Abstract
The study aimed for addressing the expression of soluble Fas (sFas) and soluble Fas Ligand (sFasL) in human nucleus pulposus (NP) and its attendant relationship with disc degeneration. Human NP samples were collected from patients with disc degeneration and cadavers as degenerate and normal groups, respectively. Subsequently, NP cells were cultured in monolayer. ELISA was performed to identify the expression levels of sFas and sFasL in the supernatant of NP cell cultures in vitro. Quantitative real-time PCR was used to detect the expression of sFas and sFasL in human NP cells in mRNA solution. The study comprised 12 degenerate and 8 normal cadaveric NP samples. The concentration value of sFas in the supernatant was significantly higher from degenerate NP than that from normal NP at each time point. In contrast, sFasL was significantly lower at each time point. Moreover, the expression of sFas and sFasL reached the peak at various early stages of cell cultures and decreased thereafter. Furthermore, the mRNA level of Fas in degenerate NP cells was significantly higher than that in normal cells; whereas FasL showed an opposite pattern. The study is the first addressing the expression of sFas and sFasL in human NP cell cultures. Moreover, the expression of sFas and sFasL varies with culture time in vitro with different levels in degenerate and normal settings. These findings indicate that sFas and sFasL might play a role in intervertebral disc degeneration.
Keywords: Intervertebral disc, nucleus pulposus, soluble Fas, soluble FasL, immune privilege
Introduction
The intervertebral disc is a significant component of the spine and plays a crucial role in the support, stability and flexibility of the vertebral column [1]. As the largest avascular organ in the body, the disc forms a complex composition, with a central nucleus pulposus (NP) surrounded an outer anulus fibrosus (AF) and sandwiched with endplate cartilages [2]. Consequently, this unique structure makes the disc isolated from the host immune system. Indeed, once the NP is exposed to the immune system, autoimmune reaction is evoked, which is thought to be one of the important factors contributing to intervertebral disc degeneration (IDD) [3-5].
While physical barrier plays a crucial role in the immune privilege of the IVD, accumulating evidence has indicated that molecular mechanisms are indispensable parts [6,7]. In fact, the local expression of Fas and Fas ligand (FasL) in NP has been noted as the underlying mechanism of immune privilege of the disc. As a type II membrane protein of 36 kDa, FasL belongs to the tumor necrosis factor (TNF) family. While binding to its receptor Fas, FasL can induce apoptosis as a triggering ligand [8,9]. NP cells in normal disc express FasL, which acts as apoptosis inducer for infiltrating immune cells and consequently maintains the immune privilege of the disc [10-12]. However, the exact cell-cell interaction mechanisms remain undefined.
Recently, it is notable that soluble Fas (sFas) and soluble Fas ligand (sFasL) are closely linked with the classic membrane forms as Fas and FasL with different function. sFas can neutralize membrane FasL, which inhibits the apoptosis of cells [13]. sFasL is deficient in transducing signals upon engagement with membrane Fas as a competitive inhibitor to induce apoptosis of the Fas baring immune cells [14]. Meanwhile, NP is a gelatinous structure rich in type II collagen and proteoglycans. This unique arrangement binds water molecules and renders the NP a much higher composition of water contributing to its consistency. Accordingly, we hypothesize that sFas and sFasL might play a role in the immune privilege of the disc with particular reference to the regulation of apoptosis of the infiltrating immune cells. Therefore, the aim of this study was to address the expression of sFasL and sFasL in human NP and their attendant relationship with disc degeneration.
Materials and methods
The institutional ethics review board of Xijing Hospital approved the study (No. 20111103-7). Furthermore, we collected written informed consents from each patient. The same written informed consents were obtained from their relatives as for cadaveric donors.
Human disc cell isolation and cultures
The degeneration degree was classified according to Pfirrmann’s grading system [15]. Samples obtained from cadaveric donors are classified as normal group; grade 4 and 5 disc samples from anterior interbody fusions are included in the degenerate group. All specimens were collected from the lumbar spine (L3/4-L5/S1). Patients with diseases, including spinal tumors, infections, degenerative stenosis, lumbar disc epidural corticosteroids within 3 months were excluded. Magnetic resonance imaging (MRI) data in records were collected as for the cadavers. Both group specimens were obtained within 2 hour after surgery or anatomy. Following washed with Hank balance salt solution, NP and AF tissues were identified and separated by a stereotaxic microscope. The NP samples were digested for 40 minutes in 0.2% pronase (Gibco-BRL, Carlsbad, CA, USA) and washed with Hank balance salt solution, the incubated in 0.25% type II collagenase (type I collagenase was used for the AF tissue) (Gibco-BRL, Carlsbad, CA, USA) at 37°C under gentle agitation. The remaining tissue debris was removed through a 45-μm pore-size nylon mesh after 4 hours. NP and AF cells were centrifuged at 200 g for 8 minutes and seeded in culture flasks with DMEM/F12-based culture solution (containing 10% FBS, 1% P/S) in 5% CO2 and 20% oxygen incubator, respectively.
Culture supernatant collection
Disc cells from normal and degenerate groups were cultured in 1×106/cm3. Supernatant of cultured medium was collected at day 2, day 4, day 6 and day 8. Briefly, at each time point, cells were centrifuged at 200 g for 8 minutes. Supernatant was collected and the pellet was then resuspended. Following washed in phosphate buffered saline (PBS), cells were re-seeded and cultured in the same environment.
Quantification of sFasL and sFas in the supernatant of disc cells
According to the manufacturer’s instructions, enzyme-linked immunosorbent assay (ELISA) kits with an antibody that recognizes human FasL (Rand D Systems, Minneapolis, USA) was used to determine the concentration of FasL in the supernatant of cultured NP cells (1×106 cells/well). Natural human FasL provided by the supplier was used to construct a standard curve and to obtain absolute values for calibration. The concentration was determined in triplicate in each sample and the average measurement was considered to be the final concentration.
As for the measurement of sFas, the same procedure was performed using an ELISA kit with an antibody to Fas (Rand D Systems, Minneapolis, USA) according to the manufacturer’s instructions. Supernatant of AF cells were used as a negative control.
Quantitative real-time PCR (qRT-PCR) analysis of Fas and FasL
Following the manufacturers’ instructions, RNA from NP cells of each group was extracted using Trizol reagent (Invitrogen, Carlsbad, CA). Reverse transcription to cDNA was performed Using a High-Capacity cDNA Archive Kit (ABI, Foster City, CA, USA). RNA concentrations were measured using a NanoDrop instrument (NanoDrop, Wilmington, DE, USA). The levels of mRNA were normalized to GAPDH mRNA controls. All reactions were performed three times in a GeneAmp PCR 9700 Thermocycler (ABI). The relative amounts of mRNA were calculated by the comparative Ct (2−ΔΔCt) method. Predesigned primers were purchased from Sangon (Sangon, Shanghai, China) as follows: Fas: forward 5’-TGTGTGCACAAGGCTGGCGC-3’, reverse 5’-TGCATCTGTCACTGCACTTACCACCA-3’; FasL: forward 5’-CAATGAAAATGAACACATTG-3’, reverse 5’-CCCACTTTAGAAATTAGATC-3’ [16].
Statistical analysis
Student’s t-test was used to compare two-group parameters (degenerate group and normal group). A p value less than 0.05 was considered statistical significant. The SPSS statistical package (SPSS, Chicago, IL, USA) for statistical analysis was used.
Results
The study comprised degenerate samples from 12 patients undergoing discectomy (age 32.2 ± 7.1 years, range 23-46 years) and normal samples from 8 cadavers (age 31.6 ± 7.4 years, range 21–42 years) (Table 1).
Table 1.
Summary of demographic data included in this study
| Patients NO. | Age | Gender | Level | Degree* |
|---|---|---|---|---|
| Degenerate group | ||||
| 1 | 32 | M | L45 | V |
| 2 | 23 | M | L45 | IV |
| 3 | 41 | M | L45 | V |
| 4 | 33 | F | L45 | IV |
| 5 | 34 | M | L45 | V |
| 6 | 28 | F | L5S1 | IV |
| 7 | 29 | F | L5S1 | IV |
| 8 | 24 | M | L45 | IV |
| 9 | 46 | M | L45 | IV |
| 10 | 26 | F | L5S1 | V |
| 11 | 28 | M | L45 | IV |
| 12 | 42 | F | L5S1 | IV |
| Normal group | ||||
| 13 | 42 | M | L45 | I |
| 14 | 25 | M | L45 | I |
| 15 | 33 | F | L45 | I |
| 16 | 27 | M | L45 | I |
| 17 | 40 | M | L45 | I |
| 18 | 39 | F | L45 | I |
| 19 | 21 | M | L45 | I |
| 20 | 26 | M | L45 | I |
Pfirrmann’s grading system.
The level of sFas in degenerate NP cells culture supernatant was significantly higher than that in the normal NP supernatant in each time point (p < 0.0001, p < 0.0001, p = 0.017, p = 0.026). On day 4, the level of sFas hit the highest point, and decreased with time in both groups. The rank of sFasL was significantly lower in degenerate group than that in normal group (p < 0.0001, p < 0.0001, p = 0.009, p = 0.018). The Concentration values of sFasL achieved the highest level on day 4 and day 2 in degenerate group and normal group, respectively. Thereafter, concentrations of both groups decreased with time. As negative control, sFas and sFasL were undetectable in supernatant of AF cells culture medium. The concentration values of sFas and sFasL of the two groups in different time point were shown (Figures 1 and 2).
Figure 1.

sFas expression of normal and degenerate NP cell culture supernatant in different time points. The expression of sFas is in higher level in degenerate NP supernatant than that of normal NP. After reaching peak values on day 4 of cultures, the expression of sFas decreases with time. Data are representative of the average concentration values of at least three samples of each group. Error bars represent SEM. *p < 0.05.
Figure 2.
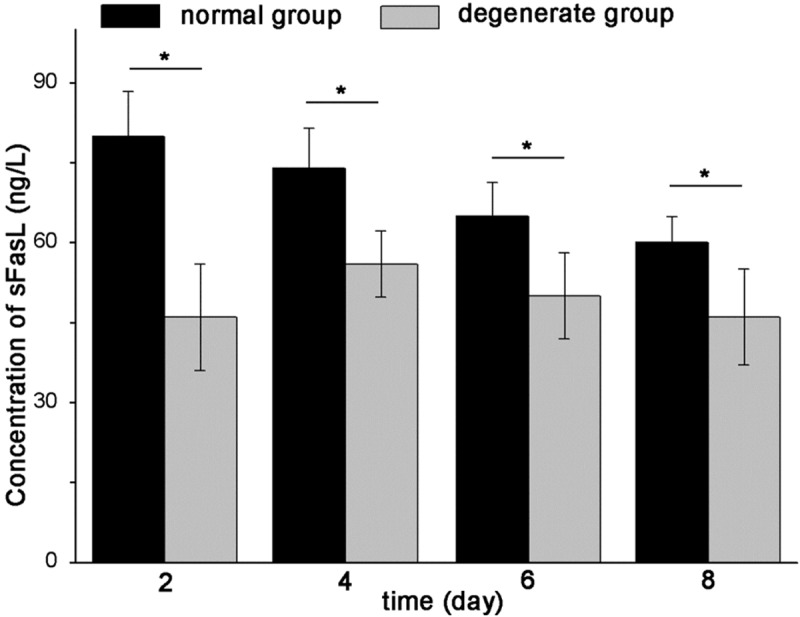
sFasL expression of normal and degenerate NP cell culture supernatant in different time points. The expression of sFasL is lower in degenerate NP supernatant than that of normal NP. The concentration values of sFasL achieve the highest level on day 4 and day 2 in degenerate group and normal group, respectively. Thereafter, the concentration values of both groups decrease with time. Data are representative of the average concentration values of at least three samples of each group. Error bars represent SEM. *p < 0.05.
Furthermore, the mRNA level of Fas in degenerate NP cells was higher than that in normal cells (p < 0.0001) (Figure 3); whereas FasL showed an opposite pattern (p < 0.0001) (Figure 4).
Figure 3.

Gene expression of Fas in normal and degenerate NP cells. The mRNA level of Fas is significantly higher in degenerate NP cells than that in normal NP cells. Data are representative of three independent experiments. Error bars represent SEM. *p < 0.05.
Figure 4.
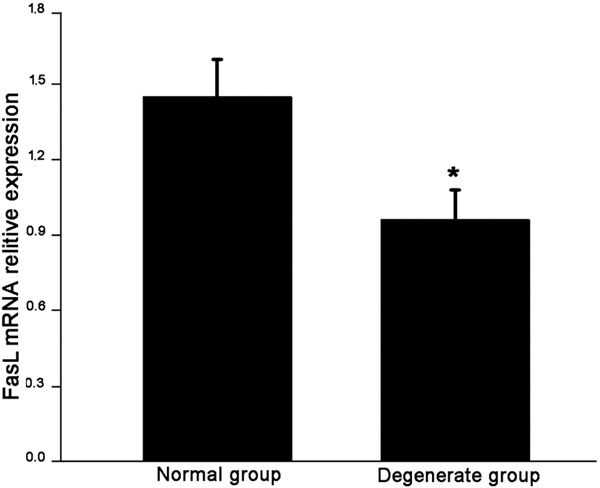
Gene expression of FasL in normal and degenerate NP cells. The mRNA level of FasL is significantly lower in degenerate NP cells than that in normal NP cells. Data are representative of three independent experiments. Error bars represent SEM. *p < 0.05.
Discussion
So far, the study is the first addressing the expression of sFas and sFasL in human NP cell cultures. Moreover, the expression of sFas and sFasL varies with culture time in vitro with different levels in degenerate and normal settings. These findings indicate that sFas and sFasL might play a role in intervertebral disc degeneration.
First, the Fas-FasL machinery is closed pertinent to cell apoptosis and plays a significant role in the maintenance of immune privilege. Various lines of evidence has shown a positive expression of FasL in normal NP cells and indicates FasL as a crucial regulator to produce pro-inflammatory cytokines [17] and induce apoptosis of infiltrating external cells [18]. However, the exact mechanisms are still unclear. As NP is highly hydrous with a low cell density, direct contact of cells is uncommon and the paracrine of cytokines could exert an important function. At this point, sFas and sFasL might be the actual functional executors of their membrane forms. In this study, significant difference is noted between degenerate and normal NP cells culture supernatant in terms of the concentration of sFas and sFasL. Our findings indicate a close link of sFas and sFasL with IDD.
Second, the increasing expression of sFas in degenerate NP cells might reduce the ability of sFasL to induce apoptosis of infiltrating immune cells. Indeed, sFas is thought to be produced by proteolytic cleavage of membrane-bound receptors or spliced mRNA. Lacking in the transmembrane domain, sFas exerts a protective function from Fas-mediated apoptosis [13]. Moreover, sFas is considered as a hallmark in numerous autoimmune and neoplastic diseases given that sFas can promote the proliferation of immune cells or tumor cells by inhibiting their apoptosis [19-22]. For NP, the increased sFas can neutralize the impact of FasL and improve the infiltration of immune cells, breaking the immune balance of NP and contributing to IDD.
Third, the decreasing sFasL of NP cells cultures is in accordance with the trend of membrane FasL in NP since sFasL is thought to be cleaved from the surface of NP cells [23]. sFasL is known to be deficient in transducing signals upon engagement with membrane Fas [14]. In fact, Han et al noted that FasL might have double roles in the regulation of apoptosis in NP [24]. FasL can act as both an immune cells apoptosis inducer and an inflammation mediator [25]. These findings reflect the complex of the function of FasL. The decreasing sFasL might both protect the infiltrating immune cells from apoptosis and worsen NP cells loss.
Although our study shed new light on the effects of sFas/sFasL on IDD, there are several drawbacks in the study. For one, we detected the expression of sFas and sFasL in cell cutures in vitro, which could not reflect the real scenarios in vivo. Nevertheless, our results provide direct evidence of the different expression levels of sFas and sFasL between degenerate and normal NP. Moreover, the mission of collecting sFas and sFasL in NP tissues might be impossible due to technical limitations nowadays.
In conclusion, the study is the first addressing the expression of sFas and sFasL in human NP cell cultures. Moreover, the expression of sFas and sFasL varies with culture time in vitro with different levels in degenerate and normal settings. These findings indicate that sFas and sFasL might play a role in intervertebral disc degeneration.
Acknowledgments
This work was supported by Chinese National Natural Science Foundation Grants (No.30901509, No.81270028, and No.81171747). We thank Dan Li for helping obtain cadaveric specimens and all of the members of the Orthopaedic Laboratory of Xijing Hospital for valuable work and discussions.
Disclosure of conflict of interest
The authors have declared that no competing interests exist.
References
- 1.Mirza SK, White AA 3rd. Anatomy of intervertebral disc and pathophysiology of herniated disc disease. J Clin Laser Med Surg. 1995;13:131–142. doi: 10.1089/clm.1995.13.131. [DOI] [PubMed] [Google Scholar]
- 2.Hughes SP, Freemont AJ, Hukins DW, McGregor AH, Roberts S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J Bone Joint Surg Br. 2012;94:1298–1304. doi: 10.1302/0301-620X.94B10.28986. [DOI] [PubMed] [Google Scholar]
- 3.Peng B, Hao J, Hou S, Wu W, Jiang D, Fu X, Yang Y. Possible pathogenesis of painful intervertebral disc degeneration. Spine (Phila Pa 1976) 2006;31:560–566. doi: 10.1097/01.brs.0000201324.45537.46. [DOI] [PubMed] [Google Scholar]
- 4.Geiss A, Larsson K, Rydevik B, Takahashi I, Olmarker K. Autoimmune properties of nucleus pulposus: an experimental study in pigs. Spine (Phila Pa 1976) 2007;32:168–173. doi: 10.1097/01.brs.0000251651.61844.2d. [DOI] [PubMed] [Google Scholar]
- 5.Sun Z, Zhang M, Zhao XH, Liu ZH, Gao Y, Samartzis D, Wang HQ, Luo ZJ. Immune cascades in human intervertebral disc: the pros and cons. Int J Clin Exp Pathol. 2013;6:1009–1014. [PMC free article] [PubMed] [Google Scholar]
- 6.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 7.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–2. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury I, Tharakan B, Bhat GK. Current concepts in apoptosis: the physiological suicide program revisited. Cell Mol Biol Lett. 2006;11:506–525. doi: 10.2478/s11658-006-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 10.Takada T, Nishida K, Doita M, Kurosaka M. Fas ligand exists on intervertebral disc cells: a potential molecular mechanism for immune privilege of the disc. Spine (Phila Pa 1976) 2002;27:1526–1530. doi: 10.1097/00007632-200207150-00009. [DOI] [PubMed] [Google Scholar]
- 11.Park JB, Kim KW, Han CW, Chang H. Expression of Fas receptor on disc cells in herniated lumbar disc tissue. Spine (Phila Pa 1976) 2001;26:142–146. doi: 10.1097/00007632-200101150-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kaneyama S, Nishida K, Takada T, Suzuki T, Shimomura T, Maeno K, Kurosaka M, Doita M. Fas ligand expression on human nucleus pulposus cells decreases with disc degeneration processes. J Orthop Sci. 2008;13:130–135. doi: 10.1007/s00776-007-1204-4. [DOI] [PubMed] [Google Scholar]
- 13.Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, Barr PJ, Mountz JD. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science. 1994;263:1759–1762. doi: 10.1126/science.7510905. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 15.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Tang T, Yang H, Yao X, Chen L, Liu W, Li T. The expression of Fas ligand on normal and stabbed-disc cells in a rabbit model of intervertebral disc degeneration: a possible pathogenesis. J Neurosurg Spine. 2007;6:425–430. doi: 10.3171/spi.2007.6.5.425. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto J, Maeno K, Takada T, Kakutani K, Yurube T, Zhang Z, Hirata H, Kurakawa T, Sakai D, Mochida J, Doita M, Kurosaka M, Nishida K. Fas ligand plays an important role for the production of pro-inflammatory cytokines in intervertebral disc nucleus pulposus cells. J Orthop Res. 2013;31:608–615. doi: 10.1002/jor.22274. [DOI] [PubMed] [Google Scholar]
- 18.Park JB, Lee JK, Cho ST, Park EY, Riew KD. A biochemical mechanism for resistance of intervertebral discs to metastatic cancer: Fas ligand produced by disc cells induces apoptotic cell death of cancer cells. Eur Spine J. 2007;16:1319–1324. doi: 10.1007/s00586-007-0463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulukaya E, Acilan C, Yilmaz M, Yilmaztepe-Oral A, Ari F, Zik B, Ursavas A, Tokullugil AH. sFas levels increase in response to cisplatin-based chemotherapy in lung cancer patients. Cell Biochem Funct. 2010;28:565–570. doi: 10.1002/cbf.1689. [DOI] [PubMed] [Google Scholar]
- 20.Murata J, Abe R. Soluble Fas ligand: is it a critical mediator of toxic epidermal necrolysis and Stevens-Johnson syndrome? J Invest Dermatol. 2007;127:744–745. doi: 10.1038/sj.jid.5700693. [DOI] [PubMed] [Google Scholar]
- 21.Fersching DM, Nagel D, Siegele B, Salat C, Heinemann V, Holdenrieder S, Stoetzer OJ. Apoptosis-related biomarkers sFAS, MIF, ICAM-1 and PAI-1 in serum of breast cancer patients undergoing neoadjuvant chemotherapy. Anticancer Res. 2012;32:2047–2058. [PubMed] [Google Scholar]
- 22.Vysotskii MM, Digaeva MA, Kushlinskii NE, Abbasova SG, Laktionov KP, Ermilova VD, Bakhoeva KA, Kryuk YV, Manukhin IB. Serum sFas, leptin, and VEGF in patients with ovarian cancer and benign tumors. Bull Exp Biol Med. 2009;148:810–814. doi: 10.1007/s10517-010-0823-5. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han D, Ding Y, Liu SL, Wang G, Si IC, Wang X, Cui L, Huang D. Double role of Fas ligand in the apoptosis of intervertebral disc cells in vitro. Acta Biochim Biophys Sin (Shanghai) 2009;41:938–947. doi: 10.1093/abbs/gmp087. [DOI] [PubMed] [Google Scholar]
- 25.Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase 1-independent IL-1beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]


