Abstract
Surgical procedures involving the rehabilitation of the maxillofacial region frequently require bone grafts; the aim of this research was to evaluate the interface between recipient and graft with cortical or cancellous contact. 6 adult beagle dogs with 15 kg weight were included in the study. Under general anesthesia, an 8 mm diameter block was obtained from parietal bone of each animal and was put on the frontal bone with a 12 mm 1.5 screws. Was used the lag screw technique from better contact between the recipient and graft. 3-week and 6-week euthanized period were chosen for histometric evaluation. Hematoxylin-eosin was used in a histologic routine technique and histomorphometry was realized with IMAGEJ software. T test was used for data analyses with p<0.05 for statistical significance. The result show some differences in descriptive histology but non statistical differences in the interface between cortical or cancellous bone at 3 or 6 week; as natural, after 6 week of surgery, bone integration was better and statistically superior to 3-week analyses. We conclude that integration of cortical or cancellous bone can be usefully without differences.
Keywords: Bone defects, bone grafting, animal study
Introduction
Surgical procedures involving the rehabilitation of the maxillofacial region frequently require bone grafts [1]. This graft incorporation happens dynamically through reabsorption and apposition of the new bone tissue [2], and it can be influenced by factors that are inherent to the patient or external factors [3-5].
One factor that can interfere with the incorporation process is the graft’s structural characteristic [6] that can be cancellous, cortical or a mixture of them [7]. Medullary grafts are typically revascularized faster involving the entire region, whereas cortical grafts are slower and incomplete [7].
Thus, the bone incorporation is made harder because of the fewer blood vessels in the graft region. This reduces the number of precursor osteoblast cells [8]. As a result, the cortical graft incorporation process is slower than the cancellous one, and at the end of the process there are still regions that did not undergo any bone tissue formation and they show remaining islands of grafted material [9]. Despite that, several studies show the efficacy for both grafts in bone reconstruction before implant insertion [10-12].
Since that cortical and cancellous bone has morphological and chemical differences [13], the object of this study is to assess autogenous graft block incorporation through a histometric analysis when grafts are of the cortical-medullary or cortical-cortical contact.
Material and method
A descriptive research in an animal model was designed for histological analyses. This research was approved by the Ethic Commission for Animal Experimentation of Campinas State University under protocol number 1343-1.
Preoperative
Were selected 6 adult beagles dog (15 kg approximately) for the study. Thirty minutes before the procedure the animals received intramuscular benzathine benzylpenicillin (0.1 ml/kg) and dexamethasone (0.5 mg/kg). Before the surgical procedures, the animals were sedated with the anesthetic inductor ketamine chlorohydrate (0.15 mL/kg) and underwent general anesthesia receiving a 3% pentobarbital sodium (30 mg/kg) intravenous.
Surgical procedure
The bone graft was obtained from de parietal bone. With a trephine of 8 mm diameter was realized the bone cut until the dura mater which preserved with a carefully dissection. With a hand piece (10.000 rpm), two cortical-cancellous grafts (from each side of parietal bone) measuring 5 mm high (2 mm cortical and 3 mm cancellous bone) and 8 mm diameter were removed. Bone fragments that were removed were fixed on the frontal bone region without gap between the graft and recipient area, using 12 mm (1.5 mm system) metallic screws with a compression (lag screw) technique. Recipient site was not decorticalized (Figure 1).
Figure 1.
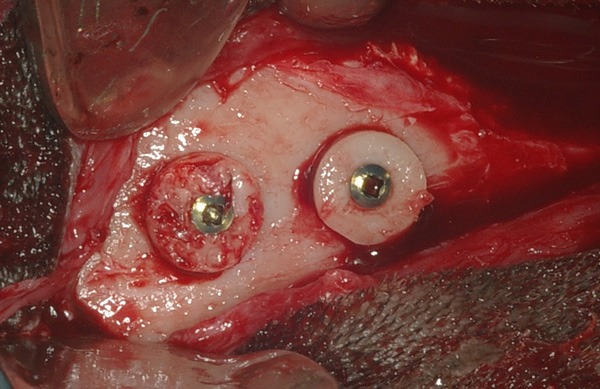
Intraoperatory image of graft position being one with cortical-bone-contact recipient and another with cancellous-bone-contact recipient. All graft was fixed with one compression 12 mm screws.
Blocks fixtures were randomly; one of them was fixed with the cortical region to recipient contact (Group I) and another one was fixed with the cancellous region to the recipient contact (Group II). After bone graft’s stabilization and fixation, periosteum and temporal muscles were approximated with sutures by using absorbable stitch (polyglactin 910). Superficial planes were sutured with monofilament 4-0 nylon stitch.
Euthanasia
Animals were randomly divided into two groups corresponding to the two euthanasia periods: the first group consisted of 3 animals which were euthanized three weeks after surgical procedure; the second group consisted of 3 animals which were euthanized six weeks after surgical procedure, which corresponds to 1 and 2 months in humans, i.e., (primary and secondary repair according to Turner et al. [14]). Euthanasia took place with a 19.1% potassium chloride intravenous overdose until cardiorespiratory arrest. Following this stage, access to the animal’s skull was created and the grafted region exposed.
Bone blocks were obtained by cross and coronal sectioning of the bone with a 702 tapered drill in high-speed turbine under constant saline solution irrigation with a 5 mm to 10 mm safety margin for the previously operated areas.
Histological analysis
Specimens were immersed in 4% buffered formalin. Posteriorly, the specimens were subsequently dehydrated in an ascending series of ethyl alcohols and infiltrated with methylmethacrylate. The hardened blocks were position-ed in a microtome (Microslice 2, Ultra Tec, Santa Ana, CA) and sectioned perpendicularly to in-terface recipient-graft to obtain sections of about 30 μm; then were stained using he-matoxylin-eosinophile for light mi-croscopy analysis. Finally, were obta-ined 4 slices by each graft area.
Histological analysis was done using a light microscope (DMLB, Leica, Bensheim, Germany), and for histomorphometric analys-is the images were acquired in X4 magnification using a camera (CCD IRIS; Sony, Tokyo, Japan) coupled to a light microscope (BX50; Olympus, Tokyo, Japan). Each area was quantified using the public domain image-processing program IMAGEJ (National Institutes of Health, Bethesda, MD); the type of tissue was identified manually, marked and assigned to a color. In detail, the connective tissue, blood vessel and old bone were described and areas of new bone formation were calculated per total bone defect area and expressed as a percentage [15,16].
Statistical analysis
Descriptive statistics were used for histological analysis referring the presence or not of blood vessel, connective tissue and mineralized tissue formation. For 48 examined slices with histomorphometric analysis, t test for paired analysis was used (Biostat 5.0 software) with a 5% significance level.
Results
3 weeks
Descriptive analyses noted on the interface for both group the presence of connective tissue fulfilling the interface between the graft and recipient area, as well as the mineralized tissue formation and blood vessels. The recipient area and graft were well delimited as shows Figure 2. Table 1 resumes the 3 and 6 weeks period hi-stological descriptive analyses.
Figure 2.

A, B: Histological analysis (10x) in a 3 week period. A: Group I - Cortical interface (CoG). B: Group II - Cancellous interface (CaG). Calcified material inside (MT), blood vessel (BV) and connective tissue (CT) are observed.
Table 1.
Descriptive histological analyses for both groups in 3 and 6 week
| Group | Connective tissue | Inflammatory process | Mineralized tissue formation | Blood vessels | Recipient area |
|---|---|---|---|---|---|
| I – 3 week | Present | Absent | Present | Present | Well delimited |
| II – 3 week | Present | Absent | Present | Present | Well delimited |
| I – 6 week | Present | Absent | Present | Present | Not delimited |
| II – 6 week | Present | Absent | Present | Present | Not delimited |
The mean values for mineralized tissue formation (MTF) on the interface graft and recipient area in the group I (cortical interface) was 11.57% (± 8.60% of standard error mean) and for group II (cancellous interface) was 11.62% (± 5.11% of standard error mean) as shows Table 2. It was not observed statistically significant difference (p=0.961). Mineralized tissue formation showed statistically the same results in each group (Figure 3).
Table 2.
Cortical vs. cancellous bone interface analyses for both groups on 3 and 6 week period
| Period | Interface | n (slides) | Mean (%) | SD (%) | Standard Error Mean (%) | P value |
|---|---|---|---|---|---|---|
| 3 week | Cortica | 12 | 11.57 | 29.80 | 8.60 | 0.961 |
| Cancellous | 12 | 11.62 | 17.71 | 5.11 | ||
| 6 week | Cortical | 12 | 30.26 | 30.51 | 8.80 | 0.365 |
| Cancellous | 12 | 28.72 | 49.04 | 14.15 |
Figure 3.

Cortical and cancellous interface standard error deviation values on 3 weeks period.
6 weeks
Descriptive analyses noted on the interface in both groups the presence of blood vessels and less connective tissue fulfilling the interface between the graft and recipient area, being this region almost fulfilled by mineralized tissue formation. The recipient area and graft were not evidenced as shows Figure 4.
Figure 4.

A, B: Histological analysis (10x) in a 6 week perior. A: Group I - Cortical interface (CoG). B: Group II - Cancellous interface (CaG). Is observed more calcified material inside (MT) than 3 weeks period, blood vessel (BV) and connective tissue (CT).
The mean values for MTF on the interface graft and recipient area for the group I was 30.26% (± 8.80% of standard error mean) and for the group II was 28.72% (± 14.15% of standard error mean) as shows Table 2. It was not observed significant difference (p=0.365). MTF showed statistically the same results in each group (Figure 5).
Figure 5.

Cortical and cancellous interface standard error deviation values on 6 weeks period.
When analyzed the comparison between the 6 weeks and 3 weeks periods, both groups, cortical and cancellous interface, presented significant difference (p=0.000) for MTF as shows Table 3.
Table 3.
Comparison between 3 week vs. 6 week period for cortical and cancellous interface
| Interface | Week Period | n (slides) | Mean (%) | SD (%) | Standard Error Mean (%) | P value |
|---|---|---|---|---|---|---|
| Cortical | 3 week period | 12 | 11.57 | 29.80 | 29.80 | 0.000 |
| 6 week period | 12 | 30.26 | 30.51 | 30.51 | ||
| Cancellous | 3 week period | 12 | 11.62 | 17.71 | 5.11 | 0.000 |
| 6 week period | 12 | 28.72 | 49.04 | 14.15 |
Discussion
The use of bone graft for treatment of congenital or acquired bone deficiencies has increased in line with dental implant therapy [17,18]. This study used autogenous bone, since this is the gold standard because of its properties of osteoconduction, osteoinduction, and osteogenesis. Also, this material is the most used to treat these kinds of defects [19].
The lag screw technique reduces the space between the graft and recipient area [20]. This technique, used on the present research, reduced the gap on the interface graft-recipient area, improving the incorporation of the graft probably because it promotes primary reparation.
Amongst the parameters for assessing graft incorporation is the presence of conjunctive tissue, blood vessels, and newly formed mineralized tissue [21]. This study observed in both groups the same kind of cells in both euthanasia periods. In both cases there was graft incorporation at the 6-week assessment, which corresponds to 2 months in human beings as described by Turner et al. [14]. Gerressen et al. [22], in a human sinus floor elevation surgery research used iliac bone with pure cancellous in one sinus and a mixture of cortical and cancellous in another sinus; the results demonstrate non differences between density or quality in both maxillary sinus; also, don’t show differences between 4 or 7 month of evaluation.
Besides some authors noted that decorticalization of receipt area have influence on bone graft healing, improving their integration [4,5,7], we observed that both of interface were integrated without any preparation of recipient area, suggesting that it does not influence on bone graft integration.
Patient factors can influence the graft incorporation process [5,7]. External factors include the graft’s structural characteristics [6], the cancellous-type showing a better repair process when compared to cortical grafts because of a greater angiogenesis. This supposedly facilitates osteoblast precursor cell migration to the graft region, thus speeding up the incorporation due to greater absorption and new mineralized connective tissue formation [7]. Despite that, this study could not observe any statistic difference between the two groups in mineralized tissue formation for both euthanasia periods. This way, it may be suggest that, when analyze the probability to relapse, both grafts are similar, in agreed with other authors suggests [6,7].
Based in our result, it may be concluded that both the cancellous or cortical graft interface in autogenous block does not influence in bone graft incorporation.
Disclosure of conflict of interest
None.
References
- 1.Jensen J, Sindet-Pedersen S. Autogenous mandibular bone grafts and osseointegrated implants for reconstruction of the severely atrophied maxilla: a preliminary report. J Oral Maxillofac Surg. 1991;49:1277–87. doi: 10.1016/0278-2391(91)90303-4. [DOI] [PubMed] [Google Scholar]
- 2.Schliephake H. Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg. 2002;31:469–84. doi: 10.1054/ijom.2002.0244. [DOI] [PubMed] [Google Scholar]
- 3.Issa MJP, Tiossi R, Pitol DL, Mello SAS. TGF-ß and new bone formation. Int J Morphol. 2006;24:399–405. [Google Scholar]
- 4.Jensen SS, Broggini N, Hjørting-Hansen E, Schenk R, Buser D. Bone healing and graft resorption of autograft, anorganic bovine bone and beta-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin Oral Implants Res. 2006;17:237–43. doi: 10.1111/j.1600-0501.2005.01257.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang YN, Feng CC, Liu JL, Li F, Fu L, Wang Z. Transforming Growth Factor beta Alleviates Acute Graft-versus-Host-Disease after Allogeneic Bone Marrow Transplantation in Murine Model. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008;16:1135–9. [PubMed] [Google Scholar]
- 6.Tong L, Buchman SR. Facial bone grafts: contemporary science and thought. J Craniomaxillofac Trauma. 2000;6:31–41. [PubMed] [Google Scholar]
- 7.Nunamaker DM. Experimental models of fracture repair. Clin Orthop Relat Res. 1998;355(Suppl):S56–65. doi: 10.1097/00003086-199810001-00007. [DOI] [PubMed] [Google Scholar]
- 8.Bhatt KA, Chang EI, Warren SM, Lin SE, Bastidas N, Ghali S, Thibboneir A, Capla JM, McCarthy JG, Gurtner GC. Uniaxial Mechanical Strain: An In Vitro Correlate to Distraction Osteogenesis. J Surg Res. 2007;143:329–36. doi: 10.1016/j.jss.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Kübler A, Neugebauer J, Oh J, Scheer M, Zöller JE. Growth and proliferation of human osteoblasts on different bone graft substitutes. An in vitro study. Implant Dent. 2004;13:171–9. doi: 10.1097/01.id.0000127522.14067.11. [DOI] [PubMed] [Google Scholar]
- 10.Serra E Silva FM, Ricardo de Albergaria-Barbosa J, Mazzonetto R. Clinical evaluation of association of bovine organic osseous matrix and bovine bone morphogenetic protein versus autogenous bone graft in sinus floor augmentation. J Oral Maxillofac Surg. 2006;64:931–5. doi: 10.1016/j.joms.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Silva FM, Cortez AL, Moreira RW, Mazzonetto R. Complications of intraoral donor site for bone grafting prior to implant placement. Implant Dent. 2006;15:420–6. doi: 10.1097/01.id.0000246225.51298.67. [DOI] [PubMed] [Google Scholar]
- 12.Sverzut AT, Stabile GA, de Moraes M, Mazzonetto R, Moreira RW. The influence of tobacco on early dental implant failure. J Oral Maxillofac Surg. 2008;66:1004–9. doi: 10.1016/j.joms.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn LT, Grynpas MD, Rey CC, Wu Y, Ackerman JL, Glimcher MJ. A comparison of the physical and chemical differences between cancellous and cortical bovine bone mineral at two ages. Calcif Tissue Int. 2008;83:146–154. doi: 10.1007/s00223-008-9164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner AS. Animal models of osteoporosis-necessity and limitations. Eur Cell Mater. 2001;1:66–81. doi: 10.22203/ecm.v001a08. [DOI] [PubMed] [Google Scholar]
- 15.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 16.Recker RR. Bone Histomorphometry: Techniques and Interpretation. Boca Raton, FL: CRC; 1983. [Google Scholar]
- 17.Callan DP, Rohrer MD. Use of bovine derived hydroxiapatite in the treatment of edentulous ridge deffects: a human clinical and histologic case report. J Periodontol. 1993;64:575–82. doi: 10.1902/jop.1993.64.6.575. [DOI] [PubMed] [Google Scholar]
- 18.Pallesen L, Schou S, Aaboe M, Hjørting-Hansen E, Nattestad A, Melsen F. Influence of particle size of autogenous bone grafts on the early stages of bone regeneration: a histologic and stereologic study in rabbit calvarium. Int J Oral Maxillofac Implants. 2002;17:498–506. [PubMed] [Google Scholar]
- 19.Chaves Netto HDM, Olate S, Chaves MMGA, Barbosa AJR, Mazzonetto R. Análisis histológico del proceso de reparación en defectos óseos. Reconocimiento de defectos críticos. Int J Morphol. 2009;27:1121–1127. [Google Scholar]
- 20.Ellis E 3rd. Is lag screw fixation superior to plate fixation to treat fractures of the mandibular Symphysis? J Oral Maxillofac Surg. 2012;70:875–882. doi: 10.1016/j.joms.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 21.Franceschi C. Genes involved in immune response/inflammation, IGF1/insulin pathway and response to oxidative stress play a major role in the genetics of human longevity: the lesson of centenarians. Mech Ageing Dev. 2005;126:351–61. doi: 10.1016/j.mad.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Gerressen M, Hermanns-Sachweh B, Riediger D, Hilgers RD, Spiekermann H, Ghassemi A. Purely cancellous vs. corticocancellous bone in sinus floor augmentation with autogenous iliac crest: a prospective clinical trial. Clin Oral Implants Res. 2009;20:109–115. doi: 10.1111/j.1600-0501.2008.01619.x. [DOI] [PubMed] [Google Scholar]


