Abstract
Exercise training (ExT) normalizes the increased sympathetic outflow in chronic heart failure (HF). The underlying mechanisms are not clearly understood. We hypothesized that ExT normalized the blunted central component of the baroreflex control of renal sympathetic nerve activity (RSNA) in HF. Four groups of rats [sham operated (sham)-sedentary (Sed), sham-ExT, HF-Sed, and HF-ExT] were used. HF was induced by left coronary artery ligation, and ExT consisted of 3 wk of treadmill running. In anesthetized rats, the decrease in RSNA in response to aortic depressor nerve stimulation (5–40 Hz) in the HF-Sed group was significantly lower than that in the sham-Sed group (−37 ± 7% vs. −63 ± 8% at 40 Hz, P < 0.05). In the HF-ExT group, responses in RSNA, mean arterial pressure (MAP), and heart rate (HR) were not significantly different from those in the sham-Sed or sham-ExT groups. ExT normalized blunted RSNA, MAP, and HR responses to bicuculline microinjections into the paraventricular nucleus (PVN) in rats with HF. Activation of the PVN by blockade of GABA receptors with bicuculline in normal control rats blunted the centrally component of the baroreflex arc. GABAA-α1 and -β1 receptor protein expression were significantly lower (by 48% and 30%) in the HF-Sed group, but ExT normalized this difference between the HF and sham groups. These data suggest that one mechanism by which ExT alleviates elevated sympathetic outflow in HF may be through normalization of central integrative mechanisms, perhaps via improving the inhibitory GABAergic mechanism within the PVN, on the baroreflex arc.
Keywords: paraventricular nucleus, aortic depressor nerve, blood pressure, sympathetic outflow, γ-aminobutyric acid receptor
a salient feature of heart failure (HF) is increased sympathoexcitation, which correlates with the severity of the disease as well as complications and mortality (31). The source of the increased sympathoexcitation associated with HF is not entirely understood. Although several lines of evidence point to a role for altered baroreflex function as a possible cause for the increased sympathoexcitation (53), other studies have pointed against this contention (3, 22). This contrary evidence is based on the observation that baroreceptor denervation does not prevent the increase in sympathoexcitation. Sinoaortic (3) or cardiovagal denervation (22) does not normalize plasma norepinephrine (NE) levels in denervated compared with intact HF dogs. Moreover, plasma NE is also not altered in HF dogs by β-adrenergic blockade (22). However, a study (1) in hypertensive rats, monitoring renal sympathetic nerve activity (RSNA) in conscious animals over a period of weeks, has shown that there is a tonic baroreceptor-mediated component in sympathoexcitation during hypertension. A similar contribution of an altered central baroreceptor mechanism in HF may contribute to the increased sympathoexcitation. Furthermore, stimulation of the aortic depressor nerve (ADN) produced altered lumbar sympathetic nerve activity in rats with HF compared with sham rats (14). These data indicate that an alteration within the central nervous system is one of the causes for the increased sympathoexcitation observed in HF (3, 14, 22).
Within the central nervous system, there are various hierarchical sites that are known to participate in the generation of sympathetic tone. One of the highest among them is the paraventricular nucleus (PVN) of the hypothalamus (41). The PVN is reciprocally connected to various other areas of the brain stem that are known to be involved in the control of cardiovascular function (16). The PVN also contains preautonomic neurons, which project to sympathetic preganglionic neurons within the intermediolateral cell column of the spinal cord both directly and indirectly via the rostral ventrolateral medulla (RVLM) (40). Previous data from this laboratory and others (36, 43, 47) have suggested that there is increased activity of PVN neurons associated with sympathoexcitation during HF. We also found that an altered GABAergic mechanism within the PVN may be involved in the regulation of sympathetic outflow in HF and that this altered inhibitory mechanism may contribute to the increased sympathetic nerve activity in this disease state (46). The increased activity within the PVN may be part of the altered central component of the baroreflex arc that results in increased overall sympathetic nerve activity in HF.
Generally, exercise training (ExT) has been shown to improve baroreflex sensitivity and reduce sympathetic outflow in both humans and rats (13, 19, 20, 27). ExT in HF patients increases survival, decreases complications, and decreases the overall neurohumoral drive, including muscle sympathetic nerve activity (39). In a rat coronary ligation model of HF, 3- to 4-wk ExT reduced plasma levels of NE and ANG II (18). Similarly, in a rapid pacing model of HF in rabbits, ExT decreased RSNA and plasma levels of NE to those of normal rabbits (23). ExT also increases arterial baroreflex sensitivity in HF rabbits and rats (23, 24, 33). However, the mechanism by which ExT normalizes sympathetic outflow in HF is not entirely known. In the present study, we hypothesized that one mechanism by which ExT restores sympathetic outflow to normal levels in HF is by normalizing the central GABAergic inhibitory mechanism within the PVN influencing the central component of the baroreflex response. Specifically, we tried to determine whether ExT attenuates blunted RSNA response to ADN stimulation and whether activation of the PVN, using the GABA antagonist bicuculline, blunts the RSNA response to ADN stimulation. Furthermore, we wanted to determine whether ExT improves the blunted GABAergic tone within the PVN of rats with HF.
METHODS
Animals
Male Sprague-Dawley rats weighing 220–250 g (Sasco Breeding Laboratories, Omaha, NE) were fed and housed according to institutional guidelines. Protocols were approved by the Institutional Animal Care and Use Committee of the University of Nebraska and were in accordance with the American Physiological Society's “Guiding Principles in the Care and Use of Laboratory Animals.” Rats were given rat chow and water ad libitum and were housed in a room with a 12:12-h light-dark cycle. Rats were allowed to acclimatize for 1 wk before cardiac surgery.
Induction of HF
Rats were randomly assigned to either a sham-operated (sham) control group or a HF group. HF was induced by ligation of the left coronary artery. Echocardiograms were performed (under a mild anesthetic condition with 3–4% isoflurane) on all groups of rats. Left ventricular (LV) dysfunction was assessed using hemodynamic and anatomic criteria. LV end-diastolic pressure (LVEDP) was measured using a Mikro-Tip catheter (Millar Instruments, Houston, TX) inserted into the LV via the right carotid artery at the time of the terminal experiment. Infarct size (in %) was determined by dividing the size of the infarcted area by the total size of the LV. Rats with elevated LVEDP (≥15 mmHg) and an infarct size > 30% of the total LV wall were considered to be in HF and used in this study.
ExT
Three weeks after coronary artery ligation surgery, rats were randomly assigned to either an ExT group or a sedentary (Sed) group to produce the following four total experimental groups: sham-Sed, HF-Sed, sham-ExT, and HF-ExT. For ExT, rats ran for a specific timed protocol on a motor-driven treadmill (Columbus Instruments, Columbus, OH) for a period of 3 wk according to a modified protocol of Musch and Terrell (18, 28). Initially, low speed (10 m/min) and grade (0%) and short duration (10 min/day) were used to familiarize the rats with running on the treadmill. The speed, duration, and grade were gradually increased to 20–25 m/min, 60 min/day, and 5–10%, respectively, to ensure that a significant endurance effect was produced. This level of exercise is considered moderate for sham rats. Only rats that ran steadily with little or no prompting were used in the study. Experiments were done at least 24 h after the last bout of ExT.
General Surgery for Hemodynamic and RSNA Measurements and Microinjection
Rats were anesthetized with urethane (0.75 g/kg ip) and α-chloralose (70 mg/kg ip) and instrumented for recordings of arterial pressure and heart rate (HR) as previously described (46). The left femoral vein was cannulated with polyethylene tubing (PE-50) for injection of supplemental anesthesia. The left femoral artery was cannulated and connected via a pressure transducer (Gould P23 1D) to a computer-based data recording and analyzing program (PowerLab, ADInstruments) to record mean arterial blood pressure (MAP) and HR.
The left kidney was exposed through a retroperitoneal flank incision. A branch of the renal nerve was isolated from fat and connective tissue. The central end of the nerve was placed on thin bipolar platinum electrodes. The nerve-electrode junction was fixed and electrically insulated from surrounding tissues with a Wacker Silgel (Wacker, St. Louis, MO) mixture. The electrical signal was amplified with a Grass amplifier with high- and low-frequency cutoffs of 1,000 and 100 Hz, respectively. The rectified output from the amplifier was displayed using the PowerLab system to record and integrate the raw nerve discharge. Basal nerve activity was determined at the beginning of the experiment, and background noise was determined by nerve activity recorded at the end of the experiment (after the rat was euthanized). Nerve activity during the experiment was calculated by subtracting the background noise from the recorded value. The RSNA response was expressed as the percent change from the basal value.
For the placement of microinjection cannulas into the PVN, anesthetized rats were placed in a stereotaxic apparatus (David Kopf Instruments, Tujanga, CA). A longitudinal incision was made on the head, and the bregma was exposed. A small burr hole was made in the skull to allow access to the PVN. The coordinates for the PVN, determined using the Paxinos and Watson atlas (37), were 1.5 mm posterior to the bregma, 0.4 mm lateral to the midline, and 7.8 mm ventral to the dura. A thin needle (0.2-mm outer diameter) connected to a 0.5-μl microsyringe (Hamilton, Reno, NV) was lowered into the PVN for microinjections.
The Methodological Approach to Stimulate the Left ADN
In urethane- and α-chloralose-anesthetized rats, a 4- to 6-mm length of the left ADN was isolated below its junction with the superior laryngeal nerve and placed on a bipolar platinum electrode with an interelectrode distance of 2 mm. Correct identification of the nerve was confirmed by its typical pattern of discharge synchronous with arterial pulse pressure. The ADN was covered with a Wacker Silgel mixture. A 30-min period was allowed for complete polymerization of the silicone impression material, and the activity of the nerve was recorded again to verify the integrity of the signal. The fine platinum wires of the electrodes were then exteriorized and connected to the electrical stimulator (World Precision Instruments, Sarasota, FL).
Experimental Protocol
Experiment 1.
Experiment 1 was performed 7–8 wk after HF or sham surgery. In four groups of rats (sham-Sed, HF-Sed, sham-ExT, and HF-ExT, n = 5 rats/group), RSNA, MAP, and HR were recorded for at least 15 min before electrical stimulation of the ADN. Each ADN stimulation (5, 10, 20, and 40 Hz, constant current: 1 mA) was applied for 5 s at intervals of 2–3 min. Maximum changes in each of the parameters elicited by the increasing frequency of electrical stimulation were quantified.
Experiment 2.
RSNA, MAP, and HR were measured in the four groups of rats (sham-Sed, HF-Sed, sham-ExT, and HF-ExT, n = 5 rats/group). Bicuculline was injected into the PVN (100 and 200 pmol in 100 nl). Maximum changes in RSNA, MAP, and HR were determined.
Experiment 3.
In separate groups of sham and HF rats (n = 5 rats/group), RSNA, MAP, and HR responses to ADN stimulation (5, 10, and 40 Hz) were recorded with or without microinjection of the GABA receptor antagonist bicuculline (200 pmol in 100 nl) into the PVN. After microinjection experiments, Chicago blue dye (50 nl) was injected into the brain to histologically verify that the injection site was located within the PVN.
Brain Histology
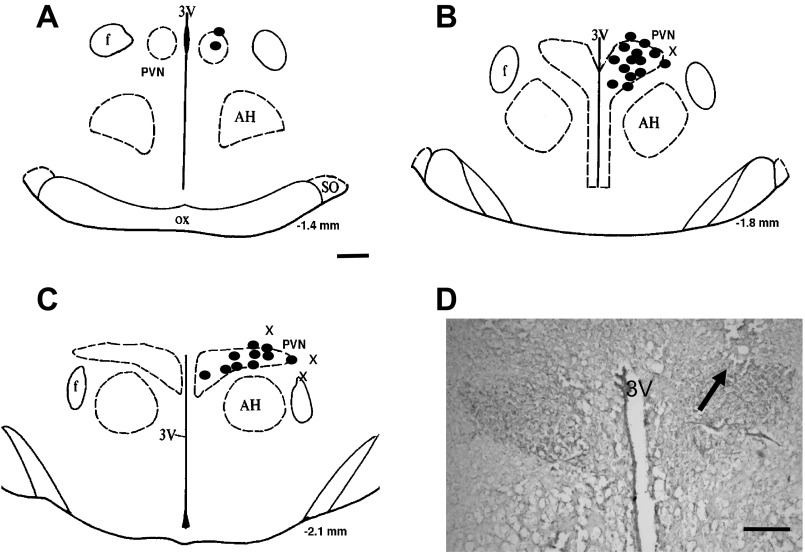
At the end of the experiment, the brain was carefully removed and fixed in 4% formaldehyde. The brain was then frozen, and serial transverse sections (30 μm) were cut using a cryostat. Sections were thaw mounted on slides and stained using 1% aqueous neutral red. The presence of blue dye within the PVN was determined using a light microscope. Dye that was located in the PVN was considered to be “hits” of the PVN. The results of these injections are shown in Fig. 6.
Fig. 6.
A–C: schematic representations of serial sections from the rostral (−1.4) to caudal (−2.1) extent of the region of the PVN. The distance (in mm) posterior to the bregma is shown for each section. Solid circles represent the site of termination of an injection that is considered to be within the PVN region. “x” represents the site of termination of an injection that is outside the PVN region. D: histological photo showing the injection site (arrow) in the PVN of one rat. AH, anterior hypothalamic nucleus; f, fornix; 3V, third ventricle; OX, optic tract; SO, supraoptic nucleus. Bar = 200 μm.
Microdissection of the PVN and Isolation of Protein for Western Blot Measurements
The following experiments were performed in a separate group of animals from those used in the PVN microinjection experiments described above. After the animal was euthanized by pentobarbital (150 mg/kg), the brain was removed and quickly frozen on dry ice. The PVN was bilaterally punched as previously described (50, 51). For each brain, 12 punches for each brain were placed in 100 μl of protein extraction buffer (10 mM Tris, 1 mM EDTA, 1% SDS, Triton X-100, and 1 mM PMSF), sonicated, and incubated for 30 min at 37°C to extract protein.
Western Blot Measurement of GABAA Receptor Protein
The total protein concentration from the extracted protein described above was measured with a bicinchoninic acid assay kit (Pierce). Protein samples were loaded onto a 7.5% SDS-PAGE gel. Gels were subjected to electrophoresis and electrophoretically transferred to a polyvinylidene difluoride membrane (Millipore). The membrane was probed with primary antibody [rabbit anti-GABAA-α1 (1:500, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-GABAA-β1 (1:500, Abcam), or rabbit anti-GAPDH (1:2,000, Santa Cruz Biotechnology)], washed with Tris-buffered saline-Tween 20, and then probed with secondary antibody (peroxidase-conjugated anti-rabbit IgG, 1:5,000 dilution, Pierce). An enhanced chemiluminescence substrate (Pierce) was applied to the membrane for 5 min followed by a 1- to 3-min exposure within an Epi Chemi II Darkroom (UVP BioImaging) for visualization with the Worklab digital imaging system. Kodak 1D software (Kodak) was used to quantify the signal. The expression of protein was calculated as the ratio of intensity of the GABAA receptor relative to the intensity of the GAPDH band.
Data Analysis
Data are presented as mean ± SE. Data were subjected to one-way or two-way ANOVA followed by comparison for individual group differences using the Newman-Keuls test for post hoc analysis of significance (Statview II, Abacus, Berkeley, CA). Statistical significance was indicated by P values of <0.05.
RESULTS
General Data
Table 1 shows baseline MAP and HR data as well as morphological characteristics and LV function data for the four experimental groups. Sham rats had no visible myocardial damage. Echo data showed that there was a significantly decreased ejection fraction in rats with HF regardless of ExT. LVEDP was significantly increased in the HF-Sed group compared with both sham groups and the HF-ExT group. While LVEDP was only partially normalized by ExT, the values in the HF-ExT group were nonetheless significantly different from those in either of the sham groups. Taken together, these data confirm that rats in the HF groups were experiencing cardiac dysfunction and that ExT did not normalize cardiac function per se.
Table 1.
Basal characteristics of sham and HF rats
| Sham-Sed Group | HF-Sed Group | Sham-ExT Group | HF-ExT Group | |
|---|---|---|---|---|
| Body weight, g | 397 ± 15 | 403 ± 23 | 365 ± 11† | 379 ± 10 |
| Heart weight, g | 1.3 ± 0.1 | 2.0 ± 0.2* | 1.2 ± 0.1 | 1.9 ± 0.2* |
| Infarct size, % epicardial LV | 0 | 36 ± 5* | 0 | 37 ± 4* |
| LV end-diastolic pressure, mmHg | 1.3 ± 0.5 | 27.7 ± 4.5* | 1.0 ± 0.4 | 21.2 ± 4.1* |
| Ejection fraction, % | 82 ± 2 | 49 ± 3* | 83 ± 2 | 50 ± 4* |
| Basal mean blood pressure, mmHg | 94 ± 4 | 92 ± 3 | 90 ± 3 | 89 ± 4 |
| Basal heart rate, beats/min | 346 ± 15 | 362 ± 21 | 331 ± 25 | 351 ± 24 |
Values are means ± SE; n = 10 rats/group. Sham, sham operation; Sed, sedentary; HF, heart failure; ExT, exercise training; LV, left ventricular.
P < 0.05 vs. the respective sham rats;
P < 0.05 vs. the respective Sed rats.
RSNA and Hemodynamic Responses to Electrical Stimulation of the ADN
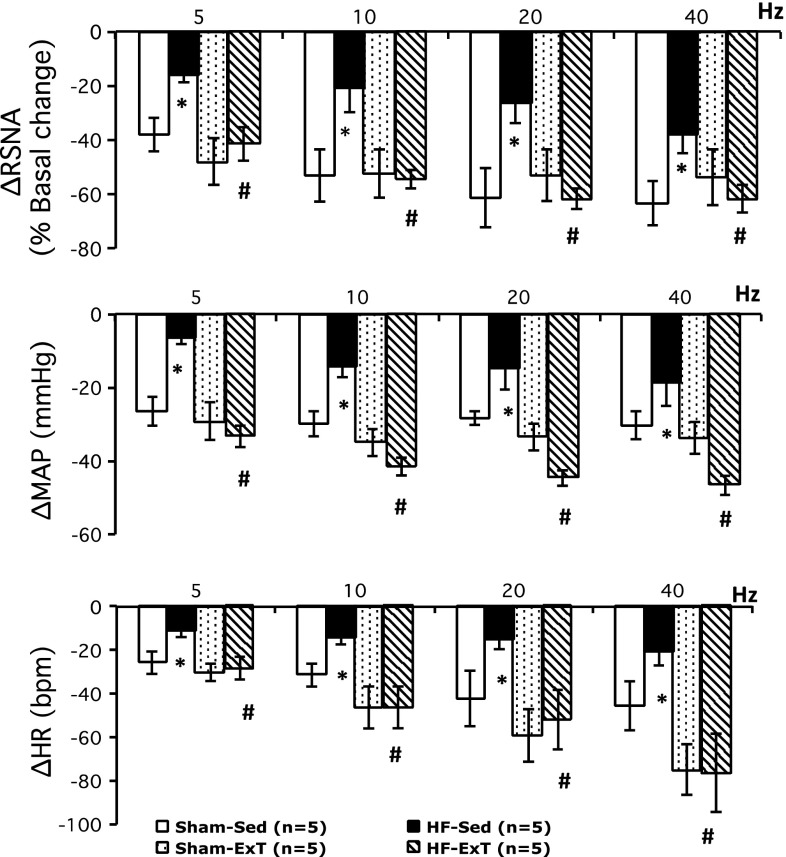
Figure 1 shows examples of responses of RSNA, MAP, and HR elicited by electrical stimulation (5, 10, 20, and 40 Hz) of the ADN in each of the four experimental groups. Group data for the changes in RSNA, MAP, and HR elicited by electrical stimulation of the ADN are shown in Fig. 2. ADN stimulation caused significant frequency-dependent decreases in RSNA, MAP, and HR in all four experimental groups. The decreases in RSNA in response to ADN stimulation in the HF-Sed group were significantly smaller than those of the sham-Sed group (−37 ± 7% vs. −63 ± 8% at 40 Hz, P < 0.05). Three weeks of ExT significantly improved RSNA, MAP, and HR responses to ADN stimulation in rats with HF compared with the HF-Sed group (ΔRSNA: −61 ± 5% vs. −37 ± 7% at 40 Hz, P < 0.05). Frequency-dependent reflex decreases in RSNA, MAP, and HR in response to ADN stimulation were not significantly different in the HF-ExT group versus sham-Sed or sham-ExT groups. The slopes of the relationship between RSNA, MAP, and HR versus frequency were significantly steeper in the sham-Sed group compared with the HF-Sed group. There were significant differences in slopes between the sham groups and the HF-ExT group for each of the parameters. This suggests that the “sensitivity” index of the baroreflex (slope) was significantly reduced in HF and that 3 wk of ExT ameliorated this difference.
Fig. 1.
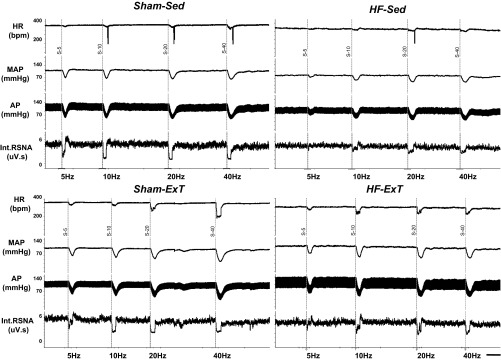
Typical tracing from a rat from each group [sham operated (sham)-sedentary (Sed), heart failure (HF)-Sed, sham-exercise training (ExT), and HF-ExT] showing heart rate [HR; in beats/min (bpm)], mean arterial pressure (MAP), pulsatile arterial pressure (AP), and integrated renal sympathetic nerve activity (Int RSNA) responses to electrical aortic depressor nerve (ADN) stimulation at 5, 10, 20, and 40 Hz. Bar = 1 min.
Fig. 2.
Changes in RSNA, MAP, and HR in response to electrical ADN stimulation at 5, 10, 20, and 40 Hz in four groups of rats (sham-Sed, HF-Sed, sham-ExT, and HF-ExT). *P < 0.05 vs. the sham group; #P < 0.05 vs. the Sed group.
RSNA and Hemodynamic Responses to Bicuculline Microinjection Into the PVN
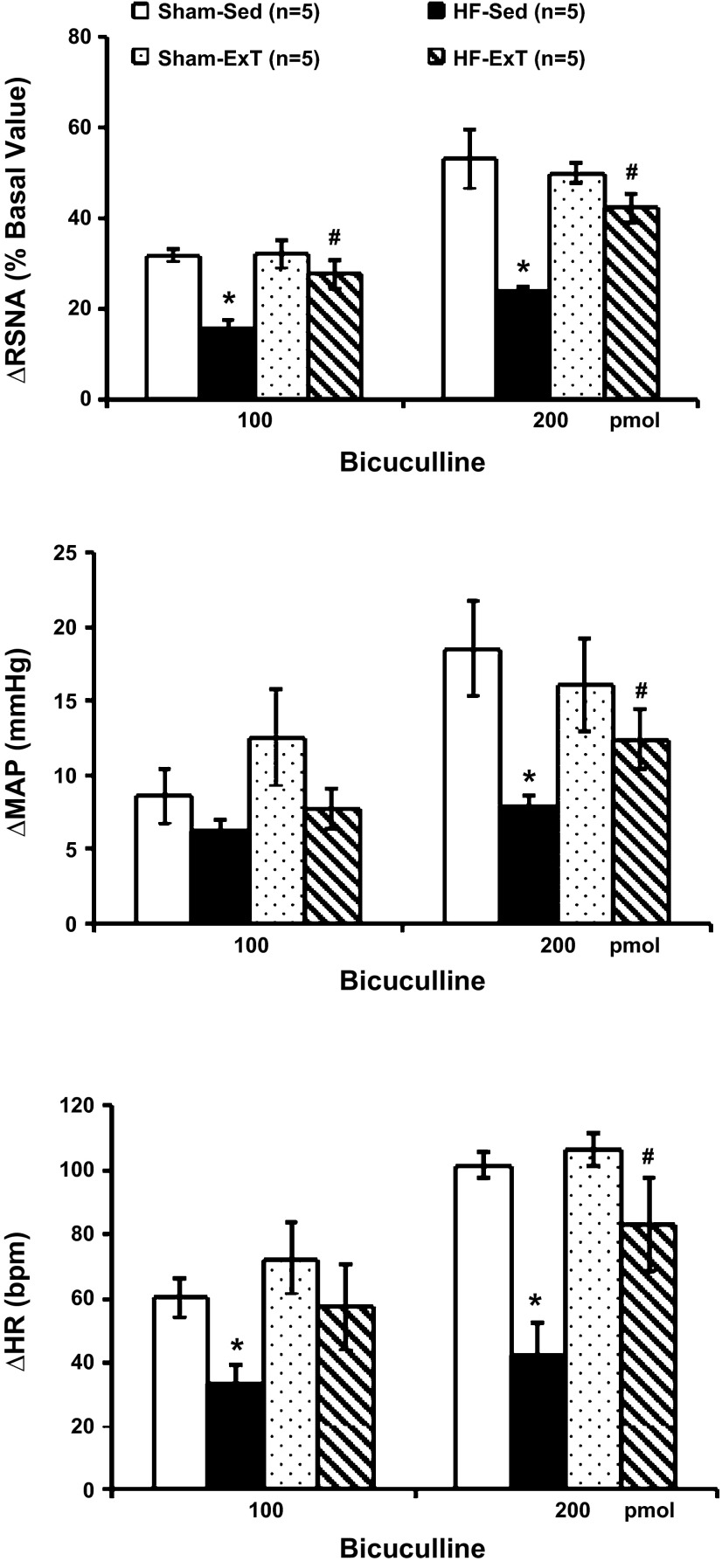
Bicuculline injected into the PVN elicited increases in RSNA, MAP, and HR in each of the four experimental groups (Fig. 3). Responses in HF-Sed rats were significantly smaller than in sham-Sed rats, consistent with previous data (46). In the HF-ExT group, this response (RSNA: 50 ± 2%, n = 5) was significantly larger (P < 0.05) than in the HF-Sed group (24 ± 1%) and not different from either of the sham groups (sham-Sed and sham-ExT). These data indicate that ExT normalizes the RSNA response to bicuculline microinjection into the PVN in rats with HF.
Fig. 3.
Changes in RSNA, MAP, and HR in response to bicuculline (Bic) microinjection (100 and 200 pmol) into the paraventricular nucleus (PVN) of four groups of rats (sham-Sed, HF-Sed, sham-ExT, and HF-ExT). *P < 0.05 vs. the sham group; #P < 0.05 vs. the Sed group.
Effects of the GABA Receptor Antagonist Bicuculline on Responses to Electrical Stimulation of the ADN
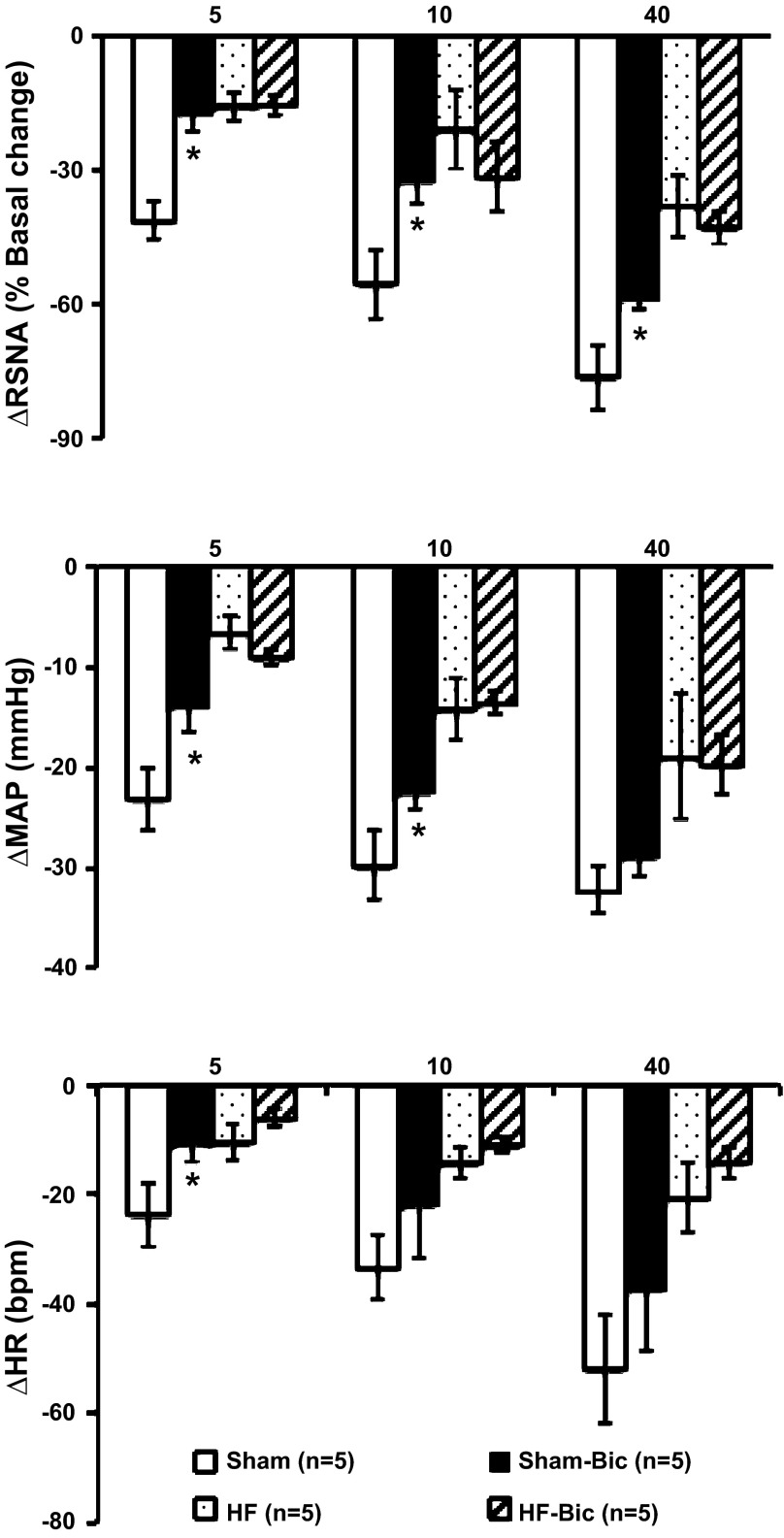
Group data for changes in RSNA, MAP, and HR elicited by electrical stimulation of the ADN with or without inhibition of GABA receptors with bicuculline in the PVN are shown in Fig. 4. In sham rats, stimulation of the ADN produced frequency-dependent decreases in RSNA, which was blunted by premicroinjection of bicuculline within the PVN (−76 ± 7% vs. −59 ± 2% at 40-Hz stimulation, P < 0.05). Microinjection of bicuculline in the PVN significantly attenuated baroreflex-mediated decreases in MAP and HR at lower stimulation frequencies (5 and 10 Hz). Bicuculline did not significantly influence baroreflex-mediated decreases in MAP and HR at the higher stimulation frequency (40 Hz). In HF rats, GABA antagonism with bicuculline did not blunt the baroreflex any further, as seen in sham rats.
Fig. 4.
Changes in RSNA, MAP, and HR in response to electrical ADN stimulation at 5, 10, and 40 Hz in sham and HF rats with or without Bic microinjection into the PVN. *P < 0.05 vs. rats without Bic.
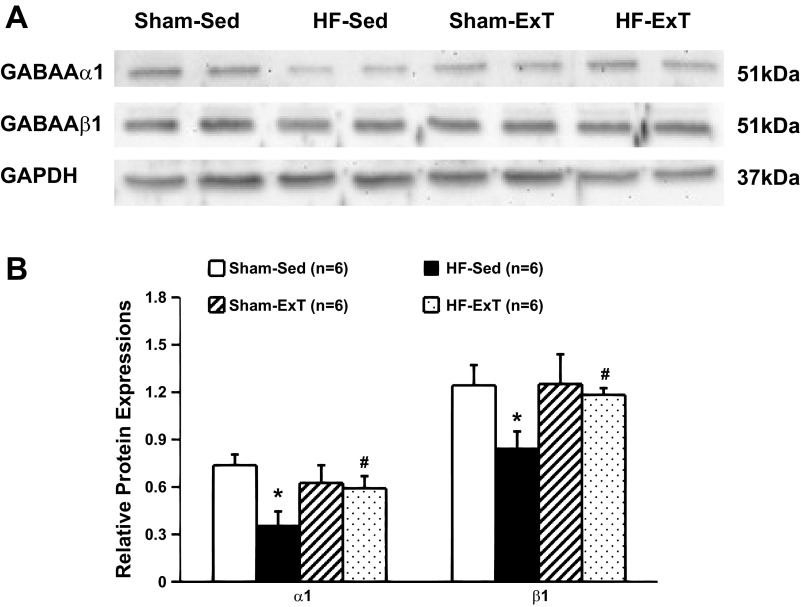
Expression of GABAA Receptor Protein Within the PVN
The rodent brain contains several forms of each GABA subunit, including six α-subunits (α1–α6), three β-subunits (β1–β3), and three γ-subunits (γ1–γ3). An electrophysiological study (12) has indicated that different subunit combinations may mediate different physiological or pharmacological properties. Within the PVN, the α1-, β1-, β3-, and γ2-subunits predominate. Previously, we (46) observed that bicuculline responses were blunted in rats with HF. Subsequently, we examined various subtypes of GABA receptor subtypes to see if there were changes in these subtypes in HF and observed that α1- and β1-subunits were reduced in rats with HF. GABAA-α1 receptor and GABAA-β1 receptor protein expressions, as measured by Western blot analysis, are shown in Fig. 5. Sample gels showing GABAA-α1 receptor, GABAA-β1 receptor, and GAPDH protein in the four experimental groups are shown in Fig. 5A. Figure 6B shows composite data for GABAA-α1 and GABAA-β1 receptor expression within the PVN, calculated as the ratio of the density of the GABAA receptor band to the density of the GAPDH band. The level of GABAA-α1 receptor protein expression in the HF-Sed group was significantly lower than in the sham-Sed group (0.36 ± 0.09 vs. 0.74 ± 0.07, P < 0.05). In the HF-ExT group, GABAA-α1 receptor protein expression (0.59 ± 0.08) was significantly higher than in the HF-Sed group and was not different from either the sham-Sed group or the sham-ExT group. GABAA-β1 receptor protein levels within the PVN were significant lower in the HF-Sed group compared with the sham-Sed group (0.85 ± 0.10 vs. 1.24 ± 0.13, P < 0.05). ExT significantly increased GABAA-β1 receptor protein levels within the PVN in HF rats (1.14 ± 0.05).
Fig. 5.
A: example of visualized bands of GABAA-α1 and GABAA-β1 receptor and GAPDH in the PVN in the four groups of rats (sham-Sed, HF-Sed, sham-ExT and HF-ExT). B: mean data of band densities normalized by GAPDH in the four groups of rats (sham-Sed, HF-Sed, sham-ExT and HF-ExT). *P < 0.05 vs. the sham group; #P < 0.05 vs. the Sed group.
DISCUSSION
In the present study, we found that the baroreflex-mediated decreases in RSNA, MAP, and HR responses to ADN stimulation are blunted in HF-Sed rats. This indicates that the central component of the baroreflex arc is altered in HF. Furthermore, ExT normalized the blunted decreases in RSNA, MAP, and HR responses to ADN stimulation in rats with HF. Application of bicuculline within the PVN, to disinhibit the PVN (i.e., activate the PVN), in normal control rats caused blunted RSNA, MAP, and HR responses. ExT also improved RSNA, MAP, and HR responses to microinjection of bicuculline as well as GABAA receptor protein expression in the PVN of rats with HF. These observations, taken together with previous results (36, 43, 47), indicate that one mechanism by which ExT normalizes sympathetic outflow in HF may be through normalization of the central inhibitory component of the baroreflex arc, possibly by improving the contribution of a GABAergic sympathoinhibitory mechanism at the level of the PVN.
The sympathetic hyperactivity observed in HF is closely related to abnormalities in cardiovascular reflexes. Sympathoinhibitory cardiovascular reflexes such as the arterial baroreceptor reflex (44) and volume reflex (48) are significantly suppressed, whereas sympathoexcitatory reflexes, including the cardiac sympathetic afferent reflex (52) and arterial chemoreceptor reflex, are augmented (42). Although these reflexes are altered during HF, the relative contributions of the central component of these reflexes are less well studied. It has long been assumed that altered baroreceptor function does not contribute to hypertension since baroreceptor denervation does not appreciably alter arterial pressure; rather, it increases the liability of arterial pressure and HR (5). However, recent evidence from chronic recording of RSNA in hypertensive rats implicates baroreceptors in the development and maintenance of hypertension (30). Baroreceptors are chronically reset to a higher “set point” when arterial pressure is chronically elevated, resulting in increased sympathetic nerve activity. It is possible that in chronic HF, similar to chronic hypertension, there is a change in the set point of sympathoexcitation such that there is a chronic increase in sympathetic nerve activity of central origin in HF. This study demonstrated that the central component of the baroreflex is altered in rats with HF. It clearly identified an altered central component in the RSNA response to activation of baroreceptors in HF.
So what specific mechanisms or substrates within this central component of the baroreflex arc are altered in HF? The central nervous system receives input from a variety of sources that play a major role in the final sympathetic outflow. For example, cardiopulmonary input has been known to alter baroreflex function (11). It is possible that various other altered afferents may influence the final sympathetic nerve activity in HF. A variety of humoral substances that are altered during HF may contribute to alterations of the central gain of the baroreflex. For example, infusion of ANG II produces a blunting of the baroreflex (8, 9). Baroreflex sensitivity is altered in many experimental models of hypertension, and its impairment appears to precede the onset of hypertension and involve central modulation/remodeling within various sites. Also, in HF, the neuronal hierarchy may undergo similar remodeling, and specific autonomic areas that dictate sympathetic drive (one of which is the PVN) may be altered.
The PVN is involved in the baroreflex control of sympathetic outflow, fluid balance, and vasopressin release (2). The PVN receives afferent input from cardiac vagal neurons (25) and is reciprocally connected to other cardiovascular control regions within the brain (16). The PVN is involved in the increased sympathetic activity associated with HF (34). The results of the present study demonstrate that the central component of baroreflex inhibition of RSNA is blunted in rats with HF, which is consistent with a previous report (4) showing a blunted response of inhibition of lumbar sympathetic nerve activity in response to ADN stimulation. In the HF condition, the PVN is activated (36, 43, 47). The PVN is known to influence resting sympathetic nerve activity and baroreflex-mediated changes in sympathetic nerve activity (10, 26, 35). We therefore examined if disinhibition of the PVN, by microinjection of bicuculline into the PVN, would mimic the increased activation of PVN seen in HF and thus blunt baroreflex-mediated changes in RSNA. The results demonstrate that activation of the PVN in normal rats blunts baroreflex-mediated inhibition of RSNA. Similar disinhibition of the PVN in rats with HF did not produce any further blunting of the baroreflex, further corroborating the notion that blunted GABAergic input within the PVN may contribute to the baroreflex dysfunction seen in the Sed-HF group. These data suggest that the activation of the PVN observed in rats with HF, possibly due to blunted GABA inhibition, may contribute to the blunted central component of the baroreflex in rats with HF. Furthermore, our data indicates that ExT normalized RSNA, MAP, and HR responses to bicuculline microinjection and GABAA receptor protein expression in the PVN in rats with HF. These observations indicate that ExT-induced normalization of sympathetic outflow in HF may be through improving the GABAergic sympathoinhibitory mechanism originating within the PVN.
Previous work from others investigating the regulatory role of GABA inputs in the PVN on baroreflex control using pharmacologic techniques (intravenous infusion of nitroprusside and phenylephrine) documented an increase in baroreflex sensitivity after GABAa inhibition with bicuculline, and this was specifically due to changes in baroreflex sensitivity during the hypotensive state induced by the intravenous infusion of nitroprusside (32). Also, intravenous nitroprusside may change nitric oxide levels, potentially affecting GABA mechanisms. To examine the central component of the baroreflex, it is appropriate to provide the same signal to the brain to assess the central component. With the pharmacological manipulation of MAP, changes in afferent signals at the actual baroreceptors are also involved. To avoid this afferent component, we used the ADN stimulation technique of assessing the central component of the baroreflex. The hypotensive phase was basically removing the baroreceptor input in the previous study. In the present study, we activated the baroreceptors.
Three to four weeks of ExT have been shown to increase arterial baroreflex sensitivity in a chronic HF group (23, 24, 33). To compare with a longer-term training regimen, 3–4 wk of ExT only partially improved the increased LVEDP and did not improve the decreased ejection fraction associated with HF, indicating that ExT did not normalize cardiac function per se in this model of HF. In our experience with this model of HF, we have observed that the higher the severity of the infarct (>50%), the lower the possibility of reversal of cardiac function parameters with ExT. This, combined with the relatively short duration of ExT (3–4 wk), leads to some changes in centrally mediated autonomic function, but the cardiac remodeling remains relatively limited (18). It is of interest to note that the HF-ACTION study (29) done in patients with a LV ejection fraction of only 25% demonstrated that ExT resulted in nonsignificant reductions in the primary end point of all-cause mortality but improved quality of life. This lack of effect on end-point mortality may be indicative of an improvement of the neurohumoral drive but not cardiac impairment per se.
In the present study, we demonstrated that ExT improves the central component of the baroreflex in rats with HF. This observation is consistent with the proposed mechanism of improvement in the arterial baroreflex, for the beneficial effects of ExT in patients with HF (21). These data are also consistent with observations by others (38) showing improvements of the arterial baroreflex in HF with ExT. Kajekar et al. (15) have shown that a single bout of ExT upregulates GABA signaling at sympathetic cardiovascular RVLM neurons, leading to a decreased neuronal output that may contribute to the decrease in sympathetic outflow for a period of 10 h. In the present study, it should be noted that the measurements were conducted 24 h after the last bout of exercise and are probably due to the long-term effects of ExT rather than the acute effects of ExT.
The possible mechanism by which ExT improves the central component of the baroreflex in HF is perhaps through a GABAergic mechanism. However, the observation that the sympathoexcitatory drive from the PVN is increased in HF and that such an increase in drive, demonstrated in this study by activation of the PVN in normal rats, blunts the central component of the baroreflex in normal rats suggests that normalization of the central drive from the PVN in HF may have a beneficial effect on baroreflex responses. Consistent with these observations, we (49) have previously demonstrated that ExT improves the sympathoinhibitory drive from the PVN by normalization of the blunted nitric oxide-mediated sympathoinhibition in rats with HF. Furthermore, we (18, 51) have shown that ExT improves the sympathoexcitatory drive from the PVN by normalization of the glutamatergic and angiotensinergic drives to sympathoexcitation in rats with HF. Recently, the role of the nonclassical pathway of renin-angiotensin system genes such as angiotensin-converting enzyme 2 and the Mas receptor in the central nervous system and their participation in central sympathetic activation have been also been widely addressed (6, 7, 17, 45). Changes in these inhibitory pathways may also be involved in the improvement of the central component of the baroreflex in rats with HF, but this remains to be examined. Overall, the ExT-induced improvement/reduction in sympathetic drive from the PVN in rats with HF may be the underlying contributing mechanism for the improvement in the central component of the baroreflex.
Perspectives
The results of the present study demonstrate that ExT attenuates the blunted decreases in RSNA in response to activation of the baroreceptors in rats with HF. Normalization of the sympathoexcitatory/inhibitory balance mediated by the PVN may underlie this effect. These findings underscore the importance of ExT to restore sympathetic outflow in HF. This is perhaps through normalization of a central component of the baroreflex by correcting the excitatory/inhibitory balance within the PVN dictating the baroreceptor reflex.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-62222.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.P.P., H.C.S., and H.Z. conception and design of research; K.P.P., H.C.S., X.L., and H.Z. interpreted results of experiments; K.P.P. and H.Z. drafted manuscript; K.P.P., H.C.S., and H.Z. edited and revised manuscript; K.P.P., H.C.S., X.L., and H.Z. approved final version of manuscript; X.L. and H.Z. performed experiments; X.L. and H.Z. analyzed data; X.L. and H.Z. prepared figures.
ACKNOWLEDGMENTS
The authors thank Dr. Kurtis Cornish and Jaci Castania for technical assistance.
REFERENCES
- 1. Barrett CJ, Guild SJ, Ramchandre R, Malpas SC. Baroreceptor denervation prevents sympathoinhibition during angiotensin II-induced hypertension. Hypertension 46: 168–172, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Bennett T, Gardiner SM. Involvement of vasopressin in cardiovascular regulation. Cardiovasc Res 19: 57–68, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Brändle M, Patel KP, Wang W, Zucker IH. Hemodynamic and norepinephrine responses to pacing-induced heart failure in conscious sinoaortic-denervated dogs. J Appl Physiol 81: 1855–1862, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Claassen DE, Morgan DA, Hirai T, Kenney MJ. Nonuniform sympathetic nerve responses after sustained elevation in arterial pressure. Am J Physiol Regul Integr Comp Physiol 271: R1264–R1269, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Cowley AWJ, Guyton AC. Baroreceptor reflex effects on transient and steady-state hemodynamics of salt-loading hypertension in dogs. Circ Res 36: 536–546, 1975 [DOI] [PubMed] [Google Scholar]
- 6. Diz DI, Garcia-Espinosa MA, Gegick S, Tommasi EN, Ferrario CM, Ann Tallant E, Chappell MC, Gallagher KP. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii reduce baroreceptor reflex sensitivity for heart rate control in rats. Exp Physiol 93: 694–700, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng Y, Yue X, Xia H, Bindom SM, Hickman PJ, Filipeanu CM, Wu G, Lazartigues E. Angiotensin-converting enzyme 2 overexpression in the subfornical organ prevents the angiotensin II-mediated pressor and drinking responses and is associated with angiotensin II type 1 receptor downregulation. Circ Res 102: 729–736, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol 288: H2271–H2279, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Guo GB, Abboud FM. Angiotensin II attenuates baroreflex control of heart rate and sympathetic activity. Am J Physiol Heart Circ Physiol 246: H80–H89, 1984 [DOI] [PubMed] [Google Scholar]
- 10. Haselton JR, Goering J, Patel KP. Parvocellular neurons of the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. J Auton Nerv Syst 50: 1–11, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Hasser EM, Bishop VS, Hay M. Interactions between vasopressin and baroreflex control of the sympathetic nervous system. Clin Exp Pharmacol Physiol 24: 102–108, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Huang R, Dillon G. Functional characterization of GABAA receptors in neonatal hypothalamic brain slice. Neuroscience 88: 1655–1663, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Iellamo F, Legramante JM, Massaro M, Raimondi G, Galante A. Effects of a residential exercise training on baroreflex sensitivity and heart rate variability in patients with coronary artery disease: a randomized, controlled study. Circulation 102: 2588–2592, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Jung R, Dibner-Dunlap ME, Gilles MA, Thames MD. Cardiorespiratory reflex control in rats with left ventricular dysfunction. Am J Physiol Heart Circ Physiol 268: H218–H225, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Kajekar R, Chen CY, Mutoh T, Bonham AC. GABAA receptor activation at medullary sympathetic neurons contributes to postexercise hypotension. Am J Physiol Heart Circ Physiol 282: H1615–H1624, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Kannan H, Yamashita H. Connections of neurons in the region of the nucleus tractus solitarius with the hypothalamic paraventricular nucleus: their possible involvement in neural control of the cardiovascular system in rats. Brain Res 329: 205–212, 1985 [DOI] [PubMed] [Google Scholar]
- 17. Kar S, Gao L, Zucker IH. Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. J Appl Physiol 108: 923–932, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol 294: R1863–R1872, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Komine H, Sugawara J, Hayashi K, Yoshizawa M, Yokoi T. Regular endurance exercise in young men increases arterial baroreflex sensitivity through neural alteration of baroreflex arc. J Appl Physiol 106: 1499–1505, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Krieger EM, Da Silva GJ, Negrao CE. Effects of exercise training on baroreflex control of the cardiovascular system. Ann NY Acad Sci 940: 338–347, 2001 [DOI] [PubMed] [Google Scholar]
- 21. La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation 106: 945–949, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Levett JM, Marinelli CC, Lund DD, Pardini BJ, Nader S, Scott BD, Augelli NV, Kerber RE, Schmid PG., Jr Effects of β-blockade on neurohumoral responses and neurochemical markers in pacing-induced heart failure. Am J Physiol Heart Circ Physiol 266: H468–H475, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure–a role for angiotensin II. Circulation 102: 1854–1862, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Liu JL, Kulakofsky J, Zucker IH. Exercise training enhances baroreflex control of heart rate by a vagal mechanism in rabbits with heart failure. J Appl Physiol 92: 2403–2408, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Lovick TA, Coote JH. Electrophysiological properties of paraventriculo-spinal neurones in the rat. Brain Res 454: 123–130, 1988 [DOI] [PubMed] [Google Scholar]
- 26. Lovick TA, Malpas S, Mahoney MT. Renal vasodilatation in respone to acute volume load is attenuated following lesions of parvocellular neurones in the paraventricular nucleus in rats. J Auton Nerv Syst 43: 247–256, 1993 [DOI] [PubMed] [Google Scholar]
- 27. McDonald MP, Sanfilippo AJ, Savard GK. Baroreflex function and cardiac structure with moderate endurance training in normotensive men. J Appl Physiol 74: 2469–2477, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol Heart Circ Physiol 262: H411–H419, 1992 [DOI] [PubMed] [Google Scholar]
- 29. O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL, Investigators HA. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301: 1439–1450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Osbron JW, Jacob F, Guzman P. A neural set point for the long-term control of arterial pressure: beyond the arterial baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 288: R846–R855, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Packer M. Neurohormonal interactions and adaptations in congestive heart failure. Circulation 77: 721–730, 1988 [DOI] [PubMed] [Google Scholar]
- 32. Page MC, Cassaglia PA, Brooks VL. GABA in the paraventricular nucleus tonically suppresses baroreflex function: alterations during pregnancy. Am J Physiol Regul Integr Comp Physiol 300: R1452–R1458, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan YX, Gao L, Wang WZ, Zheng H, Liu D, patel KP, Zucker IH, Wang W. Exercise training prevents arterial baroreflex dysfunction in rats treated with central angiotensin II. Hypertension 49: 519–527, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel KP. Role of paraventrivular nucleus in mediating sympathetic outflow in heart failure. Heart Failure Rev 5: 73–86, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Patel KP, Schmid PG. Role of the paraventricular nucleus (PVH) in baroreflex-mediated changes in lumbar sympathetic nerve activity and heart rate. J Auton Nerv Syst 22: 211–219, 1988 [DOI] [PubMed] [Google Scholar]
- 36. Patel KP, Zhang PL, Krukoff TL. Alterations in brain hexokinase activity associated with heart failure in rats. Am J Physiol Regul Integr Comp Physiol 265: R923–R928, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Orlando, FL: Academic, 1986 [Google Scholar]
- 38. Rondon E, Brasileiro-Santos MS, Moreira ED, Rondon MU, Mattos KC, Coelho MA, Silva GJ, Brum PC, Fiorino P, Irigoyen MC, Krieger EM, Middlekauff HR, Negrao CE. Exercise training improves aortic depressor nerve sensitivity in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol 291: H2801–H2806, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrao CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol 42: 854–860, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801: 239–243, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res 491: 156–162, 1989 [DOI] [PubMed] [Google Scholar]
- 42. Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced activity of carotid body chemoreceptors in rabbits with heart failure: role of nitric oxide. J Appl Physiol 86: 1273–1282, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Vahid-Ansari F, Leenen FHH. Pattern of neuronal activation in rats with CHF after myocardial infarction. Am J Physiol Heart Circ Physiol 275: H2140–H2146, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Wang Y, Patel KP, Cornish KG, Channon KM, Zucker IH. nNOS gene transfer to RVLM improves baroreflex function in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 285: H1660–H1667, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension 49: 926–931, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol 282: R1006–R1015, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol 283: H423–H433, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Zheng H, Li YF, Zucker IH, Patel KP. Exercise training improves renal excretory responses to acute volume expansion in rats with heart failure. Am J Physiol Renal Physiol 291: F1148–F1156, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Zheng H, Li YF, Cornish KG, Zucker IH, Patel KP. Exercise training improves endogenous nitric oxide mechanisms within the paraventricular nucleus in rats with heart failure. Am J Physiol Heart Circ Physiol 288: H2332–H2341, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Zheng H, Liu X, Patel KP. Angiotensin-converting enzyme 2 overexpression improves central nitric oxide mediated sympathetic outflow in chronic heart failure. Am J Physiol Heart Circ Physiol 301: H2402–H2412, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zheng H, Sharma NM, Liu X, Patel KP. Exercise training normalizes enhanced sympathetic activation from the paraventricular nucleus in chronic heart failure: role of angiotensin II. Am J Physiol Regul Integr Comp Physiol 303: R387–R394, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu GQ, Gao L, Patel KP, Zucker IH, Wang W. ANG II in the paraventricular nucleus potentiates the cardiac sympathetic afferent reflex in rats with heart failure. J Appl Physiol 97: 1746–1754, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Zucker IH, Wang W, Brändle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis 37: 397–414, 1995 [DOI] [PubMed] [Google Scholar]