Abstract
Currently, there are discrepancies in the interpretation between cervical liquid-based cytology (LBC) and histologic diagnoses. The aim of our study was to evaluate the utility of p16INK4a (p16) and IMP3 staining of LBC specimens to increase the concordance rate. A total of 98 cell blocks with biopsy results, including 37 low-grade squamous intraepithelial lesions (LSIL), 36 high-grade squamous intraepithelial lesions (HSIL), and 25 squamous cell carcinomas (SCC), were selected for the immunocytochemical analysis of p16 and IMP3. The LBC diagnoses corresponded with histological diagnoses for 59.5% (22/37), 63.9% (23/36), and 88.0% (22/25) of LSIL, HSIL, and SCC lesions, respectively. We found a high frequency of p16 positivity in HSIL (72.2%) and SCC (100%), but not LSIL (29.7%). IMP3 was frequently expressed in SCC (84.0%), but rarely in LSIL (8.1%) and HSIL (25.0%). Cervical intraepithelial neoplasia 1 (CIN1) was negative for both p16 and IMP3, CIN2/3 tended to be positive for p16 and negative for IMP3, and SCC was positive for both p16 and IMP3. The combination of p16 and IMP3 immunostaining had a higher sensitivity and specificity for detecting CIN1 and CIN2/3 than cytology. For detecting SCC, p16/IMP3 had a higher sensitivity than cytology, but a lower specificity. IMP3 is a useful diagnostic immunomarker that can be used to identify SCC and the combination of p16/IMP3 expression was found to improve the discrepant results between cytologic and histologic diagnoses.
Keywords: Liqui-PREP, cell blocks, immunocytochemistry, p16INK4a, IMP3
Introduction
Cervical cytologic diagnoses for unequivocal squamous epithelial abnormalities include low-grade squamous intraepithelial lesions (LSIL), high-grade squamous intraepithelial lesions (HSIL), and squamous cell carcinoma (SCC) [1,2]. This classification scheme provides standardized terminology and guidance for clinical management [2]. Despite the clear guidelines for interpretation of Pap tests, many specimens contain cells that cannot be easily placed into the accepted schema [1-3]. The diagnostic dilemma of overestimating LSIL or underestimating HSIL is made more difficult by the fact that the Pap test specimen is one of the most litigated of all pathologic specimens [2]. To reduce the discordance rate between cytology diagnosis and follow-up biopsies, a variety of objective and reliable diagnostic tools, including immunocytochemistry, have been investigated [4-13].
p16INK4a (p16), a tumor suppressor protein that regulates cell proliferation by inhibiting cell cycle G1 progression, has been demonstrated to be strongly overexpressed in almost all high-grade precursor lesions and invasive cancers of the cervix uteri. In cervical cytology, p16 also has been used as a surrogate marker for the presence of HSIL or more advanced lesions [5-12]. Because HSIL and SCC consistently express p16, immunostaining for this marker has no practical utility in distinguishing SCC from HSIL.
IMP3 is a member of the insulin-like growth factor (IGF) mRNA binding protein (IMP) family that consists of IMP1, IMP2, and IMP3. It is also known in the literature as KOC, IGF2BP3, and L523S [14]. Expression of IMP3 in adults is normally limited to the placenta, lymph node germinal centers, and endocervical mucosa. It has also been identified in a number of malignant tumors, including non-small cell carcinoma of the lung, high-grade neuroendocrine carcinomas of the lung, extrapulmonary small cell carcinomas, mesothelioma, renal cell carcinoma, endometrioid adenocarcinoma, colon cancer, leiomyosarcoma, and melanoma [14-30]. Recent studies have demonstrated that the upregulation of IMP3 is associated with tumor metastasis, a more aggressive clinical course, and a poorer prognosis [28-30].
Immunohistochemical analysis of IMP3 expression in a number of organ systems can be used to differentiate benign lesions and low-grade dysplasia from high-grade dysplasia and invasive carcinomas [14-25]. A recent study has found that IMP3 expression is negative in normal tissue and cervical intraepithelial neoplasia I (CINI, 0%), CINII (1%), CINIII (8%) and SCC (96%) and that IMP3 expression differentiated a group of patients with SCC on cervical biopsy [28]. Therefore, IMP3 has the potential to aid in evaluation of liquid-based cytology (LBC) for SCC.
To the best of our knowledge, there are no prior studies examining IMP3 expression on cervical cytology specimens. The goals of this study were to investigate the diagnostic value of IMP3 expression in cell blocks from residual LBC and to determine whether p16 and IMP3 can serve as a molecular biomarkers to reduce the discordance rate between LBC and follow-up biopsies.
Materials and methods
Case selection
This retrospective study was conducted using data from the archives of the Third Affiliated Hospital during the years of 2008–2010. Problematic cases, including LSIL, HSIL, and SCC, were selected to prepare cell blocks from residual Liqui-PREP samples (LGM International, Fort Lauderdale, FL, USA). A total of 173 cases were reviewed. Of the 173 cases, 75 cases were excluded due to low cellularity or absence of biopsy confi rmation. This left 98 biopsy-confi rmed cases for review and analysis. For the purposes of the current study, all LBC slides corresponding to the cell blocks were reviewed and cytological diagnoses (according to the 2001 Bethesda System) were confirmed or reassigned based on a consensus reached by two pathologists (Z. Tong and W. Qing Zhu). The cytological diagnoses consisted of 37 LSIL, 36 HSIL, and 25 SCC. The histopathologic diagnoses were classified according to the WHO criteria as a normal cervix, CIN1, CIN2/CIN3, or SCC; and were used as the gold standard for the study. The histologic diagnosis of CIN1 was considered equivalent to the cytologic diagnosis of LSIL, the CIN2/3 diagnosis was considered equivalent to HSIL, and the SCC diagnosis was considered equivalent to SCC. A discordant case was defined as “a cytology-histology pair with differential diagnosis [31].”
Liqui-PREP processing
All cervical samples were collected using a cytobrush, for which the head of the brush was immediately removed after collection and put in a collection vial. Liqui-PREP cleaning solution was poured into a 15.0 mL polystyrene conical centrifuge tube (Fisher HealthCare, USA) and, after vortexing, 3.0 mL from the collection vial was added to the centrifuge tube with the cleaning solution. The tube was then centrifuged for 10 min at 1,000 x g in a SLW centrifuge (Shanghai LW Scientific Co., Ltd, China), and the supernatant was discarded. Liqui-PREP Cellular Base (500 μL) was added to the cellular pellet, which was resuspended by vortexing for 10 s. The suspension (50 μL) was pipetted onto a slide to form a 2.0-cm diameter circle. The slide was stained with the Papanicolaou stain.
Cell block preparation
Paraffin-embedded cell blocks were prepared as previously published [13]. Briefly, the Liquid-PREP tube was used to centrifuge the residual preservative fl uid for 10 min at 1,000 x g and the supernatant was decanted. The remaining cell pellet was carefully wrapped in lens paper, transferred to a tissue cassette, and embedded in paraffin.
Immunohistochemistry
Immunohistochemical stains were performed on 4-μm thick sections from paraffin-embedded cell block sections. Briefl y, the cell blocks were sectioned, mounted on poly-L-lysine-coated slides, deparaffinized in xylene, rehydrated in graded alcohols, and subjected to heat-induced antigen retrieval (at 95°C for 15 min in citrate buffer, pH 6.0). Endogenous peroxidase activity was then inhibited with a 3% hydrogen peroxide solution.
Sections were immunostained using the following primary antibodies: a monoclonal mouse anti-human antibody against IMP3 (clone 69.1, Dako North America, Carpentaria, CA, USA; dilution 1:100) and p16 (Dako) The EnVision+ System (DAKO) and 3,3’-diamino-benzidine (DAB) were used for detection of antibody-conjugated peroxidase activity. Pancreatic carcinoma cases were used as positive controls for IMP3 expression. Cases of CIN3 were used as positive controls for p16. Negative control sections were prepared by substituting non-immune IgG for the primary antibody.
Interpretation of p16 and IMP3
A positive cellular reaction for p16 was determined by nuclear signal with or without cytoplasm staining. For the purposes of this study, p16-positive endometrial cells, metaplastic cells, or tubal metaplasia were excluded during the analysis [10]. A positive stain for IMP3 was defined as cytoplasmic immunostaining. Specimens containing positive abnormal cells were classified as positive, regardless of the number of abnormal cells and the density of the immunostaining [28].
Statistical analysis
The sensitivity and specificity of p16 and IMP3 were calculated. The designations of “true” and “false” were based on the study hypothesis that p16 is expressed in CIN2/3 and SCC, but not in CIN1; and that IMP3 is expressed in all SCC, but not in CIN1 and CIN2/3.
Results
A summary of the cytologic and histologic diagnoses is presented in Table 1. The 98 total cases were diagnosed histopathologically as follows: 12 normal cervix, 29 CIN1, 32 CIN2/CIN3, and 25 SCC. Review of histologic diagnoses revealed that a single cytologic diagnostic category often contained a number of different cervical lesions. In samples that were diagnosed as LSIL, HSIL and SCC, the concordance rate between the cytologic and histologic diagnosis were 59.5% (22/37), 63.9% (23/36), and 88.0% (22/25), respectively. The overall concordance rate was 68.4% (67/98). Also, the concordance rate between cytologic and histologic diagnoses was higher for SCC than for HSIL and LSIL.
Table 1.
Correlation between cytological diagnosis and histological diagnosis
| Histological diagnosis | Cytological diagnosis | Total | ||
|---|---|---|---|---|
|
| ||||
| LSIL | HSIL | SCC | ||
| Normal | 9 | 3 | 0 | 12 |
| CIN1 | 22 | 7 | 0 | 29 |
| CIN2/3 | 6 | 23 | 3 | 32 |
| SCC | 0 | 3 | 22 | 25 |
| Total | 37 | 36 | 25 | 98 |
Abbreviations: LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; SCC, squamous cell carcinoma; CIN, cervical intraepithelial neoplasia.
The p16 protein was immunocytochemically detectable in 63.3% (62/98) of samples, whereas IMP3 was detectable in only 33.7% (33/98) of samples. Of note, only p16-positive cases showed IMP3 immunoreactivity and 46.8% (29/62) of p16-positive cases were negative for IMP3 expression. In total, p16 was detected in 29.7% (11/37) of LSIL, 72.2% (26/36) of HSIL, and 100% (25/25) of SCC.
IMP3 immunoreactivity was observed in 8.1% (3/37) of LSIL, 25.0% (9/36) of HSIL, and 84.0% (21/25) of SCC. IMP3 is frequently expressed in SCC, with only limited expression in LSIL and HSIL. Three of the twenty-five SCC (12.0%) lesions diagnosed on biopsy displayed HSIL on LBC evaluation. Of the three cases diagnosed as HSIL by cytology, two cases were positive for IMP3 expression. Conversely, only 1 of the 22 (4.5%) SCC cases identified by LBC were negative for IMP3 expression (Table 2).
Table 2.
Summary of immunostaining results
| Histological diagnosis | p16+ | Total | IMP3+ | Total | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| LSIL | HSIL | SCC | LSIL | HSIL | SCC | |||
| Normal (n = 12) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CIN1 (n = 29) | 5 | 2 | 0 | 7 | 2 | 1 | 0 | 3 |
| CIN2/3 (n = 32) | 6 | 21 | 3 | 30 | 1 | 6 | 0 | 7 |
| SCC (n = 25) | 0 | 3 | 22 | 25 | 0 | 2 | 21 | 23 |
| Total | 11 | 26 | 25 | 62 | 3 | 9 | 21 | 33 |
The staining pattern using a combination of p16 and IMP3 immunoreactivity was divided into the following three categories: p16-/IMP3-, p16+/IMP3-, or p16+/IMP3+. In general, p16-/IMP3- was associated with CIN1 (Figure 1), p16+/IMP3- was associated with CIN2/CIN3 (Figure 2), and p16+/IMP3+ was associated with SCC (Figure 3). The histologic diagnoses were used as the gold standard for calculating the sensitivity and specificity of cytology and the staining patterns CIN1, CIN2/3 and SCC (Tables 3 and 4). The combination of p16 and IMP3 immunostaining had a higher sensitivity and specificity than cytology for detecting CIN1 (81.4% and 96.5% vs. 59.1% and 90.1%) and CIN2/3 (71.9% and 88.9% vs. 63.9% and 73.6%) and a higher sensitivity than cytology for detecting SCC (92.0% vs. 88.0%). Cytology, however, had a higher specificity for detecting SCC, compared to p16/IMP3 staining (95.5% vs. 83.6%).
Figure 1.
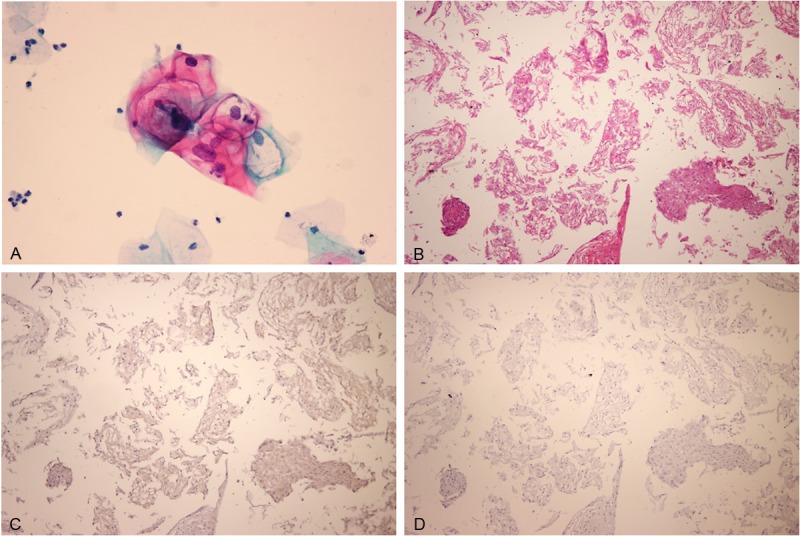
Low-grade squamous intraepithelial lesion (LSIL) viewed with (A) liquid-based cytology or (B) section from a cell block. LSIL showed negative staining for (C) p16 and (D) IMP3.
Figure 2.
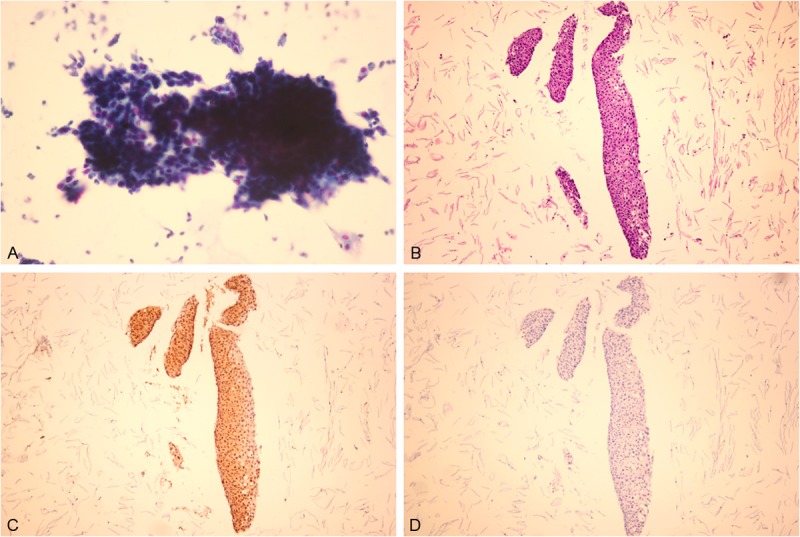
High-grade squamous intraepithelial lesion (HSIL) viewed with (A) liquid-based cytology or (B) section from a cell block. HSIL showed (C) positive staining for p16 and (D) negative staining for IMP3.
Figure 3.
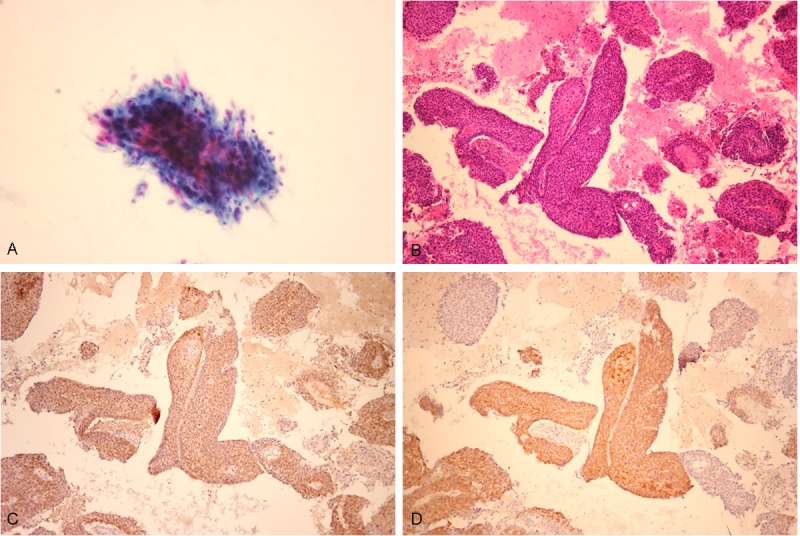
Squamous cell carcinoma (SCC) viewed with (A) liquid-based cytology or (B) section from a cell block. SCC showed positive staining for (C) p16 and (D) IMP3.
Table 3.
Sensitivity and specificity of cytologic diagnosis
| Cytological diagnosis | Sensitivity (%) | Specifi city (%) |
|---|---|---|
| LSIL | 59.5 | 90.1 |
| HSIL | 63.9 | 73.6 |
| SCC | 88.0 | 95.9 |
Abbreviations: LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; SCC, squamous cell carcinoma; CIN, cervical intraepithelial neoplasia.
Table 4.
Sensitivity and specificity of histological diagnoses from p16 and IMP3
| Histological diagnosis | Staining pattern | Sensitivity (%) | Specifi city (%) |
|---|---|---|---|
| CIN1 | p16-/IMP3- | 81.4 | 96.5 |
| CIN2/3 | p16+/IMP3- | 71.9 | 88.9 |
| SCC | p16+/IMP3+ | 92.0 | 83.6 |
Abbreviations: p16, p16INK4a; -, negative; +, positive; CIN, cervical intraepithelial neoplasia.
Discussion
Cervical cytology and biopsy correlation is used by laboratories for the evaluation of their cytodiagnostic capabilities, as a part of overall laboratory quality improvement [32]. The frequency of discrepancies between cervical cytology diagnoses and follow-up biopsies was reported to occur within the range of 11.5–50% [6,32]. In the current study, the concordance rate of LSIL, HSIL, and SCC with histological diagnosis were 59.5% (22/37), 63.9% (23/36), and 88.0% (22/25), respectively. Therefore, there were significant discrepancies between the cytological and histological diagnoses, especially for LSIL and HSIL. Similarly, Yoshida et al. [9] found that the concordance rates between LSIL, HSIL, and invasive carcinoma and histologic diagnoses were 25% (3/12), 32.9% (24/73), and 73.3% (11/15), respectively while, in a larger study, Alsharif et al. [1] demonstrated that the concordance rates between LSIL and HSIL and histologic diagnoses were 47.6% (1,093/2,297) and 69.0% (468/678), respectively. These results are consistent with our studies. Therefore, there are a certain proportion of discrepant results between cytologic and histologic diagnoses and a new biomarker for dysplastic cervical cells is greatly needed to aid in the improved evaluation of a patient’s diagnosis.
p16 has already been established as a useful and reliable marker of high-grade precursor lesions and invasive cancer of the cervix [5-12]. In the present study, we found a high frequency of p16 expression in 72.2% (26/36) of HSIL and 100% (25/25) of SCC, while a low frequency of expression in 29.7% (11/37) of LSIL. Our findings are consistent with most studies [5-12], which report that p16 immunostaining is significantly associated with HSIL or SCC. Therefore, there is a usefulness of using p16 immunostaining to discriminate HSIL and SCC from LSIL in LBC, but there is no practical utility for its use in distinguishing SCC from HSIL. Thus, there exists a need to identify additional biomarkers that are capable of identifying SCC in LBC.
IMP3 is an mRNA-binding protein that regulates the transcription of IGF-II. While several studies found that the IMP3 antibody is a highly specific marker for malignant lesions in biopsy and effusion samples [14-30], other studies showed that positive IMP3 staining does not discriminate invasive carcinoma from high-grade dysplasia. Riener et al. [2] found that IMP3 was strongly expressed in high-grade dysplasia of the extrahepatic biliary tract and bile duct carcinomas, while normal, infl amed, and bile ducts with low-grade dysplasia were only negative or weakly positive. Feng et al. [18] showed that low-grade esophageal columnar dysplasia displayed only focal, weak IMP3 expression, in contrast to high-grade esophageal columnar dysplasia, which showed a more intense diffuse expression of IMP3. Li et al. demonstrated that IMP3 may prove to be a useful and important biomarker for the diagnosis of adenocarcinoma in situ in equivocal or borderline cases [16]. A recent study found that IMP3 expression is negative in normal and CINI (0%), CINII (1%), CINIII (18%), and invasive cancer (96%) and IMP3 expression could be used to identify a group of patients with SCC [28]. In agreement, we found that IMP3 was frequently expressed in SCC, with only limited expression in LSIL and HSIL. Therefore, IMP3 can be a useful biomarker for the detection of SCC in LBC specimens. We also found that there were differences in IMP3 expression between squamous and glandular cell lesions. To our knowledge, this is the first report examining immunohistochemical IMP3 expression in LBC specimens.
In the current study, only p16-positive cases showed IMP3 immunoreactivity. The staining patterns of p16 and IMP3 were divided into the following three categories: p16-/IMP3-, p16+/IMP3-, or p16+/IMP3+. Our data showed that CIN1, CIN2/CIN3, and SCC lesions all displayed distinct staining patterns of p16 and IMP3. SCC was frequently p16+/IMP3+, CIN2/3 was frequently p16+/IMP3-, and p16-/IMP3- was more frequently seen in CIN1.
When we analyzed the sensitivity and specificity for using p16/IMP3 to distinguish between the lesions, we found that both measures for CIN1 and CIN2/3 were significantly improved, compared with measures obtained using cytology. Also, for SCC, we observed a higher sensitivity, but a lower specificity, for p16+/IMP3+ analysis compared to cytology. Based on these data, we propose the use of a decision tree for the diagnosis of unequivocal squamous epithelial abnormalities (Figure 4). The combination of p16 and IMP3 immunostaining appears to be useful to improve upon the discrepancies between cytologic and histologic diagnoses.
Figure 4.
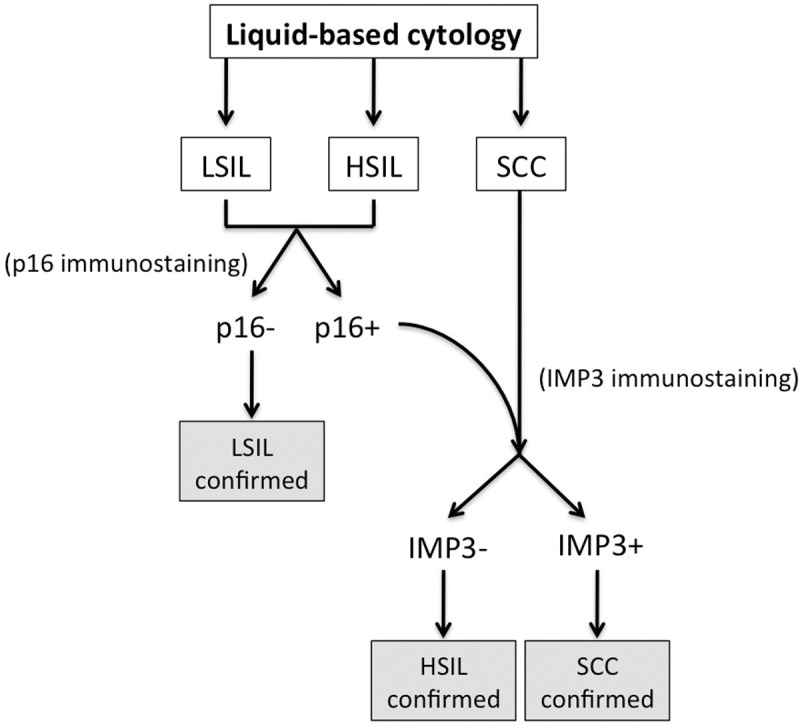
Decision tree for the diagnosis of unequivocal squamous epithelial abnormalities using p16 and IMP3 on LBC specimens.
In summary, the results of this study demonstrate that IMP3 is a useful diagnostic immunomarker to identify SCC on LBC. Our study suggests the use of p16 and IMP3 markers as a panel to improve the discrepant results between cytologic and histologic diagnoses for unequivocal squamous epithelial abnormalities.
Disclosure of conflict of interest
We do not have competing commercial interests.
References
- 1.Alsharif M, Kjeldahl K, Curran C, Miller S, Gulbahce HE, Pambuccian SE. Clinical significance of the diagnosis of low-grade squamous intraepithelial lesion, cannot exclude high-grade squamous intraepithelial lesion. Cancer. 2009;117:92–100. doi: 10.1002/cncy.20004. [DOI] [PubMed] [Google Scholar]
- 2.Owens CL, Moats DR, Burroughs FH, Gustafson KS. “Low-grade squamous intraepithelial lesion, cannot exclude high-grade squamous intraepithelial lesion” is a distinct cytologic category: histologic outcomes and HPV prevalence. Am J Clin Pathol. 2007;128:398–403. doi: 10.1309/QRDNMWWAKJQTVJGF. [DOI] [PubMed] [Google Scholar]
- 3.Stelow EB, Skeate R, Wahi MM, Kjeldahl K, McKeon D, Larkin S, Woronzoff K, Pambuccian SE. Pap test discrepancies and follow-up histology. Diagn Cytopathol. 2003;29:111–115. doi: 10.1002/dc.10332. [DOI] [PubMed] [Google Scholar]
- 4.Tambouret RH, Misdraji J, Wilbur DC. Longitudinal clinical evaluation of a novel antibody cocktail for detection of high-grade squamous intraepithelial lesions on cervical cytology specimens. Arch Pathol Lab Med. 2008;132:918–925. doi: 10.5858/2008-132-918-LCEOAN. [DOI] [PubMed] [Google Scholar]
- 5.Pinto AP, Degen M, Villa LL, Cibas ES. Immunomarkers in gynecologic cytology: the search for the ideal ‘biomolecular Papanicolaou test’. Acta Cytol. 2012;56:109–121. doi: 10.1159/000335065. [DOI] [PubMed] [Google Scholar]
- 6.David O, Cabay RJ, Pasha S, Dietrich R, Leach L, Guo M, Mehrotra S. The role of deeper levels and ancillary studies (p16(Ink4a) and ProExC) in reducing the discordance rate of Papanicolaou findings of high-grade squamous intraepithelial lesion and follow-up cervical biopsies. Cancer. 2009;117:157–166. doi: 10.1002/cncy.20020. [DOI] [PubMed] [Google Scholar]
- 7.Bergeron C, Ordi J, Schmidt D, Trunk MJ, Keller T, Ridder R. Conjunctive p16INK4a testing significantly increases accuracy in diagnosing high-grade cervical intraepithelial neoplasia. Am J Clin Pathol. 2010;133:395–406. doi: 10.1309/AJCPXSVCDZ3D5MZM. [DOI] [PubMed] [Google Scholar]
- 8.Shin EK, Lee SR, Kim MK, Kang EJ, Ju W, Lee SN, Han WS, Kim SC. Immunocytochemical staining of p16(ink4a) protein as an adjunct test in equivocal liquid-based cytology. Diagn Cytopathol. 2008;36:311–316. doi: 10.1002/dc.20786. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida T, Fukuda T, Sano T, Kanuma T, Owada N, Nakajima T. Usefulness of liquid-based cytology specimens for the immunocytochemical study of p16 expression and human papillomavirus testing: a comparative study using simultaneously sampled histology materials. Cancer. 2004;102:100–108. doi: 10.1002/cncr.20046. [DOI] [PubMed] [Google Scholar]
- 10.Guo M, Warriage I, Mutyala B, Patel S, Lin E, Gong Y, Sneige N. Evaluation of p16 immunostaining to predict high-grade cervical intraepithelial neoplasia in women with Pap results of atypical squamous cells of undetermined significance. Diagn Cytopathol. 2011;39:482–488. doi: 10.1002/dc.21415. [DOI] [PubMed] [Google Scholar]
- 11.Benevolo M, Vocaturo A, Mottolese M, Mariani L, Vocaturo G, Marandino F, Sperduti I, Rollo F, Antoniani B, Donnorso RP. Clinical role of p16INK4a expression in liquid-based cervical cytology: correlation with HPV testing and histologic diagnosis. Am J Clin Pathol. 2008;129:606–612. doi: 10.1309/BEPQXTCQD61RGFMJ. [DOI] [PubMed] [Google Scholar]
- 12.Yu L, Wang L, Zhong J, Chen S. Diagnostic value of p16INK4A, Ki-67, and human papillomavirus L1 capsid protein immunochemical staining on cell blocks from residual liquid-based gynecologic cytology specimens. Cancer Cytopathol. 2010;118:47–55. doi: 10.1002/cncy.20061. [DOI] [PubMed] [Google Scholar]
- 13.Wei Q, Liu J, Zhang Z, Yang Q, Zhao T. Morphological features of cell blocks prepared from residual liqui-PREP samples can distinguish between high-grade squamous intraepithelial lesions and squamous cell carcinomas. Acta Cytol. 2011;55:245–250. doi: 10.1159/000323312. [DOI] [PubMed] [Google Scholar]
- 14.Findeis-Hosey JJ, Xu H. The use of insulin like-growth factor II messenger RNA binding protein-3 in diagnostic pathology. Hum Pathol. 2011;42:303–314. doi: 10.1016/j.humpath.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Levy M, Lin F, Xu H, Dhall D, Spaulding BO, Wang HL. S100P, von Hippel-Lindau gene product, and IMP3 serve as a useful immunohistochemical panel in the diagnosis of adenocarcinoma on endoscopic bile duct biopsy. Hum Pathol. 2010;41:1210–1219. doi: 10.1016/j.humpath.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Rock KL, Woda BA, Jiang Z, Fraire AE, Dresser K. IMP3 is a novel biomarker for adenocarcinoma in situ of the uterine cervix: an immunohistochemical study in comparison with p16(INK4a) expression. Mod Pathol. 2007;20:242–247. doi: 10.1038/modpathol.3800735. [DOI] [PubMed] [Google Scholar]
- 17.Wachter DL, Schlabrakowski A, Hoegel J, Kristiansen G, Hartmann A, Riener MO. Diagnostic value of immunohistochemical IMP3 expression in core needle biopsies of pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2011;35:873–877. doi: 10.1097/PAS.0b013e3182189223. [DOI] [PubMed] [Google Scholar]
- 18.Feng W, Zhou Z, Peters JH, Khoury T, Zhai Q, Wei Q, Truong CD, Song SW, Tan D. Expression of insulin-like growth factor II mRNA-binding protein 3 in human esophageal adenocarcinoma and its precursor lesions. Arch Pathol Lab Med. 2011;135:1024–1031. doi: 10.5858/2009-0617-OAR2. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda K, Tate G, Suzuki T, Kitamura T, Mitsuya T. IMP3/L523S, a novel immunocytochemical marker that distinguishes benign and malignant cells: the expression profiles of IMP3/L523S in effusion cytology. Hum Pathol. 2010;41:745–750. doi: 10.1016/j.humpath.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 20.Riener MO, Fritzsche FR, Clavien PA, Pestalozzi BC, Probst-Hensch N, Jochum W, Kristiansen G. IMP3 expression in lesions of the biliary tract: a marker for high-grade dysplasia and an independent prognostic factor in bile duct carcinomas. Hum Pathol. 2009;40:1377–1383. doi: 10.1016/j.humpath.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Yantiss RK, Cosar E, Fischer AH. Use of IMP3 in identification of carcinoma in fine needle aspiration biopsies of pancreas. Acta Cytol. 2008;52:133–138. doi: 10.1159/000325470. [DOI] [PubMed] [Google Scholar]
- 22.Hanley KZ, Facik MS, Bourne PA, Yang Q, Spaulding BO, Bonfiglio TA, Xu H. Utility of anti-L523S antibody in the diagnosis of benign and malignant serous effusions. Cancer. 2008;114:49–56. doi: 10.1002/cncr.23254. [DOI] [PubMed] [Google Scholar]
- 23.Hart J, Parab M, Mandich D, Cartun RW, Ligato S. IMP3 immunocytochemical staining increases sensitivity in the routine cytologic evaluation of biliary brush specimens. Diagn Cytopathol. 2012;40:321–326. doi: 10.1002/dc.21571. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda K, Tate G, Suzuki T, Kitamura T, Mitsuya T. Diagnostic usefulness of EMA, IMP3, and GLUT-1 for the immunocytochemical distinction of malignant cells from reactive mesothelial cells in effusion cytology using cytospin preparations. Diagn Cytopathol. 2011;39:395–401. doi: 10.1002/dc.21398. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt MT, Himmelfarb EA, Shafi H, Lin F, Xu H, Wang HL. Use of IMP3, S100P, and pVHL Immunopanel to Aid in the Interpretation of Bile Duct Biopsies With Atypical Histology or Suspicious for Malignancy. Appl Immunohistochem Mol Morphol. 2012;20:478–87. doi: 10.1097/PAI.0b013e318245e05b. [DOI] [PubMed] [Google Scholar]
- 26.Findeis-Hosey JJ, Yang Q, Spaulding BO, Wang HL, Xu H. IMP3 expression is correlated with histologic grade of lung adenocarcinoma. Hum Pathol. 2010;41:477–484. doi: 10.1016/j.humpath.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Cornejo K, Shi M, Jiang Z. Oncofetal protein IMP3: a useful diagnostic biomarker for leiomyosarcoma. Hum Pathol. 2012;43:1567–1572. doi: 10.1016/j.humpath.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Lu D, Yang X, Jiang NY, Woda BA, Liu Q, Dresser K, Mercurio AM, Rock KL, Jiang Z. IMP3, a New Biomarker to Predict Progression of Cervical Intraepithelial Neoplasia Into Invasive Cancer. Am J Surg Pathol. 2011;35:1638–1645. doi: 10.1097/PAS.0b013e31823272d4. [DOI] [PubMed] [Google Scholar]
- 29.Li D, Yan D, Tang H, Zhou C, Fan J, Li S, Wang X, Xia J, Huang F, Qiu G. IMP3 is a novel prognostic marker that correlates with colon cancer progression and pathogenesis. Ann Surg Oncol. 2009;16:3499–506. doi: 10.1245/s10434-009-0648-5. [DOI] [PubMed] [Google Scholar]
- 30.Szarvas T, vom Dorp F, Niedworok C, Melchior-Becker A, Fischer JW, Singer BB, Reis H, Bánkfalvi Á, Schmid KW, Romics I, Ergün S, Rübben H. High insulin-like growth factor mRNA-binding protein 3 (IMP3) protein expression is associated with poor survival in muscle-invasive bladder cancer. BJU Int. 2012;110:E308–17. doi: 10.1111/j.1464-410X.2012.11149.x. [DOI] [PubMed] [Google Scholar]
- 31.Izadi-Mood N, Sarmadi S, Sanii S. Quality control in cervicovaginal cytology by cytohistological correlation. Cytopathology. 2013;24:33–38. doi: 10.1111/j.1365-2303.2011.00926.x. [DOI] [PubMed] [Google Scholar]
- 32.Cioc AM, Julius CJ, Proca DM, Tranovich VL, Keyhani-Rofagha S. Cervical biopsy/cytology correlation data can be collected prospectively and shared clinically. Diagn Cytopathol. 2002;26:49–52. doi: 10.1002/dc.10036. [DOI] [PubMed] [Google Scholar]


