Abstract
B. anthracis edema toxin (ET) and lethal toxin (LT) are each composed of protective antigen (PA), necessary for toxin uptake by host cells, and their respective toxic moieties, edema factor (EF) and lethal factor (LF). Although both toxins likely contribute to shock during infection, their mechanisms are unclear. To test whether ET and LT produce arterial relaxation, their effects on phenylephrine (PE)-stimulated contraction in a Sprague-Dawley rat aortic ring model were measured. Rings were prepared and connected to pressure transducers. Their viability was confirmed, and peak contraction with 60 mM KCl was determined. Compared with PA pretreatment (control, 60 min), ET pretreatment at concentrations similar to those noted in vivo decreased the mean (±SE) maximum contractile force (MCF; percent peak contraction) in rings generated during stimulation with increasing PE concentrations (96.2 ± 7.0 vs. 57.3 ± 9.1) and increased the estimated PE concentration producing half the MCF (EC50; 10−7 M, 1.1 ± 0.3 vs. 3.7 ± 0.8, P ≤ 0.002). ET inhibition with PA-directed monoclonal antibodies, selective EF inhibition with adefovir, or removal of the ring endothelium inhibited the effects of ET on MCF and EC50 (P ≤ 0.02). Consistent with its adenyl cyclase activity, ET increased tissue cAMP in endothelium-intact but not endothelium-denuded rings (P < 0.0001 and 0.25, respectively). LT pretreatment, even in high concentrations, did not significantly decrease MCF or increase EC50 (all P > 0.05). In rings precontracted with PE compared with posttreatment with PA (90 min), ET posttreatment produced progressive reductions in contractile force and increases in relaxation in endothelium-intact rings (P < 0.0001) but not endothelium-denuded rings (P = 0.51). Thus, ET may contribute to shock by producing arterial relaxation.
Keywords: anthrax, edema toxin, lethal toxin, arterial contraction, aortic ring model
concern over Bacillus anthracis infection has increased in the developed world with outbreaks of inhalational infection in the United States in 2001 and soft tissue infection in Europe in 2009 as well as with this bacteria's potential as a bioterrorist agent (4, 17, 18). Lethality with B. anthracis is high, and the development of shock during infection, even with aggressive treatment, is associated with a particularly poor prognosis. All patients with shock in the United States outbreak and >70% of those needing vasopressors in the European outbreak died (17, 18).
While the mechanisms underlying shock with B. anthracis appear complex, understanding them may improve the clinical management of infection. As a gram-positive bacteria, B. anthracis can stimulate the excessive inflammatory response associated with shock caused by more commonly encountered bacteria (8). However, anthrax also produces lethal toxin (LT) and edema toxin (ET), which likely contribute to shock. Both are binary type toxins with a protective antigen (PA) component necessary for host cell uptake of the toxic moieties, lethal factor (LF) for LT and edema factor (EF) for ET (15, 27, 40). LF is a metalloproteinase that inhibits MAPKKs and stimulates host inflammasome formation (27, 28). EF is a calmodulin-dependent adenyl cyclase that increases intracellular cAMP to high levels (20).
While both LT and ET produce hypotension in animal models, the mechanisms for their effects are unclear (6, 10, 45). We (39) have shown in a sedated, instrumented, and mechanically ventilated canine model that hypotension with either toxin was associated with significant reductions in systemic vascular resistance. These changes, while slow to develop with LT (i.e., 24–48 h) but rapid with ET (i.e., 1–2 h), suggest that both toxins might produce direct arterial relaxation. To further investigate the potential contributions of ET and LT to shock with B. anthracis, we tested their effects on arterial contraction using a rat aortic ring model. In initial experiments, pretreatment with ET but not LT inhibited the contractile response of aortic rings to phenylephrine (PE) stimulation. In subsequent experiments directed at ET, we examined whether PA-directed monoclonal antibody (mAb) or adefovir, a nucleoside that selectively blocks EF-stimulated cAMP production, inhibited the effects of the toxin and whether ET's relaxant effects required an intact arterial endothelium (3, 36, 37). Also, to determine whether ET reverses catecholamine-induced arterial contraction, we measured its relaxant effects on aortic rings precontracted with PE. Finally, we investigated the effects of ET on aortic tissue cAMP levels.
MATERIALS AND METHODS
Animal Care
This study was reviewed and approved by the Animal Care and Use Committee of the Clinical Center of the National Institutes of Health.
Study Design for Experiments Testing the Effects of ET or LT on Aortic Ring Contraction
In one group of experiments, Sprague-Dawley rat aortic rings were prepared (as described below) and pretreated with ET or LT alone or in combination with other interventions, and their contractile responses to increasing PE concentrations were then tested (Fig. 1 and Table 1) (2, 5, 33). In another group of experiments, rings were precontracted with PE, and their relaxation in response to ET was then tested. On each study day, up to four rings were prepared from a single animal.
Fig. 1.

Summary of the study design and sequence of interventions for each of the experiments performed. PE, phenylephrine; ET, edema toxin; mAb, monoclonal antibody; LT, lethal toxin.
Table 1.
Summary of aortic ring experiments
| Treatment* |
Intervention |
|||||
|---|---|---|---|---|---|---|
| Experiment | Primary Measure(s) | Type | Dose | Drug or manipulation | Dose | Number of Rings Tested |
| Aortic rings treated with or without interventions before stimulation with increasing PE doses | ||||||
| Effect of pretreatment with diluent, PA, or ET | MCF and EC50 | Diluent | 8 | |||
| PA | 1,600 | 9 | ||||
| ET | 800† | 7 | ||||
| Effect of pretreatment with differing doses of ET | MCF and EC50 | PA | 5 | |||
| ET | 50 | 1 | ||||
| ET | 100 | 3 | ||||
| ET | 200 | 5 | ||||
| ET | 400 | 5 | ||||
| ET | 800 | 3 | ||||
| Effect of PA mAb on ET pretreatment | MCF and EC50 | PA | 1,600 | PA mAb | 10ׇ | 10 |
| PA | 1,600 | NS mAb | 10 | |||
| ET | 800 | PA mAb | 10× | 12 | ||
| ET | 800 | NS mAb | 12 | |||
| Effect of adefovir on ET pretreatment | MCF and EC50 | PA | 1,600 | Diluent | 4 | |
| PA | 1,600 | Adefovir | 1.6 μM | 4 | ||
| ET | 800 | Diluent | 12 | |||
| ET | 800 | Adefovir | 1.6 μM | 12 | ||
| Effect of ET pretreatment comparing intact and denuded aortic rings | MCF and EC50 | PA | 1,600 | Intact ring | 8 | |
| ET | 800 | Intact ring | 8 | |||
| PA | 1,600 | Denuded ring | 8 | |||
| ET | 800 | Denuded ring | 8 | |||
| Effect of pretreatment with differing doses of LT | MCF and EC50 | PA | 5 | |||
| LT | 40 | 1 | ||||
| LT | 80 | 5 | ||||
| LT | 160 | 5 | ||||
| LT | 320 | 5 | ||||
| LT | 640 | 1 | ||||
| Aortic rings treated with or without intervention after contraction with a single dose of PE | ||||||
| Effect of ET with differing doses of PE precontraction | Percent relaxation | |||||
| 1 μM PE | PA | 1,600 | 11 | |||
| ET | 800 | 9 | ||||
| 10 μM PE | PA | 1,600 | 4 | |||
| ET | 800 | 4 | ||||
| 100 μM PE | PA | 1,600 | 2 | |||
| ET | 800 | 2 | ||||
| Effect of ET comparing intact and denuded aortic rings | Percent relaxation | PA | 1,600 | Intact ring | 6 | |
| ET | 800 | Intact ring | 6 | |||
| PA | 1,600 | Denuded ring | 5 | |||
| ET | 800 | Denuded ring | 5 | |||
PE, phenylephrine; ET, edema toxin; MCF, maximal contraction during the PE dose response; EC50, dose of PE producing 50% of maximum contraction; PA, protective antigen; NS mAb, nonspecific (control) monoclonal antibody; PA mAb, PA-directed mAb; LT, lethal toxin.
Treatment was either before (i.e., pretreatment) or after stimulation with PE.
Dose of ET shown represents the amount of edema factor administered (see materials and methods).
Dose of PA mAb represents 10× the PA molar dose, whereas the dose of NS mAb used had the same protein content as PA mAb.
Effect of ET pretreatment.
Aortic rings were incubated for 60 min with 800 ng/ml ET [i.e., EF (800 ng/ml) combined with PA (1,600 ng/ml), n = 7], 1,600 ng/ml PA (n = 9), or diluent only (n = 8). After a wash and equilibration (30 min), the contractile force of each ring to increasing concentrations of PE (10−9–10−5 M) was measured. At least one of each of the pretreatments was studied concurrently. In other experiments, aortic rings were incubated for 60 min with one of four ET concentrations (800 ng/ml, n = 3, 400 ng/ml, n = 5, 200 ng/ml, n = 5, or 100 ng/ml, n = 3) or PA alone (1,600 ng/ml, n = 5). After a wash and equilibration, the contractile force of each ring to PE was measured.
Effect of PA mAb on pretreatment with ET.
To determine the involvement of PA-mediated cellular uptake of ET on the toxin's effects, aortic rings were incubated for 60 min with ET (800 ng/ml) combined with either a mAb directed against PA (PA mAb, n = 12) or a nonspecific (NS) mAb (n = 12). Other rings were incubated with PA (1,600 ng/ml) combined with PA mAb (n = 10) or NS mAb (n = 10). After a wash and equilibration, the contractile force of each ring to PE was measured.
Effect of adefovir on pretreatment with ET.
To determine the involvement of EF in the effect of ET, aortic rings were incubated for 30 min with adefovir (1.6 μM) or diluent only. After a wash and equilibration, rings were incubated for 60 min with either ET (800 ng/ml, n = 12 adefovir-treated rings and 12 diluent-treated rings) or PA (1,600 ng/ml, n = 4 adefovir-treated rings and 4 diluent-treated rings). After a wash and equilibration, the contractile force of each ring to PE was measured.
Effect of pretreatment with ET in endothelium-intact versus endothelium-denuded rings.
Each study day, four aortic rings from the same animal were prepared. The endothelium of two rings was then removed, and the viability and maximal contractile force (MCF) of all rings was tested (as described below). After a wash and equilibration, the two rings with endothelium (intact rings) and two rings without (denuded rings) were incubated with either ET (800 ng/ml) or PA (1,600 ng/ml) for 60 min (i.e., n = 8 rings/group total). After a wash and equilibration, the contractile force of rings to PE was measured.
Effect of ET on rings precontracted with PE.
Aortic rings were contracted with PE (1, 10, or 100 μM) for 10 min. Rings were then incubated for 90 min with ET (800 ng/ml) or PA (1,600 ng/ml, n = 9 and 11 rings after 1 μM PE, respectively, 4 and 4 rings after 10 μM PE, respectively, and 2 and 2 rings after 100 μM PE, respectively). The contractile force of each ring was measured, and the percent relaxation from immediately before the start of ET or PA was then calculated.
Effect of ET on endothelium-intact versus endothelium-denuded rings precontracted with PE.
On each study day, two intact and two denuded aortic rings were prepared. After a wash and equilibration, all rings were contracted with 1 μM PE for 10 min. Intact rings were incubated with either ET (800 ng/ml, n = 6) or PA (1,600 ng/ml, n = 6), and denuded rings were incubated with ET (800 ng/ml, n = 5) or PA (1,600 ng/ml, n = 5) for 90 min. After incubation, the contractile force of each ring was measured, and the percent relaxation from immediately before the start of ET or PA was then calculated. As a control, the effects of incubation with forskolin, an agent reported to have equally relaxant effects in endothelium-intact and -denuded rings, was compared with diluent in intact rings [forskolin (0.8 μM): n = 8 or diluent: n = 8] and denuded rings [forskolin (0.8 μM): n = 8 or diluent: n = 8].
Effect of pretreatment with LT.
Aortic rings were incubated for 120 min with one of five increasing concentrations of LT (40 ng/ml, n = 1, 80 ng/ml, n = 5, 160 ng/ml, n = 5, 320 ng/ml, n = 5, and 640 ng/ml, n = 1) or PA alone (640 ng/ml, n = 5). After a wash and equilibration, the contractile force of each ring to PE was measured.
Study Design of Experiments Testing the Effects of ET on Aortic Tissue cAMP Levels
In daily experiments, the aorta was excised from one Sprague-Dawley rat and sectioned into 3-mm lengths (rings). Up to eight rings could be obtained from an animal. In initial experiments, rings were incubated with either ET in increasing concentrations (200, 400, or 800 ng/ml, n = 6 rings/concentration) or PA alone (1,600 ng/ml, n = 6) for 90 min, after which tissue cAMP levels were measured. In a second set of experiments, rings were incubated with ET (800 mg/ml) or PA (1,600 ng/ml) and then taken for cAMP measures at 5 min (n = 4 rings each for ET or PA), 15 min (n = 4), 30 min (n = 8), 60 min (n = 9), or 90 min (n = 8) after the start of incubation. Additional rings that were randomized to but not incubated with ET or PA served as baseline controls (time = 0 min, n = 7 rings/group). In a final set of experiments, endothelium-intact or -denuded rings were incubated with either ET (800 ng/ml, n = 6 intact rings and 6 denuded rings) or PA (1,600 ng/ml, n = 6 intact rings and 6 denuded rings) for 90 min, after which tissue cAMP levels were measured.
Aortic Ring Preparation and Contractile Measures
Male Sprague-Dawley rats weighing 250 g on average and at 8–10 wk of age were anesthetized with isoflorane, and their thoracic aortas were isolated, removed, and cleaned of adventitia and fat (2, 5, 33). Sections of aorta 4 mm in length (aortic rings) were cut and mounted between two tungsten hooks in baths containing modified Krebs-Henseleit (KH) buffer (16) at 37°C and continuously bubbled with 95% O2-5% CO2 at pH 7.4. Suspended rings were connected to a force transducer, and their contractile responses recorded on a polygraph (Lab Chart, ADInstruments, Colorado Springs, CO). Rings were equilibrated until their resting tension stabilized at 1.5 g. After equilibration, the functional integrity of each ring was assessed by measuring its contractile response to 10−7 M PE followed by its relaxation with 10−5 M ACh. A tension increase of ≥1 g to PE and a subsequent relaxation of >90% to ACh in endothelium-intact rings were considered as evidence of a ring's functional integrity. Rings not meeting these criteria were not studied. After a subsequent 30-min equilibration period, each ring's contractile response was measured by exposure for 4 min to 60 mM KCl. This measure was performed three times, and the highest value was determined to be the ring's peak contractile force. In subsequent PE dose-response experiments, MCF of each ring was calculated as the percentage of its peak contraction with 60 mM KCl. The estimated dose of PE producing 50% of the MCF was designated the half-maximal effective concentration (EC50; ×10−7 M). MCF and EC50 were calculated by standard curve analysis with SigmaPlot software (Systat Software, San Jose, CA). In experiments in precontracted rings, relaxation represented the percent reduction in maximal contraction achieved with PE immediately before incubation with ET, PA, forskolin, or diluent. Endothelium-denuded rings were prepared by gently rubbing the luminal side of a ring with plastic tubing and removing the vascular endothelium. Only rings with relaxation <10% with ACh treatment were used. The endothelium was judged as removed if rings contracted adequately with PE but did not relax with 10−5 M ACh.
Aortic Tissue Preparation and cAMP Measures
Aortic rings 3 mm in length were preincubated in KH buffer for 30 min at 37°C. Rings were then incubated with PA or ET as described above. At the end of incubation, rings were immediately weighed and put in liquid nitrogen. Frozen rings were homogenized in iced sample diluent (100 mg/ml). The diluent used was supplied with the assay kit (cAMP Chemiluminescent Immunoassay Kit, Arbor Assays, Ann Arbor, MI). The sample was then centrifuged at 15,000 rpm at 4°C for 15 min, and the supernatant was collected and stored at −70°C. cAMP levels were measured based on the manufacturer's instructions, and the cAMP content is expressed as picomoles per milligram of wet tissue.
Toxin and Treatment Preparation
Toxin components (PA, LF, and EF) were recombinant proteins prepared from Escherichia coli as previously described (6, 7). ET and LT were composed of EF or LF with PA at ratios of 1:2 on the basis of weight. The concentration of ET and LT reported for each experiment reflects the concentration of EF or LF used. The lipopolysaccharide (LPS) content of PA, LF, and EF was 0.001, 0.002, and 0.006 ng/μg, respectively. The PA mAb used (Raxibacumab, supplied by Human Genome Sciences, Rockville, MD) was a human mAb directed against PA (24), whereas the placebo was an inactive, nonspecific mAb. The dose of PA mAb used was 10 times the molar PA dose used during incubation with ET. This ratio of PA mAb to toxin PA content has been previously shown to improve survival in toxin-challenged rats (37). Adefovir dipivoxil (Sigma-Aldrich) was dissolved in ethanol (1 mg/ml) and then diluted to a working solution using KH buffer (36). Forskolin (Sigma-Aldrich) was dissolved in ethanol (100 μM) and then diluted to a working solution using KH buffer. The final concentrations of ethanol in the baths were 0.008% with adefovir and forskolin. A similar ethanol vehicle was used as a control in experiments with adefovir and forskolin.
Statistical Analysis
Measures of MCF, EC50 (Figs. 2–4 and 7), relaxation at 90 min (Figs. 5 and 6), and cAMP levels (Fig. 7) were analyzed with ANOVA accounting for challenge (ET, LT, PA, or diluent) or treatment (PA mAb, adefovir, or endothelium removal) or observation time point using PROC MIXED in SAS (version 9.3) software (SAS Institute, Cary, NC). In relaxation experiments (Figs. 5 and 6), repeated-measures ANOVA accounting for challenge (ET or PA and forskolin or diluent), intact or denuded endothelium, and time was performed using PROC MIXED to determine the effects of ET or forskolin across observation time. For clarity in Fig. 4, E and F, the effects of ET compared with PA (i.e., ET − the PA control) are shown. Data are presented as means ± SE. Two-sided P values of ≤0.05 were considered significant.
Fig. 2.
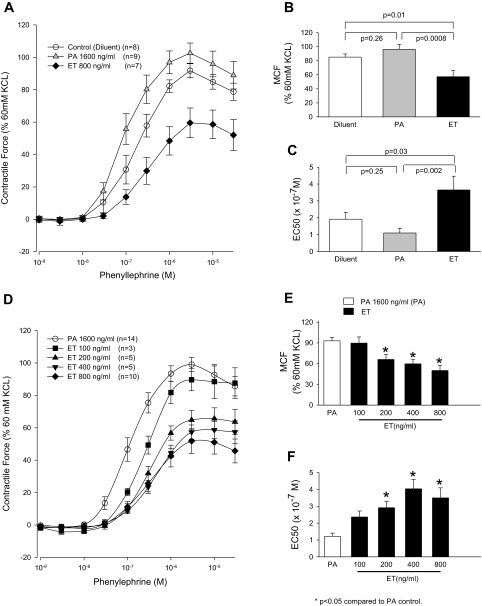
A–C: effects of pretreatment of aortic rings for 60 min with diluent, PA alone (1,600 ng/ml), or ET (800 ng/ml) on mean (±SE) contractile force that the rings subsequently generated during stimulation with increasing PE concentrations (A), the mean (±SE) maximal contractile force (MCF) they developed during PE stimulation (B), and the mean (±SE) estimated concentration of PE producing 50% of the MCF (EC50; C). D–F: the same measures for rings pretreated for 60 min with PA alone (1,600 ng/ml) or with four increasing concentrations of ET (100, 200, 400, and 800 ng/ml). The contractile force shown in A and C and MCF shown in B and E were calculated as a percentage of peak contractile force rings generated when exposed initially to 60 mM KCl (see materials and methods). The number of rings used in each experiment (n) is shown.
Fig. 4.
A–C: effects of pretreatment of either endothelium-intact or -denuded aortic rings for 60 min with PA alone (1,600 ng/ml) or with ET (800 ng/ml) on the mean (±SE) contractile force that the rings subsequently generated during stimulation with increasing PE concentrations (A), the mean (±SE) MCF they developed during PE stimulation (B), and the mean (±SE) EC50 (C). D and E: mean (±SE) effects of ET (see materials and methods for calculation) in intact versus denuded rings on MCF (D) and EC50 (E). The contractile force shown in A and MCF shown in B were calculated as a percentage of peak contractile force rings generated when exposed initially to 60 mM KCl (see materials and methods). The number of rings used in each experiment (n) is shown.
Fig. 7.
A: mean (±SE) cAMP levels measured in aortic rings (in pmol/mg tissue) incubated for 90 min with diluent, PA (1,600 ng/ml) alone, or increasing concentrations of ET (200, 400, or 800 ng/ml). B: mean (±SE) cAMP measured in aortic rings (in pmol/mg tissue) at baseline (time 0) or after 5, 15, 30, 60, or 90 min of incubation with PA alone (1,600 ng/ml) or ET (800 ng/ml). C: mean (±SE) cAMP measured in endothelium-intact or -denuded aortic rings (in pmol/mg tissue) incubated for 90 min with PA alone (1,600 ng/ml) or ET (800 ng/ml). The number of rings (n) used in each experiment is shown in A–C.
Fig. 5.
A–C: serial mean (±SE) contractile force in rings precontracted for 10 min with 1, 10, or 100 μM PE generated during 90 min of subsequent treatment with PA (1,600 ng/ml) or ET (800 ng/ml). D–F: serial mean (±SE) percent relaxation calculated for the respective rings shown in A–C. G–I: mean (±SE) relaxation recorded at 90 min in rings treated with PA alone versus ET. The contractile forces shown in A–C were calculated as a percentage of peak contractile force that rings generated when exposed initially to 60 mM KCl (see materials and methods). The number of rings used in each experiment (n) is shown in A–C.
Fig. 6.
A: serial mean (±SE) contractile force in endothelium-intact or -denuded rings precontracted for 10 min with 1 μM PE generated during 90 min of subsequent treatment with PA (1,600 ng/ml) or ET (800 ng/ml). B: serial mean (±SE) contractile force in endothelium-intact or -denuded rings precontracted for 10 min with 1 μM PE generated during 90 min of subsequent treatment with diluent or forskolin (0.8 μM). C and D: serial mean (±SE) percent relaxation calculated for the respective rings shown in A and B. E: mean (±SE) relaxation recorded at 90 min in intact rings treated with PA alone versus ET and in denuded rings treated with PA alone versus ET. F: mean (±SE) relaxation recorded at 90 min in intact rings treated with diluent alone versus forskolin and in denuded rings treated with diluent alone versus forskolin. The contractile forces shown in A and B were calculated as a percentage of peak contractile force that rings generated when exposed initially to 60 mM KCl (see materials and methods). The number of rings used in each experiment (n) is shown in A and B.
RESULTS
Experiments Testing the Effects of ET or LT on Aortic Ring Contraction
Effect of ET pretreatment.
In aortic rings pretreated with diluent alone, cumulatively increasing PE concentrations produced a mean (±SE) MCF of 85.2 ± 4.5, whereas the EC50 (× 10−7 M) was 1.9 ± 0.4 (Fig. 2, A–C). Compared with diluent, with PA pretreatment (1,600 ng/ml), MCF (96.2 ± 7.0) and EC50 (1.1 ± 0.3) did not differ significantly. Compared with diluent or PA, with ET pretreatment (800 ng/ml), MCF was decreased (57.3 ± 9.1) and EC50 was increased (3.7 ± 0.8, P ≤ 0.03 for all comparisons). In subsequent experiments, only PA was used as a control. Compared with PA (1,600 ng/ml), increasing ET pretreatment concentrations (100 vs. 200 vs. 400 vs. 800 ng/ml) produced progressive decreases in MCF and increases in EC50 (P ≤ 0.0002 for the effect of increasing ET doses on changes in MCF or EC50; Fig. 2, D–F).
Effect of PA mAb on ET pretreatment.
These experiments used PA mAb to examine whether PA-mediated binding was necessary for ET's inhibition of PE-stimulated contraction. Mean (±SE) MCF and EC50 during PE stimulation did not differ significantly when rings pretreated with PA combined with NS mAb (89.6 ± 8.1 and 1.6 ± 0.2, respectively) versus PA mAb (79.5 ± 7.0 and 1.7 ± 0.2, respectively) were compared (Fig. 3, A–C). Compared with PA with either antibody, rings pretreated with ET and NS mAb had an impaired contractile response to PE with reduced MCF (49.8 ± 6.0) and increased EC50 (6.5 ± 1.2, P ≤ 0.0001). However, rings pretreated with ET combined with PA mAb had MCF (81.7 ± 5.8) and EC50 (2.1 ± 0.5) values that were not significantly different from PA-pretreated rings but were different from rings pretreated with ET and NS mAb (P ≤ 0.001). Thus, blockade of PA reversed ET inhibition of PE-stimulated contraction.
Fig. 3.
A–C: effects of pretreatment of aortic rings for 60 min with PA (1,600 ng/ml) in combination with either NS mAb or PA mAb or with ET (800 ng/ml) in combination with either of these two mAbs on the mean (±SE) contractile force that the rings subsequently generated during stimulation with increasing PE doses (A), the mean (±SE) MCF they developed during PE stimulation (B), and the mean (±SE) EC50 (C). D–F: the same measures for rings initially pretreated for 30 min with diluent or adefovir (ADF) followed by additional treatment for 60 min with PA alone (1,600 ng/ml) or with ET (800 ng/ml). The contractile force shown in A and C and MCF shown in B and E were calculated as a percentage of peak contractile force rings generated when exposed initially to 60 mM KCl (see materials and methods). The number of rings used in each experiment (n) is shown.
Effect of adefovir on ET pretreatment.
These experiments used adefovir to examine whether EF was necessary for ET's inhibition of PE-stimulated contraction. Mean (±SE) MCF and EC50 during PE stimulation did not differ significantly when rings pretreated with PA after diluent (96.0 ± 3.1 and 1.1 ± 0.3, respectively) versus after adefovir (104.2 ± 8.6 and 0.7 ± 0.2, respectively) were compared (Fig. 3, D–F). Compared with PA with either diluent or adefovir, rings pretreated with ET after diluent showed a significantly impaired contractile response to PE with reduced MCF (62.2 ± 6.4) and increased EC50 (2.6 ± 0.3, P ≤ 0.008). However, rings pretreated with ET after adefovir had MCF (91.0 ± 6.2) and EC50 (1.7 ± 0.2) values that were not significantly different from PA-pretreated rings but were different from rings pretreated with ET after diluent (P ≤ 0.02). Thus, blockade of EF reversed ET inhibition of PE-stimulated contraction.
Effect of ET pretreatment on endothelium-intact versus endothelium-denuded rings.
These experiments examined the role of the endothelium in ET's inhibition of PE-stimulated contraction. In rings pretreated with PA, mean (±SE) MCF was greater and EC50 was reduced during PE stimulation in denuded versus intact rings (P = 0.11 and P = 0.01, respectively; Fig. 4). This difference was likely due to the spontaneous release of endothelium-derived relaxing factor (EDRF) during stretch or contraction of intact but not denuded rings and has been previously described (2, 30). As observed in prior experiments in the present study, in intact rings, compared with MCF and EC50 after PA pretreatment (102.7 ± 7.1 and 0.9 ± 0.1, respectively), contraction was significantly impaired in ET-pretreated rings (72.1 ± 2.8 and 3.6 ± 0.4, respectively, P < 0.0001 for both measures). In contrast, in denuded rings, compared with MCF and EC50 after pretreatment with PA (112.7 ± 2.5 and 0.2 ± 0.02, respectively), contraction after ET pretreatment (105.8 ± 2.8 and 0.3 ± 0.1, respectively), although reduced, was not significantly different (P = 0.26 for MCF and P = 0.56 for EC50). Overall, the inhibitory effects of ET on PE-stimulated contraction (i.e., reduction in MCF and increase in EC50) was significantly greater when intact versus denuded rings were compared (P = 0.009 and P < 0.0001, respectively).
Effect of ET on rings precontracted with PE.
Stimulation with PE (1, 10, or 100 μM for 10 min each) increased the contractile force of rings (Fig. 5, A–C). Compared with subsequent treatment with PA alone (1,600 ng/ml), treatment with ET (800 ng/ml) produced progressive reductions in contractile force and increases in relaxation over 90 min of observation (P < 0.0001, <0.0001, and 0.008, respectively, for the changes in relaxation over time; Fig. 5, D–F). At 90 min, compared with treatment with PA, ET produced increases in mean (±SE) relaxation that were significant in rings precontracted with 1 and 10 μM PE and approached being significant in rings precontracted with 100 μM PE (46.5 ± 8.7 vs. 83.5 ± 6.2, P = 0.004, 33.6 ± 10.3 vs. 80.7 ± 6.7, P = 0.009, and 63.8 ± 10.6 vs. 95.8 ± 4.1, P = 0.10 respectively; Fig. 5, G–I). Relaxation with ET did not differ when the three PE concentrations were compared (P = 0.8).
Effect of ET on endothelium-intact versus endothelium-denuded rings precontracted with PE.
These experiments examined the role of the endothelium in ET's relaxant effects in rings precontracted with PE. Stimulation for 10 min with 1 μM PE increased the contractile force in both intact and denuded rings (Fig. 6A). Compared with subsequent treatment with PA alone (1,600 ng/ml), treatment with ET (800 ng/ml) produced progressive reductions in contractile force and increases in relaxation over 90 min of observation in intact rings (P = 0.01 and <0.0001, respectively, for the changes over time) but not in denuded rings (P = 0.99 and 0.71, respectively; Fig. 6, A and C). At 90 min, compared with treatment with PA, ET produced increases in relaxation that were significant in intact rings (32.3 ± 11.2 vs. 89.4 ± 10.4, P < 0.0001) but not in denuded rings (2.0 ± 2.8 vs. 9.7 ± 3.7, P = 0.51; Fig. 6E). These effects of ET differed significantly when intact and denuded rings were compared (P = 0.004).
As a positive control, in additional experiments, forskolin, an agent reported to have relaxant effects in both intact and denuded rings, was examined. The concentration of forskolin investigated had previously been shown in a pilot study to produce relaxation in intact rings comparable with the relaxation noted in intact rings treated with ET (800 ng/ml; data not shown). Different from ET, compared with treatment with diluent alone, forskolin produced progressive reductions in contraction and increases in relaxation in both intact and denuded rings (P ≤ 0.0005 for the changes over time; Fig. 6, B and D). At 90 min, compared with treatment with diluent, forskolin produced increases in relaxation that were significant in both intact rings (45.3 ± 9.6 vs. 94.4 ± 2.6, P < 0.0001) and denuded rings (1.5 ± 3.5 vs. 48.4 ± 9.3, P = 0.0003; Fig. 6F). These effects of forskolin did not differ significantly when intact and denuded rings were compared (P = 0.90).
Effect of LT pretreatment.
Compared with MCF and EC50 after pretreatment with PA (76.8 ± 7.4 and 1.5 ± 0.2, respectively, n = 5), pretreatment with LT at several increasing concentrations (40 ng/ml, n = 1, 80 ng/ml, n = 5, 160 ng/ml, n = 5, 320 ng/ml, n = 5, and 640 ng/ml, n = 1) did not produce significant decreases in MCF (104.1 ± 0.0, 93.7 ± 9.7, 102.0 ± 5.8, 95.9 ± 6.3, and 105.9 ± 0.0, respectively) or increases in EC50 (0.9 ± 0.0, 1.0 ± 0.1, 1.1 ± 0.3, 1.2 ± 0.2, and 2.2 ± 0.0, respectively, all P > 0.05). The doses of LT tested were comparable with or substantially higher than doses shown to produce cardiovascular changes in vivo (6).
Experiments Testing the Effects of ET on Aortic Tissue cAMP Levels
Compared with diluent (n = 6), incubation of aortic tissue for 90 min with PA (n = 7) did not increase cAMP levels significantly (Fig. 7A). Compared with diluent and PA, incubation with increasing concentrations of ET (200 ng/ml, n = 6, 400 ng/ml, n = 6, and 800 ng/ml, n = 6) produced progressive increases in cAMP levels (all P < 0.0001). Compared with PA, incubation with ET for increasing periods from 5 to 60 min produced progressive increases in cAMP levels that may have begun to decline by 90 min (P < 0.0001 for the time interaction; Fig. 7B). Compared with PA, ET significantly increased cAMP levels in endothelium-intact rings (P < 0.0001) but not endothelium-denuded rings (P = 0.25; Fig. 7C).
DISCUSSION
In this study, treatment of rat aortic rings with B. anthracis ET decreased their contractile response to subsequent stimulation with PE and produced relaxation in rings already contracted with PE. Reduction in PE-stimulated contraction by ET was dependent on uptake of toxin by host cells, since PA-directed mAb, but not NS mAb, blocked the inhibitory effects of ET. Reduction in contractile force with ET was also dependent on EF-mediated cAMP production since it was blocked by adefovir (36). Consistent with this, ET produced dose-dependent increases in cAMP levels in aortic tissue. Finally, ET's relaxant and cAMP-stimulatory effects in aortic tissue appeared in part dependent on the vascular endothelium since both were significantly reduced in denuded aortic rings. In contrast to ET, pretreatment of aortic rings with B. anthracis LT, even in high doses, did not interfere with the contractile response to PE.
Growing evidence has implicated ET in the pathogenesis of shock and lethality associated with B. anthracis, and the present findings, in combination with previous findings, provide a mechanism for such a contribution. Animals challenged with mutant B. anthracis strains lacking ET are less lethal than wild-type bacteria, and mAbs directed against the toxin are protective in spore-challenged mice (21, 31). Lethality with ET has appeared in part related to its cardiovascular effects. Purified ET challenge in mouse, rat, and canine models produces rapid reductions in systemic blood pressure and, in the canine, reductions in systemic vascular resistance and central venous pressure (6, 39, 44). These cardiovascular changes are followed by organ dysfunction and death when sufficient ET doses are used (39). Even nonlethal doses of ET can produce reductions in blood pressure (6). The present findings suggest that these hypotensive effects of ET are related, at least in part, to its ability to stimulate arterial relaxation. Such an effect would be highly consistent with the central role increases in intracellular cAMP have on producing relaxation in vascular smooth muscle and would provide a basis for the rapidity with which ET challenge produces hypotension and reductions in systemic vascular resistance in animal models (1, 23, 29). We (16) have previously shown that ET administration in an isolated perfused rat heart model resulted in significant increases in coronary artery flow rates as well as in myocardial tissue and effluent cAMP levels and speculated that this was due to cAMP-mediated coronary artery dilation by ET. The present findings support these prior speculations. An effect of ET on the smooth muscle of venous capacitance vessels could also explain the rapid reductions in central venous pressure in the absence of evidence of hemoconcentration or extravascular fluid collections noted in a canine model (39).
An early study (38) with ET showed that its subcutaneous administration produced localized edema formation. This property led to the toxin's name and has been proposed as a contributor to the substantial extravascular fluid collections noted in patients with B. anthracis infection. Based on this effect, some have proposed that hypotension with ET is related to increases in endothelial permeability, extravasation of fluid, and loss of intravascular volume (10, 22, 44). However, increases in endothelial cell cAMP levels are broadly associated with improved rather than reduced endothelial cell integrity (14, 35). On the one hand, it is possible that either the high intracellular cAMP levels that occur with ET or that their location results in paradoxical disruption of endothelial barrier function (14, 35). However, in vitro studies (9, 14, 22, 41) examining the effect of ET on the endothelium have shown inconsistent results, with some reporting evidence of toxin-associated increases in permeability and others decreases. The findings in the present study showing that ET can produce direct arterial relaxation provide an alternative explanation to hypotension with the toxin that does not require it to necessarily have an adverse effect on endothelial barrier function.
Although blood concentrations of ET have not been reported from either clinical or laboratory models of B. anthracis infection, the concentrations of toxin used in the present study do not appear greater than those that may occur with infection. The maximal concentrations of PA and EF aortic rings were incubated with (1.6 and 0.8 μg/ml, respectively) were less than concentrations of PA noted in rabbits or nonhuman primates with infection (9.07 and 66.4 μg/ml) or of LF concentrations noted in a human with B. anthracis infection (800 μg/ml) (24, 42). Also, the range of PA concentrations in the ET doses used in the present study that inhibited aortic ring contraction with PE (0.4–1.6 μg/ml) were very close to blood concentrations of PA (0.5–1.2 μg/ml) associated with shock and lethality in canines challenged with 24-h ET infusions (39).
The present findings highlight several areas for future study. While it is likely that the vasorelaxant effects of ET noted here are initiated by EF's effects on intracellular cAMP levels, whether these are then mediated via cAMP protein kinase, exchange protein activated by cAMP, or other pathways requires investigation (1, 23, 29). Also, while EF's predominant intracellular effect appears to be on cAMP, it has been reported to stimulate the production of other cyclic nucleotides, which could contribute to the toxin's vasorelaxant effects (12). The observation that ET's effects on reducing contractile force and stimulating cAMP release were, in contrast to forskolin, significantly greater in intact compared with denuded aortic tissue suggests that ET's effects on relaxation were, in part, endothelium dependent. In this regard, it is possible that the distribution of anthrax toxin receptors 1 and 2 may be greater on the endothelium than in smooth muscle cells in the aortic ring preparations used. It is also possible that although in vivo challenge with ET is not associated with marked changes in circulating nitric oxide (NO) levels, at the cellular level, NO release may contribute to ET's effects in the aortic ring model and possibly in vivo (6). Both these points also require testing by possibly comparing anthrax toxin receptor density on the endothelium and muscle or by examining the effects of NO synthase inhibition in the aortic ring assay.
Although LT has also been implicated in shock during B. anthracis infection and LT challenge produces hypotension in animal models, in the present study, this toxin, even at high concentrations, did not interfere with PE-stimulated aortic ring contraction (7, 26, 39). This may be because, even with longer incubation with LT than ET (120 vs. 60 min), the aortic ring preparation used did not provide sufficient time for LT to elicit inhibitory effects on vascular smooth function. In an animal study (39), hypotension with LT is not as rapid as with ET. Also, while there is growing evidence that LT may alter myocardial function, this also takes several hours to days to develop dependent on the animal model studied (11, 25, 39, 45). Finally, in in vitro studies (13, 19, 43), the effects of LT on endothelial dysfunction have taken time to develop. Thus, whether hypotension with LT results from a direct effect of this toxin on arterial contraction remains to be elucidated.
The present findings regarding ET have potential clinical implications. Use of catecholamine vasopressors is standard for hemodynamic support in patients with severe infection and shock who are unresponsive to fluid therapy alone. Patients with shock during the recent outbreak of anthrax in Scotland were observed to be relatively resistant to conventional hemodynamic support, including vasopressors (4, 17). Our present findings provide one potential basis for this resistance. Circulating ET in these patients could have contributed to this shock by causing arterial relaxation directly as well as by inhibiting the vasoconstrictor action of endogenous or therapeutically administered catecholamines. Possibly consistent with this, in a sedated and mechanically ventilated canine model, a regimen of fluid and norepinephrine support, which improved survival in LT-challenged animals, was ineffective with a similarly lethal ET challenge (3, 34).
A potential limitation of this study is that the effects of ET and LT were measured in the aorta, a conductance vessel, and not in resistance vessels, which might be more relevant with respect to the hypotensive effects of the two toxins. It is possible that the sensitivity to ET and LT of resistance vessels may differ from the aorta. Comparing anthrax toxin receptor density in differing vascular tissues would be one way to address whether such differences exist. Examining the effects of ET and LT on blood flow across isolated organ beds might be another way.
Although PA and EF were each associated with trace amounts of LPS (see materials and methods), this likely did not contribute to the observed effects of ET for several reasons. First, the relaxant effects of ET were negated by PA-directed mAb and adefovir, which selectively inhibited PA and EF, respectively. These two agents would not have altered the effects of LPS. Second, in prior in vivo experiments, administration of the amounts of LPS associated with lethal doses of ET in rats was not associated with evidence of inflammatory cytokine or NO release (32).
In conclusion, while the pathogenesis of shock with B. anthracis infection appears complex and is likely related to the production of both toxin and nontoxin bacterial components, the present findings suggest that ET-stimulated arterial relaxation could make an important pathogenic contribution. Further defining this contribution during B. anthracis infection itself, as well as its underlying mechanisms, may improve the management of patients with anthrax and shock in the future.
GRANTS
This work was supported by the Intramural Program of the National Institutes of Health, Clinical Center, Critical Care Medicine Department.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.L., X.C., S.B.S., K.R., and P.Q.E. conception and design of research; Y.L., X.C., and Y.F. performed experiments; Y.L., X.C., and P.Q.E. analyzed data; Y.L., X.C., and P.Q.E. interpreted results of experiments; Y.L., X.C., and P.Q.E. prepared figures; Y.L., X.C., and P.Q.E. drafted manuscript; Y.L., X.C., and P.Q.E. edited and revised manuscript; Y.L., X.C., and P.Q.E. approved final version of manuscript.
REFERENCES
- 1. Akata T. Cellular and molecular mechanisms regulating vascular tone. Part 2: regulatory mechanisms modulating Ca2+ mobilization and/or myofilament Ca2+ sensitivity in vascular smooth muscle cells. J Anesth 21: 232– 242, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Auguet M, Delaflotte S, Pirotzky E, Clostre F, Braquet P. Role of endothelium on phenylephrine-triggered contractile events in aorta of spontaneously hypertensive rats. Fundam Clin Pharmacol 3: 655– 664, 1989 [DOI] [PubMed] [Google Scholar]
- 3. Barochia AV, Cui X, Sun J, Li Y, Solomon SB, Migone TS, Subramanian GM, Bolmer SD, Eichacker PQ. Protective antigen antibody augments hemodynamic support in anthrax lethal toxin shock in canines. J Infect Dis 205: 818– 829, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Booth MG, Hood J, Brooks TJ, Hart A. Anthrax infection in drug users. Lancet 375: 1345– 1346, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Callera GE, Yeh E, Tostes RC, Caperuto LC, Carvalho CR, Bendhack LM. Changes in the vascular β-adrenoceptor-activated signalling pathway in 2kidney-1clip hypertensive rats. Br J Pharmacol 141: 1151– 1158, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cui X, Li Y, Li X, Laird MW, Subramanian M, Moayeri M, Leppla SH, Fitz Y, Su J, Sherer K, Eichacker PQ. Bacillus anthracis edema and lethal toxin have different hemodynamic effects but function together to worsen shock and outcome in a rat model. J Infect Dis 195: 572– 580, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Cui X, Moayeri M, Li Y, Li X, Haley M, Fitz Y, Correa-Araujo R, Banks SM, Leppla SH, Eichacker PQ. Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am J Physiol Regul Integr Comp Physiol 286: R699– R709, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Cui X, Su J, Li Y, Shiloach J, Solomon S, Kaufman JB, Mani H, Fitz Y, Weng J, Altaweel L, Besch V, Eichacker PQ. Bacillus anthracis cell wall produces injurious inflammation but paradoxically decreases the lethality of anthrax lethal toxin in a rat model. Intensive Care Med 36: 148– 156, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ebrahimi CM, Sheen TR, Renken CW, Gottlieb RA, Doran KS. Contribution of lethal toxin and edema toxin to the pathogenesis of anthrax meningitis. Infect Immun 79: 2510– 2518, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Firoved AM, Miller GF, Moayeri M, Kakkar R, Shen Y, Wiggins JF, McNally EM, Tang WJ, Leppla SH. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am J Pathol 167: 1309– 1320, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Golden HB, Watson LE, Lal H, Verma SK, Foster DM, Kuo SR, Sharma A, Frankel A, Dostal DE. Anthrax toxin: pathologic effects on the cardiovascular system. Front Biosci 14: 2335– 2357, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Gottle M, Dove S, Kees F, Schlossmann J, Geduhn J, Konig B, Shen Y, Tang WJ, Kaever V, Seifert R. Cytidylyl and uridylyl cyclase activity of bacillus anthracis edema factor and Bordetella pertussis CyaA. Biochemistry 49: 5494– 5503, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gozes Y, Moayeri M, Wiggins JF, Leppla SH. Anthrax lethal toxin induces ketotifen-sensitive intradermal vascular leakage in certain inbred mice. Infect Immun 74: 1266– 1272, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guichard A, Nizet V, Bier E. New insights into the biological effects of anthrax toxins: linking cellular to organismal responses. Microbes Infect 14: 97– 118, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hicks CW, Cui X, Sweeney DA, Li Y, Barochia A, Eichacker PQ. The potential contributions of lethal and edema toxins to the pathogenesis of anthrax associated shock. Toxins (Basel) 3: 1185– 1202, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hicks CW, Li Y, Okugawa S, Solomon SB, Moayeri M, Leppla SH, Mohanty A, Subramanian GM, Mignone TS, Fitz Y, Cui X, Eichacker PQ. Anthrax edema toxin has cAMP-mediated stimulatory effects and high-dose lethal toxin has depressant effects in an isolated perfused rat heart model. Am J Physiol Heart Circ Physiol 300: H1108– H1118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hicks CW, Sweeney DA, Cui X, Li Y, Eichacker PQ. An overview of anthrax infection including the recently identified form of disease in injection drug users. Intensive Care Med 38: 1092– 1104, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, Tapper M, Fisk TL, Zaki S, Popovic T, Meyer RF, Quinn CP, Harper SA, Fridkin SK, Sejvar JJ, Shepard CW, McConnell M, Guarner J, Shieh WJ, Malecki JM, Gerberding JL, Hughes JM, Perkins BA. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis 7: 933– 944, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirby JE. Anthrax lethal toxin induces human endothelial cell apoptosis. Infect Immun 72: 430– 439, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leppla S. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci USA 79: 6, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leysath CE, Chen KH, Moayeri M, Crown D, Fattah R, Chen Z, Das SR, Purcell RH, Leppla SH. Mouse monoclonal antibodies to anthrax edema factor protect against infection. Infect Immun 79: 4609– 4616, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maddugoda MP, Stefani C, Gonzalez-Rodriguez D, Saarikangas J, Torrino S, Janel S, Munro P, Doye A, Prodon F, Aurrand-Lions M, Goossens PL, Lafont F, Bassereau P, Lappalainen P, Brochard F, Lemichez E. cAMP signaling by anthrax edema toxin induces transendothelial cell tunnels, which are resealed by MIM via Arp2/3-driven actin polymerization. Cell Host Microbe 10: 464– 474, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Metrich M, Berthouze M, Morel E, Crozatier B, Gomez AM, Lezoualc'h F. Role of the cAMP-binding protein Epac in cardiovascular physiology and pathophysiology. Pflügers Arch 459: 535– 546, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Migone TS, Subramanian GM, Zhong J, Healey LM, Corey A, Devalaraja M, Lo L, Ullrich S, Zimmerman J, Chen A, Lewis M, Meister G, Gillum K, Sanford D, Mott J, Bolmer SD. Raxibacumab for the treatment of inhalational anthrax. N Engl J Med 361: 135– 144, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Moayeri M, Crown D, Dorward DW, Gardner D, Ward JM, Li Y, Cui X, Eichacker P, Leppla SH. The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS). PLoS Pathog 5: e1000456, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moayeri M, Haines D, Young HA, Leppla SH. Bacillus anthracis lethal toxin induces TNF-α-independent hypoxia-mediated toxicity in mice. J Clin Invest 112: 670– 682, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moayeri M, Leppla SH. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol Aspects Med 30: 439– 455, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moayeri M, Sastalla I, Leppla SH. Anthrax and the inflammasome. Microbes Infect 14: 392– 400, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morgado M, Cairrao E, Santos-Silva AJ, Verde I. Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cell Mol Life Sci 69: 247– 266, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakaki T, Otsuka Y, Kato R. Tension-induced release of endothelium-derived relaxing factor; possible role in establishment of desensitization of norepinephrine-induced contraction in rat aorta. Jpn J Pharmacol 54: 491– 494, 1990 [DOI] [PubMed] [Google Scholar]
- 31. Pezard C, Berche P, Mock M. Contribution of individual toxin components to virulence of Bacillus anthracis. Infect Immun 59: 3472– 3477, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qiu P, Li Y, Shiloach J, Cui X, Sun J, Trinh sc L-B, Kubler-Kielb J, Vinogradov E, Mani H, Al-Hamad M, Fitz Y, Eichacker PQ. B. anthracis cell wall peptidoglycan but not lethal or edema toxins produces changes consistent with disseminated intravascular coagulation in a rat model. J Infect Dis. June 3, 2013. 10.1093/infdis/jit247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rascado RR, Bendhack LM. Activation of α2-adrenoceptors is necessary to induce nitric oxide release in isoprenaline-induced relaxation. Vascul Pharmacol 42: 63– 68, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Remy KE, Cui X, Solomon SB, Sun J, Migone TS, Bolmer SD, Al-Hamad M, Li Y, Fitz Y, Eichacker PQ. Hemodynamic support and early treatment with protective antigen directed monoclonal antibody together improve survival in a canine model of anthrax edema toxin induced shock (Abstract). The International Conference of American Thoracic Society, Philadelphia, PA, May 17–22, 2013. [Google Scholar]
- 35. Sayner SL. Emerging themes of cAMP regulation of the pulmonary endothelial barrier. Am J Physiol Lung Cell Mol Physiol 300: L667– L678, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen Y, Zhukovskaya NL, Zimmer MI, Soelaiman S, Bergson P, Wang CR, Gibbs CS, Tang WJ. Selective inhibition of anthrax edema factor by adefovir, a drug for chronic hepatitis B virus infection. Proc Natl Acad Sci USA 101: 3242– 3247, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sherer K, Li Y, Cui X, Li X, Subramanian M, Laird MW, Moayeri M, Leppla SH, Fitz Y, Su J, Eichacker PQ. Fluid support worsens outcome and negates the benefit of protective antigen-directed monoclonal antibody in a lethal toxin-infused rat Bacillus anthracis shock model. Crit Care Med 35: 1560– 1567, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Stanley JL, Smith H. Purification of factor I and recognition of a third factor of the anthrax toxin. J Gen Microbiol 26: 49– 63, 1961 [DOI] [PubMed] [Google Scholar]
- 39. Sweeney DA, Cui X, Solomon SB, Vitberg DA, Migone TS, Scher D, Danner RL, Natanson C, Subramanian GM, Eichacker PQ. Anthrax lethal and edema toxins produce different patterns of cardiovascular and renal dysfunction and synergistically decrease survival in canines. J Infect Dis 202: 1885– 1896, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sweeney DA, Hicks CW, Cui X, Li Y, Eichacker PQ. Anthrax infection. Am J Respir Crit Care Med 184: 1333– 1341, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tessier J, Green C, Padgett D, Zhao W, Schwartz L, Hughes M, Hewlett E. Contributions of histamine, prostanoids, and neurokinins to edema elicited by edema toxin from Bacillus anthracis. Infect Immun 75: 1895– 1903, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walsh JJ, Pesik N, Quinn CP, Urdaneta V, Dykewicz CA, Boyer AE, Guarner J, Wilkins P, Norville KJ, Barr JR, Zaki SR, Patel JB, Reagan SP, Pirkle JL, Treadwell TA, Messonnier NR, Rotz LD, Meyer RF, Stephens DS. A case of naturally acquired inhalation anthrax: clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor. Clin Infect Dis 44: 968– 971, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Warfel JM, Steele AD, D'Agnillo F. Anthrax lethal toxin induces endothelial barrier dysfunction. Am J Pathol 166: 1871– 1881, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Watson LE, Kuo SR, Katki K, Dang T, Park SK, Dostal DE, Tang WJ, Leppla SH, Frankel AE. Anthrax toxins induce shock in rats by depressed cardiac ventricular function. PLos One 2: e466, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Watson LE, Mock J, Lal H, Lu G, Bourdeau RW, Tang WJ, Leppla SH, Dostal DE, Frankel AE. Lethal and edema toxins of anthrax induce distinct hemodynamic dysfunction. Front Biosci 12: 4670– 4675, 2007 [DOI] [PubMed] [Google Scholar]