Abstract
Acute hyperglycemia (AHG) decreases the availability of nitric oxide (NO) and impairs anesthetic preconditioning (APC)-elicited protection against myocardial infarction. We investigated whether D-4F, an apolipoprotein A-1 mimetic, rescues the myocardium by promoting APC-induced endothelial NO signaling during AHG. Myocardial infarct size was measured in mice in the absence or presence of APC [isoflurane (1.4%)] with or without AHG [dextrose (2 g/kg ip)] and D-4F (0.12 or 0.6 mg/kg ip). NO production, superoxide generation, protein compartmentalization, and posttranslational endothelial NO synthase (eNOS) modifications were assessed in human coronary artery endothelial cells cultured in 5.5 or 20 mM glucose with or without isoflurane (0.5 mM) in the presence or absence of D-4F (0.5 μg/ml). Myocardial infarct size was significantly decreased by APC (36 ± 3% of risk area) compared with control (54 ± 3%) in the absence, but not presence, of AHG (49 ± 4%). D-4F restored the cardioprotective effect of APC during AHG (36 ± 3% and 30 ± 3%, 0.12 and 0.6 mg/kg, respectively), although D-4F alone had no effect on infarct size (53 ± 3%). Isoflurane promoted caveolin-1 and eNOS compartmentalization within endothelial cell caveolae and eNOS dimerization, concomitant with increased NO production (411 ± 28 vs. 68 ± 10 pmol/mg protein in control). These actions were attenuated by AHG (NO production: 264 ± 18 pmol/mg protein). D-4F reduced superoxide generation and enhanced caveolin-1 and eNOS caveolar compartmentalization and posttranslational eNOS modifications, thus restoring NO production during isoflurane and AHG (418 ± 36 pmol/mg protein). In conclusion, D-4F restored the cardioprotective effect of APC during AHG, possibly by decreasing superoxide generation, which promoted isoflurane-induced eNOS signaling and NO biosynthesis.
Keywords: anesthetic preconditioning, cardioprotection, D-4F, hyperglycemia, nitric oxide, endothelial nitric oxide synthase
acute hyperglycemia (AHG) alone, in the presence or absence of overt diabetes, is a major predictor of cardiovascular morbidity and mortality (18, 21, 23, 30), but the mechanisms that contribute to increased risk are poorly understood. Recent evidence strongly implicates perioperative hyperglycemia, in the absence of diabetes, as an independent predictor of death after noncardiac surgery (15). AHG increases the production of ROS (7, 34), decreases the availability of nitric oxide (NO) (20), impairs endothelial function (31), and attenuates coronary microcirculatory responses to myocardial ischemia (25). Evidence from our laboratory also indicates that AHG markedly attenuates cardioprotective signal transduction produced by pharmacological agents such as volatile anesthetics (1, 24). The American College of Cardiology/American Heart Association 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery recommend the use of volatile anesthetic agents in patients at risk for myocardial ischemia due to their cardioprotective effects (13). As the beneficial effects of these agents may be impaired by AHG (1, 53), additional strategies to address the adverse consequences of perioperative AHG are required.
Apolipoprotein (Apo)A-1 is the major component of high-density lipoprotein, and ApoA-1 mimetics that scavenge oxidized lipids and modulate cholesterol transport to membrane microdomains (52) have been suggested to possess properties that may decrease cardiovascular risk during diabetes and AHG (19). Membrane (lipid) rafts containing cholesterol serve an important function to organize signaling molecules, such as endothelial NO synthase (eNOS), into temporally and spatially regulated caveolar microdomains that facilitate signal transduction during anesthetic cardioprotection (22, 29). We hypothesized that AHG adversely modulates eNOS function during cardioprotective signaling and that, conversely, the ApoA-I mimetic D-4F enhances isoflurane-induced intracellular compartmentalization and activation of eNOS within caveolae, thereby increasing NO and restoring the cardioprotective effects of anesthetic preconditioning (APC).
MATERIALS AND METHODS
D-4F.
D-4F (Ac-DWFKAFYDKVAEKFKEAF-NH2) was synthesized using all d-amino acids as previously described (32, 36); it was dissolved in PBS before administration.
Animals.
Male C57BL/6J mice (weight: 26.9 ± 2.0 g, age: 9–12 wk) were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were housed in an environmentally controlled room and maintained on a 12:12-h light-dark cycle. All experimental procedures used in this study were approved by the Animal Care and Use Committee of the Medical College of Wisconsin (Milwaukee, WI) and conformed with the “Guiding Principles in the Care and Use of Laboratory Animals” of the American Physiological Society and were in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Myocardial ischemia-reperfusion injury in vivo.
We have previously described the in vivo murine model of myocardial ischemia-reperfusion injury (16, 17). Briefly, mice were anesthetized by an intraperitoneal injection of pentobarbital sodium with an initial dose of 80 mg/kg and ventilated using a positive-pressure mouse ventilator (Minivent type 845, Hugo-Sachs Eletronik-Harvard Apparatus, March, Germany). Additional doses of pentobarbital (15 mg/kg) were given during the experiment (1 h after the initial dose) as needed to maintain anesthesia (movement to noxious stimulation). Body temperature was maintained between 36.8 and 37.5°C. Myocardial ischemia was produced by occluding the left coronary artery, and reperfusion was initiated by loosening the suture. At the conclusion of each experiment, mice were killed with intraperitonal pentobarbital (80 mg/kg) and intravenous 50 mM KCl (0.1 ml). The infarct area was delineated by perfusing the coronary arteries with 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich, St. Louis, MO) via the aortic root, and the area at risk (AAR) was determined by perfusing phthalo blue dye (Heath Acrylics, Effingham, IL) into the aortic root after ligation of the coronary artery at the site of previous occlusion. As a result of these procedures, the nonischemic portion of the left ventricle was stained dark blue. Viable myocardium within the AAR was stained bright red, and infarcted tissue was light yellow.
Experimental protocol.
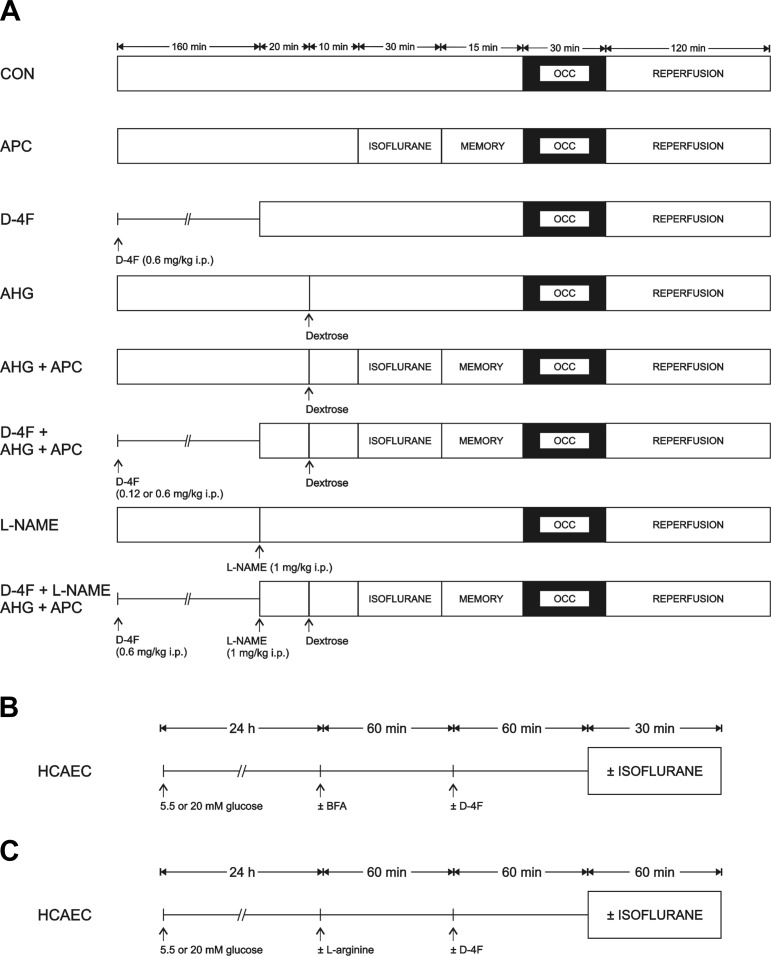
The concentration-dependent effects of D-4F (0.12 or 0.6 mg/kg ip) or vehicle (control) to restore APC [1.0 minimum alveolar concentration of isoflurane (1.40%) (51) administered for 30 min followed by 15 min of washout] in the presence or absence of hyperglycemia [dextrose (2 g/kg ip)] were determined in C57BL/6J mice randomly assigned to six separate experimental groups. In two additional groups, the contribution of enhanced NO production to D-4F protection during hyperglycemia was evaluated in mice treated with the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 1 mg/kg ip). All mice were subjected to 30 min of coronary occlusion followed by 2 h of reperfusion (Fig. 1A). Blood glucose levels were monitored throughout the experiment, and heart rate was monitored by electrocardiogram. AAR for infarction and myocardial infarct size were determined after each experiment.
Fig. 1.
Experimental protocols used to determine myocardial infarct size in C57BL/6J mice (A); O2·− formation, endothelial nitric oxide (NO) synthase (eNOS) dimerization, and eNOS, phosphorylated (p-)eNOS, and caveolin (Cav)-1 compartmentalization in human coronary artery endothelial cells (HCAECs; B), and NO production in HCAECs (C). AHG, acute hyperglycemia; APC, anesthetic preconditioning; BFA, brefeldin A; CON, control; D-4F, Ac-DWFKAFYDKVAEKFKEAF-NH2 (apolipoprotein A-1 mimetic); l-NAME, NG-nitro-l-arginine methyl ester (NO synthase inhibitor); OCC, occlusion.
Human coronary artery endothelial cells.
Human coronary artery endothelial cells (HCAECs; Cell Applications, San Diego, CA) isolated from healthy coronary arteries were purchased and cultured in growth medium (Cells Applications) as previously described (1). To assess the effects isoflurane and high glucose concentrations on HCAECs, 24 h before experimentation, growth medium was replaced with normal 5.5 mM media (containing 5.5 mM d-glucose, 14.5 mM mannitol, 81 mM NaCl, 4.0 mM KCl, and 1.6 mM CaCl2; pH 7.4) or high 20.0 mM glucose media (containing 20.0 mM d-glucose, 81 mM NaCl, 4.0 mM KCl, and 1.6 mM CaCl2; pH 7.4) media. HCAECs were also pretreated with 0.5 μg/ml D-4F for 1 h before 1 minimum alveolar concentration isoflurane exposure for 30 min using continuous air flow as previously described (1) to determine if D4-F mitigated the adverse effects of high glucose concentrations on HCAECs. Additional experiments were completed using 4 μg/ml brefeldin A (B5936, Sigma-Aldrich) for 1 h before D-4F to inhibit protein trafficking. Brefeldin A has previously been shown to impair cholesterol and caveolin (Cav) transport to caveolae (45) (Fig. 1B).
Ozone chemiluminescence.
To determine the effects of isoflurane on NO production with and without high glucose, nitrite concentration, an index of NO production, was measured in cell culture media by ozone-based chemiluminescence as previously described (56). Samples (30 μl) were refluxed in reaction solution (50 mg KI in 1 ml of double-distilled water) mixed with glacial acetic acid (4 ml). Nitrite concentrations were calculated after the subtraction of background levels and normalized to protein concentration (Bradford method). As NO production in HCAECs has previously been shown to peak at 60 min after stimulation (8), NO measurements were performed after 60 min of exposure to isoflurane. Additional experiments were completed using 1 mM l-arginine (A8094, Sigma-Aldrich) for 1 h before isoflurane exposure to stimulate NO synthase activity that could be substrate limited, particularly during high glucose. Since gas flow can induce shear-stress-dependent NO release, control groups were exposed to air and CO2 alone at the same flow rate (Fig. 1C).
O2·− analysis in living HCAECs.
To evaluate the effects of high glucose on ROS production during isoflurane, the formation of O2·− was assessed in living HCAECs with dihydroethidium (10 μM, Invitrogen, Carlsbad, CA), which, upon oxidation, yields red fluorescent ethidium. Dihydroethidium was added to cell media 5 min before isoflurane. Controls using antimycine A (10 μM, Sigma-Aldrich) were positive, and those using DMSO Hybri-Max (D2650, Sigma-Aldrich) alone were negative. Quantification of ethidium fluorescence intensity was performed at the end of the experiment using a laser-scanning confocal microscope (Nikon Eclipse TE2000-U, Tokyo, Japan) with a ×60/1.4 oil-immersion objective (Nikon) (46). Ethidium fluorescence (excitation wavelength/emission wavelength = 543/570–610) was acquired, and the data were analyzed using ImageJ software (NIH, Bethesda, MD).
Western blot analysis.
Cells were lysed with 250 μl buffer (20 mM MOPS, 5 mM EDTA, 2 mM EGTA, and 0.5% Nonidet P-40) and protease and phosphatase inhibitor cocktails as previously described (1). Protein concentration was determined using the Bradford method, and equivalent amounts of protein (25 μg) were resolved by SDS-PAGE. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane, blocked, and exposed overnight to primary antibodies against Cav-1 (1:1,000, ab17052, Abcam, Cambridge, MA), eNOS (1:5,000, sc-654, Santa Cruz Biotechnology, Santa Cruz, CA), and phosphorylated Ser1177 eNOS (p-eNOS; 1:1,000, Cell Signaling Technology, Danvers, MA). Blots were washed and incubated with secondary antibodies (Cav-1: 1:2,000, goat anti-mouse, Bio-Rad, Hercules, CA, and eNOS and p-eNOS: 1:10,000, donkey anti-rabbit, GE Healthcare). To investigate eNOS homodimer formation in endothelial cells as an index of coupled eNOS activity during isoflurane, low-temperature SDS-PAGE was performed with nonreducing Laemmli buffer. Equivalent amounts of protein (15 ug) were loaded, transferred to a PVDF membrane, blocked, and then probed with eNOS antibody (1:500, Santa Cruz). After being stripped, blots were reprobed with CD-31 antibody (1: 2,000, ab28364, Abcam). The secondary antibody was donkey anti-rabbit (eNOS: 1:2,000 and CD-31:1:8,000). Immunoreactive bands were detected by enhanced chemiluminescence followed by densitometric analysis using ImageJ software (NIH). eNOS dimer and monomer band densities were normalized to CD-31, and the change in the dimer-to-monomer ratio during isoflurane was expressed relative to control conditions in the absence of isoflurane.
Discontinuous sucrose gradient.
To investigate the actions of isoflurane with or without high glucose to modulate the intracellular localization of eNOS-related proteins, buoyant fractions, membrane fractions enriched in caveolae and lipid rafts, were isolated on a discontinuous sucrose gradient as previously described (35). HCAECs were washed, scraped into 2 ml of 500 mM Na2CO3, and homogenized on ice. Cell homogenates (600 μg protein/sample) were fractionated by sucrose density ultracentrifugation for 18 h. Fractions (1 ml) were collected, and an equal volume of each fraction was used for eNOS, p-eNOS, and Cav-1 Western blot analyses. All fractions were probed for the specific endoplasmic reticulum marker calreticulin (1:500, ab 4, Abcam).
Statistics.
Data are expressed as means ± SE. Comparison of two means was performed using Student's t-test. Comparison of several means was performed using one-way (one factor tested) or two-way (two factors tested) ANOVA, when appropriate, and the Newman-Keuls post hoc test. Hemodynamic data were analyzed with repeated-measures ANOVA. Changes within and between the groups were considered statistically significant at P < 0.05 (two-tailed). Statistical analysis was performed using NCSS 2007 software (Statistical Solutions, Cork, Ireland).
RESULTS
D-4F restored the cardioprotective effect of APC in mice with AHG.
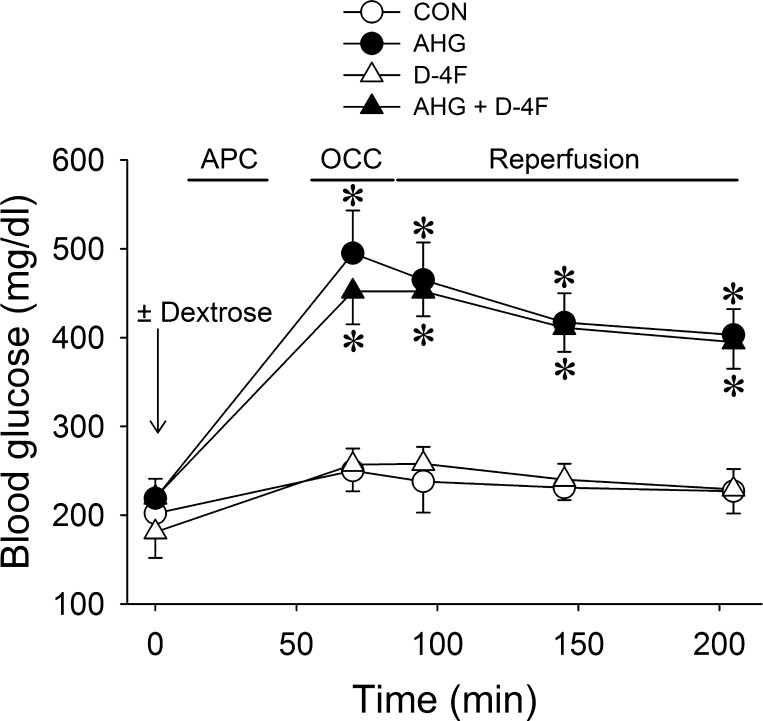
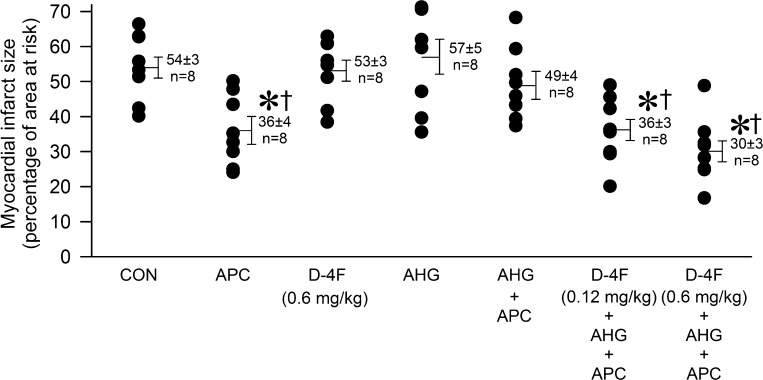
Ninety-two mice were instrumented to obtain seventy successful experiments. Twenty-two mice died during the experiment and were excluded from analysis [control: 2 mice, APC: 2 mice, D-4F: 2 mice, AHG + APC: 2 mice, AHG: 5 mice, D-4F (0.12 mg/kg) + AHG + APC: 3 mice, D-4F (0.6 mg/kg) + AHG + APC: 2 mice, l-NAME: 2 mice, and D-4F + AHG + APC + l-NAME: 2 mice]. Heart rate at baseline and after occlusion and reperfusion was similar among groups (Table 1), and blood glucose levels were not affected by APC or D-4F throughout the experiment (Fig. 2). There were no differences in AARs for infarction among groups (Table 2). Myocardial infarct size (Fig. 3) was significantly (P < 0.05) decreased by APC (36 ± 3% of AAR) compared with control experiments (54 ± 3% of AAR), and this action was blocked by AHG (49 ± 4% of AAR). Pretreatment of mice with D-4F at 0.12 and 0.6 mg/kg restored APC during AHG (36 ± 3% and 30 ± 3% of AAR, respectively), although D-4F alone had no effect on infarct size (53 ± 3% of AAR). l-NAME alone had no effect on infarct size (63 ± 5%, n = 7) but partially attenuated the beneficial effects of D-4F during APC and AHG (44 ± 3% of AAR, n = 7) compared with D-4F + APC + AHG in the absence of NO synthase inhibition (30 ± 3% of AAR).
Table 1.
Heart rates in mice during in vivo experiments
| Reperfusion |
|||||
|---|---|---|---|---|---|
| Experimental Group | Baseline | Occlusion | 30 min | 60 min | 120 min |
| Control | 407 ± 15 | 420 ± 11 | 418 ± 10 | 416 ± 12 | 404 ± 11 |
| AHG | 401 ± 14 | 410 ± 12 | 411 ± 12 | 412 ± 12 | 419 ± 13 |
| APC | 413 ± 17 | 383 ± 27 | 424 ± 19 | 417 ± 14 | 431 ± 15 |
| D-4F | 402 ± 8 | 387 ± 11 | 419 ± 11 | 404 ± 10 | 402 ± 14 |
| AHG + APC | 434 ± 22 | 407 ± 10 | 415 ± 12 | 424 ± 13 | 438 ± 13 |
| D-4F (0.12 mg/kg) + AHG + APC | 420 ± 12 | 412 ± 15 | 405 ± 19 | 433 ± 10 | 417 ± 13 |
| D-4F (0.6 mg/kg) + AHG + APC | 429 ± 15 | 408 ± 18 | 398 ± 14 | 389 ± 11 | 399 ± 10 |
| l-NAME | 388 ± 12 | 386 ± 9 | 398 ± 13 | 394 ± 12 | 392 ± 12 |
| D-4F (0.6 mg/kg) + l-NAME + AHG + APC | 422 ± 16 | 420 ± 18 | 427 ± 18 | 414 ± 15 | 418 ± 16 |
Data are means ± SE (in beats/min). AHG, acute hyperglycemia; APC, anesthetic preconditioning; D-4F, Ac-DWFKAFYDKVAEKFKEAF-NH2 (apolipoprotein A-I mimetic); l-NAME, NG-nitro-l-arginine methyl ester (nitric oxide synthase inhibitor).
Fig. 2.
Blood glucose measurements in C57BL/6J mice with or without AHG [dextrose (2 g/kg ip)] in the presence or absence of the apolipoprotein A-1 mimetic D-4F (0.6 mg/kg ip) during APC. Data are means ± SE; n = 6 mice/group. *P < 0.05 compared with the CON group.
Table 2.
Areas at risk for infarction
| Experimental Group | Area at Risk, % of the Left Ventricle |
|---|---|
| Control | 49 ± 11 |
| AHG | 50 ± 12 |
| APC | 50 ± 9 |
| D-4F | 51 ± 12 |
| AHG + APC | 53 ± 12 |
| D-4F (0.12 mg/kg) + AHG + APC | 52 ± 11 |
| D-4F (0.6 mg/kg) + AHG + APC | 51 ± 10 |
| l-NAME | 53 ± 3 |
| D-4F (0.6 mg/kg) + l-NAME + AHG + APC | 52 ± 3 |
Data are means ± SE.
Fig. 3.
Myocardial infarct size shown as a percentage of the area at risk for infarction in C57BL/6J mice. Circles are individual infarct size measurements (with means ± SE). *P < 0.05 compared with the CON group; †P < 0.05 compared with the AHG + APC group.
D-4F enhanced isoflurane-stimulated NO production during high glucose.
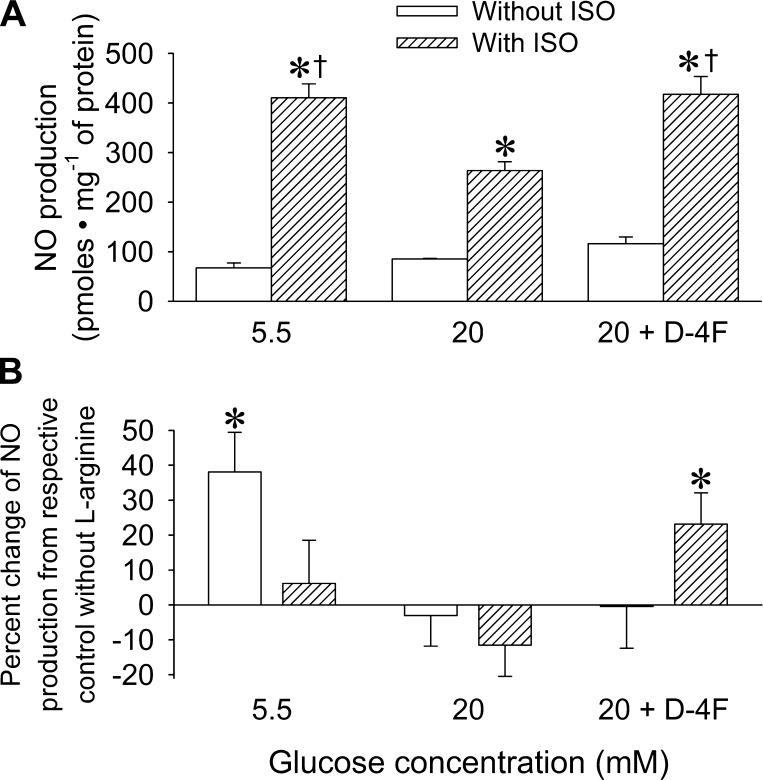
Isoflurane significantly (P < 0.05) increased NO production in HCAECs (Fig. 4A) cultured under normal glucose conditions (411 ± 28 pmol/mg protein, n = 3). This was attenuated by high glucose (264 ± 18 pmol/mg protein, n = 3) and restored when HCAECs were pretreated with D-4F (418 ± 36 pmol/mg protein, n = 5). l-Arginine enhanced NO production in unstimulated HCAECs (n = 8; Fig. 4B) but not in those stimulated by isoflurane (n = 6). High glucose prevented l-arginine-induced increases in NO with (n = 7) or without (n = 9) isoflurane. Interestingly, during high glucose, combined treatment of cells with D-4F and isoflurane (n = 9) substantially enhanced the ability of eNOS to use the substrate and increased NO production, whereas this did not occur with D-4F alone (n = 6).
Fig. 4.
A: NO production in HCAECs cultured in normal (5.5 mM) media or high glucose (20 mM) media. Data are means ± SE; n = 3–5 experiments/group. *P < 0.05 compared with the respective CON group without isoflurane (ISO); †P < 0.05 compared with 20 mM glucose + ISO. B: NO production in HCAECs after the addition of l-arginine to the media. Data are expressed as mean percent changes from the respective CON group without l-arginine (means ± SE; n = 6–9 experiments/group). *P < 0.05 compared with the respective CON group.
D-4F attenuated O2·− formation during high glucose.
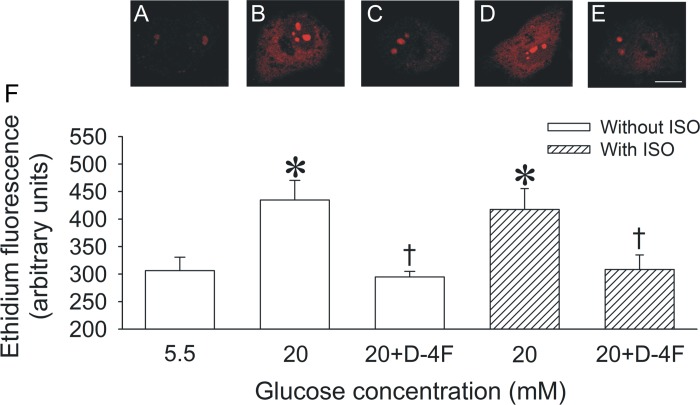
High glucose significantly (P < 0.05) increased O2·− formation (435 ± 36 arbitrary units, n = 6; Fig. 5) compared with normal glucose conditions (306 ± 25 arbitrary units, n = 7). Isoflurane had no effect on O2·− formation during high glucose (417 ± 38 arbitrary units, n = 6). However, D-4F decreased O2·− formation to levels observed during normal glucose in either the absence (295 ± 10 arbitrary units, n = 6) or presence (308 ± 26 arbitrary units, n = 6) of isoflurane.
Fig. 5.
O2·− formation measured with dihydroethidium, which, upon oxidation, yields red fluorescent ethidium in living HCAECs cultured in normal (5.5 mM) or high glucose (20 mM) media. Scale bar = 10 μm. Confocal images of ethidium fluorescence are shown for the following groups: 5.5 mM glucose CON (A), 20 mM glucose (B), 20 mM glucose + D-4F (C), 20 mM glucose + ISO (D), and 20 mM glucose + D-4F + ISO (E). F: quantification of ethidium fluorescence in HCAECs. Data are means ± SE; n = 6 experiments/group. *P < 0.05 compared with the 5.5 mM CON group; †P < 0.05 compared with the 20 mM glucose group.
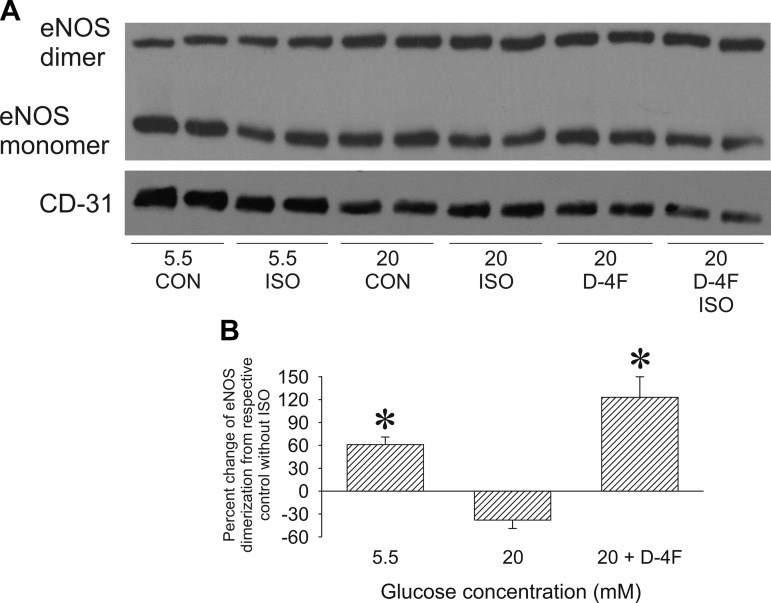
D-4F enhanced isoflurane-stimulated eNOS homodimerization during high glucose.
Isoflurane significantly (61 ± 10%, n = 5, P < 0.05) increased eNOS dimerization (Fig. 6) during normal glucose conditions compared with unstimulated cells (n = 4), whereas high glucose blocked this effect (−38 ± 11%, n = 5). D-4F enhanced the ability of isoflurane to increase the eNOS protein dimer-to-monomer ratio (123 ± 27%, n = 5).
Fig. 6.
eNOS protein dimer-to-monomer ratio in HCAECs. A: representative Western blot showing eNOS dimers (280 kDa), eNOS monomers (140 kDa), and CD-31 (140 kDa) protein expression in HCAECs. B: histogram showing the eNOS protein dimer-to-monomer ratio normalized to CD-31 and expressed as the percent change from the respective CON group without ISO. Data are means ± SE; n = 5 experiments/group. *P < 0.05 compared with the respective CON group without ISO.
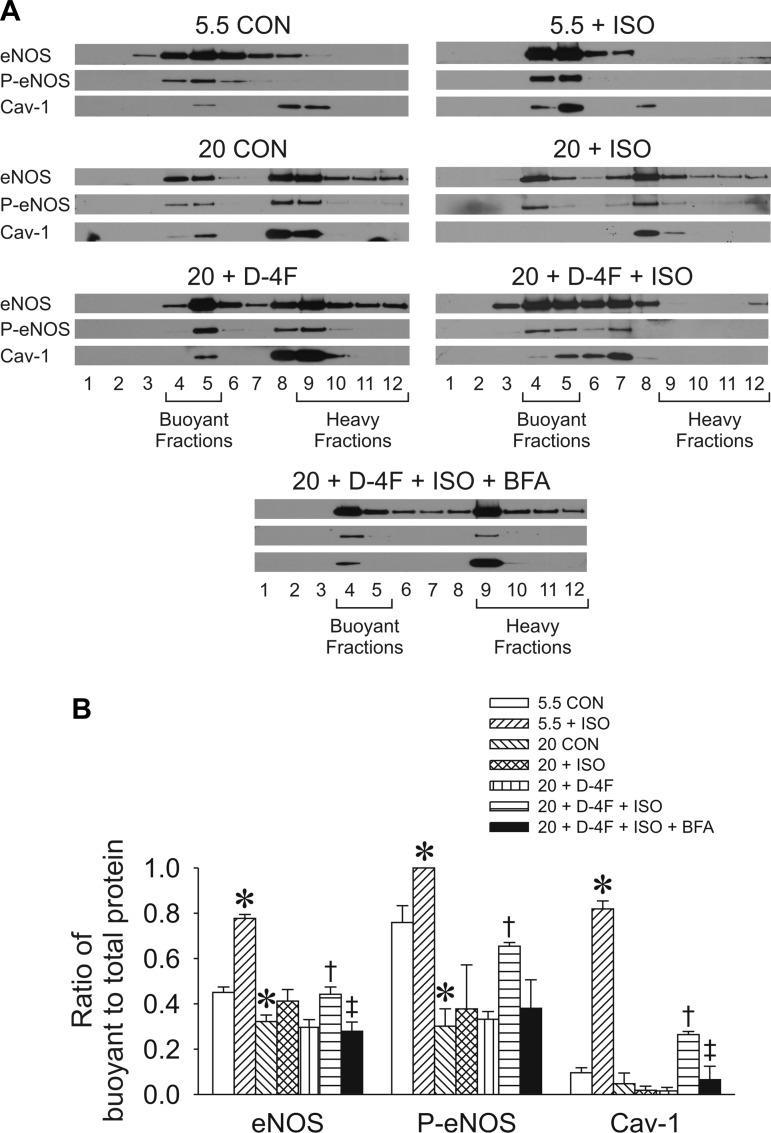
D-4F promoted isoflurane-induced Cav-1, eNOS, and p-eNOS caveolar compartmentalization.
During unstimulated, normal glucose conditions, Cav-1 was located in heavy/noncaveolar fractions, eNOS was equally distributed between caveolar and noncaveolar compartments, and Ser1177 phosphorylation occurred predominantly in buoyant/caveolar fractions (fractions 4 and 5; n = 3 per condition; Fig. 7). Isoflurane significantly (P < 0.05) promoted the redistribution of eNOS and Cav-1 from noncaveolar to caveolar fractions and increased Ser1177 phosphorylation of eNOS exclusively in caveolar fractions. In contrast, high glucose enhanced the retention of eNOS, p-eNOS, and Cav-1 in noncaveolar fractions. Isoflurane or D-4F alone had no effect on protein redistribution toward caveolae during high glucose. However, combined D-4F and isoflurane treatment significantly (P < 0.05) increased the redistribution of Cav-1, eNOS, and p-eNOS to buoyant fractions, with the latter being blocked by brefeldin A, a pharmacological inhibitor of protein trafficking at a dose that had no effect on cell viability measured with trypan blue (data not shown).
Fig. 7.
Subcellular localization of eNOS, p-eNOS, and Cav-1 in HCAECs evaluated by discontinuous sucrose gradient fractionation. Fractions 4 and 5 represent buoyant caveolar microdomains, whereas fractions 9–12 represent heavy membrane fractions. A: representative sucrose density gradient separations by Western blot analysis. B: quantification of the ratio of buoyant to total protein expression in HCAECs. Data are means ± SE; n = 3 experiments with 5 dishes/group. *P < 0.05 compared with the 5.5 mM glucose CON group; †P < 0.05 compared with the 20 mM glucose group; ‡P < 0.05, 20 mM glucose + D-4F + ISO + BFA group compared with 20 mM + D-4F + ISO group.
DISCUSSION
AHG and diabetes have been demonstrated to abrogate the cardioprotective effects of APC (1, 24, 44, 53); however, the responsible mechanisms have been incompletely elucidated. Hyperglycemia is well known to impair the bioavailability of NO, an important trigger and mediator of APC (2, 9). We (1) have previously shown that hyperglycemia adversely modulates NO signaling during pharmacological protection with volatile anesthetics, such as isoflurane, by attenuating heat shock protein 90 interactions with eNOS and by decreasing tetrahydrobiopterin concentrations. Recently, the contribution of lipid rafts to intracellular signaling has emerged as a critical component of pharmacological cardioprotection (38, 54); however, the role of high glucose to adversely impact NO production through interactions with lipid rafts has not been investigated. The results of this study indicate that volatile anesthetics modulate intracellular compartmentalization and posttranslational modifications of eNOS in lipid rafts, that this process is sensitive to glucose concentration, and that the adverse effects of AHG were ameliorated by an ApoA-1 mimetic.
Lipid/membrane rafts are sterol-, sphingolipid-, and cholesterol-enriched microdomains present in the plasma membrane, endoplasmic reticulum, and mitochondria that allow for the dynamic, temporal regulation of protein trafficking and control of intracellular signal transduction events (43). Caveolae are a subclass of membrane rafts distinguished by the presence of the scaffolding proteins Cav-1, Cav-2, and Cav-3 (10). Cav-1 and Cav-2 are differentially expressed in adipocytes, endothelial cells, and fibroblasts, whereas Cav-3 is expressed predominantly in skeletal, cardiac, and smooth muscle cells. The number of caveolae and functional signal transduction events that occur in caveolae are indirectly regulated by Cav expression. The fluidity of membrane rafts is modulated by Cavs through the binding of cholesterol, which, in turn, alters membrane composition and signaling effects (4). Although high glucose has been shown to impair the organization of lipid rafts in pancreatic β-cells (50), the effects of high glucose to disrupt the compartmentalization of proteins involved in APC signal transduction have not been investigated.
The present findings confirm and extend recent evidence showing that lipid rafts and Cavs play an important role in cardioprotection. Cav-1−/− mice have been demonstrated to be resistant to the cardioprotective effects of isoflurane (38). Isoflurane increased the formation of caveolae in wild-type adult mouse cardiomyocytes and enhanced the localization of Cav-1 to buoyant membrane fractions in the myocardium in vivo (38). Similarly, isoflurane induced the compartmentalization of Cav-1 and eNOS within endothelial cell caveolae in the present investigation, with concurrent increased Ser1177 phosphorylation (2) and production of NO. Cav-1 has been shown to regulate eNOS activity through protein-protein interaction events that are localized in specific membrane domains (11). Intracellular compartmentalization of eNOS was thus correlated with eNOS activity, and eNOS activation was sevenfold greater in the plasma membrane fraction compared with the cytosolic fraction. In addition, NO synthase activity was ninefold greater in caveolar versus noncaveolar membranes (47). Paradoxically, basal activity of eNOS was negatively regulated through direct interactions with Cav-1. However, eNOS localization within the caveolae was essential for eNOS activation in response to physiological stimuli (11). During pathophysiological conditions, such as increased oxidative stress, oxidation of cholesterol in caveolae induced the translocation of Cav-1 away from the plasma membrane to the Golgi apparatus (49). Increased levels of oxidized low-density lipoprotein (LDL) that caused the depletion of caveolar cholesterol also promoted Cav-1 and eNOS redistribution to the cytosolic compartment with subsequent eNOS inactivation (4, 42). Diabetes has also been shown to downregulate membrane-associated Cav-1 expression in the renal cortex concomitantly with a decrease in p-eNOS (27). Our results similarly demonstrated that high glucose impaired isoflurane-induced Cav-1 compartmentalization within caveolae and Ser1177 phosphorylation of eNOS. Taken together, the previous and present findings implicate AHG as a source of oxidative stress that impairs eNOS signaling by disrupting normal caveolar compartmentalization of proteins.
D-4F is an 18-amino acid peptide composed of d-amino acids that contains four phenylalanine residues (4F) and has no sequence homology with ApoA-1 but shares the lipid-associating structural ApoA-1 motif (3). ApoA-1 mimetics have been shown to possess antiatherogenic and anti-inflammatory effects and to produce favorable vascular effects in animal models of type I diabetes (28, 40), type II diabetes and obesity (39, 41), atherosclerosis (32), and hypercholesterolemia (36). The 4F peptides are proposed to exert anti-inflammatory effects by binding and sequestering proinflammatory oxidized phospholipids and fatty acid hydroperoxides (3), thereby reducing LDL oxidation (55). ApoA-1 or its mimetic have also been demonstrated to modulate cellular cholesterol metabolism (12, 48, 55), thus rendering plausible the assumption that D-4F facilitates eNOS compartmentalization. A single oral dose of D-4F was safe, well tolerated, and improved an in vitro inflammatory index in patients with coronary heart disease (5). The efficacy of ApoA-1 mimetics to improve outcomes in patients with coronary artery disease, AHG, or diabetes, however, has never been tested.
The present results demonstrated that a dose of D-4F, which by itself was not cardioprotective, restored APC during AHG. D-4F normalized isoflurane-stimulated NO production during high glucose, which was accompanied by enhanced translocation of Cav-1 and eNOS to the caveolar membrane fraction. High glucose increased the formation of O2·− in endothelial cells, although the intracellular source of ROS was not identified. High glucose has been demonstrated to increase ROS generation from multiple enzymatic sources, including mitochondria (40). Nevertheless, under conditions of oxidative stress and tetrahydrobiopterin deficiency, eNOS fails to produce NO and can itself generate O2·−, a condition referred to as eNOS “uncoupling” (14). Uncoupling of eNOS has been linked to monomerization of the protein, a process exacerbated by high glucose and diabetes (6, 57). To our knowledge, the present results are the first to show that isoflurane increases eNOS coupling and that high glucose abrogates isoflurane-stimulated dimerization of eNOS and the production of NO even under conditions of excess substrate availability. Interestingly, D-4F ameliorated O2·− formation during high glucose with or without isoflurane and enhanced eNOS dimerization and NO production in the presence of isoflurane, consistent with an action of D-4F to promote coupled eNOS activity. This is consistent with previous evidence showing that the ApoA-1 mimetic L-4F enhanced NO and decreased O2·− generation in endothelial cells treated with LDL (37). Finally, the beneficial actions of D-4F were not entirely abolished by l-NAME in vivo; thus, D4-F may have additional effects on lipid oxidation and cholesterol metabolism that could contribute to cardioprotection. This hypothesis requires further investigation to confirm, however.
Our findings should be interpreted within the constraints of several potential limitations. Heart rates were similar during coronary artery occlusion in mice with or without prior exposure to isoflurane or D-4F. Thus, it is unlikely that differences in hemodynamics among the groups contributed to the observed results. However, myocardial O2 consumption was not measured in the present investigation. In vitro experiments were performed to elucidate the detailed mechanisms contributing to isoflurane-stimulated NO regulation in endothelial cells during high glucose. To recapitulate the in vivo setting as precisely as possible, the time course of drug and glucose administration during these experiments was different. For example, the pharmacokinetics of D-4F (time to peak concentration: 1.5–2 h after application) (33) and the expected in vivo duration of the peak drug effect after intraperitoneal injection required a more prolonged application of D-4F in vivo than in vitro. AHG in mice was produced by an intraperitioneal injection of dextrose compared with the intravenous administration of dextrose previously performed in a large animal species (26). Canine, rabbit, and rodent species are acutely sensitive to short-term exposure to high glucose in vivo; however, preliminary experiments indicated that isoflurane-stimulated NO production in HCAECs was insensitive to short-term (<24 h) culture in high glucose media. The latter observation is consistent with other reports in the literature investigating the effects of high glucose on cells in culture. Our cellular model of NO-related signaling mechanisms during APC has consistently replicated findings in vivo using models of AHG and diabetes (1, 9).
In conclusion, these results demonstrate that AHG impairs isoflurane-stimulated eNOS activity and subsequent cardioprotection by interfering with intracellular protein compartmentalization, phosphorylation, and homodimerization and that this action is mitigated by the ApoA-1 mimetic D-4F. AHG portends a worse clinical outcome in the perioperative period in patients with or without diabetes. Thus, further studies are needed to define strategies for improving eNOS function during AHG as a potential means for decreasing cardiovascular morbidity and mortality.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-063705 (to J. R. Kersten) and P01-GM-066730 (to J. R. Kersten).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.B., Z.-D.G., F.S., D.C.W., and J.R.K. conception and design of research; I.B., Z.-D.G., F.S., A.C., and D.W. performed experiments; I.B., Z.-D.G., F.S., A.C., D.W., and J.R.K. analyzed data; I.B., Z.-D.G., F.S., D.W., and J.R.K. interpreted results of experiments; I.B. and J.R.K. drafted manuscript; I.B., Z.-D.G., F.S., A.C., D.W., D.C.W., and J.R.K. approved final version of manuscript; Z.-D.G., D.W., D.C.W., and J.R.K. edited and revised manuscript; F.S. prepared figures.
ACKNOWLEDGMENTS
The authors thank M. Paterson (Medical College of Wisconsin, Milwaukee, WI), D. A. Schwabe (Medical College of Wisconsin, Milwaukee, WI), and A. R. Billstrom (Medical College of Wisconsin, Milwaukee, WI) for technical assistance and H. Loebel (Medical College of Wisconsin, Milwaukee, WI) for manuscript preparation.
Part of this work was presented at the 2010 Annual Meeting of the American Society Anesthesiologists.
REFERENCES
- 1.Amour J, Brzezinska AK, Jager Z, Sullivan C, Weihrauch D, Du J, Vladic N, Shi Y, Warltier DC, Pratt PF, Jr, Kersten JR. Hyperglycemia adversely modulates endothelial nitric oxide synthase during anesthetic preconditioning through tetrahydrobiopterin- and heat shock protein 90-mediated mechanisms. Anesthesiology 112: 576–585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amour J, Brzezinska AK, Weihrauch D, Billstrom AR, Zielonka J, Krolikowski JG, Bienengraeber MW, Warltier DC, Pratt PF, Jr, Kersten JR. Role of heat shock protein 90 and endothelial nitric oxide synthase during early anesthetic and ischemic preconditioning. Anesthesiology 110: 317–325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anantharamaiah GM, Mishra VK, Garber DW, Datta G, Handattu SP, Palgunachari MN, Chaddha M, Navab M, Reddy ST, Segrest JP, Fogelman AM. Structural requirements for antioxidative and anti-inflammatory properties of apolipoprotein A-I mimetic peptides. J Lipid Res 48: 1915–1923, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem 274: 32512–32519, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res 49: 1344–1352, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai S, Khoo J, Channon KM. Augmented BH4 by gene transfer restores nitric oxide synthase function in hyperglycemic human endothelial cells. Cardiovasc Res 65: 823–831, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Ceriello A. Acute hyperglycaemia and oxidative stress generation. Diabet Med 14, Suppl 3: S45–S49, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Chen JX, Lawrence ML, Cunningham G, Christman BW, Meyrick B. HSP90 and Akt modulate Ang-1-induced angiogenesis via NO in coronary artery endothelium. J Appl Physiol 96: 612–620, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Chiari PC, Bienengraeber MW, Weihrauch D, Krolikowski JG, Kersten JR, Warltier DC, Pagel PS. Role of endothelial nitric oxide synthase as a trigger and mediator of isoflurane-induced delayed preconditioning in rabbit myocardium. Anesthesiology 103: 74–83, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Cohen AW, Combs TP, Scherer PE, Lisanti MP. Role of caveolin and caveolae in insulin signaling and diabetes. Am J Physiol Endocrinol Metab 285: E1151–E1160, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Feron O, Kelly RA. The caveolar paradox: suppressing, inducing, and terminating eNOS signaling. Circ Res 88: 129–131, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Fielding CJ, Fielding PE. Intracellular cholesterol transport. J Lipid Res 38: 1503–1521, 1997 [PubMed] [Google Scholar]
- 13.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, Freeman WK, Froehlich JB, Kasper EK, Kersten JR, Riegel B, Robb JF, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Md RN, Ornato JP, Page RL, Tarkington LG, Yancy CW. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation 116: 1971–1996, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Forstermann U. Janus-faced role of endothelial NO synthase in vascular disease: uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biol Chem 387: 1521–1533, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, Hudson M, Mendoza J, Johnson R, Lin E, Umpierrez GE. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 33: 1783–1788, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge ZD, Peart JN, Kreckler LM, Wan TC, Jacobson MA, Gross GJ, Auchampach JA. Cl-IB-MECA [2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methylcarboxamide] reduces ischemia/reperfusion injury in mice by activating the A3 adenosine receptor. J Pharmacol Exp Ther 319: 1200–1210, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Ge ZD, Pravdic D, Bienengraeber M, Pratt PF, Jr, Auchampach JA, Gross GJ, Kersten JR, Warltier DC. Isoflurane postconditioning protects against reperfusion injury by preventing mitochondrial permeability transition by an endothelial nitric oxide synthase-dependent mechanism. Anesthesiology 112: 73–85, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerstein HC, Pais P, Pogue J, Yusuf S. Relationship of glucose and insulin levels to the risk of myocardial infarction: a case-control study. J Am Coll Cardiol 33: 612–619, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Getz GS, Wool GD, Reardon CA. Biological properties of apolipoprotein a-I mimetic peptides. Curr Atheroscler Rep 12: 96–104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giugliano D, Marfella R, Coppola L, Verrazzo G, Acampora R, Giunta R, Nappo F, Lucarelli C, D'Onofrio F. Vascular effects of acute hyperglycemia in humans are reversed by l-arginine. Evidence for reduced availability of nitric oxide during hyperglycemia. Circulation 95: 1783–1790, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 100: 1134–1146, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Horikawa YT, Patel HH, Tsutsumi YM, Jennings MM, Kidd MW, Hagiwara Y, Ishikawa Y, Insel PA, Roth DM. Caveolin-3 expression and caveolae are required for isoflurane-induced cardiac protection from hypoxia and ischemia/reperfusion injury. J Mol Cell Cardiol 44: 123–130, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Kurisu S, Nishioka K, Umemura T, Nakamura S, Yoshida M. Impact of acute hyperglycemia on left ventricular function after reperfusion therapy in patients with a first anterior wall acute myocardial infarction. Am Heart J 146: 674–678, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Kehl F, Krolikowski JG, Mraovic B, Pagel PS, Warltier DC, Kersten JR. Hyperglycemia prevents isoflurane-induced preconditioning against myocardial infarction. Anesthesiology 96: 183–188, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Kersten JR, Brooks LA, Dellsperger KC. Impaired microvascular response to graded coronary occlusion in diabetic and hyperglycemic dogs. Am J Physiol Heart Circ Physiol 268: H1667–H1674, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Kersten JR, Schmeling TJ, Orth KG, Pagel PS, Warltier DC. Acute hyperglycemia abolishes ischemic preconditioning in vivo. Am J Physiol Heart Circ Physiol 275: H721–H725, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Komers R, Schutzer WE, Reed JF, Lindsley JN, Oyama TT, Buck DC, Mader SL, Anderson S. Altered endothelial nitric oxide synthase targeting and conformation and caveolin-1 expression in the diabetic kidney. Diabetes 55: 1651–1659, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kruger AL, Peterson S, Turkseven S, Kaminski PM, Zhang FF, Quan S, Wolin MS, Abraham NG. D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation 111: 3126–3134, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Lohr N, Kersten JR. Man overboard! Rescuing myocardium with membrane rafts. Anesthesiology 112: 1076–1078, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marfella R, Nappo F, De Angelis L, Siniscalchi M, Rossi F, Giugliano D. The effect of acute hyperglycaemia on QTc duration in healthy man. Diabetologia 43: 571–575, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Marfella R, Verrazzo G, Acampora R, La Marca C, Giunta R, Lucarelli C, Paolisso G, Ceriello A, Giugliano D. Glutathione reverses systemic hemodynamic changes induced by acute hyperglycemia in healthy subjects. Am J Physiol Endocrinol Metab 268: E1167–E1173, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, Lallone R, Fogelman AM. Oral administration of an Apo A-I mimetic peptide synthesized from d-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation 105: 290–292, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Navab M, Reddy ST, Anantharamaiah GM, Imaizumi S, Hough G, Hama S, Fogelman AM. Intestine may be a major site of action for the apoA-I mimetic peptide 4F whether administered subcutaneously or orally. J Lipid Res 52: 1200–1210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Ostrom RS, Insel PA. Methods for the study of signaling molecules in membrane lipid rafts and caveolae. Methods Mol Biol 332: 181–191, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Ou J, Wang J, Xu H, Ou Z, Sorci-Thomas MG, Jones DW, Signorino P, Densmore JC, Kaul S, Oldham KT, Pritchard KA., Jr Effects of D-4F on vasodilation and vessel wall thickness in hypercholesterolemic LDL receptor-null and LDL receptor/apolipoprotein A-I double-knockout mice on Western diet. Circ Res 97: 1190–1197, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ou Z, Ou J, Ackerman AW, Oldham KT, Pritchard KA., Jr L-4F, an apolipoprotein A-1 mimetic, restores nitric oxide and superoxide anion balance in low-density lipoprotein-treated endothelial cells. Circulation 107: 1520–1524, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, Huang D, Moreno AL, Patel PM, Insel PA, Roth DM. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J 21: 1565–1574, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Peterson SJ, Drummond G, Kim DH, Li M, Kruger AL, Ikehara S, Abraham NG. L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J Lipid Res 49: 1658–1669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterson SJ, Husney D, Kruger AL, Olszanecki R, Ricci F, Rodella LF, Stacchiotti A, Rezzani R, McClung JA, Aronow WS, Ikehara S, Abraham NG. Long-term treatment with the apolipoprotein A1 mimetic peptide increases antioxidants and vascular repair in type I diabetic rats. J Pharmacol Exp Ther 322: 514–520, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Peterson SJ, Kim DH, Li M, Positano V, Vanella L, Rodella LF, Piccolomini F, Puri N, Gastaldelli A, Kusmic C, L'Abbate A, Abraham NG. The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res 50: 1293–1304, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peterson TE, Poppa V, Ueba H, Wu A, Yan C, Berk BC. Opposing effects of reactive oxygen species and cholesterol on endothelial nitric oxide synthase and endothelial cell caveolae. Circ Res 85: 29–37, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res 47: 1597–1598, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Raphael J, Gozal Y, Navot N, Zuo Z. Hyperglycemia inhibits anesthetic-induced postconditioning in the rabbit heart via modulation of phosphatidylinositol-3-kinase/Akt and endothelial nitric oxide synthase signaling. J Cardiovasc Pharmacol 55: 348–357, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Sciaky N, Presley J, Smith C, Zaal KJ, Cole N, Moreira JE, Terasaki M, Siggia E, Lippincott-Schwartz J. Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J Cell Biol 139: 1137–1155, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sepac A, Sedlic F, Si-Tayeb K, Lough J, Duncan SA, Bienengraeber M, Park F, Kim J, Bosnjak ZJ. Isoflurane preconditioning elicits competent endogenous mechanisms of protection from oxidative stress in cardiomyocytes derived from human embryonic stem cells. Anesthesiology 113: 906–916, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem 271: 6518–6522, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Smart EJ, Ying Y, Donzell WC, Anderson RG. A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem 271: 29427–29435, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Smart EJ, Ying YS, Conrad PA, Anderson RG. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol 127: 1185–1197, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Somanath S, Barg S, Marshall C, Silwood CJ, Turner MD. High extracellular glucose inhibits exocytosis through disruption of syntaxin 1A-containing lipid rafts. Biochem Biophys Res Commun 389: 241–246, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Sonner JM, Gong D, Eger EI., 2nd Naturally occurring variability in anesthetic potency among inbred mouse strains. Anesthesia Analgesia 91: 720–726, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Sviridov D, Fidge N, Beaumier-Gallon G, Fielding C. Apolipoprotein A-I stimulates the transport of intracellular cholesterol to cell-surface cholesterol-rich domains (caveolae). Biochem J 358: 79–86, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanaka K, Kehl F, Gu W, Krolikowski JG, Pagel PS, Warltier DC, Kersten JR. Isoflurane-induced preconditioning is attenuated by diabetes. Am J Physiol Heart Circ Physiol 282: H2018–H2023, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Tsutsumi YM, Kawaraguchi Y, Horikawa YT, Niesman IR, Kidd MW, Chin-Lee B, Head BP, Patel PM, Roth DM, Patel HH. Role of caveolin-3 and glucose transporter-4 in isoflurane-induced delayed cardiac protection. Anesthesiology 112: 1136–1145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wool GD, Reardon CA, Getz GS. Apolipoprotein A-I mimetic peptide helix number and helix linker influence potentially anti-atherogenic properties. J Lipid Res 49: 1268–1283, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc Natl Acad Sci USA 101: 7891–7896, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou MH, Shi C, Cohen RA. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 109: 817–826, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]