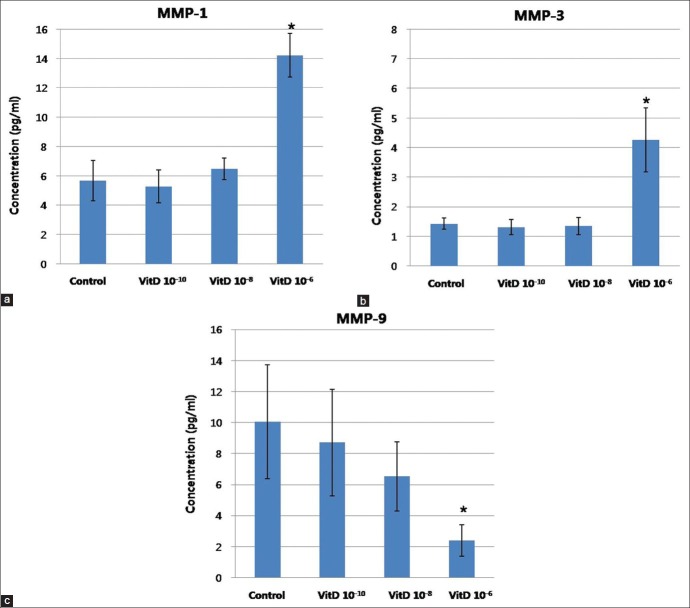
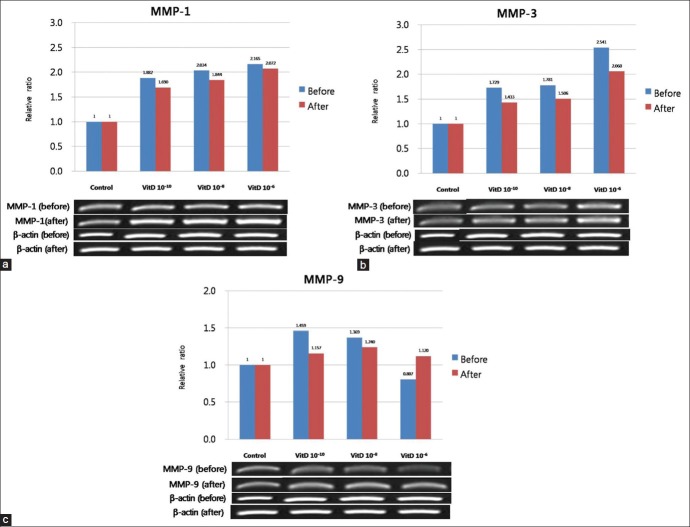
Expression of MMP-1 and MMP-3 in cultured sebocytes increased after vitamin D treatment, whereas MMP-9 expression decreased
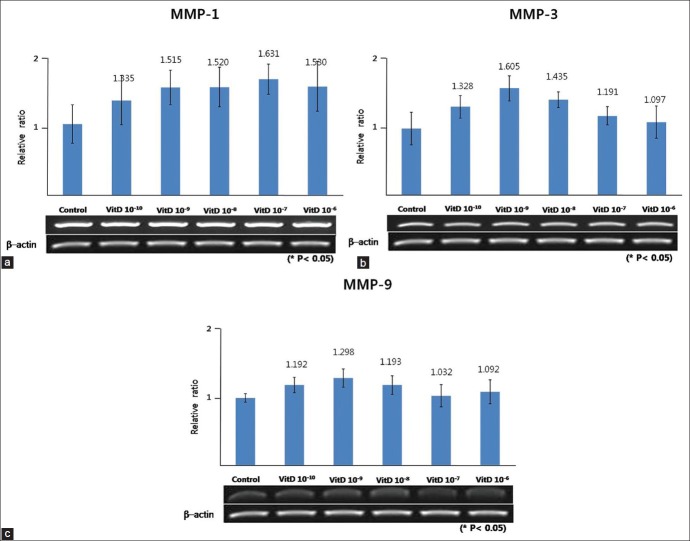
The expression of MMP-1, MMP-3, and MMP-9 in cultured sebocytes did not show significant changes after treatment with vitamin D [Figure 4]. However, in ELISA, release of MMP-1 and MMP-3 from cultured sebocytes showed a significant increase after treatment with vitamin D (10-6 M) (P < 0.05) [Figure 5]. On the contrary, ELISA analysis of MMP-9 from cultured sebocytes showed a significant decrease after treatment with vitamin D (10−6 M) (P < 0.05) [Figure 5]. The gene expression of MMP-1, MMP-3, and MMP-9 in the VDR siRNA-treated sebocytes was not blocked after treatment with vitamin D (10−10, 10−8, and 10−6 M) [Figure 6].
Figure 4.
Effect of vitamin D on the gene expression of MMP-1 (a), MMP-3 (b), and MMP-9 (c) in cultured sebocytes
Figure 5.
ELISA of MMP-1 (a), MMP-3 (b), and MMP-9 (c) in cultured sebocytes
Figure 6.
Effect of VDR siRNA on the gene expression of MMP-1 (a), MMP-3 (b), and MMP-9 (c) in cultured sebocytes after treatment with vitamin D
Discussion
Acne vulgar is is a chronic inflammatory and multifactorial disease caused by an alteration in the pattern of keratinization within the pilosebaseous follicles, resulting in comedo formation, increase in sebum production, which is influenced by androgens, proliferation of P. acnes, and peri-follicular inflammation.[1] It is well known that P. acnes GroEL (heat-shock protein HSP60), bacterial cell wall factors, stimulates the production of the proinfammatory cytokines IL-1β and TNF-γ from human keratinocytes.[6] Similar to keratinocytes, human sebocytes also express functional receptors for various substances such as corticotropin-releasing hormone.[7] The biological function of sebocytes is further regulated by several factors, including ligands of receptors expressed in sebocytes, such as androgens and estrogens, PPAR (peroxisome proliferator-activated receptor) ligands and neuropeptides, liver-X receptor ligands, histamines, retinoids, and vitamin D. The ligand–receptor complexes activate pathways involving cell proliferation, differentiation, lipogenesis, hormone metabolism, and cytokine and chemokine release.[8] Abnormal colonization by P. acnes has been implicated in the occurrence of acne via induction of inflammatory mediators. It stimulates the production of inflammatory cytokines, including IL-1β, IL-8, IL-12, and TNF-α, from both keratinocytes and sebocytes.[9,10,11,12] Both cell types can be activated by P. acnes via Toll-like receptors (TLRs), CD14, and CD1 molecules.[11] Expression of TLR2, TLR4, TLR6, and CD14 has already been documented in SZ95 sebocytes.[13] Recently, several studies presented some results that could reduce acne development by modulating sebocytes. TNF-α was revealed to induce lipogenesis in SZ95 human sebocytes through the c- Jun N-terminal kinase (JNK) and phosphoinositide-3-kinase/Akt pathways.[14] Regulation of stearoyl-coenzyme A desaturase and fatty acid delta-6 desaturase-2 expression by treating SZ95 human sebocytes with linoleic acid and arachidonic acid leads to enhancement of proinflammatory activity but did not affect lipogenesis in human sebocytes.[15]
Our former study showed that slightly increased immunoreactivity to IL-1β, IL-6, and TNF-α was observed after adding substance P and dihydrotestosterone to cultured sebocytes.[4,5] In general, the effects of vitamin D are not only important in calcium homeostasis, but also in immune regulation, cell growth, and cell differentiation. Binding of vitamin D to vitamin D receptor stimulates the proliferation of sebocytes, and inhibits the differentiation and lipid synthesis of sebocytes.[16] According to our experimental data, we report that vitamin D shows a decrease in the production of inflammatory biomarkers, especially IL-6, IL-8, and MMP-9, from cultured sebocytes. Likely to our result, Krδmer et al.[17] reported that vitamin D reduced the secretion of IL-6 and IL-8 in the supernatant of SZ95 sebocytes. Zhang et al.[18] also demonstrated that vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting mitogen-activated protein kinase (MAPK) phosphatase-1. Vitamin D can modulate MMP expression.[19] Especially, MMP-9 is decreased by treatment with vitamin D.[20] Based on our study, different from other inflammatory biomarkers, expression of MMP-1 and MMP-3 was increased by treatment with vitamin D. Moreover, it is suggested that blockade of VDR did not show a significant change in the gene expression of inflammatory biomarkers in cultured sebocytes after treatment with vitamin D. It is reported that the repressed cytokine production by vitamin D could be explained partly by the reduced cell membrane expression of TLRs.[21]
In conclusion, expression of IL-6, IL-8, and MMP-9 from cultured sebocytes decreased by treatment with vitamin D. On the contrary, expression of MMP-1 and MMP-3 from cultured sebocytes increased by treatment with vitamin D. Therefore, it is thought that the effect of vitamin D on sebocytes should be further evaluated for applying it as a promising therapeutic agent in acne vulgaris.
What is new?
1. Expression of IL-6, IL-8, and MMP-9 from cultured sebocytes decreased by treatment with vitamin D.
2. Expression of MMP-1 and MMP-3 from cultured sebocytes increased by treatment with vitamin D. On the contrary, expression of MMP-9 decreased.
3. The gene expression of inflammatory biomarkers in VDR siRNA-treated sebocytes was not blocked after treatment with vitamin D. Therefore, it was suggested that cultured sebocytes were not influenced through VDR.
Footnotes
Source of Support: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2007017), the Anti-Aging and Well-being Research Center of Kyungpook National University and AmorePacific Corporation
Conflict of Interest: Nil.
References
- 1.Winston MH, Shalita AR. Acne vulgaris. Pathogenesis and treatment. Pediatr Clin North Am. 1991;38:889–903. doi: 10.1016/s0031-3955(16)38158-5. [DOI] [PubMed] [Google Scholar]
- 2.Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;22:360–6. doi: 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Reichrath J. Vitamin D and the skin: An ancient friend, revisited. Exp Dermatol. 2007;16:618–25. doi: 10.1111/j.1600-0625.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee WJ, Jung HD, Lee HJ, Kim BS, Lee SJ, Kim do W. Influence of substance-P on cultured sebocytes. Arch Dermatol Res. 2008;300:311–6. doi: 10.1007/s00403-008-0854-1. [DOI] [PubMed] [Google Scholar]
- 5.Lee WJ, Jung HD, Chi SG, Kim BS, Lee SJ, Kim do W, et al. Effect of dihydrotestosterone on the upregulation of inflammatory cytokines in cultured sebocytes. Arch Dermatol Res. 2010;302:429–33. doi: 10.1007/s00403-009-1019-6. [DOI] [PubMed] [Google Scholar]
- 6.Marta Guarna M, Coulson R, Rubinchik E. Anti-inflammatory activity of cationic peptides: Application to the treatment of acne vulgaris. FEMS Microbiol Lett. 2006;257:1–6. doi: 10.1111/j.1574-6968.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 7.Slominski A, Ermak G, Hwang J, Chakroborty A, Mazurkiewicz JE, Mihm M. Proopiomelanocortin, corticotropin releasing hormone and corticotropin releasing hormone receptor genes are expressed in human skin. FEBS Lett. 1995;374:113–6. doi: 10.1016/0014-5793(95)01090-2. [DOI] [PubMed] [Google Scholar]
- 8.Zouboulis CC, Schagen S, Alestas T. The sebocyte culture: A model to study the pathophysiology of the sebaceous gland in sebostasis, seborrhoea and acne. Arch Dermatol Res. 2008;300:397–413. doi: 10.1007/s00403-008-0879-5. [DOI] [PubMed] [Google Scholar]
- 9.Nagy I, Pivarcsi A, Kis K, Koreck A, Bodai L, McDowell A, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and inflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8:2195–205. doi: 10.1016/j.micinf.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Kurokawa I, Danby FW, Ju Q, Wang X, Xiang LF, Xia L, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821–32. doi: 10.1111/j.1600-0625.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim J. Review of the innate immune response in acne vulgaris: Activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193–8. doi: 10.1159/000087011. [DOI] [PubMed] [Google Scholar]
- 12.Koreck A, Pivarcsi A, Dobozy A, Kemeny L. The role of innate immunity in the pathogenesis of acne. Dermatology. 2003;206:96–105. doi: 10.1159/000068476. [DOI] [PubMed] [Google Scholar]
- 13.Georgel P, Crozat K, Lauth X, Makrantonaki E, Seltmann H, Sovath S, et al. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with Gram-positive bacteria. Infect Immun. 2005;73:4512–21. doi: 10.1128/IAI.73.8.4512-4521.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi JJ, Park MY, Lee HJ, Yoon DY, Lim Y, Hyun JW, et al. TNF-α increases lipogenesis via JNK and PI3K/Akt pathways in SZ95 human sebocytes. J Dermatol Sci. 2012;65:179–88. doi: 10.1016/j.jdermsci.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Zouboulis CC, Angres S, Seltmann H. Regulation of stearoyl-coenzyme A desaturase and fatty acid delta-6 desaturase-2 expression by linoleic acid and arachidonic acid in human sebocytes leads to enhancement of proinflammatory activity but does not affect lipogenesis. Br J Dermatol. 2011;165:269–76. doi: 10.1111/j.1365-2133.2011.10340.x. [DOI] [PubMed] [Google Scholar]
- 16.Clemens TL, Adams JS, Horiuchi N, Gilchrest BA, Cho H, Tsuchiya Y, et al. Interaction of 1,25-dihydroxyvitamin-D3 with keratinocytes and fibroblasts from skin of normal subjects and a subject with vitamin-D-dependent rickets, type II: A model for study of the mode of action of 1,25-dihydroxyvitamin D3. J Clin Endocrinol Metab. 1983;56:824–30. doi: 10.1210/jcem-56-4-824. [DOI] [PubMed] [Google Scholar]
- 17.Krämer C, Seltmann H, Seifert M, Tilgen W, Zouboulis CC, Reichrath J. Characterization of the vitamin D endocrine system in human sebocytes in vitro. J Steroid Biochem Mol Biol. 2009;113:9–16. doi: 10.1016/j.jsbmb.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–35. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang GY, Cheng T, Luan Q, Liao T, Nie CL, Zheng X, et al. Vitamin D: A novel therapeutic approach for keloid, an in vitro analysis. Br J Dermatol. 2011;164:729–37. doi: 10.1111/j.1365-2133.2010.10130.x. [DOI] [PubMed] [Google Scholar]
- 20.Wasse H, Cardarelli F, De Staercke C, Hooper C, Veledar E, Guessous I. 25-hydroxyvitamin D concentration is inversely associated with serum MMP-9 in a cross-sectional study of African American ESRD patients. BMC Nephrol. 2011;12:24. doi: 10.1186/1471-2369-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoo AL, Chai LY, Koenen HJ, Sweep FC, Joosten I, Netea MG, et al. Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clin Exp Immunol. 2011;164:72–9. doi: 10.1111/j.1365-2249.2010.04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]