Abstract
The presence of urocortins (UCNs) and corticotropin-releasing factor (CRF) receptors has been reported in the hypothalamic arcuate nucleus (ARCN). We have previously reported that UCNs are involved in central cardiovascular regulation. Based on this information, we hypothesized that the ARCN may be one of the sites where UCNs exert their central cardiovascular actions. Experiments were done in artificially ventilated, adult male Wistar rats anesthetized with urethane. Unilateral microinjections (30 nl) of UCN1 (0.12–2 mM) elicited decreases in mean arterial pressure (MAP) and heart rate (HR). Maximum cardiovascular responses were elicited by a 1 mM concentration of UCN1. Microinjections of UCN2 and UCN3 (1 mM each) into the ARCN elicited similar decreases in MAP and HR. UCN1 was used as a prototype for the other experiments described below. HR responses elicited by UCN1 were significantly attenuated by bilateral vagotomy. Prior microinjections of NBI-27914 (CRF-1 receptor antagonist) and astressin (CRF-1 receptor and CRF-2 receptor antagonist) (1 mM each) into the ARCN significantly attenuated the cardiovascular responses elicited by UCN1 microinjections at the same site. Microinjections of UCN1 into the ARCN decreased efferent renal sympathetic nerve activity. It was concluded that microinjections of UCN1, UCN2, and UCN3 into the ARCN elicited decreases in MAP and HR. Decreases in MAP, HR, and renal sympathetic nerve activity elicited by UCN1 microinjections into the ARCN were mediated via CRF receptors. Bradycardic responses to UCN1 were mediated via the activation of vagus nerves, and decreases in MAP may be mediated via decreases in sympathetic nerve activity.
Keywords: blood pressure, heart rate, microinjection, N-methyl-d-aspartic acid, sympathetic nerve activity
corticotropin-releasing factor (CRF), also known as corticotropin-releasing hormone, is released in the hypothalamus in response to stress (52). CRF reaches the anterior pituitary via the hypophyseal portal system and releases adrenocorticotropic hormone into the circulation. Adrenocorticotropic hormone then elicits the secretion of cortisol from the adrenal cortex. Cortisol mobilizes protein and fat and promotes gluconeogenesis to make adjustments for stress-induced responses (21). Initial stress-induced responses also include increases in blood pressure (BP) and heart rate (HR) (13).
Urocortins (UCNs), which include UCN1, UCN2, and UCN3, are recent additions to the CRF peptide family. Intracerebroventricular or intravenous injections of UCN1 elicit decreases in water and food intake (42) and in BP and increases in anxiogenic behavior (35). UCN2 (also known as stresscopin-related peptide) has been implicated in the central regulation of appetite and autonomic functions (47). UCN3 (structurally closely related to stresscopin) plays a role in the regulation of feeding and the recovery phase of stress (25).
There are two major subtypes of CRF receptors (CRFRs): CRF-1 and CRF-2 (1, 13, 18). These plasma membrane receptors have a seven-transmembrane domain, are coupled to Gs protein, and stimulate adenylate cyclase activity. UCN1 mediates its actions via both CRF-1 and CRF-2 receptors (CRF1Rs and CRF2Rs, respectively), whereas UCN2 and UCN3 mediate their actions primarily via CRF2Rs (14, 18, 19, 28, 47).
UCNs have been implicated in the central regulation of cardiovascular function (9, 11, 17, 31, 38, 39). Microinjections of UCNs into different brain areas elicit diverse cardiovascular responses. In the medial nucleus tractus solitarius (mNTS), microinjections of UCNs elicit decreases in mean arterial pressure (MAP) and HR (38, 39, 58), and similar microinjections into the nucleus ambiguus (nAmb) elicit bradycardia (9, 10). In the hypothalamic paraventricular nucleus (PVN), microinjections of UCN3 elicit pressor and tachycardic responses (31). Information regarding the effects of UCNs in other brain areas is incomplete.
Recently, the ARCN has been implicated in the central regulation of cardiovascular function (49). Stimulation of the ARCN in rats, by microinjections of N-methyl-d-aspartic acid (NMDA), elicits decreases as well as increases in BP and sympathetic nerve activity (SNA) (23, 24, 37). The type of BP and SNA response (i.e., increase or decrease) elicited from the ARCN depends on the level of baroreceptor activity, which, in turn, is determined by the baseline BP (23). In rats with normal baroreceptor activity at normal baseline BP, decreases in BP and SNA elicited by microinjections of NMDA into the ARCN are mediated via GABAA, neuropeptide Y1 (NPY1), and opiate receptors in the PVN (23). The increases in BP and SNA elicited by microinjections of NMDA into the ARCN in rats with baseline BP lower than normal are mediated via spinal ionotropic glutamate (Glu) receptors (iGLURs) (37). In barodenervated rats, microinjections of NMDA into the ARCN elicit increases in BP and SNA, which are mediated via iGLURs and melanocortin 3 and 4 receptors in the PVN (24). Increases in HR elicited by microinjections of NMDA into the ARCN are mediated via the activation of sympathetic inputs as well as inhibition of parasympathetic inputs to the heart (37). The ARCN has also been reported to be involved in the mediation of cardiovascular actions of some circulating hormones, like ANG II and ANG-(1–12) (2, 6, 46, 57).
The presence of UCNs and CRFRs has been reported in the ARCN (15, 56). UCNs regulate autonomic, endocrine, emotional, and behavioral responses to stress (26). As mentioned above, UCNs are also involved in central cardiovascular regulation. Based on this information, we hypothesized that the ARCN may be one of the sites where UCNs exert their central cardiovascular actions. This hypothesis was tested in the present study by direct microinjections of UCNs into the ARCN.
METHODS
General procedures.
Adult male Wistar rats (n = 92, Charles River Laboratories, Wilmington, MA) weighing 300–360 g were used in this study. They were housed under controlled conditions with a 12:12-h light-dark cycle. Food and water were available to the animals ad libitum. The experimental protocols, designed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey.
Details of all the procedures used in the present study have been reported in our previous publications (2, 23, 24, 37). Rats were initially anesthetized with isoflurane (2–3% in 100% oxygen) to cannulate the trachea and a femoral vein and a femoral artery. Urethane (1.2–1.4 g/kg) was then injected intravenously in eight to nine aliquots, and the administration of isoflurane was discontinued. The appropriate depth of anesthesia was indicated by the absence of an increase in BP and/or withdrawal of the limb in response to pinching of a hindpaw. Rats were artificially ventilated, and end-tidal CO2 was maintained at 3.5–4.5%. Rectal temperature was continuously monitored and maintained at 37.0 ± 0.5°C. BP and HR were recorded on computer hard drive using 1401 plus analog-to-digital converter and Spike 2 software (Cambridge Electronic Design, Cambridge, UK).
Microinjections into the ARCN.
Rats were placed in a prone position in a stereotaxic instrument with a bite bar 18 mm below the interaural line. Multibarreled glass micropipettes (tip size: 20–40 μm) were used for microinjections. Micropipettes were placed at an angle (112°) pointing caudally with reference to the bregma. A hole (8–10 mm in diameter) was drilled in the midline at the junction of the two parietal bones caudal to the bregma. Glass micropipettes were inserted into the brain tissue through this hole on either side of the midline. For microinjections into the ARCN, the coordinates were 1.72–4.36 mm caudal to the bregma, 0.2–1 mm lateral to the midline, and 8.8–10.1 mm ventral to the dura. Microinjections of NMDA (10 mM) were used to identify the ARCN (37). In this and other series of experiments, unless otherwise indicated, the duration of microinjections was 5–10 s, the volume of the microinjection was 30 nl, microinjections of artificial cerebrospinal fluid (aCSF; pH 7.4) were used as controls, and all microinjections were unilateral.
Microinjections into the nAmb.
Microinjections into the nAmb were made using a dorsal approach (9, 10). The coordinates for the nAmb were 0.12 caudal to 0.64 mm rostral with reference to the calamus scriptorius, 1.8–2 mm lateral to the midline, and 2–2.4 mm deep from the dorsal surface of the medulla. The procedure for microinjections into the nAmb was similar to that described for microinjections into the ARCN except that l-Glu (5 mM) instead of NMDA was used to identify the nAmb sites eliciting bradycardia.
Microinjections into the NTS.
The dorsal medulla was exposed, and microinjections were made into the mNTS using the following coordinates: 0.5–0.6 mm rostral and 0.5–0.8 mm lateral to the calamus scriptorius and 0.5–0.7 mm deep from the dorsal medullary surface. mNTS sites eliciting depressor and bradycardic responses were identified by microinjections of l-Glu (5 mM) (8). The rest of the procedure was similar to that described for the ARCN.
Nerve recordings.
The renal nerve was exposed using a retroperitoneal approach and sectioned at its entry into the kidney, and a few millimeter length of the distal end was desheathed. Renal SNA (RSNA) was recorded using standard techniques (11, 37, 46). RSNA was inhibited by an increase in BP elicited by a bolus injection of l-phenylephrine (PE; 10 μg/kg iv), indicating that this activity was barosensitive. At the end of the experiment, the renal nerve was sectioned rostrally, and the residual activity was considered to be the noise level, which was subtracted from the whole nerve activity to calculate the basal nerve activity. Changes in RSNA were expressed as percent changes from the basal nerve activity.
Histological verification of microinjection sites.
At the end of the experiments, diluted (1: 50) green retrobeads (Lumafluor, Durham, NC) were microinjected (30 nl) into the ARCN to mark the microinjection site. In some of these rats (n = 10), green retrobeads diluted (1: 50) with NMDA solution (10 mM) were microinjected to estimate if the diffusion of NMDA was within the boundaries of the ARCN. In each procedure, animals were then perfused and fixed with 2% paraformaldehyde. Serial sections of the medulla were cut (40 μm), mounted on slides, covered with Citifluor Mountant media, and coverslipped. Microinjection sites were identified using a standard atlas (43).
Drugs and chemicals.
The following drugs and chemicals were used in this study: astressin (highly potent antagonist for CRF1Rs and CRF2Rs) (33), d-(−)-2-amino-7-phosphonoheptanoic acid (d-AP7; NMDA receptor antagonist) (44), l-Glu monosodium, isoflurane, 5-chloro-N-(cyclopropylmethyl)-2-methyl-N-propyl-N′-(2,4,6-trichloro-phenyl)-4,6-pyrimidinediamine hydrochloride (NBI-27914; specific CRF1R antagonist) (7), muscimol hydrobromide (GABAA receptor agonist), 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) disodium salt (non-NMDA receptor antagonist) (36), NMDA, PE hydrochloride, UCN1, UCN2, UCN3, and urethane. All of the solutions for the microinjections were freshly prepared in aCSF (pH 7.4). The concentrations mentioned for l-Glu, NBI-27914, muscimol, NBQX, and PE refer to their salts used in this study. Sources of the drugs were as follows: UCNs (American Peptide, Sunnyvale, CA), astressin, d-AP7, NBI-27914, and NBQX (R&D Systems, Minneapolis, MN), and isoflurane (Piramal Critical Care, Bethlehem, PA). All other drugs were purchased from Sigma-Aldrich (St. Louis, MO).
Statistical analyses.
Maximum changes in MAP and HR are expressed as means ± SE. One-way ANOVA followed by Tukey-Kramer's multiple-comparison test was used to evaluate the significance of maximum changes in MAP and HR in different groups of rats. All statistical analyses were done using a Student's paired t-test. The amplitude of integrated RSNA activity was averaged over a period of 30 s just before the microinjection of a substance into the ARCN or intravenous injection of PE. Changes in RSNA were expressed as percent changes from the basal value of this activity. Student's paired t-test was used to compare the mean values of the integrated nerve signals. Differences were considered significant at P < 0.05 in all cases.
RESULTS
Baseline values for MAP and HR in urethane-anesthetized rats were 99 ± 4 mmHg and 414 ± 14 beats/min, respectively (n = 92).
Microinjections of UCN1 into the ARCN.
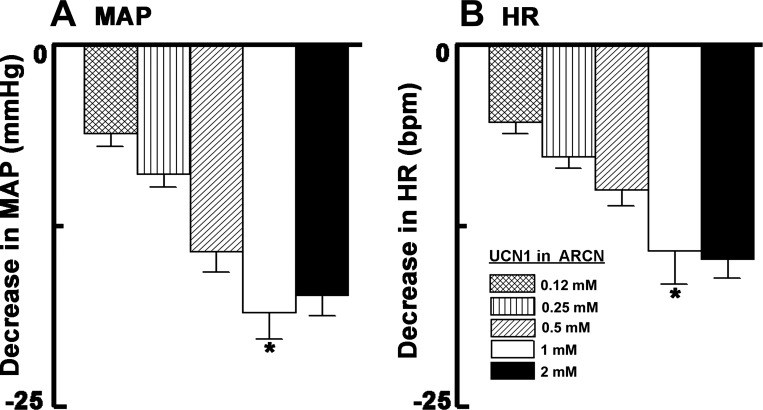
In this and other series of experiments, the ARCN was always identified by microinjections of NMDA (10 mM), which elicited decreases in MAP (19 ± 5 mmHg) and increases in HR (43 ± 7 beats/min, n = 15). The interval between microinjections of NMDA and other agents was at least 5 min. The decreases in MAP and HR elicited by microinjections (30 nl) of 0.12, 0.25, 0.5, 1.0, and 2.0 mM concentrations of UCN1 into the ARCN are shown in Fig. 1. Cardiovascular responses elicited by microinjections of UCN1 (1 mM) into the ARCN were greater than those elicited by 0.12 and 0.25 mM concentrations of UCN1 (P < 0.01–0.001; Fig. 1). The differences in depressor and bradycardic responses elicited by 1 and 2 mM concentrations of UCN1 were not significantly different (P > 0.05). Therefore, the 1 mM concentration of UCN1 was selected for other experiments. The onset and duration of cardiovascular responses to microinjections of UCN1 (1 mM) were 30 ± 4 s and 7 ± 1 min, respectively. The peak effect was observed at 3 ± 1 min. Maximum decreases (19 ± 5 mmHg) in MAP elicited by microinjections of NMDA (10 mM) into the ARCN were not significantly different (P > 0.05) from the decreases (14 ± 2 mmHg) elicited by UCN1 (1 mM) in this nucleus. In this and other experiments, microinjections of aCSF (30 nl) into different brain sites (the ARCN, nAmb, and NTS) elicited no cardiovascular responses. Unless indicated otherwise, all microinjections were unilateral.
Fig. 1.
Cardiovascular responses of different concentrations of urocortin 1 (UCN1). A: mean arterial pressure (MAP). *P < 0.01 compared with 0.12 and 0.25 mM. B: heart rate [HR; in beats/min (bpm)]. Microinjections (30 nl) of 0.12, 0.25, 0.5, 1.0, and 2.0 mM concentrations of UCN1 into the arcuate nucleus (ARCN) elicited decreases of 5 ± 1, 8 ± 1, 10 ± 1, 14 ± 2, and 15 ± 1 mmHg in MAP, respectively. The same concentrations of UCN1 at the same site elicited decreases of 6 ± 1, 9 ± 1, 14 ± 1, 19 ± 2, and 17 ± 1 beats/min in HR, respectively. *P < 0.001 compared with 0.12 and 0.25 mM.
Microinjections of UCN2 and UCN3 into the ARCN.
The decreases in MAP elicited by microinjections of UCN2 and UCN3 (1 mM each) were 10 ± 1 and 12 ± 2 mmHg, respectively. The depressor responses elicited by UCN2 and UCN3 were not significantly different (P > 0.05) from the depressor responses elicited by UCN1 (15 ± 2 mmHg). The decreases in HR elicited by microinjections of UCN2 and UCN3 (1 mM each) were 12 ± 1 and 13 ± 2 beats/min, respectively. Although the decreases in HR elicited by both UCN2 and UCN3 were smaller than that elicited by UCN1 (19 ± 2 beats/min), only the difference between UCN1 and UCN2 reached statistical significance (P < 0.05). Because the responses elicited by UCN2 and UCN3 were qualitatively similar to those elicited by UCN1, detailed experiments on the mechanisms of action were carried out only for UCN1.
Site specificity of UCN1-induced responses.
Microinjections of UCN1 (1 mM) into the ARCN elicited decreases in MAP (14 ± 2 mmHg) and HR (19 ± 3 beats/min, n = 5). On the other hand, microinjections of the same concentration of UCN1 into the adjacent hypothalamic ventromedial nucleus (VMN) elicited increases in MAP (7 ± 1 mmHg) and HR (14 ± 2 beats/min). Both ARCN and VMN sites were previously identified by microinjections of NMDA (10 mM); microinjections of NMDA into the ARCN elicited decreases in MAP (19 ± 5 mmHg) and increases in HR (43 ± 7 beats/min), whereas similar microinjections into the VMN elicited increases in MAP (22 ± 11 mmHg) and HR (39 ± 9 beats/min).
Reproducibility of UCN1-induced responses.
The decreases in MAP in response to three consecutive microinjections of UCN1 (1 mM) into the ARCN at 60-min intervals were 16 ± 3, 18 ± 3, and 14 ± 3 mmHg, respectively, and the decreases in HR were 17 ± 1, 16 ± 1, and 19 ± 2 beats/min, respectively (n = 5). Repeated-measures ANOVA showed that these values were not statistically different (P > 0.05). Therefore, in all experiments, the interval between the microinjections of UCN1 was at least 60 min to avoid tachyphylaxis.
Effect of NBI-27914 on UCN1-induced responses.
Microinjections of UCN1 (1 mM) into an ARCN site (previously identified by microinjections of NMDA) elicited depressor and bradycardic responses (n = 6). After an interval of 60 min, NBI-27914 (0.5 mM/30 nl, specific CRF1R antagonist) was microinjected at the same site. NBI-27914 by itself did not elicit any changes in BP and HR. Two minutes later, the decreases in MAP and HR elicited by microinjections of UCN1 (1 mM) at the same site were significantly (P < 0.05) attenuated (Table 1). In another group of rats (n = 6), a higher concentration of NBI-27914 (1 mM/30 nl) elicited a more pronounced attenuation (P < 0.001) of UCN1-induced decreases in BP and HR (Table 1). The UCN1-induced decreases in MAP and HR did not recover to initial values within 60 min after the microinjection of NBI-27914 (1 mM) into the ARCN. Microinjections of NBI-27914 did not alter the decreases in MAP and increases in HR elicited by microinjections of NMDA into the ARCN.
Table 1.
Blockade of UCN1-induced responses in the ARCN
| UCN1-Induced Decreases in MAP, mmHg |
UCN1-Induced Decreases in HR, beats/min |
|||
|---|---|---|---|---|
| Drug Dose and Site of Injection | Before | After | Before | After |
| NBI-27914 in the ARCN (0.5 mM) | 12 ± 1 | 6 ± 1* | 15 ± 3 | 8 ± 2* |
| NBI-27914 in the ARCN (1 mM) | 12 ± 1 | 4 ± 1‡ | 16 ± 1 | 5 ± 1‡ |
| Astressin in the ARCN (0.5 mM) | 13 ± 2 | 5 ± 1* | 17 ± 2 | 5 ± 2† |
| Astressin in the ARCN (1 mM) | 13 ± 1 | 3 ± 1‡ | 18 ± 2 | 3 ± 1‡ |
| Muscimol in the nAmb (1 mM) | 17 ± 3 | 13 ± 1 | 21 ± 3 | 6 ± 2§ |
| NBQX (2 mM) + d-AP7 (5 mM) in the NTS | 20 ± 3 | 9 ± 2* | 23 ± 4 | 6 ± 1† |
Values are means ± SE; n = 6 animals/group, except nAmb group where n = 9. UCN1, urocortin; MAP, mean arterial pressure; HR, heart rate; NBI-27914, 5-chloro-N-(cyclopropylmethyl)-2-methyl-N-propyl-N′-(2,4,6-trichloro-phenyl)-4,6-pyrimidinediamine hydrochloride [corticotropin-releasing factor (CRF)-1 receptor antagonist]; NBQX, 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione; d-AP7, d-(−)-2-amino-7-phosphonoheptanoic acid (ionotropic glutamate receptor antagonist); ARCN, hypothalamic arcuate nucleus; nAmb, nucleus ambiguus; mNTS, medial nucleus tractus solitarius. Astressin was used as a CRF-1 receptor and CRF-2 receptor antagonist, and muscimol was used as a GABAAreceptor agonist (inhibits neurons).
P < 0.05;
P < 0.01;
P < 0.001;
P < 0.0001.
Effect of astressin on UCN1-induced responses.
In this group of rats (n = 6), microinjections of UCN1 (1 mM) into the ARCN site elicited depressor and bradycardic responses. After an interval of 60 min, astressin (0.5 mM, CRF1R and CRF2R antagonist) was microinjected at the same site; astressin by itself did not elicit any changes in BP and HR. Two minutes later, the decreases in MAP and HR elicited by microinjections of UCN1 (1 mM) at the same site were significantly (P < 0.05–0.01) attenuated (Table 1). In another group of rats (n = 6), a higher concentration of astressin (1 mM) elicited a more pronounced (P < 0.001) attenuation of UCN1-induced decreases in MAP and HR (Table 1). The UCN1-induced decreases in MAP and HR did not recover to initial values within 60 min after the microinjection of astressin (1 mM) into the ARCN. Microinjections of astressin did not alter the decreases in MAP and increases in HR elicited by microinjections of NMDA into the ARCN.
Effect of microinjections of UCN1 into the ARCN on renal nerve activity.
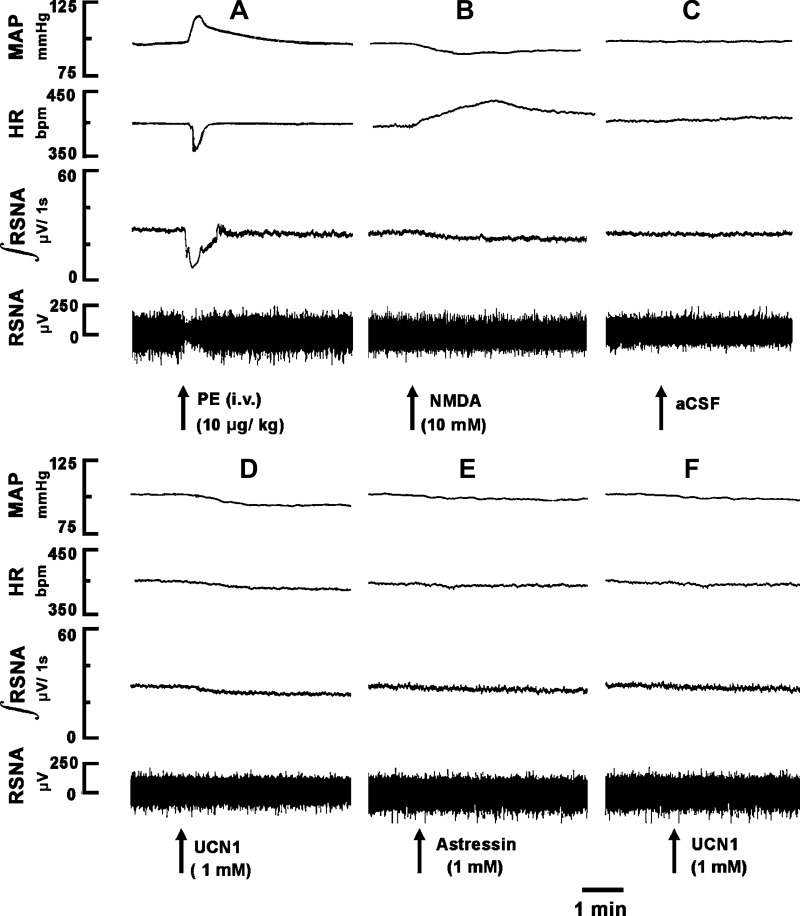
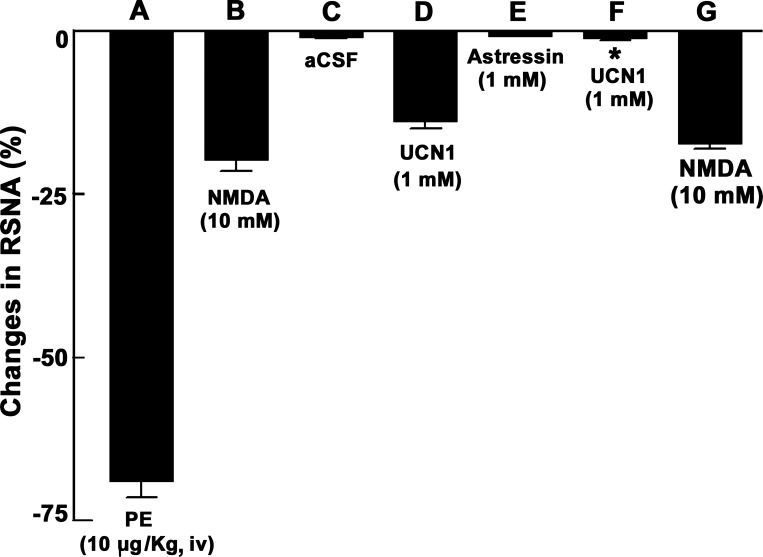
A bolus injection of PE (10 μg/kg iv) increased MAP, which, in turn, elicited reflex bradycardia and inhibition of efferent RSNA lasting for 26 ± 4 s (n = 5; Fig. 2A). Ten minutes later, when RSNA recovered to baseline, a microinjection of NMDA (10 mM) into the ARCN elicited a decrease in RSNA (Fig. 2B). After an interval of 20 min, microinjection of aCSF (30 nl) into the same ARCN site did not alter RSNA (Fig. 2C). Five minutes later, microinjection of UCN1 (1 mM) into the ARCN elicited a decrease in efferent RSNA (Fig. 2D). Sixty minutes later, astressin (1 mM) was microinjected into the ARCN; no changes in RSNA were elicited (Fig. 2E). Two minutes later, UCN1 (1 mM) was again microinjected at the same site; the effect of UCN1-induced inhibition of RSNA was blocked (Fig. 2F). Microinjection of NMDA (10 mM) continued to elicit decreases in efferent RSNA (not shown). Group data for this experiment are shown in Fig. 3. All changes in RSNA were compared with basal nerve activity. Intravenous bolus injection of PE (10 μg/kg) decreased RSNA significantly (69 ± 3%, P < 0.05; Fig. 3A). Microinjections of NMDA (10 mM) into the ARCN elicited a significant (P < 0.05) decrease in RSNA (20 ± 2%; Fig. 3B). At the same site, microinjection of aCSF did not elicit significant changes in RSNA (Fig. 3C). Five minutes later, microinjections UCN1 (1 mM) into the ARCN elicited a significant (P < 0.05) decrease (14 ± 1%) in RSNA (Fig. 3D). Sixty minutes later, astressin (1 mM) was microinjected at the same site, and no significant changes in RSNA were elicited (Fig. 3E). Two minutes later, the decrease in RSNA elicited by microinjection of UCN1 (1 mM) into the ARCN was significantly attenuated (1 ± 1%, P < 0.05; Fig. 3F) compared with the UCN1-induced decrease before the microinjection of astressin (Fig. 3D). After microinjection of astressin, NMDA (10 mM) microinjected into the ARCN continued to elicit a decrease (17 ± 1%) in RSNA (Fig. 3G), which was not significantly different compared with the NMDA-induced decrease before the microinjection of astressin (P > 0.05; Fig. 3B).
Fig. 2.
Effect of microinjection of UCN1 (1 mM) into the ARCN on renal sympathetic nerve activity (RSNA). The top trace shows MAP (in mmHg), the middle top trace shows HR (in beats/min), the middle bottom trace shows integrated RSNA (∫RSNA; in μV/1s), and the bottom trace shows whole RSNA (in μV). A: phenylephrine (PE; 10 μg/kg iv) elicited a pressor response accompanied by reflex inhibition of HR and RSNA. B: 10 min later, microinjection of N-methyl-d-aspartic acid (NMDA; 10 mM) into the ARCN decreased MAP, integrated RSNA, and whole RSNA and increased HR. C: no responses were observed after microinjection of artificial cerebrospinal fluid (aCSF) into the ARCN. D: 20 min later, microinjection of UCN1 (1 mM) into the ARCN decreased MAP, HR, integrated RSNA, and whole RSNA. E: 60 min later, microinjection of astressin (1 mM) into the ARCN did not elicit any response. F: 2 min later, microinjection of UCN1 (1 mM) at the same site failed to elicit a response.
Fig. 3.
Group data showing changes in RSNA elicited by microinjection of UCN1 (1 mM) into the ARCN and its blockade by astressin. A: effect of PE (10 μg/kg iv). B: microinjection of NMDA (10 mM) into the ARCN. C: microinjection of aCSF into the ARCN. D: UCN1 (1 mM)-induced decreases in RSNA. E: effect of Astressin (1 mM) on RSNA. F: blockade of UCN1 (1 mM)-induced inhibition of RSNA by astressin (1 mM). G: astressin did not alter NMDA-induced decreases in RSNA. *P < 0.05 compared with D.
Effect of bilateral vagotomy on UCN1-induced responses.
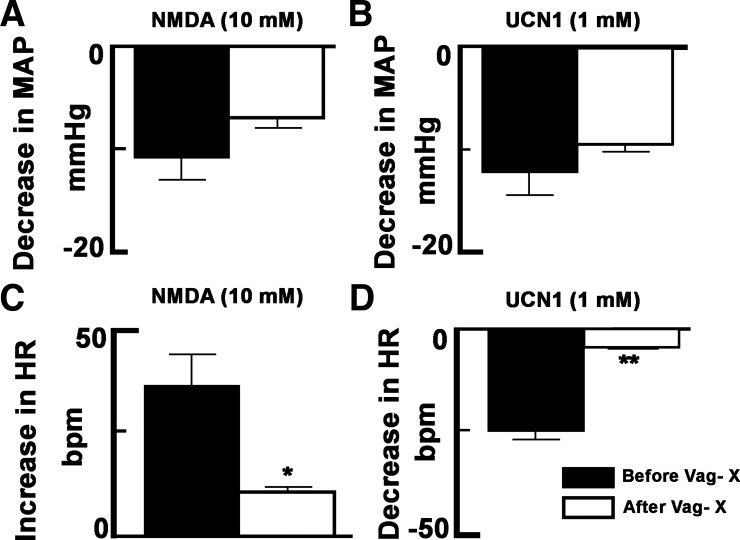
Baseline values of MAP before and after vagotomy were 102 ± 3 and 97 ± 2 mmHg, respectively; there was no significant change (P > 0.05) in MAP after vagotomy. Baseline values for HR before and after vagotomy were 404 ± 3 and 437 ± 8 beats/min, respectively; there was a significant (P < 0.001) increase in baseline HR (33 ± 7 beats/min) after vagotomy. Bilateral vagotomy did not alter the decreases in MAP elicited by microinjections of either NMDA (10 mM) or UCN1 (1 mM) into the ARCN (n = 5). The decreases in MAP elicited by NMDA (10 mM) before and after vagotomy were 11 ± 2 and 7 ± 1 mmHg, respectively (P > 0.05; Fig. 4A), and the decreases in MAP elicited by UCN1 (1 mM) before and after vagotomy were 12 ± 2 and 10 ± 1 mmHg, respectively (P > 0.05; Fig. 4B). However, the increases in HR elicited by the same concentrations of NMDA in the ARCN and decreases in HR elicited by UCN1 in the ARCN were significantly reduced after vagotomy. The increases in HR elicited by NMDA (10 mM) before and after vagotomy were 36 ± 7 and 11 ± 1 beats/min, respectively (P < 0.01; Fig. 4C), and the decreases in HR elicited by UCN1 (1 mM) before and after vagotomy were 25 ± 2 and 5 ± 1 beats/min, respectively (P < 0.001; Fig. 4D).
Fig. 4.
Effect of bilateral vagotomy (Vag-X) on NMDA- and UCN1-induced cardiovascular responses elicited from the ARCN. A: the decreases in MAP elicited by microinjections of NMDA (10 mM) into the ARCN before and after Vag-X were 11 ± 2 and 7 ± 1 mmHg, respectively. B: the decreases in MAP elicited by microinjections of UCN1 (1 mM) into the ARCN before and after Vag-X were 12 ± 2 and 10 ± 1 mmHg, respectively. C: the increases in HR elicited by microinjections of NMDA (10 mM) into the ARCN before and after Vag-X were 36 ± 7 and 11 ± 1 beats/min, respectively. *P < 0.01 compared with before Vag-X. D: the decreases in HR elicited by microinjections of UCN1 (1 mM) into the ARCN before and after Vag-X were 25 ± 2 and 5 ± 1 beats/min, respectively. **P < 0.001 compared with before Vag-X.
Effect of inhibition of the nAmb on responses elicited by UCN1 in the ARCN.
In this group of rats (n = 9), microinjections of UCN1 (1 mM) into the ARCN elicited decreases in MAP and HR (Table 1). After an interval of 60 min, l-Glu (5 mM, 30 nl) was microinjected into the nAmb; a decrease in HR was elicited without any changes in MAP, indicating that the site was in the nAmb. Five minutes later, microinjections of aCSF into the nAmb elicited no cardiovascular responses. Two minutes later, muscimol (1 mM) was microinjected into the nAmb; an increase in baseline HR was elicited. Within 5 min, UCN1 (1 mM) was again microinjected into the ARCN; the decreases in HR elicited by microinjection of UCN1 (1 mM) into the ARCN were significantly attenuated (P < 0.0001; Table 1). On the other hand, the decreases in MAP elicited by microinjection of UCN1 (1 mM) into the ARCN were not significantly altered by microinjections of muscimol into the nAmb (Table 1). UCN1-induced decreases in HR into the ARCN did not completely recover to initial values within 60 min of the microinjection of muscimol into the nAmb. Typical tracings of these results are shown in Fig. 5.
Fig. 5.
Effect of inhibition of the nucleus ambiguus (nAmb) on UCN1-induced bradycardic responses elicited from the ARCN. The top trace shows pulsatile arterial pressure (PAP; in mmHg), the middle trace shows MAP (in mmHg), and the bottom trace shows HR (in beats/min). A: microinjection of NMDA (10 mM) into the ARCN elicited decreases in PAP and MAP and increases in HR. After blood pressure (BP) and HR recovered to baseline levels, aCSF was microinjected into the ARCN, and no changes were observed (not shown). B: 20 min later, microinjection of UCN1 (1 mM) into the ARCN elicited decreases in PAP, MAP, and HR. C: 60 min later, the nAmb was identified with l-glutamate (l-Glu; 5 mM), and a decrease in HR was elicited with no changes in PAP and MAP. After the recovery of HR, aCSF was microinjected into the nAmb, and no changes were observed (not shown). Five minutes later, muscimol (1 mM) was microinjected into the nAmb, and an increase in HR was elicited (not shown): D: 5 min later, microinjection of UCN1 (1 mM) into the ARCN failed to elicit decreases in HR, whereas decreases in PAP and MAP persisted.
Effect of blockade of iGLURs in the NTS on UCN1-induced responses in the ARCN.
Microinjection of UCN1 (1 mM) into the ARCN elicited decreases in MAP and HR (n = 6; Table 1). Sixty minutes later, sequential microinjections of NBQX (2 mM) and d-AP7 (5 mM), delivered within 1 min, into the ipsilateral NTS elicited no significant changes on MAP and HR. Within 2 min of the microinjections of these iGLUR antagonists into the NTS, the decreases in MAP and HR elicited by microinjections of UCN1 into the ipsilateral ARCN were significantly (P < 0.05–0.01) attenuated (Table 1). The responses to UCN1 did not recover after a 60-min waiting period.
Effect of microinjection of naloxone into the ARCN on UCN1-induced responses.
Microinjection of UCN1 (1 mM) into an ARCN site elicited decreases in MAP (20 ± 3 mmHg) and HR (25 ± 5 beats/min, n = 6). After an interval of 60 min, microinjections of naloxone (10 mM) into the same site elicited no significant changes in MAP and HR. Within 2 min, the HR decreases (1 ± 1 beats/min) elicited by microinjections of UCN1 (1 mM) at the same ARCN site were significantly attenuated (P < 0.001). Furthermore, after a delay of 2–3 min after the UCN1 microinjection, HR increased (16 ± 5 beats/min). The bradycardic responses elicited by microinjections of UCN1 into the ARCN before the microinjection of naloxone did not recur for at least 60 min after the naloxone microinjection. Although microinjections of naloxone into the ARCN attenuated the decreases in MAP elicited by microinjections of UCN1 into the ARCN, the difference did not reach statistical significance; UCN1-induced decreases in MAP before and after the microinjection of naloxone were 20 ± 3 and 16 ± 2 mmHg, respectively (P > 0.05).
Effect of barodenervation on UCN1-induced responses.
Baseline values of MAP before and after barodenervation were 100 ± 2 and 124 ± 2 mmHg, respectively; there was a significant (P < 0.001) increase in baseline MAP (24 ± 2 mmHg) after barodenervation. Baseline values of HR before and after barodenervation were 402 ± 10 and 420 ± 12 beats/min, respectively; there was a significant (P < 0.001) increase in baseline HR (18 ± 2 beats/min) after barodenervation. In bilaterally barodenervated rats (n = 7), microinjection of NMDA (10 mM) into the ARCN elicited increases in MAP (21 ± 4 mmHg) and HR (26 ± 6 beats/min). At the same site, microinjection of a maximally effective dose of UCN1 (1 mM) into the ARCN elicited increases in MAP (11 ± 2 mmHg) and HR (14 ± 2 beats/min), respectively.
Histology.
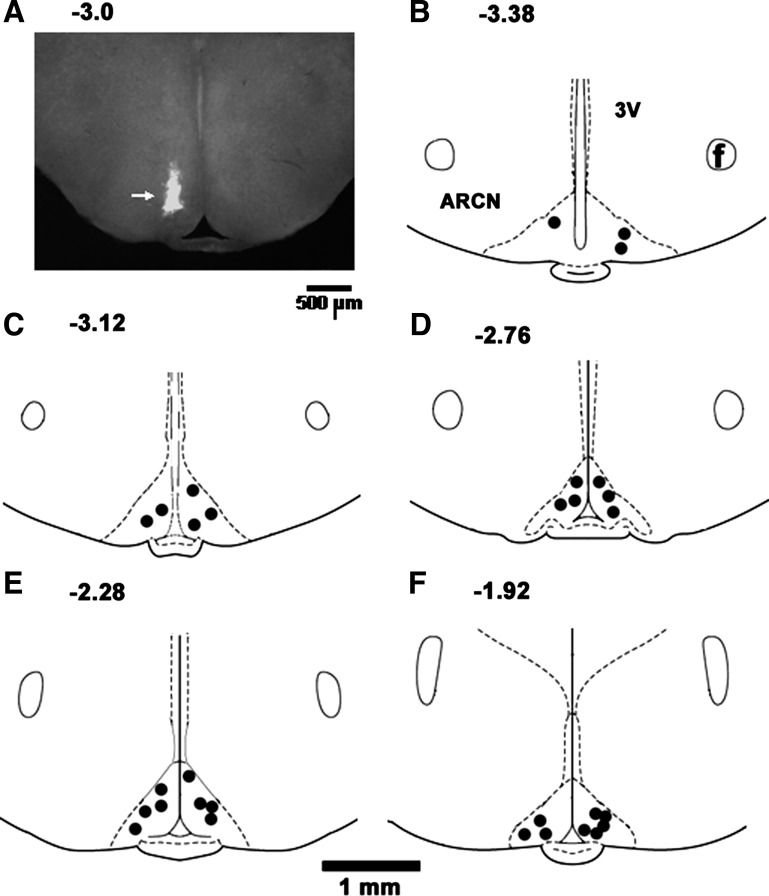
A typical ARCN site marked by microinjection (30 nl) of diluted (1:50) green retrobeads containing NMDA (10 mM) is shown in Fig. 6A. Composite diagrams of marked ARCN sites that were located 3.48–1.92 mm caudal to the bregma are shown in Fig. 6, B–F (n = 30; overlapping sites are not shown). Rats in which the diffusion sphere of the marker was not within the boundaries of the ARCN, as shown in a standard atlas (43), were not included in the study.
Fig. 6.
Histological identification of microinjection sites in the ARCN. A: coronal section at a level of 3.0 mm caudal to the bregma showing a microinjection site in the ARCN, which was marked by a microinjection of diluted (1:50) green retrobeads IX containing NMDA (10 mM, 30 nl, arrow). The center of the spot was 0.35 mm lateral to the midline and 9.5 mm deep from the dura. B–F: drawings of coronal sections 3.48 (B), 3.12 (C), 2.76 (D), 2.28 (E), and 1.92 mm (F) caudal to the bregma showing the ARCN microinjection sites as dark spots. Each spot represents a site in one animal; the microinjection sites were located in the ARCN, 0.1–0.6 mm lateral to the midline and 9.8–10.1 mm deep from the dura. 3V, third ventricle; f, fornix.
DISCUSSION
The main observations of this study were as follows: 1) microinjections of UCN1 into the ARCN elicited decreases in MAP, HR, and RSNA; 2) blockade of either CRF1Rs or both CRF1Rs and CRF2Rs in the ARCN attenuated cardiovascular responses to microinjections of UCN1 into the ARCN; 3) bilateral vagotomy attenuated the decreases in HR elicited by UCN1 into the ARCN; 4) inhibition of the nAmb by microinjections of muscimol attenuated the decreases in HR but not MAP elicited by microinjections of UCN1 into the ipsilateral ARCN; 5) blockade of iGLURs in the ipsilateral NTS attenuated the decreases in MAP and HR elicited by microinjections of UCN1 into the ipsilateral ARCN; 6) blockade of opiate receptors in the ARCN attenuated the decreases in HR elicited by microinjections of UCN1 at the same site, and HR was increased after a short delay; 7) in barodenervated rats, microinjections of UCN1 into the ARCN elicited increases (instead of decreases) in MAP and HR; and 8) microinjections of UCN2 and UCN3 into the ARCN also elicited decreases in MAP and HR.
All microinjections were made unilaterally because bilateral microinjections would require repeated withdrawal and insertion of the micropipette into the ARCN on two sides, which would cause undesirable tissue damage and the reliability of making injections at the same site would be reduced. Unilateral microinjections allowed us to keep the micropipette at the injection site for the duration of the experiment once the site of microinjection in the ARCN, NTS, or nAmb was identified. Moreover, the projections of the ARCN to cardiovascular regulatory brain regions, such as the PVN, NTS, caudal ventrolateral medullary depressor area (CVLM), rostral ventrolateral medullary pressor area (RVLM), and spinal intermediolateral cell column (IML), are predominantly ipsilateral (29, 30, 49). There are no direct projections from the ARCN to the nAmb (12, 49). Although direct projections from the ARCN to the NTS, CVLM, RVLM, and IML exist, these projections are sparse (49). Cardiovascular responses elicited from the ARCN are primarily mediated via the ipsilateral PVN (23, 24). Direct projections of the PVN to the NTS, nAmb, CVLM, RVLM, and IML exist and are predominantly ipsilateral (12, 22, 49). This anatomic arrangement allowed us to microinject UCN1 into the ARCN on one side and study the alterations in the responses elicited from the ARCN by microinjections of antagonists for different receptors into the ipsilateral NTS or nAmb.
The possibility that the spread of UCN1 to the adjacent VMN may have contributed to the cardiovascular responses elicited from the ARCN was excluded because in the VMN microinjections of UCN1 elicited increases in MAP and HR, whereas similar microinjections into the ARCN elicited decreases in MAP and HR. Distortion of the brain tissue or any other nonspecific effects did not contribute to the observed cardiovascular responses because microinjections of aCSF into the ARCN did not elicit any cardiovascular responses. Consistent with our previous observations on the effects of UCNs in other brain areas such as the NTS and nAmb, UCN1 showed a bell-shaped concentration response (9, 10, 38, 39). This type of concentration response can be explained by homotropic allostery, i.e., the agonist at higher concentrations binds to a modulator site, which affects the receptor function, resulting in attenuated responses (5).
Prior microinjections of specific CRF1R and nonselective CRF1R and CRF2R antagonists into the ARCN significantly attenuated UCN1-induced responses, confirming that these cardiovascular changes were mediated through both CRF1Rs and CRF2Rs. The interval between two microinjections of UCN1 was at least 60 min. At this time interval, no tachyphylaxis of cardiovascular responses to microinjections of UCN1 was observed.
The pathways that mediate cardiovascular responses elicited from the ARCN are complex because of the diversity of chemical phenotypes of ARCN neurons and multiple connections of the ARCN with other brain areas regulating BP and HR (50). Based on a recent study (49) on the role of the ARCN in cardiovascular regulation, the mechanism of cardiovascular responses elicited by UCN1 microinjections into the ARCN can be speculated as described below.
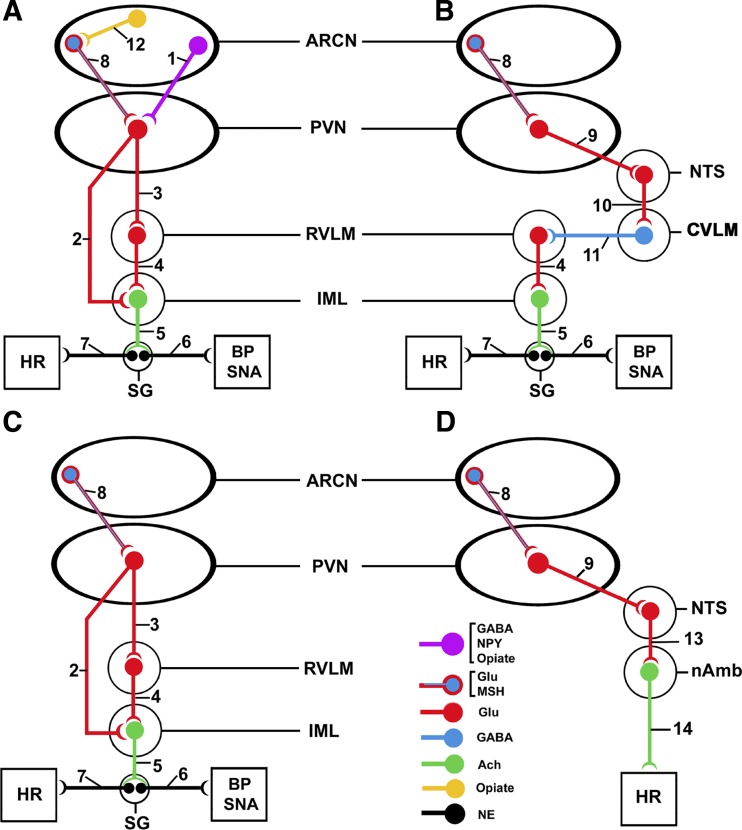
The decreases in MAP, RSNA, and HR elicited by microinjections of UCN1 into the ARCN may involve the following pathways. Microinjections of UCN1 into the ARCN may excite neurons containing NPY, GABA, and opiates that project to the PVN (49, 50). Stimulation of these neurons results in the release of inhibitory neurotransmitters (NPY, GABA, and opiates) in the PVN (Fig. 7A, pathway 1). The activity of PVN glutamatergic neurons projecting to the IML directly (Fig. 7A, pathway 2) or via the RVLM (Fig. 7A, pathways 3–4) is reduced, resulting in decreased activity of sympathetic preganglionic neurons (SPGNs) located in the IML (32). The reduction in the activity of SPGNs causes decreases in BP, RSNA, and HR (Fig. 7A, pathways 5–7) (48).
Fig. 7.
Schematic diagram showing the possible pathways mediating the cardiovascular responses to UCN1 in the ARCN (see details of pathways in the discussion). PVN, paraventricular nucleus; RVLM, rostral ventrolateral medullary pressor area; IML, spinal intermediolateral cell column; SNA, sympathetic nerve activity; NTS, nucleus tractus solitarius; CVLM, caudal ventrolateral medullary depressor area; SG, sympathetic ganglion; NPY, neuropeptide Y; MSH, α-melanocyte-stimulating hormone; NE, norepinephrine.
Decreases in MAP, RSNA, and HR elicited by microinjections of UCN1 into the ARCN may also involve the stimulation of ARCN neurons containing l-Glu and α-melanocyte-stimulating hormone (α-MSH; Fig. 7B, pathway 8) (23, 24). The release of l-Glu and/or α-MSH in the PVN may activate glutamatergic neuronal projections to the NTS (Fig. 7B, pathway 9) (22). Stimulation of glutamatergic NTS neurons results in the activation of GABAergic neurons in the CVLM (Fig. 7B, pathway 10). The increase in the activity of GABAergic CVLM neurons causes the release of GABA in the RVLM, decreasing the activity of RVLM neurons (Fig. 7B, pathway 11) with consequent decreases in BP, RSNA, and HR via pathways 4–7, as shown in Fig. 7B (48).
Microinjections of UCN1 into the ARCN of barodenervated rats elicited increases in MAP and HR. Barodenervation increases the excitability of RVLM neurons due to removal of tonic inhibitory input from the CVLM. Activation of ARCN neurons containing l-Glu/α-MSH by microinjections of UCN1 (Fig. 7C, pathway 8) results in increased activity of PVN glutamatergic neurons, which, in turn, causes increases in MAP and HR via the activation of SPGNs in the IML (Fig. 7C, pathways 2–7).
Bilateral vagotomy attenuated bradycardic responses elicited by microinjection of UCN1 into the ARCN. As described above, there are no direct projections from the ARCN to the nAmb (12, 49). However, it is known that ARCN neurons project to the PVN, which projects to the NTS (22, 23, 24). The NTS is known to project to the nAmb (34). UCN1-induced activation of l-Glu/α-MSH neurons in the ARCN may result in the stimulation of NTS neurons (Fig. 7D, pathways 8 and 9). The neurotransmitter released in the NTS may be predominantly l-Glu because blockade of iGLURs in the NTS attenuated UCN1-induced bradycardia elicited from the ARCN. Increased activity of NTS neurons results in the stimulation of preganglionic vagal neurons in the nAmb (Fig. 7D, pathway 13), and HR is decreased by increasing the vagal input to the heart (Fig. 7D, pathway 14) (48).
The preganglionic vagal neurons innervating the heart are located predominantly in the subcompact formation surrounding the nAmb (34, 41, 51). It was, therefore, hypothesized that activation of ARCN neurons by UCN1 may directly or indirectly excite nAmb neurons and elicit bradycardia via vagal activation. To test this hypothesis, the effect of inhibition of neurons in the nAmb on UCN1-induced HR responses was also studied. Microinjections of muscimol into the nAmb significantly attenuated bradycardic responses elicited by UCN1 into the ipsilateral ARCN. Our results confirmed that microinjections of UCN1 into the ARCN directly or indirectly activate nAmb neurons, resulting in vagally mediated bradycardia.
Prior microinjections of naloxone (a competitive antagonist at opiate receptors) into the ARCN attenuated the decreases in HR elicited by UCN1 in the ARCN. This observation can be explained as follows. The presence of opiate (β-endorphin)-containing neurons has been reported in the ARCN (20, 27, 45). CRFRs may be located on opiate-containing neurons in the ARCN. Binding of UCN1 to CRFRs on these neurons may release an opiate in the ARCN (Fig. 7A, pathway 12). Opiate receptors may be located on l-Glu/α-MSH neurons in the ARCN. Inhibition of these l-Glu/α-MSH neurons results in a decrease in their input to glutamatergic PVN neurons (Fig. 7A, pathway 8). A decrease in the activity of PVN glutamatergic neurons results in decreased activity of projections from PVN neurons to SPGNs located in the IML (Fig. 7A, pathways 2–4). The decrease in the activity of SPGNs in the IML causes a decrease in HR (Fig. 7A, pathways 5 and 7). After microinjections of naloxone into the ARCN, UCN1 microinjections into the same nucleus elicited a delayed (within 2–3 min) increase in HR. This observation can be explained as follows. The opiate-induced decrease in the activity of l-Glu/α-MSH neurons in the ARCN (Fig. 7D, pathway 8) results in decreased activity of PVN glutamatergic neurons projecting to the nAmb (Fig. 7D, pathways 9, 13, and 14), causing an increase in HR.
Microinjections of NMDA into the ARCN always elicited an increase in HR, which was mediated via both activation of sympathetic inputs and inhibition of parasympathetic inputs to the heart (37). The neural pathways within the brain mediating the tachycardic effects of NMDA in the ARCN are not known.
Perspectives and significance.
Microinjections of CRFR antagonists (astressin or NBI-27914) alone into the ARCN did not elicit any changes in BP and HR, indicating that CRFRs in the ARCN are not involved in the tonic regulation of BP and HR under normal physiological conditions. However, CRFRs and their endogenous ligands, UCNs, may be involved in the regulation of cardiovascular function during some pathological or pathophysiological situations. One such situation is long-term stress adaptation, in which UCNs have been reported to play a role (40). Activation of CRFRs by UCNs results in a reduction of anxiety-like behavior and recovery from stress (53, 54, 55). Based on this information, it may be hypothesized that in stressful situations, UCNs released in the ARCN may oppose the cardiovascular effects of stress. Stress has been reported to elevate BP and HR (4, 13, 16). In this study, we demonstrated that UCNs microinjected into the ARCN elicit decreases in BP and HR. Thus, release of UCNs in the ARCN may be an endogenous mechanism by which cardiovascular effects of stress are ameliorated. In this context, it may be noted that the ARCN is one of brain sites involved in mediating stress responses (3). The data presented in the present investigation provide a basis on which future studies on the role of UCNs in the regulation of cardiovascular functions during stress can be designed.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-024347 and HL-076248 (to H. N. Sapru).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.C.C., K.K., and H.N.S. conception and design of research; V.C.C. and K.K. performed experiments; V.C.C., K.K., and H.N.S. analyzed data; V.C.C., K.K., and H.N.S. interpreted results of experiments; V.C.C., K.K., and H.N.S. prepared figures; V.C.C. and H.N.S. drafted manuscript; V.C.C. and H.N.S. edited and revised manuscript; V.C.C., K.K., and H.N.S. approved final version of manuscript.
REFERENCES
- 1. Aguilera G, Nikodemova M, Wynn PC, Catt KJ. Corticotropin releasing hormone receptors: two decades later. Peptides 25: 319–329, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Arakawa H, Chitravanshi VC, Sapru HN. Hypothalamic arcuate nucleus: a new site of cardiovascular action of angiotensin-(1–12) and angiotensin II. Am J Physiol Heart Circ Physiol 300: H951–H960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baffi JS, Palkovits M. Fine topography of brain areas activated by cold stress. A fos immunohistochemical study in rats. Neuroendocrinology 72: 102–113, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bedi M, Varshney VP, Babbar R. Role of cardiovascular reactivity to mental stress in predicting future hypertension. Clin Exp Hypertens 22: 1–22, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Bindslev N. A homotropic two-state model and auto-antagonism. BMC Pharmacol 4: 11–22, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol 589: 1643–1662, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen C, Dagnino R, Jr, De Souza EB, Grigoriadis DE, Huang CQ, Kim KI, Liu Z, Moran T, Webb TR, Whitten JP, Xie YF, McCarthy JR. Design and synthesis of a series of non-peptide high-affinity human corticotropin-releasing factor1 receptor antagonists. J Med Chem 39: 4358–4360, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Chitravanshi VC, Sapru HN. Mechanism of cardiovascular effects of nociceptin microinjected into the nucleus tractus solitarius of the rat. Am J Physiol Regul Integr Comp Physiol 288: R1553–R1562, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Chitravanshi VC, Sapru HN. Microinjections of urocortin1 into the nucleus ambiguus of the rat elicit bradycardia. Am J Physiol Heart Circ Physiol 300: H223–H229, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chitravanshi VC, Proddutur A, Sapru HN. Cardiovascular actions of angiotensin-(1–12) in the hypothalamic paraventricular nucleus of the rat are mediated via angiotensin II. Exp Physiol 97: 1001–1017, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chitravanshi VC, Kawabe K, Sapru HN. Bradycardic effects of microinjections of urocortin 3 into the nucleus ambiguus of the rat. Am J Physiol Regul Integr Comp Physiol 303: R1023–R1030, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ciriello J, McMurray JC, Babic T, deOliveira CVR. Collateral axonal projections from hypothalamic hypocretin neurons to cardiovascular sites in nucleus ambiguus and nucleus tractus solitarius. Brain Res 991: 133–141, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Coste SC, Quintos RF, Stenzel-Poore MP. Corticotropin-releasing hormone-related peptides and receptors: emergent regulators of cardiovascular adaptations to stress. Trends Cardiovasc Med 12: 176–182, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci 23: 71–77, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol 28: 1–27, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grippo AJ, Johnson AK. Stress, depression and cardiovascular dysregulation: a review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress 12: 1–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hashimoto K, Nishiyama M, Tanaka Y, Noguchi T, Asaba K, Hossein PN, Nishioka T, Makino S. Urocortins and corticotropin releasing factor type 2 receptors in the hypothalamus and the cardiovascular system. Peptides 25: 1711–1721, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev 55: 21–26, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med 7: 605–611, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Ibata Y, Kawakami F, Okamura H, Obata-Tsuto HL, Morimoto N, Zimmerman EA. Light and electron microscopic immunocytochemistry of β-endorphin/β-LPH-like immunoreactive neurons in the arcuate nucleus and surrounding areas of the rat hypothalamus. Brain Res 341: 233–242, 1985 [DOI] [PubMed] [Google Scholar]
- 21. Jones DN, Kortekaas R, Slade PD, Middlemiss DN, Hagan JJ. The behavioural effects of corticotropin-releasing factor-related peptides in rats. Psychopharma (Berl) 138: 124–132, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN. Cardiovascular function of a glutamatergic projection from the hypothalamic paraventricular nucleus to the nucleus tractus solitarius. Neuroscience 153: 605–617, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kawabe T, Kawabe K, Sapru HN. Cardiovascular responses to chemical stimulation of the hypothalamic arcuate nucleus in the rat: role of the hypothalamic paraventricular nucleus. PLos One 7: e45180, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawabe T, Kawabe K, Sapru HN. Effect of barodenervation on cardiovascular responses elicited from the hypothalamic arcuate nucleus of the rat. PLos One 7: e53111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klenerova V, Kaminsky O, Sida P, Hlinak Z, Krejci I, Hynie S. Impaired passive avoidance acquisition in Wistar rats after restraint/cold stress and/or stresscopin administration. Gen Physiol Biophys 22: 115–120, 2003 [PubMed] [Google Scholar]
- 26. Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res 848: 141–152, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Lantos TA, Gorcs TJ, Palkovits M. Immunohistochemical mapping of neuropeptides in the premamillary region of the hypothalamus in rats. Brain Res Rev 20: 209–249, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA 98: 7570–7575, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li P, TjenALooi SC, Longhurst JC. Excitatory projections from arcuate nucleus to ventrolateral periaqueductal gray in electroacupuncture inhibition of cardiovascular reflexes. Am J Physiol Heart Circ Physiol 290: H2535–H2542, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Li P, TjenALooi SC, Guo ZL, Fu LW, Longhurst JC. Long-loop pathways in cardiovascular electroacupuncture responses. J Appl Physiol 106: 620–630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li X, Fan M, Shen L, Cao Y, Zhu D, Hong Z. Excitatory responses of cardiovascular activities to urocortin3 administration into the PVN of the rat. Auton Neurosci 154: 108–111, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Luiten PG, Ter Horst GJ, Karst H, Steffens AB. The course of paraventricular hypothalamic efferents to autonomic structures in medulla and spinal cord. Brain Res 329: 374–378, 1985 [DOI] [PubMed] [Google Scholar]
- 33. Maecker H, Desai A, Dash R, Rivier J, Vale W, Sapolsky R. Astressin, a novel and potent CRF antagonist, is neuroprotective in the hippocampus when administered after a seizure. Brain Res 744: 166–170, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Mendelowitz D. Advances in parasympathetic control of heart rate and cardiac function. News Physiol Sci 14: 155–161, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Moreau JL, Kilpatrick G, Jenck F. Urocortin, a novel neuropeptide with anxiogenic-like properties. Neuroreport 8: 1697–1701, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Namba T, Morimoto K, Sato K, Yamada N, Kuroda S. Antiepileptogenic and anticonvulsant effects of NBQX, a selective AMPA receptor antagonist, in the rat kindling model of epilepsy. Brain Res 638: 36–44, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Nakamura T, Bhatt S, Sapru HN. Cardiovascular responses to hypothalamic arcuate nucleus stimulation in the rat: role of sympathetic and vagal efferents. Hypertension 54: 1369–1375, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Nakamura T, Kazumi K, Sapru HN. Cardiovascular responses to microinjections of urocortin 3 in the nucleus tractus solitarius of the rat. Am J Physiol Heart Circ Physiol 296: H325–H332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nakamura T, Sapru HN. Cardiovascular responses to microinjections of urocortins into the NTS: role of ionotropic glutamate receptors. Am J Physiol Heart Circ Physiol 296: H2022–H2029, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neufeld-Cohen A, Tsoory MM, Evans AK, Getselter D, Gil S, Lowry CA, Vale WW, Chen A. A triple urocortin knockout mouse model reveals an essential role for urocortins in stress recovery. Proc Natl Acad Sci USA 107: 19020–19025, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nosaka S, Yamamoto T, Yasunaga K. Localization of vagal cardioinhibitory preganglionic neurons within rat brain stem. J Comp Neurol 186: 79–91, 1979 [DOI] [PubMed] [Google Scholar]
- 42. Oki Y, Sasano H. Localization and physiological roles of urocortin. Peptides 25: 1745–1749, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (6th ed.). New York: Academic, 2007 [Google Scholar]
- 44. Perkins MN, Collins JF, Stone TW. Isomers of 2-amino-7-phosphonoheptanoic acid as antagonists of neuronal excitants. Neurosci Lett 32: 65–68, 1982 [DOI] [PubMed] [Google Scholar]
- 45. Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signaling and obesity. J Endocrinol 172: 411–421, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension 49: 647–652, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA 98: 2843–2848, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sapru HN. Glutamate circuits in selected medullo-spinal areas regulating cardiovascular function. Clin Exp Pharmacol Physiol 29: 491–496, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Sapru HN. Role of the hypothalamic arcuate nucleus in cardiovascular regulation. Auton Neurosci 175: 38–50, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Standish A, Enquist LW, Escardo JA, Schwaber JS. Central neuronal circuit innervating the rat heart defined by transneuronal transport of pseudorabies virus. J Neurosci 15: 1998–2012, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stengel A, Taché Y. Corticotropin-releasing factor signaling and visceral response to stress. Exp Biol Med (Maywood) 235: 1168–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tanaka M, Telegdy G. Antidepressant-like effects of the CRF family peptides, urocortin 1, urocortin 2 and urocortin 3 in a modified forced swimming test in mice. Brain Res Bull 75: 509–512, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Todorovic C, Radulovic J, Jahn O, Radulovic M, Sherrin T, Hippel C, Spiess J. Differential activation of CRF receptor subtypes removes stress-induced memory deficit and anxiety. Eur J Neurosci 25: 3385–3397, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res 980: 206–212, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212, 2000 [DOI] [PubMed] [Google Scholar]
- 57. Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 57: 435–41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yamazaki T, Waki H, Kohsaka A, Nakamura T, Cui H, Yukawa K, Maeda M. Microinjection of urocortin into the rat nucleus tractus solitarii decreases arterial blood pressure. Auton Neurosci 142: 51–54, 2008 [DOI] [PubMed] [Google Scholar]