Abstract
This study analyzed the relationship between pulmonary vascular resistance (PVR) and pulmonary arterial compliance (Ca) in patients with idiopathic pulmonary arterial hypertension (IPAH) and proximal chronic thromboembolic pulmonary hypertension (CTEPH). It has recently been shown that the time constant of the pulmonary circulation (RC time constant), or PVR × Ca, remains unaltered in various forms and severities of pulmonary hypertension, with the exception of left heart failure. We reasoned that increased wave reflection in proximal CTEPH would be another cause of the decreased RC time constant. We conducted a retrospective analysis of invasive pulmonary hemodynamic measurements in IPAH (n = 78), proximal CTEPH (n = 91) before (pre) and after (post) pulmonary endarterectomy (PEA), and distal CTEPH (n = 53). Proximal CTEPH was defined by a postoperative mean pulmonary artery pressure (PAP) of ≤25 mmHg. Outcome measures were the RC time constant, PVR, Ca, and relationship between systolic and mean PAPs. The RC time constant for pre-PEA CTEPH was 0.49 ± 0.11 s compared with post-PEA-CTEPH (0.37 ± 0.11 s, P < 0.0001), IPAH (0.63 ± 0.14 s, P < 0.001), and distal CTEPH (0.55 ± 0.12 s, P < 0.05). A shorter RC time constant was associated with a disproportionate decrease in systolic PAP with respect to mean PAP. We concluded that the pulmonary RC time constant is decreased in proximal CTEPH compared with IPAH, pre- and post-PEA, which may be explained by increased wave reflection but also, importantly, by persistent structural changes after the removal of proximal obstructions. A reduced RC time constant in CTEPH is in accord with a wider pulse pressure and hence greater right ventricular work for a given mean PAP.
Keywords: hypertension, pulmonary, pulmonary circulation, pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension, pulmonary endarterectomy
recent studies (12, 13, 20) have shown that there is a fixed relationship between pulmonary vascular resistance (PVR) and pulmonary artery compliance (Ca). This relationship is best studied using the time constant of the pulmonary circulation (RC time constant).
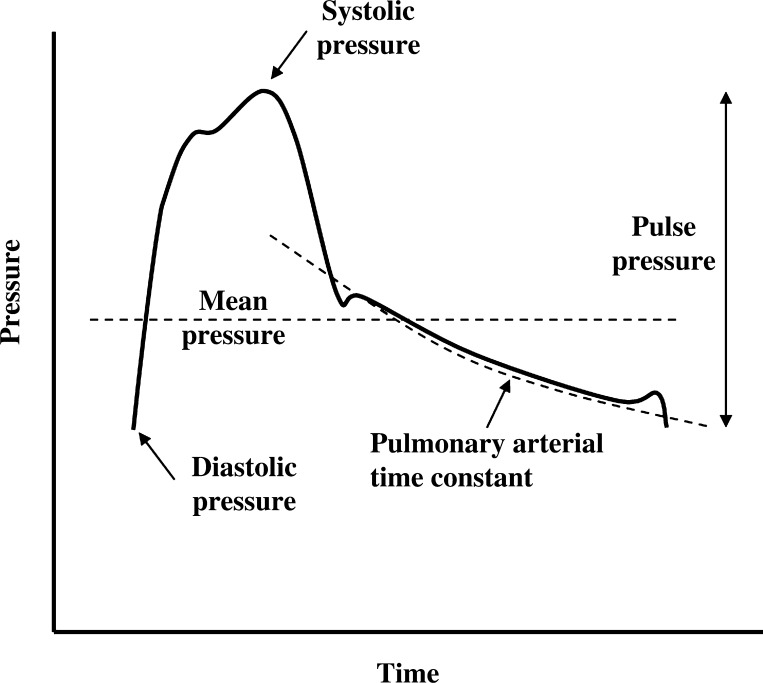
Ca represents the ability of the pulmonary circulation to stretch in response to an applied pressure. The pulmonary circulation is a low-pressure, high-compliance circuit designed to cope with the increased cardiac output associate with exercise. The RC time constant is calculated as the product of PVR by Ca and represents the exponential pressure decay in the pulmonary artery during diastole (Fig. 1). The RC time constant is potentially useful in the study of the pulmonary circulation as it is independent of the right ventricle (RV). The RC time constant has been shown to be 0.48 ± 0.17 s in patients with pulmonary hypertension (PH) or suspected PH (with a normal wedge pressure) (24). In patients with PH, the RC time constant seems unaffected by drug treatment (13). A constant relationship between PVR and Ca implies that Ca becomes a relatively more important determinant of RV afterload when PVR is normal or only moderately increased (1).
Fig. 1.
Illustration of the pulmonary artery pressure (PAP) waveform. Systolic PAP (sPAP), mean PAP (mPAP), diastolic PAP, and pulse pressure are shown. The exponential decay of pressure during diastole was characterized by the pulmonary artery time constant (RC time constant).
However, most recently, Tedford et al. (24) showed that an increased pulmonary arterial wedge pressure (PAWP) in patients with left heart failure may be a cause of a decreased RC time constant (with age also showing a smaller effect). This observation is explained by decreased Ca associated with an increased transvascular pressure at any given level of PVR (10, 16). Tedford et al. (24) suggested that PAWP amplifies peripheral pulmonary arterial pulse reflection, augmenting systolic pulmonary arterial pressure (sPAP); hence, there is a decline in total Ca.
Proximal pulmonary arterial obstruction in experimental PH has been shown to increase wave reflection, which increases pulse pressure at any given level of mean pulmonary arterial pressure (mPAP) (9). This finding has been confirmed in patients with chronic thromboembolic PH (CTEPH) (2, 17, 18), although the increase in pulse pressure was not always consistent (2), probably in relation to the inhomogeneity of the distribution of obstructive lesions along the pulmonary arterial tree typically observed in these patients.
This is at odds with the evidence indicating a fixed predictable relationship between sPAP and mPAP (4, 5, 8, 23). This fixed relationship between sPAP, diastolic pulmonary arterial pressure (dPAP), and mPAP is in contrast to the systemic circulation, where mean pressure estimates require both systolic and diastolic input and can be altered by interventions affecting either (3).
The aim of this study was to test whether proximal CTEPH [compared with idiopathic pulmonary arterial hypertension (IPAH) and distal CTEPH] demonstrated a significant difference in the RC time constant. If this were the case, would there also be a difference in pulse pressure altering the relationship between the sPAP and mPAP? Accordingly, we investigated predominantly proximal CTEPH by assessing patients who achieved a postpulmonary endarterectomy (post-PEA) decrease in mPAP to ≤25 mmHg. This group was chosen to try to determine the effect of pulmonary arterial obstructions as much as possible in isolation from a distal pulmonary vasculopathy (based on the “two-compartment model for CTEPH”) (15).
METHODS
Subjects.
This study was approved by our institutional review board. Patients were identified retrospectively from our clinical database by their diagnosis from 1999 to 2012. We searched for patients with CTEPH who had PEA, distal CTEPH, or IPAH based on the Dana point classification (21). Right heart catheter pressure measurements were obtained from a central database or from patient's notes. Patients who had a routine right heart catheterization performed at least 3 mo after PEA surgery with a significant fall in mPAP post-PEA to ≤25 mmHg were identified as predominantly proximal CTEPH. Patients with mPAP > 25 mmHg at this point were excluded. Distal CTEPH was defined as patients who had been assessed by a multidisciplinary panel (comprising a PEA surgeon, dedicated PH radiologists, and physicians) as having thrombus distributed primarily beyond the segmental divisions of the pulmonary arteries and, hence, were not candidates for PEA surgery. Patients with PAWP > 15 mmHg or where no reliable PAWP trace could be obtained were excluded.
Right heart catheter measurements.
IPAH, distal CTEPH, and pre-PEA measurements were made in incident cases at the time of diagnosis. Post-PEA measurements for each patient were performed at least 3 mo after PEA surgery. No patients were receiving pulmonary arterial hypertension licensed therapies at the time of any right heart catheterization. All catheterization measurements were performed in supine position at rest using fluoroscopic guidance with standard techniques by a specialist in PH. Pressures were measured using a quad lumen Swan-Ganz catheter (Edwards Lifesciences, Irvine, CA) connected to a Philips Haemosphere (Philips Medical Systems, Surrey, UK). Pressure measurements were taken at end expiration. Cardiac output was measured by thermodilution.
RC time constant.
The RC time constant was calculated from right heart catheter-measured parameters. Ca was calculated as stroke volume divided by pulse pressure. The RC time constant was calculated as PVR × Ca.
Statistical analysis.
Statistical analysis was performed using Graphpad Prism 3.0 (Graphpad Software, La Jolla, CA) except for the linear regression analysis, which was performed using Stata 12.1 (StataCorp). Comparison of multiple group variables was performed with ANOVA (with post hoc testing using Tukeys multiple-comparison test) or, for non-normally distributed variables, a Kruksal-Wallis test (with post hoc testing using Dunn's multiple-comparison test). Comparison between pre-PEA CTEPH and post-PEA CTEPH was performed using a paired t-test or Wilcoxon signed-rank test where appropriate. Linear regression analysis was used to adjust for the effect of different group characteristics to determine whether there was any effect on the RC time constant. PVR and Ca were normalized by log transformation for linear regression and analysis of covariance. Linear regression analysis was performed on groups of sPAP and mPAP measurements to generate the best fit line. For between-group comparisons, analysis of covariance was performed. Values in tables are means ± SD. For all statistical tests, P values of <0.05 were considered significant.
RESULTS
We identified 78 patients with IPAH, 91 patients with proximal CTEPH, and 53 patients with distal CTEPH. The IPAH group age was 53.4 ± 15.8 yr, with a male-to-female ratio of 21:57. The proximal CTEPH group age was 54.8 ± 19.0 yr, with a male-to-female ratio of 57:34. The distal CTEPH group age was 62.8 ± 14.0 yr, with a male-to-female ratio of 25:28. Right heart catheterization measurements are shown in Table 1 for IPAH, pre-PEA CTEPH, post-PEA CTEPH, and distal CTEPH. The mean interval between PEA surgery and post-PEA right heart catheterization was 120 ± 43 days.
Table 1.
Right heart catheterization results, including hemodynamics and calculated indexes (PVR, Ca, and RC time constant)
| IPAH | Distal CTEPH | Pre-PEA CTEPH | Post-PEA CTEPH | |
|---|---|---|---|---|
| Number of subjects | 59 | 53 | 91 | 91 |
| Heart rate, beats/ming | 80.9 ± 14.0 | 78.5 ± 13.1 | 79.8 ± 16.8 | 76.4 ± 13.7 |
| sPAP, mmHg | 81.8 ± 21.0 | 85.2 ± 19.0 | 76.3 ± 19.0b | 34.1 ± 6.7e |
| mPAP, mmHg | 49.9 ± 13.6 | 48.8 ± 10.6 | 42.9 ± 10.5a,b | 20.4 ± 3.3e |
| dPAP, mmHg | 32.9 ± 11.3 | 28.4 ± 7.4a | 25.0 ± 7.9a | 11.7 ± 3.2e |
| Pulse pressure, mmHg | 48.9 ± 13.4 | 56.8 ± 15.1a | 51.2 ± 14.7 | 22.4 ± 6.4e |
| Right atrial pressure, mmHg | 8.7 ± 5.2 | 9.5 ± 4.0 | 9.4 ± 5.2 | 5.6 ± 2.7e |
| Pulmonary artery wedge pressure, mmHg | 9.1 ± 3.1 | 9.8 ± 3.3 | 10.5 ± 3.8a | 10.1 ± 3.1 |
| Cardiac output, l/min | 3.7 ± 1.1 | 4.0 ± 1.2 | 3.8 ± 1.1 | 4.8 ± 1.2e |
| Stroke volume, ml | 47.2 ± 16.6 | 52.4 ± 17.8 | 49.4 ± 15.6 | 65.3 ± 20.0e |
| PVR, dyn•s−1•cm5 | 975 ± 448 | 871 ± 382 | 753 ± 369c | 182 ± 82f |
| Ca, ml/mmHg | 1.07 ± 0.58 | 0.99 ± 0.44 | 1.07 ± 0.56 | 3.07 ± 1.05f |
| RC time constant, s | 0.63 ± 0.14 | 0.55 ± 0.12c | 0.49 ± 0.11c,d | 0.38 ± 0.11f |
Values are means ± SD. IPAH, idiopathic pulmonary arterial hypertension; distal CTEPH, chronic thromboembolic pulmonary hypertension (CTEPH); pre- and post-CTEPH, proximal CTEPH before (pre) and after (post) pulmonary endarterectomy (PEA) surgery; sPAP, systolic pulmonary arterial pressure (PAP); mPAP, mean PAP; dPAP, diastolic PAP; PVR, pulmonary vascular resistance; Ca, pulmonary arterial compliance; RC time constant, time constant of the pulmonary circulation.
P < 0.05 compared with IPAH (by ANOVA),
P < 0.05 compared with distal CTEPH (by ANOVA),
P < 0.05 compared with IPAH (by Kruskal-Wallis test),
P < 0.05 compared with distal CTEPH (by Kruskal-Wallis test);
P < 0.05 compared with pre-PEA CTEPH (by t-test);
P < 0.05 compared with pre-PEA CTEPH (by Wilcoxon signed-ranked test).
ANOVA showed statistically significant differences in IPAH, distal CTEPH, and pre-PEA CTEPH groups for sPAP, mPAP, dPAP, pulse pressure, PCWP, PVR, and age. Posttest comparison of IPAH and pre-PEA CTEPH groups showed significant differences in mPAP (P < 0.001), dPAP (P < 0.001), PCWP (P < 0.05), and PVR (P < 0.01). Posttest comparison of distal CTEPH and pre-PEA CTEPH groups showed significant differences in sPAP (P < 0.05), mPAP (P < 0.05), and age (P < 0.01).
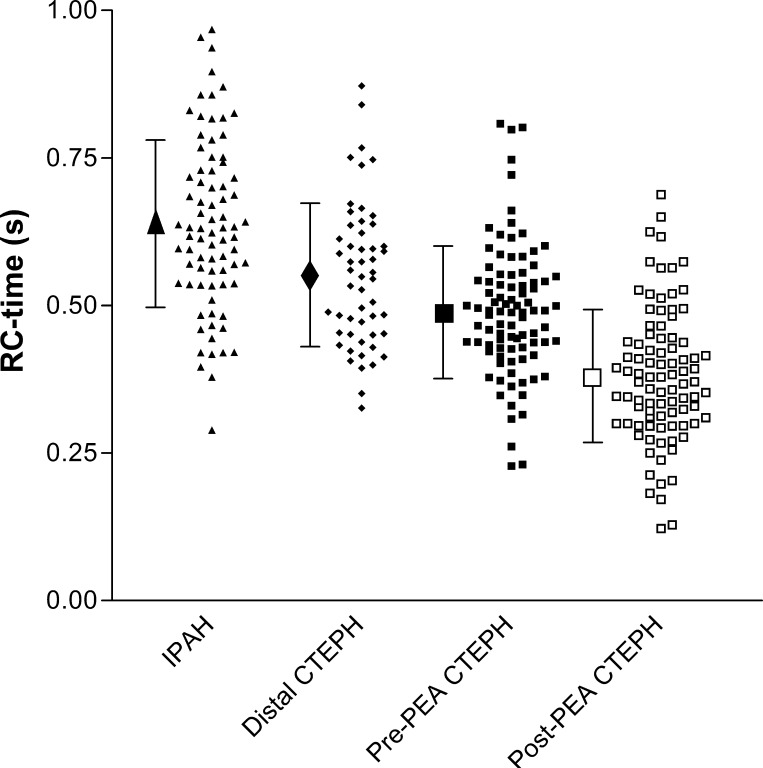
The RC time constant was calculated using the derived measurements for PVR and Ca (Table 1). Figure 2 shows a plot of the RC time constant for each group. Between-group posttest analysis of RC time constant differences showed the following differences of RC time constants: IPAH to distal CTEPH (P < 0.01), IPAH to pre-PEA CTEPH (P < 0.001), and distal CTEPH to pre-PEA CTEPH (P < 0.05). To determine the effect of age, mPAP, and PCWP on the observed differences in RC time constants across the three groups, regression analysis was performed. Independent analysis of the effect of each of the three parameters with RC time constant showed an age coefficient of −0.001 (r2 = 0.20, P = 0.031), a mPAP coefficient of −0.001 (r2 = 0.19, P = 0.103), and a PCWP coefficient of −0.009 (r2 = 0.26, P < 0.001). In a combined model (r2 = 0.30), a significant effect of PCWP (coefficient: −0.01, P < 0.001) and mPAP (coefficient: 0.002, P = 0.002) was demonstrated, with age being near significance (coefficient: −0.001, P = 0.056). However, even after adjustment for these effects, the RC time constant differences observed all remained significant (IPAH to distal CTEPH: P < 0.001, IPAH to pre-PEA CTEPH: P = 0.002, and distal CTEPH to pre-PEA CTEPH: P = 0.034).The RC time constant differed between pre-PEA CTEPH (0.49 ± 0.11 s) and post-PEA CTEPH (0.38 ± 0.11 s, P < 0.0001; Fig. 2). As expected there were significant differences in all hemodynamic parameters after successful PEA surgery.
Fig. 2.
Scatterplot of the RC time constant [the product of pulmonary vascular resistance (PVR) and pulmonary arterial compliance] for idiopathic pulmonary arterial hypertension (IPAH), proximal chronic thromboembolic pulmonary hypertension (CTEPH) at the time of diagnosis (pre-PEA) and after pulmonary endarterectomy (post-PEA), and distal CTEPH.
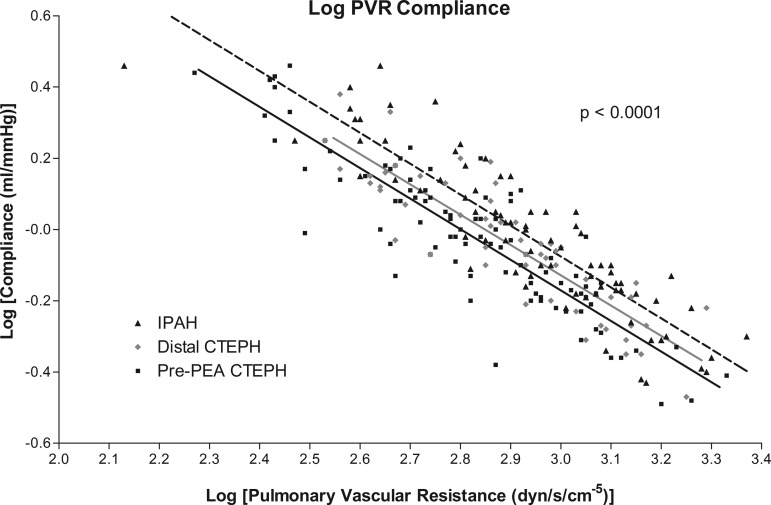
Figure 3 shows a plot of log(PVR) against log(Ca) for IPAH, distal CTEPH, and pre-PEA CTEPH groups. Log transformation gave the following values: IPAH, log(PVR) 2.94 ± 0.22 and log(Ca) −0.022 ± 0.22; distal CTEPH, log(PVR) 2.90 ± 0.19 and log(Ca) −0.042 ± 0.19; and pre-PEA CTEPH, log(PVR) 2.82 ± 0.22 and log(Ca) −0.020 ± 0.21. The lines in Fig. 3 show the best fits for IPAH (dashed line), distal CTEPH (gray line), and pre-PEA CTEPH (solid line). There were no differences in the slopes of the lines, but there were significant differences in the intercepts (P < 0.0001).
Fig. 3.
Plot of log(PVR) against log(pulmonary compliance) for IPAH, distal CTEPH, and pre-PEA CTEPH. Best-fit lines are shown for the following groups: IPAH (dashed line, r2 = 0.80), distal CTEPH (gray line, r2 = 0.77), and pre-PEA CTEPH (solid line, r2 = 0.78). Analysis of covariance showed a significant difference in the intercepts (P < 0.0001).
Relationship between sPAP and mPAP.
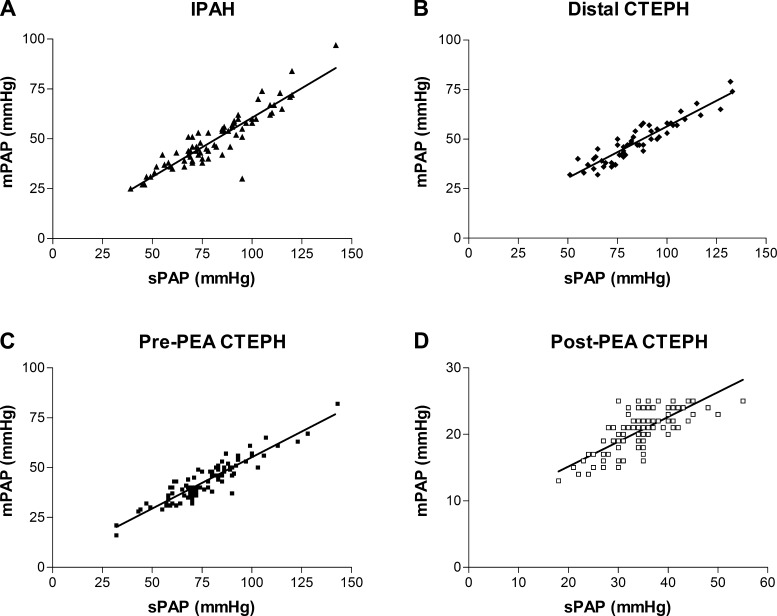
All groups demonstrated a good linear correlation between sPAP and mPAP (Table 2). Analysis of the difference in the relationships showed that the relationship between sPAP and mPAP in the IPAH group was different compared with the results for both pre-PEA CTEPH and post-PEA CTEPH groups. Figure 4 shows linear regression lines for sPAP against mPAP. The linear regression line for the IPAH group differed significantly from pre-PEA CTEPH (P = 0.034) and post-PEA CTEPH (P = 0.002) groups.
Table 2.
Linear regression analysis of sPAP and mPAP
| r2 | Linear Regression Formula | |
|---|---|---|
| IPAH | 0.84 | mPAP = 0.59(sPAP) + 1.3 |
| Distal CTEPH | 0.87 | mPAP = 0.52(sPAP) + 4.6 |
| Pre-PEA CTEPH | 0.86 | mPAP = 0.51(sPAP) + 3.7 |
| Post-PEA CTEPH | 0.57 | mPAP = 0.37(sPAP) + 7.7 |
Values are means ± SD.
Fig. 4.
Linear regression analysis of mPAP and sPAP. A: IPAH. The best-fit line (r2 = 0.84) shows mPAP = 0.59(sPAP) + 1.3. B: distal CTEPH. The best-fit line (r2 = 0.87) shows mPAP = 0.52(sPAP) + 4.6. C: pre-PEA CTEPH. The best-fit line (r2 = 0.86) shows mPAP = 0.51(sPAP) + 3.7. D: post-PEA CTEPH. The best-fit line (r2 = 0.57) shows mPAP = 0.37(sPAP) + 7.7.
DISCUSSION
The present results show that the RC time constant is different depending on the nature or cause of PH when IPAH and different types of CTEPH are compared. The difference in the RC time constant may be explained by extensive structural changes distributed in the pulmonary circulation rather than just wave reflection on a proximal site of increased resistance. A decreased RC time constant in CTEPH increases the pulsatile hydraulic load imposed on the RV and alters the prediction equations of mPAP from either sPAP or dPAP.
With the exception of Tedford et al. (24), previous studies in smaller subject groups have shown generally higher RC time constants in CTEPH than we found in our CTEPH group [Lankhaar et al. (12): non-PH, 0.79 ± 0.31 s; CTEPH, 0.74 ± 0.37 s; and IPAH, 0.69 ± 0.22 s; Lankhaar et al. (13): IPAH, 0.65 ± 0.17 s and CTEPH, 0.55 ± 0.07 s]. Tedford et al. found a RC time constant of 0.48 ± 0.17 s in a large heterogeneous PH population (n = 1,009). This population encompassed group I, III, IV, and V PH. Recently, Saouti et al. (20) demonstrated RC time constants of 0.49 s in patients investigated for CTEPH (using total pulmonary resistance) and that the RC time constant was preserved in a single lung compared with both lungs. An RC time constant recalculated with PVR would be even shorter. Saouti et al. (20) explained the observed difference in the RC time constant compared with previously published work as the result of different methods of calculating compliance. In their report (20), they did not try to identify proximal or distal distribution for the chronic thromboembolic obstructions. The presence of a higher proportion of proximal CTEPH might also explain why their RC time constant was lower than that reported by Lankhaar et al. (12).
We used the same method of calculating Ca across our patient groups and demonstrated a significant difference. Our results indicate that the RC time constant can be altered by proximal CTEPH. We avoided using a definition of proximal CTEPH based on interpretation of imaging as this is subjective and not able to identify the contribution of the distal arteries to PVR, but we did have to rely on this method for defining distal CTEPH. In ideal circumstances, our proximal and distal CTEPH patients would each behave as having only one of the “two compartments” in CTEPH affected (15).
The findings of Pagnamenta et al. (19) demonstrated that, in a dog model of CTEPH, pulmonary artery ensnarement (as a model of proximal CTEPH) gives a RC time constant of 0.30 ± 0.03 s (from a baseline RC time constant of 0.46 ± 0.07 s) compared with microembolization (as a model of distal CTEPH), which gave a RC time constant of 0.74 ± 0.05 s (from a baseline RC time constant of 0.53 ± 0.09 s). They attributed the observed differences to the effect of wave reflection caused by the ensnarement of the pulmonary artery. This supports our hypothesis that wave reflection contributes to alterations of the RC time constant in proximal CTEPH.
Perhaps the most intriguing part of our study is that the reduced resistance after PEA surgery was not matched by an equivalent increase in Ca, which resulted in a lower RC time constant compared with preoperative levels. Bonderman et al. (1) showed that, in patients with a mPAP of 19 mmHg after PEA, exercise produced a decrease in Ca; in normal subjects, exercise produced decreased PVR and increased Ca, confirming persistently altered Ca after successful proximal surgical disobstruction. Our RC time constant results suggest that the PVR and Ca relationship remains different from IPAH even after successful PEA surgery. The decrease in RC time constant between pre-PEA and post-PEA CTEPH does not help our hypothesis that wave reflection caused by proximal obstruction is the main factor responsible for differing behavior compared with IPAH, as this was removed in post-PEA CTEPH and generates more questions about how the pulmonary circulation behaves in disease states.
de Perrot et al. (6) recently reported on post-PEA improved Ca, resulting in an improvement in New York Heart Association functional class, 6-min walk distance, and RV remodeling. They did not observe any change in the RC time constant in their patient group (n = 34). Discrepant RC time constant calculations in proximal CTEPH before and after successful PEA could be explained by differences in the extent of structural changes associated with proximal thrombotic obstructions. One possible explanation for the decrease in RC time constant could be that the removal at PEA of tunica intima and part of tunica media of the pulmonary artery with subsequent healing may not leave the arterial wall with the same compliance properties as the normal pulmonary artery. The dissection in PEA surgery is a full endarterectomy from the main pulmonary artery into all segmental branches (and beyond, in some circumstances). Saouti et al. (20) suggested that total proximal arterial compliance was 19% by studying the contribution to compliance of 2 cm of the main pulmonary artery, 3 cm of the left pulmonary artery, and 3 cm of the right pulmonary artery. PEA surgery extends beyond the first 3 cm of the left and right pulmonary arteries; hence, any change to the behavior of the pulmonary artery wall could be expected to affect >19% of total Ca. A study (22) of in vitro atheroscleromatous human aortae subjected to endarterectomy demonstrated improved compliance but failure to reach the compliance levels seen in normal arteries. This seems the most likely explanation for the lack of “normalization” of the resistance-compliance relationship despite endothelialization of the denuded pulmonary arteries being a very rapid process that is normally complete within 3 wk (14).
We demonstrated in this study that IPAH and proximal CTEPH are different in the way that the pulmonary circulation responds. Our finding that the relationship between resistance and compliance is altered in proximal CTEPH indicates that studies of CTEPH should not only concentrate on PVR but also on Ca to interpret the treatment response, as the link between PVR and exercise performance is only a loose one (1, 11). In addition, PEA surgery does change the RC time constant; this implies that patients who have had PEA surgery and those who have not cannot be expected to respond in the same way in clinical studies.
RV afterload is influenced by pulsatile pressure and pulsatile flow. Three elements contribute to these parameters: PVR, Ca, and wave reflection. The effect of PEA surgery on Ca may be beneficial toward RV afterload even in situations where the reduction in PVR is marginal. There are some data that suggest that even after PEA surgery, conditional survival with a mPAP of >30 mmHg and mean PVR of 541 dyn·s−1·cm5 (with only 25% on PAH specific therapies) is comparable with patients with a mPAP of <30mmHg (7).
Our results support previous findings of widened pulse pressure in patients with proximal CTEPH compared with IPAH (17, 18). A reduced RC time constant in CTEPH compared with IPAH equates to a more rapid pressure decay in diastole; hence, for an identical mean PA pressure and heart rate, the pulse pressure in CTEPH must be wider. The effect of this is that there is a relatively greater pulsatile component to the hydraulic load on the RV, i.e., greater RV workload.
There are potential criticisms of this work. The pressure measurements were taken using quad lumen Swan-Ganz catheters. Despite this, in the IPAH cohort, we generated a remarkably similar relationship between mean and systolic pressures as those observed using high-fidelity catheters, suggesting that this has little impact on our measurements. This is a retrospective study. This has limited our method for the calculation of Ca to use only pressure and cardiac output measurements. Multiple operators were involved in performing the right heart catheterization in this study. All operators adhered to the same protocol, and all were specialist pulmonary vascular physicians. A random selection of one-third of the pressure waveforms was reviewed to check for accuracy of the clinical database. Finally, for predominantly proximal CTEPH, we were able to use a method of selection that is easily reproducible, but no such method is possible for diagnosing distal CTEPH. Despite this, the distal CTEPH group appeared to fit between IPAH and proximal CTEPH groups.
The relationship between sPAP and mPAP in our CTEPH cohort demonstrated an altered relationship compared with IPAH. Surgical intervention, proximal obstruction, and distal chronic thromboembolic disease all affect the sPAP and mPAP relationship. However, the equations to predict mPAP from sPAP (4, 5, 8, 23) used in clinical practice when sPAP is estimated from the maximum velocity of trucuspid regurgitant jet at echocardiography have to be adapted in proximal CTEPH with a lower mPAP recalculated from any estimate of sPAP.
Our results prove that it is not just alteration of the pulmonary artery afterload (through raised PAWP) that can change the coupling of PVR to Ca but also that proximal CTEPH and PEA surgery are able to change this relationship. In addition, the relationship between mean and systolic pressure is not fixed under all circumstances but can be altered by the presence of proximal thromboembolic obstructions. The change to the interaction between the pulmonary circulation and RV will affect how the RV responds to progression of the underlying disease process and the likely effect of drug therapies.
GRANTS
This work was supported by the National Institute for Health Research Cambridge Biomedical Research Centre.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.V.M.R., M.R.T., E.S., R.N., and J.P.-Z. conception and design of research; R.V.M.R. performed experiments; R.V.M.R. and M.R.T. analyzed data; R.V.M.R., M.R.T., E.S., R.N., and J.P.-Z. interpreted results of experiments; R.V.M.R. prepared figures; R.V.M.R. and M.R.T. drafted manuscript; R.V.M.R., M.R.T., E.S., R.N., and J.P.-Z. edited and revised manuscript; R.V.M.R., M.R.T., E.S., R.N., and J.P.-Z. approved final version of manuscript.
REFERENCES
- 1.Bonderman D, Martischnig AM, Vonbank K, Schäfers HJ, Jansa P, Lindner J, Simkova I, Martischnig AM, Dudczak J, Sadushi R, Skoro-Sajer N, Klepetko W, Lang IM. Right ventricular load at exercise is a cause of persistent exercise limitation in patients with normal resting pulmonary vascular resistance after pulmonary endarterectomy. Chest 139: 122–127, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Castelain V, Hervé P, Lecarpentier Y, Duroux P, Simonneau G, Chemla D. Pulmonary artery pulse pressure and wave reflection in chronic pulmonary thromboembolism and primary pulmonary hypertension. J Am Coll Cardiol 37: 1085–1092, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Chemla D, Hebert JL, Aptecar E, Mazoit JX, Zamani K, Frank R, Fontaine G, Nitenberg A, Lecarpentier Y. Empirical estimates of mean aortic pressure: advantages, drawbacks and implications for pressure redundancy. Clin Sci (Lond) 103: 7–13, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Chemla D, Castelain V, Humbert M, Hébert JL, Simonneau G, Lecarpentier Y, Hervé P. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest 126: 1313–1317, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Chemla D, Castelain V, Provencher S, Humbert M, Simonneau G, Hervé P. Evaluation of various empirical formulas for estimating mean pulmonary artery pressure by using systolic pulmonary artery pressure in adults. Chest 135: 760–768, 2009 [DOI] [PubMed] [Google Scholar]
- 6.de Perrot M, McRae K, Shargall Y, Thenganatt J, Moric J, Mak S, Granton JT. Early postoperative pulmonary vascular compliance predicts outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. Chest 140: 34–41, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Freed DH, Thomson BM, Berman M, Tsui SSL, Dunning J, Sheares KK, Pepke-Zaba J, Jenkins DP. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg 141: 383–387, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Friedberg MK, Feinstein JA, Rosenthal DN. A novel echocardiographic Doppler method for estimation of pulmonary arterial pressures. J Am Soc Echocardiogr 19: 559–562, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Furuno Y, Nagamoto Y, Fujita M, Kaku T, Sakurai S, Kuroiwa A. Reflection as a cause of mid-systolic deceleration of pulmonary flow in dogs with acute pulmonary hypertension: comparison of pulmonary artery constriction with pulmonary embolisation. Cardioavas Res 25: 118–124, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Harvey RM, Enson Y, Ferrer MI. A reconsideration of the origins of pulmonary hypertension. Chest 59: 82–94, 1971 [DOI] [PubMed] [Google Scholar]
- 11.Jaïs X, D'Armini AM, Jansa P, Torbicki A, Delcroix M, Ghofrani HA, Hoeper MM, Lang IM, Mayer E, Pepke-Zaba J, Perchenet L, Morganti A, Simmonneau G, Rubin LJ. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 52: 2127–2134, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Lankhaar JW, Westerhof N, Faes TJ, Marques KM, Marcus JT, Postmus PE, Vonk-Noordegraaf A. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am J Physiol Heart Circ Physiol 291: H1731–H1737, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Lankhaar J, Westerhof N, Faes TJC, Gan CT, Marques KM, Boonstra A, van den Berg FG, Postmus PE, Vonk-Noordegraaf A. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur Heart J 29: 1688–1695, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Moseley HS, Connell RS, Krippaehne WW. Healing of the canine aorta after endarterectomy: a scanning electron microscopy study. Ann Surg 180: 329–335, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moser KM, Braunwald NS. Successful surgical intervention in severe chronic thromboembolic pulmonary hypertension. Chest 64: 29–35, 1973 [DOI] [PubMed] [Google Scholar]
- 16.Naeije R, Vachiery JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary hypertension. Eur Respir J 41: 217–223, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Nakayama Y, Sugimachi M, Nakanishi N, Takaki H, Okano Y, Satoh T, Miyatake K, Sunagawa K. Noninvasive differential diagnosis between chronic pulmonary thromboembolism and primary pulmonary hypertension by means of doppler ultrasound measurement. J Am Coll Cardiol 31: 1367–1371, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Nakayama Y, Nakanishi N, Hayashi T, Nagaya N, Sakamaki F, Satoh N, Ohya H, Kyotani S. Pulmonary artery reflection for differentially diagnosing primary pulmonary hypertension and chronic pulmonary thromboembolism. J Am Coll Cardiol 38: 214–218, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Pagnamenta A, Vanderpool RR, Brimioulle S, Naeije R. Proximal pulmonary arterial obstruction decreases the time constant of the pulmonary circulation and increases right ventricular afterload. J Appl Physiol; 10.1152/japplphysiol.00033.2013 [DOI] [PubMed] [Google Scholar]
- 20.Saouti N, Westerhof N, Helderman F, Marcus JT, Stergiopulos N, Westerhof N, Boonstra A, Postmus PE, Vonk-Noordegraaf A. RC time constant of single equals that of both lungs together: a study in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol 297: H2154–H2160, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Simonneau G, Robbins I, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54, Suppl 1: S43–S54, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Sumner DS, Hokanson DE, Strandness DE. Arterial walls before and after endarterectomy. Arch Surg 99: 606–611, 1969 [DOI] [PubMed] [Google Scholar]
- 23.Syyed R, Reeves JT, Welsh D, Raeside D, Johnson MK, Peacock AJ. The relationship between the components of pulmonary artery pressure remains constant under all conditions in both health and disease. Chest 133: 633–639, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ, Kass DA. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 125: 289–297, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]