Abstract
In vitro studies have revealed that acute increases in transmural pressure increase lymphatic vessel contractile function. However, adaptive responses to prolonged changes in transmural pressure in vivo have not been reported. Therefore, we developed a novel bovine mesenteric lymphatic partial constriction model to test the hypothesis that lymphatic vessels exposed to higher transmural pressures adapt functionally to become stronger pumps than vessels exposed to lower transmural pressures. Postnodal mesenteric lymphatic vessels were partially constricted for 3 days. On postoperative day 3, constricted vessels were isolated, and divided into upstream (UP) and downstream (DN) segment groups, and instrumented in an isolated bath. Although there were no differences between the passive diameters of the two groups, both diastolic diameter and systolic diameter were significantly larger in the UP group than in the DN group. The pump index of the UP group was also higher than that in the DN group. In conclusion, this is the first work to report how lymphatic vessels adapt to prolonged changes in transmural pressure in vivo. Our results suggest that vessel segments upstream of the constriction adapt to become both better fluid conduits and lymphatic pumps than downstream segments.
Keywords: edema, lymphangion, lymphatic muscle, contractility
lymphatic vessels transport lymph. The lymphatic system plays a vital role in the regulation of interstitial fluid volume. It removes fluid from the interstitial space and transports this fluid as lymph to the veins of the neck via collecting lymphatic vessels (3). Collecting lymphatic vessels contain closely spaced valves that ensure unidirectional flow of lymph (21). Sharing structural similarities with veins (31, 39), lymphatic vessels were at first primarily viewed as passive conduits (38). Although lymph can flow down an axial pressure gradient (14) or be propelled by extrinsic compression of lymphatic vessels (22, 32), intrinsic rhythmic contractions of lymphatic muscle can actively propel lymph (15). Lymphangions, segments of lymphatic vessels between two valves, therefore can act as pumps that transport lymph from the low-pressure interstitial space to higher-pressure veins (7, 23). This fundamental functional duality of lymphatic vessels, as both conduit and pump (34, 35), is reflected in the expression contractile proteins characteristic of both vascular smooth muscle and cardiac muscle (28).
Functional responses of lymphatic vessels to acute increases in transmural pressure have been characterized.
Although intrinsic spontaneous contractions of lymphangions can be modulated by vasoactive mediators (19, 30) and flow (1, 2, 11, 24), contractions are particularly sensitive to changes in transmural pressure. An in vitro study (24) of bovine mesenteric lymphatic vessels has demonstrated that acute increases in transmural pressure promote lymphatic pumping by increasing both the frequency and amplitude of contractions. The physiological significance of this response becomes clear considering the fact that edemagenic stimuli, such as increased capillary pressure, not only increase microvascular filtration but also interstitial pressure and, therefore, lymphatic filling pressure (3). Acutely increased lymphangion pumping is thus recognized as a fundamental negative feedback mechanism that prevents edema by increasing lymphatic drainage in response to increased microvascular filtration (3).
Adaptive responses of lymphatic vessels to prolonged changes in pressure have not been characterized.
A prolonged increase in lymphatic transmural pressure may result from chronic venous hypertension (10, 17), lymphatic vessel obstruction (36), or chronic capillary hypertension (9). In each case, lymphatic vessels must adapt to overcome a higher transmural pressure to either maintain or increase lymph flow. Although lymphatic vessel adaptations in response to prolonged changes in transmural pressure have not yet been reported, guidance may be found in reports detailing the chronic adaptation of blood vessels and ventricles. To study adaptive responses in blood vessels, investigators have used partial constriction to raise upstream transmural pressures and lower downstream transmural pressures in the same vessel (6, 37). Therefore, we used partial vessel constriction to test the hypothesis that lymphatic vessels exposed to higher transmural pressures adapt functionally to become stronger pumps than vessels exposed to lower transmural pressures.
METHODS
Animal model.
We developed a partial constriction model to study the adaptation of bovine mesenteric lymphatic vessels. Partial constriction of lymphatic vessels was chosen as an intervention, because it allowed characterization of the response of the same vessel to two different pressures: lymphatic vessel segments upstream of the partial constriction are exposed to higher transmural pressures than downstream segments. Analysis of two sections of the same vessel also ensured that lymph flow and exposure to signaling molecules in the lymph were identical. We chose to study postnodal mesenteric lymphatic vessels in particular because they have become a standard model for acute functional studies (13, 24, 29, 43). Furthermore, mesenteric vessels propel lymph solely by intrinsic rhythmic contraction, and, thus, functional adaptation can be characterized without the confounding effects of extrinsic compression (28).
Surgical preparation and lymphatic partial constriction.
Experimental procedures and animal care were performed in compliance with protocols approved by the Institutional Animal Care and Use Committee of Texas A&M University. Healthy crossbreed cows (age: 2–8 yr) were used in this study. After 48 h of fasting, animals were anesthetized with xylazine (0.2 mg/kg), diazepam (0.01–0.03 mg/kg), and ketamine (2–6 mg/kg to effect). Each animal was then ventilated mechanically, and a surgical plane of anesthesia was maintained by inhalation using 1–3% isoflurane in 100% oxygen. Through a right laparotomy incision using aseptic techniques, the small intestine, specifically the jejunum, and associated mesentery were exteriorized. Drying of the exposed mesentery was prevented by intermittent application of warmed isotonic saline. Three large postnodal conducting lymphatic vessels (2- to 4-mm diameter) were identified in the same region of the mesentery. A 20-gauge stainless steel needle was placed parallel to each selected vessel. A 2-0 suture was tied around the needle and the vessel. The needle was then removed, leaving the vessel with a ligature loose enough to prevent total constriction but tight enough to reduce lymph flow. The exteriorized intestine and mesentery were then returned back to the abdominal cavity. The abdominal incision was closed, and the animals recovered from anesthesia. Animals were monitored during the 3-day surgical recovery period.
Tissue collection.
On postoperative day 3, animals were euthanized by captive bolt followed by exsanguination. Immediately after euthanasia, the abdomen was opened, and segments (8–10 cm long) of the three partially constricted lymphatic vessels from each animal with the ligation in the middle were carefully isolated from the surrounding fat and connective tissue. After the downstream ends were tied, each vessel was divided at the ligation into the following two segment groups: segments immediately upstream from the ligature (UP group) and segments located immediately downstream from the ligature (DN group). After being harvested, these vessel segments were transported to the nearby laboratory in albumin physiological salt solution (APSS) chilled to 4°C [which contained (in mM) 145.00 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 dextrose, 2.0 sodium pyruvate, 0.02 EDTA, and 3.0 MOPS with 10 g/l BSA; pH 7.4].
Experimental setup.
The experimental preparation and apparatus used for functional analysis were similar to those previously described by Venugopal et al. (42) with few exceptions. In brief, after the surrounding fat and connective tissue were removed, each vessel segment containing at least one set of valves was mounted in a modified vessel bath (model MAYFLOWER type 813/6, Harvard Apparatus, Holliston, MA). The vessel was submerged (1 cm below the surface level) in APSS, which was circulated in the bath at a constant rate of 2 ml/min. A syringe pump (model KDS220, KD Scientific, Holliston, MA) was connected to the upstream (inlet) end of the vessel using polyethylene tubing (PE-10). The downstream end (outlet) was connected to a glass tube open to the atmosphere using similar tubing. Fluid-filled pressure transducers (model 20, CyberSense, Nicholasville, KY) were connected at the vessel inlet and outlet. Initial equilibration flow and transmural pressure were set as previously reported (24). First, luminal flow (in APSS) was set to 0.1 ml/min. This flow was calculated to clear the content of the lumen within 30 s. Second, transmural pressure was set to 6 cmH2O by adjusting the outlet tube height. Bath and luminal APSS temperature were maintained at 37°C via thermoregulators (model Lauda E-200, Brinkman Instruments, Westbury, NY). Each vessel was then allowed to equilibrate for 20–40 min until stable contractions were observed. Those vessels that did not contract spontaneously were not used for functional analysis.
Data acquisition and measurements.
Data were recorded at 4 Hz using a custom-built real-time data-acquisition system (LabView 8.0, PCI 6036E, National Instruments, Austin, TX). Transmural pressure was determined by averaging measured inlet and outlet pressures and subtracting the APSS height in the bath (i.e., 1 cmH2O). Instantaneous lymphatic vessel diameter was determined using video calipers (LabView 8.0, National Instruments) from the acquired video (camera model ST-XC50, Sony Electronics, Park Ridge, NJ) of the lymphatic vessel in the bath.
Experimental protocol.
Lymphatic vessels that exhibited spontaneous contractions in a consistent fashion within 40 min of the initial equilibration time were used for functional analysis. Each lymphatic vessel was exposed to a predetermined low endothelial shear stress (i.e., diastolic endothelial shear stress of 8.1e−5 dyn/cm2) by adjusting the luminal flow at each transmural pressure level. This shear stress was chosen to be consistent with the diastolic wall shear stress of actively contracting lymphatic vessels (24). Lymphatic transmural pressure was set to 1, 3, 6, 9, and 12 cmH2O for 10 min by raising the outlet glass tube. After each change in pressure, luminal flow was readjusted to maintain the predetermined diastolic shear stress. Vessels were allowed to equilibrate for 7 min before data were recorded for 3 min. The sequence of transmural pressure steps was selected randomly for vessels from each cow. For consistency in analysis, all vessel segments from a given cow were analyzed using the same selected sequence of transmural pressure steps. To determine the passive response of the lymphatic vessels, luminal and bath APSS were replaced with Ca2+-free APSS. Luminal flow was set to attain a low endothelial shear stress (i.e., 8.1e−5 dyn/cm2), and transmural pressure was set to 6 cmH2O. Lymphatic vessels were then allowed to equilibrate for ∼30 min. When lymphatic vessels had relaxed completely, as evidenced by a lack of spontaneous contractions (diameter oscillations < 0.02 mm), the protocol described above was repeated, and diameter was recorded at each pressure step. Vessel segments from the second vessel were used only when one of the segments (from either UP or DN groups) from the first vessel failed to exhibit spontaneous contraction.
Data analysis.
Using the lymphatic diameter recorded during the experiment, several variables characterizing lymphatic function were calculated. Functional variables used for analysis included passive diameter, diastolic diameter, systolic diameter, contraction frequency, ejection fraction, stroke volume, normalized pump index, and conduit index. All volume variables were reported per unit length of the lymphatic vessel segment with the assumption of uniform diameter change during contraction. Frequency was recorded as the number of contractions per minute. Ejection fraction was calculated as the ratio of stroke volume to diastolic volume. The pump index, a measure of active flow generated by the vessel, was calculated as the product of contraction frequency and stroke volume normalized to passive volume. The pump index characterizes the ability of a lymphangion to propel lymph and can be viewed as the number of passive volumes per minute. The conductance index for a vessel was calculated as the ratio of fourth power of the active radius, which was determined as the average measured radius during the 3-min measurement period, to the fourth power of the passive radius. The conduit index, a measure of conductance (inverse of resistance), characterizes the ability of a lymphangion to passively conduct lymph. Only one UP/DN segment pair per cow was analyzed. In the rare event that more than one vessel segment pair exhibited spontaneous contraction, only the first completed measured pair was chosen for analysis. All data are presented as means ± SE. All statistical tests were performed using the SVS software package (SVS Institute, Cary, NC). Statistical significance was determined using mixed-model ANOVA designed for a crossover experimental model. This test was performed on the differences between parameter values from UP and DN segments dissected from the same lymphatic vessel. There was no pairing comparison between segments from different vessels. Calculated P values of <0.05 were considered significant.
RESULTS
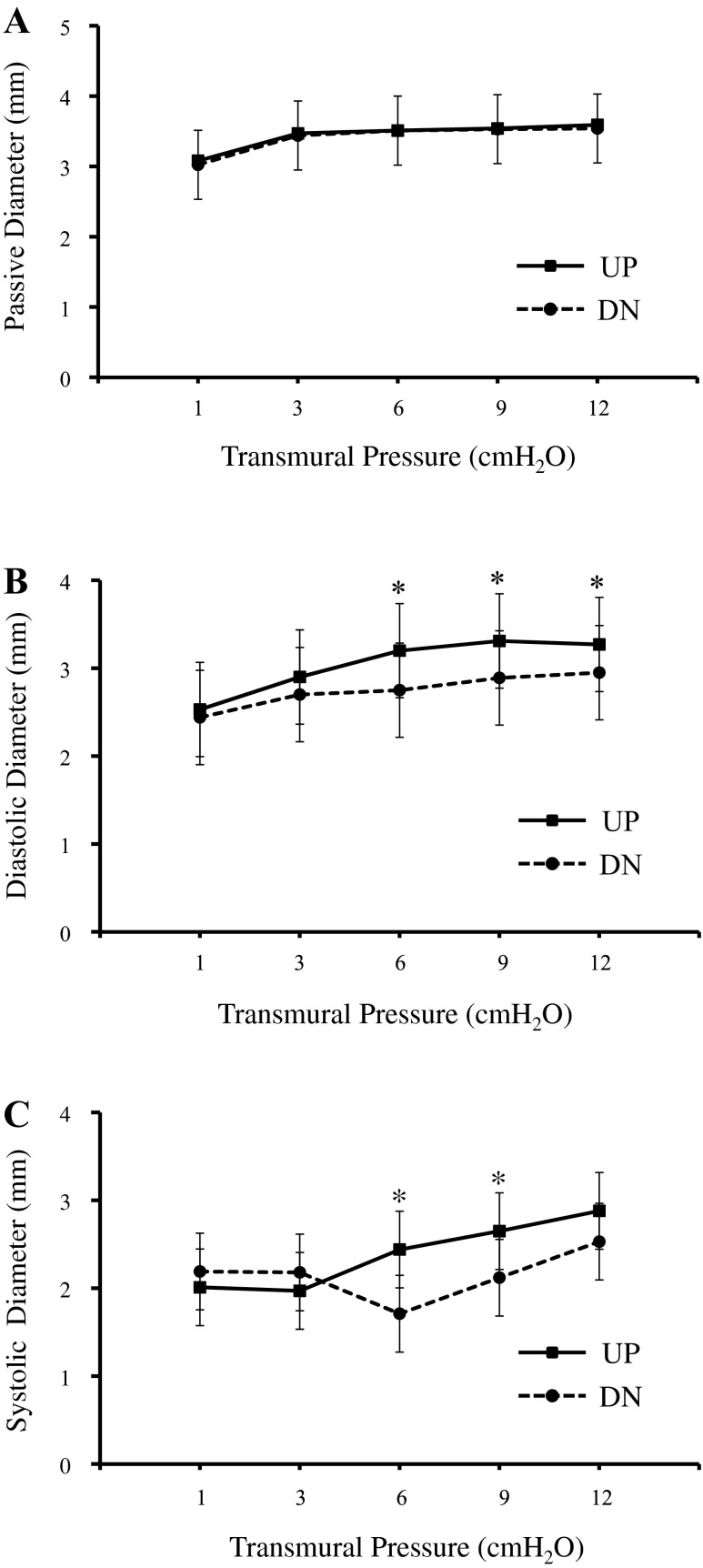
Eleven vessel segments from the UP group and eight vessel segments from the DN group exhibited consistent spontaneous contractions after the initial equilibration period. Only data from paired vessel segments (UP vs. DN group, n = 7, one pair from each cow) were analyzed to evaluate functional variables. A vessel segment pair consisted of segments from the same vessel, one vessel segment from the UP group and one from the DN group. Table 1 shows the functional variables for lymphatic vessel segments from the UP and DN groups. Contraction frequency and stroke volume of the UP group were not significantly different from those of the DN group at any transmural pressure. Ejection fractions of the two groups were not different except at a transmural pressure of 3 cmH2O, where the UP group had significantly higher ejection fraction than the DN group. Passive, diastolic, and systolic diameters of the UP and DN groups are shown in Fig. 1, A–C. Passive diameters of the UP and DN groups were not different. However, both systolic and diastolic diameters of the UP group were larger than those from the DN group at transmural pressures of 6, 9, and 12 cmH2O.
Table 1.
Lymphatic functional variables, including contraction frequency, stroke volume, and ejection fraction, for lymphatic vessel segments from the upstream and downstream groups
| Transmural Pressure |
|||||
|---|---|---|---|---|---|
| Variable | 1 cmH2O | 3 cmH2O | 6 cmH2O | 9 cmH2O | 12 cmH2O |
| Vessels in the upstream group | |||||
| Contraction frequency, contractions/min | 4.0 ± 1.37 | 4.5 ± 1.37 | 5.5 ± 1.37 | 6.8 ± 1.39 | 7.7 ± 1.35 |
| Stroke volume, mm2 | 2.94 ± 1.82 | 4.29 ± 1.82 | 4.00 ± 1.82 | 3.68 ± 1.83 | 2.12 ± 1.80 |
| Ejection fraction | 0.21 ± 0.09 | 0.52 ± 0.09* | 0.47 ± 0.09 | 0.37 ± 0.09 | 0.19 ± 0.09 |
| Vessels in the downstream group | |||||
| Contraction frequency, contractions/min | 4.2 ± 1.37 | 3.9 ± 1.37 | 6.2 ± 1.37 | 6.8 ± 1.39 | 6.9 ± 1.38 |
| Stroke volume, mm2 | 0.75 ± 1.82 | 2.14 ± 1.86 | 5.17 ± 1.82 | 4.19 ± 1.83 | 1.48 ± 1.32 |
| Ejection fraction | 0.18 ± 0.09 | 0.28 ± 0.09 | 0.57 ± 0.09 | 0.44 ± 0.09 | 0.22 ± 0.09 |
Values are means ± SE.
Significant difference between upstream and downstream groups (P < 0.05).
Fig. 1.
Vessel segments from the upstream (UP) and downstream (DN) groups had similar passive diameters (A). However, vessel segments from the UP group had larger diastolic diameters (B; at transmural pressures of 6, 9, and 12 cmH2O) and systolic diameters (C; at transmural pressures of 6 and 9 cmH2O). *Significant difference between UP and DN groups (P < 0.05).
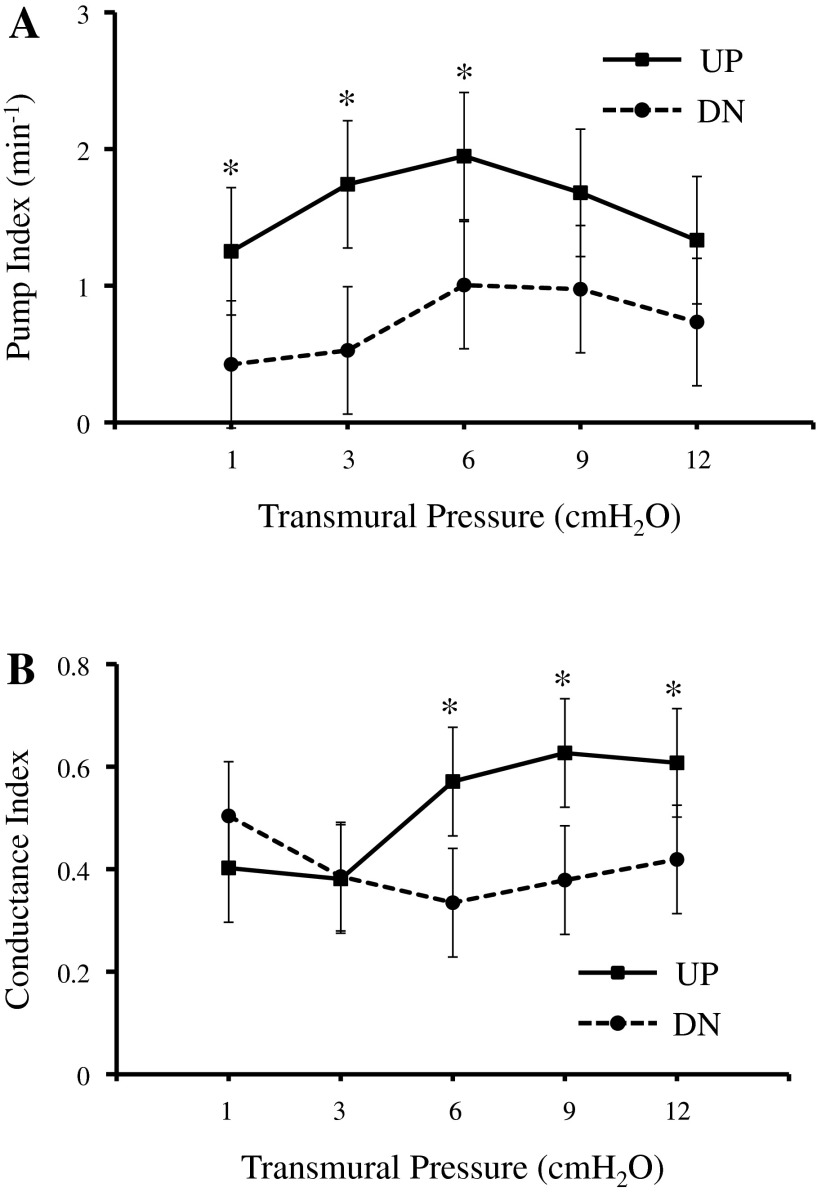
Figure 2 shows the pump index and conductance index of the UP and DN groups. The pump index of the UP group was significantly higher than that of the DN group at transmural pressures of 1, 3, and 6 cmH2O. The conductance index of the UP group was significantly higher than that of the DN group at transmural pressures of 6, 9, and 12 cmH2O.
Fig. 2.
A: the normalized pump index, a measure of ability to actively propel lymph, of UP vessel segments was higher than that of DN vessel segments at transmural pressures of 1, 3, and 6 cmH2O. B: the conductance index, a measure of conduit behavior, of UP vessel segments was higher than that of DN vessel segments at transmural pressures of 6, 9, and 12 cmH2O. *Significant difference between UP and DN groups (P < 0.05).
DISCUSSION
Summary.
The results from the present study indicate that postnodal mesenteric lymphatic vessels become stronger pumps when exposed to prolonged increases in transmural pressure. Specifically, the pump index (Fig. 2A) of lymphatic vessel segments from upstream of the constriction was higher than that of downstream segments. Such a response is consistent with the physiological necessity for negative feedback, ultimately decreasing interstitial pressure and ameliorating the increase in interstitial fluid volume (4). The adaptive responses first reported in the present work are thus consistent with the accepted understanding of interstitial volume regulation (8).
Strengths of the experimental model.
The difficulty in studying adaptation of blood vessels in vivo arises from the complication that blood pressure, blood flow, and endothelial shear stress have all been recognized as variables that stimulate growth and remodeling (26, 27, 41), and surgical interventions can alter all three stimuli. This difficulty also arises when studying lymphatic vessel adaptation. Gashev et al. (12) reported that cervical mesenteric vessels chronically adapt in rats subjected to a head-down tilt, which was believed to increase their transmural pressures and decrease their lymph flow rates. However, the individual contributions of changes in pressure and flow could not be identified. We therefore designed this study so that the effects of pressure could be independently characterized by comparing vessel segments above and below a partial constriction. The partial constriction raises one segment pressure higher than the other, whereas mean flow and lymph composition are the same in both segments (see appendix a). An additional strength of this model is that comparison of vessel segments above and below a constriction provides an ideal analysis: segments not only come from the same animal but also from the same vessel. They thus have the same initial structure, functions, and environment as well as developmental history. As a result, the statistical comparisons significantly minimize the model-based variations and highlight only the effects of the stimulus (the partial occlusion). More importantly, this model allows a comparison of absolute values of systolic and diastolic diameter as well as absolute stroke volumes and thus avoids the common practice of normalizing all functional variables by a particular diameter (11, 12). A similar statistical comparison cannot be performed with any two separate lymphatic vessels even if they are from the same region of the same animal. Moreover, interventions such as vessel ligation will most likely redistribute lymph flow to other nonligated vessels. As a result, it would be difficult to select a lymphatic vessel within the interest region to perform as a control. Nonetheless, we have provided appendix b, which describes the analysis all three groups: control, occluded UP, and occluded DN. In this comparison, mixed-model ANOVA designed for a crossover experimental model was used. Finally, although the cost and complexity of performing chronic experiments in large animals have made such experiments relatively rare, the cow model has the additional strength of allowing the comparison of our results to fairly extensive literature detailing the acute function of bovine postnodal mesenteric lymphatic vessels typically collected from an abattoir (20, 23, 24, 29, 30).
Inherent limitations of the animal model.
There are fundamental limitations to the animal model that result directly from complex physiological events as well as a lack of specifically developed technology for lymphatic research. First, a 3-day timeframe for short-term adaptation of lymphatic vessels was chosen because this is the period after the intervention that we expected no significant lymphangiogenesis to occur (5, 33). To study the effect of longer-term adaptation, this animal model needs further modification and investigation to ensure there is no complex interaction between lymphatic adaptation and other secondary factors resulting from the main insult. Second, it is not possible to measure values of critical parameters such as transmural pressure and luminal flow during the 3-day adaptation period without significantly altering them. Instrumentation of the lumen of either the UP or DN segment always significantly alters normal valve closure, transmural pressures, luminal flows, and endothelial shear stresses with current technology. Moreover, even the smallest pressure catheters are not as flexible as lymphatic vessels, and the remarkably thin wall of lymphatic vessels makes them quite easy to perforate. However, we used previously reported bovine mesenteric lymphatic vessel lymph flow and pressure data to validate our experimental model, resulting in higher transmural pressure in the upstream lymphatic segment compared with that of the lower segment (appendix a). We estimated a difference of ∼8 cmH2O across the occlusion with normal bovine lymphatic transmural pressures of 4–6 cmH2O (24). Because this difference of 8 cmH2O is distributed between an in increase in upstream pressure and a decrease in downstream pressure, it is in the expected physiological range, given the reported change in interstitial pressure (25) and cisternae chyli pressure (40).
Potential sources of variation.
There were small, but detectable, variations in diameter along the length of the vessels during contraction, which were not recorded by our diameter-tracking device. All of these variations are expected to induce no bias in comparison groups but may have decreased the ability to detect differences in some variables between the comparison groups. It was possible, however, to detect a significant difference in pump index when nonsignificant differences in contraction frequencies and stroke volumes were combined (Fig. 2, A and B). The ability to detect functional differences was improved by our ability to compare segments from the same vessel.
Implications to the regulation of interstitial fluid volume.
Contrary to expectations, lymphatic vessels exposed to prolonged increases in pressure become both better pumps (characterized by the pump index) and better conduits (characterized by the conduit index). Compared with vessel segments downstream from the occlusion, UP vessel segment did not contract at a different frequency (Table 1). Instead, the UP segment generated an evidently larger stroke volume by increasing end-diastolic diameter while maintaining end-systolic diameter. Describing this adaptive response in terms of cardiac analogies is somewhat problematic. On the one hand, this increase in end-diastolic diameter is similar functionally to increased lusitropy, although the lymphangions were not fully relaxed in diastole. In fact, passive diameters of UP segments were not different than DN segments. Perhaps more appropriate a description, increased pumping ability may be best described as negative diastolic inotropy. Nonetheless, the increased pumping ability becomes apparent when multiplying stroke volume by frequency of each vessel (Fig. 2A). At the same time, the increase in end-diastolic diameter of upstream vessel segments simultaneously created a significant reduction in the resistance to luminal flow of the vessels, leading to an increase in conductance index (Fig. 2B). Interestingly, our result shows that the adaptation to better pumps of lymphatic vessels is most evident at lower pressure ranges, whereas the adaptation to better conduit is clearer at higher pressure ranges.
Implications to disease states.
Designed primarily to elucidate the adaptive responses of lymphatic responses to altered transmural pressure, the present study nonetheless reproduces conditions that may arise from surgical obstruction. Because the adaptive response was studied only 3 days after partial constriction, it represents the first of several chronic responses, including further growth and remodeling and lymphangiogenesis. Chronic responses are particularly of interest, because surgical obstruction is believed to generate secondary lymphedema years after surgical obstruction. Although it is hazardous to extrapolate the findings of the present study to predict adaptive responses so far removed from original insult, two findings may guide future experimental work. First, although the UP vessel became a better pump relative to the DN vessel, partial occlusion may have led to a decline in function of both UP and DN segments, reflected in the significant difference in stroke volume and pump index compared with control (appendix b). Whether this decrease in function represents incomplete adaptation within a short time span or a persistent decrease in function remains unknown. Second, the increase in pumping ability of the UP vessel was accomplished primarily through enhanced relaxation during diastole. Although passive diameters may have increased somewhat (compared with control), the ability to increase diastolic diameter may be limited. Short-term adaptation, therefore, may lead to a loss of future adaptive capacity. Taken together, a decrease in function and a loss of adaptive capacity may predispose patients to secondary lymphedema after a new edemagenic challenge. Both could possibly increase the acute and chronic edemagenic gain (9), the sensitivity of interstitial fluid volume to a change in capillary pressure. Future studies relating chronic lymphatic adaptive responses to changes in edemagenic gain are certainly justified by the present study.
Potential mechanisms.
Multilevel analysis focusing on molecular, cellular, and histological changes is required to identify the mechanisms underlying the observed changes in lymphatic function. A prolonged increase in transmural pressure may have resulted in numerous alterations as diverse as altered expression of contractile proteins, apoptosis, and fibrosis (16, 18, 26). With such a diversity of potential mechanisms altering lymphangion function on the one hand, and the lack of any published data on how lymphangion function adapts to changes in pressure on the other, it was not possible to design a hypothesis-driven experiment to elucidate mechanisms of adaptation. Before investigating molecular, cellular, and histological mechanisms of adaptation to different environments, the purpose of the present study was to first determine if lymphatic vessels exhibit functional adaptation in response to the chronic stimuli induced by partial ligation and to quantify those effects. The present work provides the necessary basis to formulate hypothesis-driven experiments in future studies.
Appendix A: VALIDATION OF A PRESSURE GRADIENT ACROSS THE PARTIAL OCCLUSION
Using the Hagen-Poiseuille equation for a pressure drop in a fluid flowing through a cylindrical lumen, the pressure gradient (ΔPOC) across the occlusion could be calculated using the value of in vivo lymph flow (Qlymph), as follows:
| A1 |
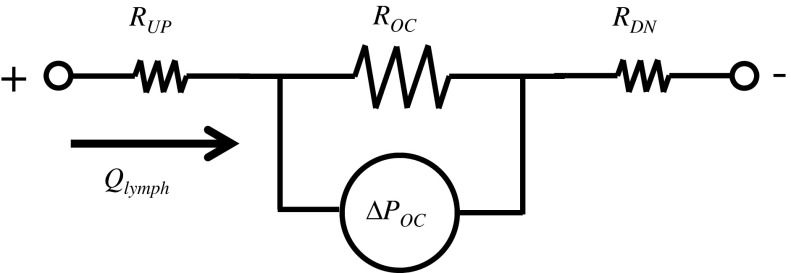
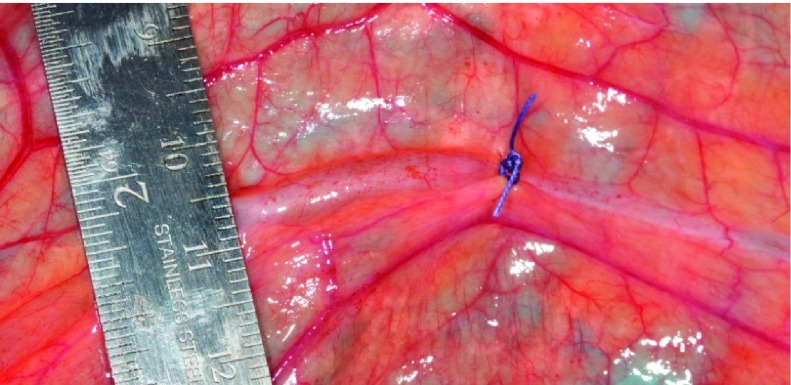
where μ is lymph viscosity, L is the length of the occlusion and equals the diameter of a 2-0 suture, and r is the radius at the occlusion and equals the sum of the radius of a 20-gauge needle and the vessel wall thickness. An analogous electrical circuit to the pressure-flow relationship of a partially occluded lymphatic vessel is shown in Fig. A1. Table A1 shows the results of calculated ΔPOC at transmural pressures of 1, 3, and 6 cmH2O. At each transmural pressure level, the value for r was chosen from our in vitro measured radius at the correspondent pressure. Values for Qlymph were obtained from a study (24) where average lymph flow was measured with no axial pressure gradient (i.e., inlet pressure is equal to outlet pressure) at different transmural pressures. Estimates of ΔPOC of the partially occluded vessels at 1 and 6 cmH2O were 2.93 ± 2.35 and 8.08 ± 5.61 cmH2O, respectively. Since normal bovine lymphatic transmural pressure is 4–6 cmH2O, these pressure gradients across the occlusion would be sufficient to substantially increase pressure in the UP vessel segment relative to the DN segment. The effect of said increases in pressure in vivo was also visually apparent immediately after the occlusion. Figure A2 shows the relative sizes of UP and DN segments of one lymphatic vessel a few minutes after the partial occlusion.
Fig. A1.
An analogous electrical circuit to the pressure-flow relationship of a partially occluded lymphatic vessel. RUP and RDN are lymph resistances in UP and DN segments from the occlusion of the lymphatic vessel. ROC represents the resistive force to lymph flow created by the partial occlusion. ΔPOC is the pressure gradient across the occlusion section. ΔPOC can determined by ROC and lymph flow (Qlymph ) through the occlusion as follows: ΔPOC = ROC × Qlymph.
Table A1.
Estimations of the pressure gradient created by partial occlusion in vivo
| Transmural Pressure |
|||
|---|---|---|---|
| 1 cmH2O | 3 cmH2O | 6 cmH2O | |
| Qlymph, ml/min | 0.38 | 1.15 | 1.38 |
| ΔPOC, cmH2O | 2.93 ± 2.35 | 8.91 ± 6.34 | 8.08 ± 5.61 |
Average lymph flows (Qlymph) at 1, 3, and 6 cmH2O were obtained from an in vitro study by McHale et al. (24), where lymphatic vessels pumped fluid at zero pressure gradient. Pressure gradients across the occlusion (ΔPOC) at transmural pressures of 1, 3, and 6 cmH2O were calculated using the Hagen-Poiseuille equation.
Fig. A2.
A bovine mesenteric lymphatic vessel immediately after being partially occluded. The UP segment to the occlusion (left) was substantially expanded compared with the DN segment (right) due to accumulated lymph.
Appendix B: THREE-GROUP ANALYSIS OF LYMPHATIC FUNCTIONAL VARIABLES
Statistical analysis.
The total number of vessels was 27 (8 vessels in the control group, 11 vessels in the UP group, and 8 vessels in the DN group). Statistical differences were determined by mixed-model ANOVA designed for a crossover experimental model, with significance designated at P < 0.05. All statistical tests were performed in SAS (SAS Institute). Analyzed parameters included passive diameter, diastolic diameter, contraction frequency, ejection fraction, stroke volume, pump index, and conduit index. To minimize the effect of size when vessels from different groups wer compared, normalized diastolic diameter, obtained by dividing diastolic diameter by its respective passive diameter, and normalized stoke volume, obtained by dividing stroke volume by its respective passive volume, were used for analyses. All volume variables were reported per unit length of the lymphatic vessel segment. Frequency was recorded as the number of contractions per minute. Ejection fraction was calculated as a fraction of diastolic volume. The pump index was calculated as the product of contraction frequency and normalized stoke volume. The pump index was used to characterize pump behavior. The conductance index for a vessel was calculated as the ratio of average instantaneous conductance during contraction to passive conductance. The conductance index was used to characterize conduit behavior and had a value of 1 when it was completely relaxed.
Results.
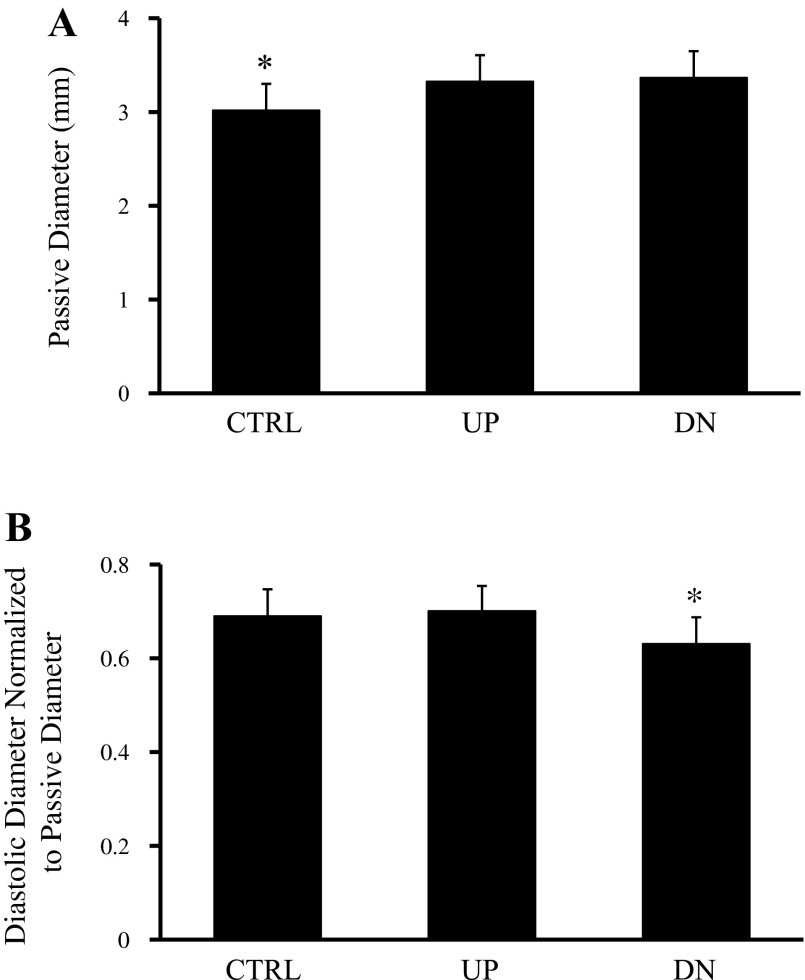
Figure B1A shows passive diameters of control vessels, and occluded UP vessels and occluded DN vessels when exposed to Ca2+-free solution. Passive diameters of the vessels from the control group (3.02 ± 0.28 mm) were smaller than passive diameters of the vessels from either occluded vessel groups, both UP (3.33 ± 0.28 mm, P < 0.05) and DN (3.37 ± 0.28 mm, P < 0.05). Normalized diastolic diameters for the vessels from the three groups are shown in Fig. A1B. Normalized diastolic diameters of vessels from the control group (0.69 ± 0.057) and UP group (0.70 ± 0.053) were not significantly different. However, normalized diastolic diameters of vessels from the DN group (0.63 ± 0.056, P < 0.05) were significantly lower than those of vessels from the control and UP groups.
Fig. B1.
Passive diameters of vessels from the control (CTRL) group and occluded vessel groups upstream and downstream from the ligation. A: passive diameters of vessels from both UP and DN groups were larger than passive diameters of vessels from the CTRL group. B: normalized diastolic diameters of vessels from the CTRL, occluded UP group, and occluded DN groups. Normalized diastolic diameters (diastolic diameter divided by the respective passive diameter) for vessels from the CTRL and UP groups were not significantly different. However, normalized diastolic diameters of vessels from the DN group were significantly lower than those of vessels from the CTRL and UP group. *Significantly different from the other two groups (P < 0.05).
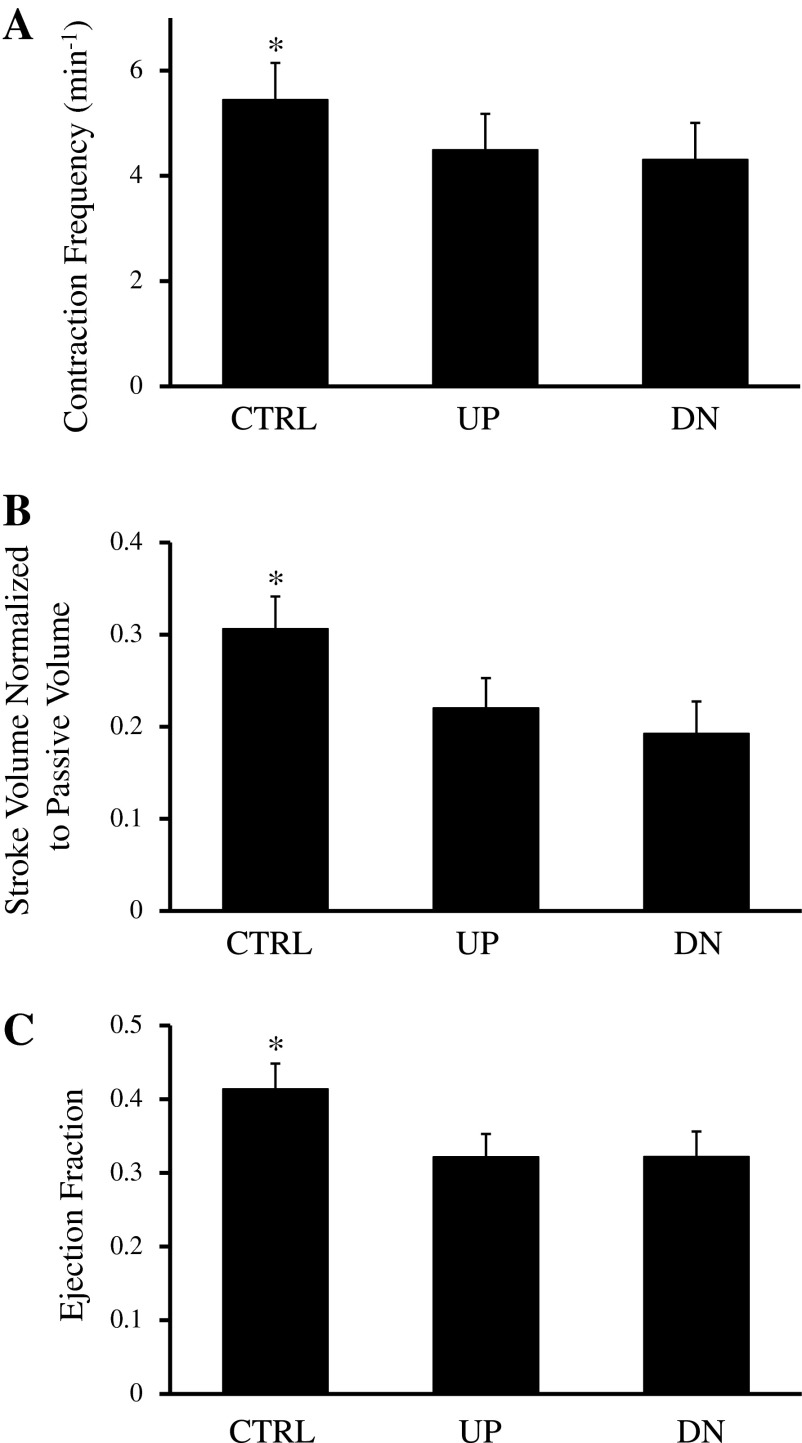
Figure B2 shows functional variables for vessels from control group, occluded UP group, and occluded DN group. Contraction frequency (Fig. B2A), normalized stroke volume (Fig. B2B), and ejection fraction (Fig. B2C) of vessels from the UP group (contraction frequency: 4.49 ± 0.68 contractions/min, normalized stroke volume: 0.22 ± 0.032, and ejection fraction: 0.32 ± 0.031) were not significantly different than those of vessels from the DN group (contraction frequency: 4.31 ± 0.69 contractions/min, normalized stroke volume: 0.19 ± 0.035, and ejection fraction: 0.32 ± 0.034). However, vessels from the control group had higher contraction frequency (5.45 ± 0.70, P < 0.05), normalized stroke volume (0.31 ± 0.035, P < 0.05), and ejection fraction (0.41 ± 0.034, P < 0.05) than those of vessels from either occluded vessel groups.
Fig. B2.
Contraction frequency (A), normalized stroke volume (B), and ejection fraction (C) of vessels from the CTRL (control group), occluded UP, and occluded DN groups. Vessels from the CTRL group had a higher contraction frequency, normalized stroke volume, and ejection fraction than vessels from the UP and DN groups. Contraction frequency, normalized stroke volume, and ejection fraction from the UP and DN groups were not significantly different. *Significantly different from the other two groups (P < 0.05).
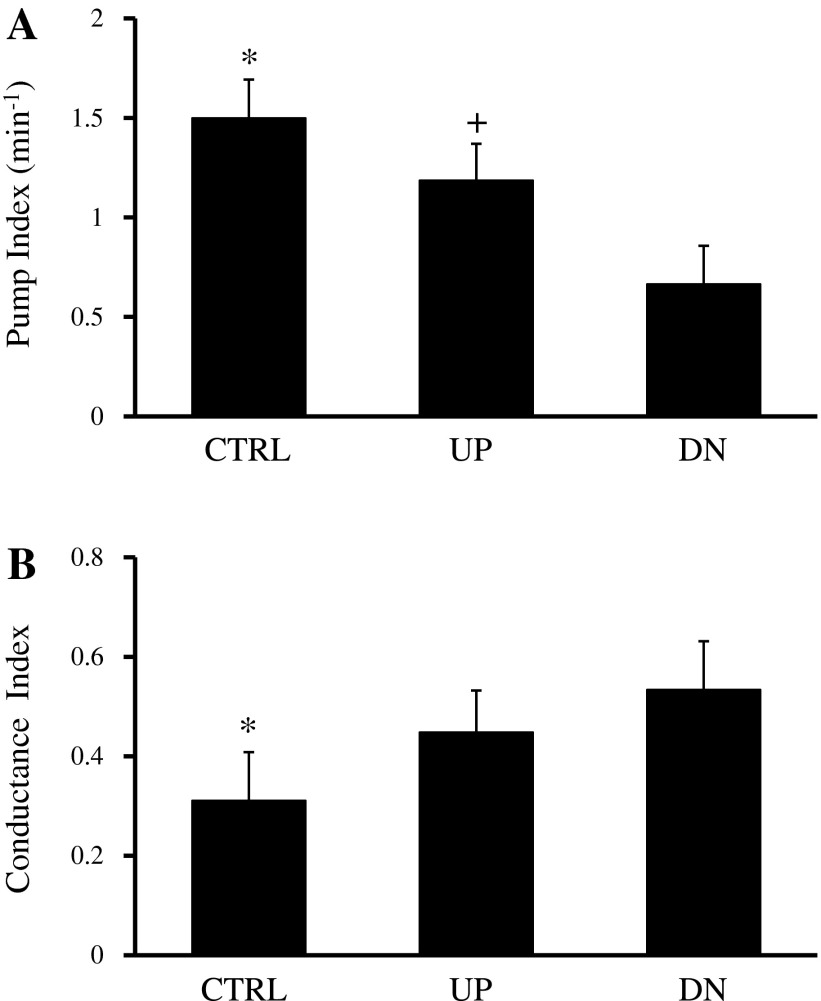
Figure B3 shows the pump index and conductance index, characterizing pump and conduit behavior, of the control, UP, and DN groups in stepped-up transmural pressure. Pump indexes for vessels from all three groups (control group: 1.50 ± 0.19 min−1, UP group: 1.19 ± 0.18 min−1, and DN group: 0.67 ± 0.19 min−1, P < 0.05) were significantly different from each other (Fig. B3A). Conductance indexes were significantly higher for vessels in the UP group (0.45 ± 0.083, P < 0.05) and DN group (0. 53 ± 0.096, P < 0.05) compared with vessels in the control group (0.31 ± 0.095; Fig. B3B).
Fig. B3.
Pump index (A) and conductance index (B), a measure of conduit behavior, of CTRL (control group), UP, and DN groups. The conductance index for a vessel was calculated from the ratio of average instantaneous conductance during contraction to passive conductance and had a value of 1 when it was completely relaxed. The pump index of vessels from the UP group was significantly higher than that of vessels from the DN group, whereas the conduit index was higher for vessels in the UP and DN groups than that of vessels in the CTRL group. *Significantly different from the other two groups (P < 0.05); +significantly different from the DN group (P < 0.05).
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-092916 (to R. H. Stewart and E. Wilson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.M.D., T.L.N., C.M.Q., G.A.L., E.W., and R.H.S. conception and design of research; R.M.D., T.L.N., and J.H. performed experiments; R.M.D., T.L.N., and R.H.S. analyzed data; R.M.D., T.L.N., C.M.Q., G.A.L., E.W., and R.H.S. interpreted results of experiments; R.M.D. and T.L.N. prepared figures; R.M.D. and T.L.N. drafted manuscript; R.M.D., T.L.N., C.M.Q., G.A.L., E.W., and R.H.S. edited and revised manuscript; R.M.D., T.L.N., C.M.Q., J.H., G.A.L., E.W., and R.H.S. approved final version of manuscript.
REFERENCES
- 1. Allen J, Iqqulden H, McHale N. β-Adrenergic inhibition of bovine mesenteric lymphatics. J Physiol 376: 401–411, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen J, Rooney B, McHale N. Effect of norepinephrine on contractility of isolated mesenteric lymphatics. Am J Physiol Heart Circ Physiol 244: H479-–H486., 1983 [DOI] [PubMed] [Google Scholar]
- 3. Aukland K, Nicolaysen G. Interstitial fluid volume: local regulatory mechanisms. Physiol Rev 61: 556–643, 1981 [DOI] [PubMed] [Google Scholar]
- 4. Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol Heart Circ Physiol 257: H2059–H2069, 1989 [DOI] [PubMed] [Google Scholar]
- 5. Boardman KC, Swartz MA. Interstitial flow as a guide for lymphangiogenesis. Circ Res 92: 801–808, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Buus NH, Kahr O, Mulvany MJ. Effect of short- and long-term heart failure on small artery morphology and endothelial function in the rat. J Cardiovasc Pharmacol 34: 34–40, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Campbell T, Heath T. Intrinsic contractility of lymphatics in sheep and in dogs. Q J Exp Physiol Cogn Med Sci 207–217, 1973 [DOI] [PubMed] [Google Scholar]
- 8. Dongaonkar RM, Laine GA, Stewart RH, Quick CM. Balance point characterization of interstitial fluid volume regulation. Am J Physiol Regul Integr Comp Physiol 297: R6–R16, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erdmann AJ, 3rd, Vaughan TR, Jr, Brigham KL, Woolverton WC, Staub NC. Effect of increased vascular pressure on lung fluid balance in unanesthetized sheep. Circ Res 37: 271–284, 1975 [DOI] [PubMed] [Google Scholar]
- 10. Fadnes HO. Effect of increased venous pressure on the hydrostatic and colloid osmotic pressure in subcutaneous interstitial fluid in rats: edema-preventing mechanisms. Scand J Clin Lab Invest 36: 371–377, 1976 [DOI] [PubMed] [Google Scholar]
- 11. Gashev AA, Davis MJ, Zawieja DC. Inhibition of active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540: 1023–1037, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gashev AA, Delp MD, Zawieja DC. Inhibition of active lymph pump by simulated microgravity in rats. Am J Physiol Heart Circ Physiol 290: H2295–H2308, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Gashev AA, Wang W, Laine GA, Stewart RH, Zawieja DC. Characteristics of the active lymph pump in bovine prenodal mesenteric lymphatics. Lymphat Res Biol 5: 71–79, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Gashev AA, Zawieja DC. Physiology of human lymphatic contractility: a historical perspective. Lymphology 34: 124–134, 2001 [PubMed] [Google Scholar]
- 15. Hall JG, Morris B, Woolley G. Intrinsic rhythmic propulsion of lymph in the unanaesthetized sheep. J Physiol 180: 336–349, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 38: 581–587, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Laine GA, Allen SJ, Katz J, Gabel JC, Drake RE. Effect of systemic venous pressure elevation on lymph flow and lung edema formation. J Appl Physiol 61: 1634–1638, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med 259: 381–392, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Mawhinney HJ, Roddie IC. The effect of catecholamines on the spontaneous contractions in bovine mesenteric lymphatics. J Physiol 219: 34P–35P, 1971 [PubMed] [Google Scholar]
- 20. Mawhinney HJ, Roddie IC. Spontaneous activity in isolated bovine mesenteric lymphatics. J Physiol 229: 339–348, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maximow AA, Bloom W. A Textbook of Histology. Philadelphia, PA: Saunders, 1947 [Google Scholar]
- 22. McGeown JG, McHale NG, Thornbury KD. The role of external compression and movement in lymph propulsion in the sheep hind limb. J Physiol 387: 83–93, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McHale N, Roddie I. Pumping activity in isolated segments of bovine mesenteric lymphatics. J Physiol 244: 70–72, 1975 [PubMed] [Google Scholar]
- 24. McHale NG, Roddie IC. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol 261: 255–269, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mortillaro NA, Taylor AE. Interstitial fluid pressure of ileum measured from chronically implanted polyethylene capsules. Am J Physiol Heart Circ Physiol 257: H62–H69, 1989 [DOI] [PubMed] [Google Scholar]
- 26. Mulvany M. Vascular remodelling in hypertension. Eur Heart J 14: C2–4, 1993 [DOI] [PubMed] [Google Scholar]
- 27. Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, Heistad DD. Vascular remodeling. Hypertension 28: 505–506, 1996 [PubMed] [Google Scholar]
- 28. Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J 17: 920–922, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Ohhashi T, Azuma T, Sakaguchi M. Active and passive mechanical characteristics of bovine mesenteric lymphatics. Am J Physiol Heart Circ Physiol 239: H88–H95, 1980 [DOI] [PubMed] [Google Scholar]
- 30. Ohhashi T, Kawai Y, Azuma T. The response of lymphatic smooth muscles to vasoactive substances. Pflügers Arch 375: 183–188, 1978 [DOI] [PubMed] [Google Scholar]
- 31. Oliver G. Lymphatic vasculature development. Nat Rev Immunol 4: 35–45, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Olszewski WL, Engeset A. Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. Am J Physiol Heart Circ Physiol 239: H775–H783, 1980 [DOI] [PubMed] [Google Scholar]
- 33. Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, Skobe M, Boardman KC, Swartz MA. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst 97: 14–21, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Quick CM, Ngo BL, Venugopal AM, Stewart RH. Lymphatic pump-conduit duality: contraction of postnodal lymphatic vessels inhibits passive flow. Am J Physiol Heart Circ Physiol 296: H662–H668, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Quick CM, Venugopal AM, Gashev AA, Zawieja DC, Stewart RH. Intrinsic pump-conduit behavior of lymphangions. Am J Physiol Regul Integr Comp Physiol 292: R1510–R1518, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Rockson SG. Lymphedema. Am J Med 110: 288–295, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Sastre E, Balfagon G, Revuelta-Lopez E, Aller MA, Nava MP, Arias J, Blanco-Rivero J. Effect of short- and long-term portal hypertension on adrenergic, nitrergic and sensory functioning in rat mesenteric artery. Clin Sci (Lond) 122: 337–348, 2012 [DOI] [PubMed] [Google Scholar]
- 38. Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol Rev 70: 987–1028, 1990 [DOI] [PubMed] [Google Scholar]
- 39. Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 21: 2422–2432, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stewart RH, Laine GA. Flow in lymphatic networks: interaction between hepatic and intestinal lymph vessels. Microcirculation 8: 221–227, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Stone PH, Coskun AU, Kinlay S, Clark ME, Sonka M, Wahle A, Ilegbusi OJ, Yeghiazarians Y, Popma JJ, Orav J, Kuntz RE, Feldman CL. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation 108: 438–444, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Venugopal A, Stewart R, Laine G, Dongaonkar R, Quick C. Lymphangion coordination minimally affects mean flow in lymphatic vessels. Am J Physiol Heart Circ Physiol 293: H1183–H1189, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Yokoyama S, Ohhashi T. Effects of acetylcholine on spontaneous contractions in isolated bovine mesenteric lymphatics. Am J Physiol Heart Circ Physiol 264: H1460–H1464, 1993 [DOI] [PubMed] [Google Scholar]