Abstract
Nonalcoholic fatty liver disease (NAFLD) is characterized by hepatic lipid accumulation which may progress towards inflammation (nonalcoholic steatohepatitis (NASH)). NAFLD is regarded as a consequence of a sedentary, food-abundant lifestyle which, in the modern world, often coincides with chronically high levels of perceived psychosocial stress. Here, we aimed to characterize the effect of chronic psychosocial stress on the development of NAFLD/NASH in male mice either fed with standard chow or NASH-inducing high fat diet. Chronic psychosocial stress was induced by chronic subordinate colony housing (CSC), a pre-clinically validated paradigm relevant for human affective and somatic disorders. Single housed (SHC) mice served as controls. Under standard chow conditions CSC mice revealed lower hepatic triglyceride levels but higher hepatic TNFα, MCP-1 and HMOX mRNA expression, while serum transaminase levels did not significantly differ from SHC mice. Under the NASH-inducing high-fat diet CSC and SHC mice showed similar body weight-gain and serum levels of glucose and adiponectin. Moreover, liver histology as well as TNFα, MCP-1 and HMOX expression were similar in CSC and SHC mice fed with HFD. Surprisingly, CSC showed even significantly lower transaminase levels than SHC mice fed with the same NASH-inducing diet. Together, these data indicate that under normal dietary conditions the CSC model induces noticeable hepatic oxidative stress and inflammation without causing manifest hepatocellular injury. In contrast, CSC exhibited a protective effect on hepatocellular injury in a dietary NASH-model. Identification of the underlying mechanisms of this phenomenon may lead to novel therapeutic strategies to prevent progression of NAFLD.
Keywords: Chronic psychosocial stress, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH)
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a pathological condition of rising clinical importance. It is characterized by hepatic lipid accumulation which starts with simple hepatic steatosis and progresses towards hepatocellular injury and inflammation (nonalcoholic steatohepatitis (NASH)) in a significant number of patients. NASH is the most common origin of abnormal liver tests in Western societies and its incidence is further increasing worldwide [1-3]. Given that obesity, type 2 diabetes, hypertension or dyslipidemia are frequently associated with NAFLD, it is considered as the hepatic manifestation of the metabolic syndrome, mainly caused by sedentary lifestyle combined with high caloric intake.
Another major risk factor for developing metabolic disturbances is chronic psychosocial stress triggered in humans, for example, by low socio-economic status or personal conflicts. Chronic stress was shown to contribute to increased weight gain and visceral adiposity in humans [4]. In support, long-term conflicts with close partners resulted in increased waist and body mass index [5]. Moreover, an association between chronic stress exposure and elevated basal sympathetic activity as well as prolonged sympathetic response to stress has been obtained in men [6,7]. A 6-years clinical trial showed that humans regarding themselves distressed had a higher prevalence developing the metabolic syndrome [8]. Chronic stress disrupts the circadian rhythm in mice and humans and has implications on metabolic deficits such as obesity, type 2 diabetes mellitus and dyslipidemia [9]. Furthermore, dysregulation of the hypothalamus-pituitary-adrenal (HPA) axis, one of the major stress systems in the body, has been described in several metabolic disorders [10]. Both, human and animal studies indicate that disrupted diurnal corticosteroid rhythms are linked to dramatic metabolic changes [11,12] and that carbohydrate metabolism, blood glucose concentration, and regulation of lipid synthesis are altered during chronic stress [13-15].
Thus, given the high comorbidity for the metabolic syndrome and NAFLD or NASH, it is likely that chronic stress indirectly promotes also the progression of chronic liver disease. This is supported by human data reporting a positive correlation between psychosocial stress and the severity of chronic hepatitis C [16]. Psychosocial stress has been further shown to exaggerate inflammatory and fibrosing changes in cirrhotic livers [17-19]. Moreover, elevated serum leptin levels were found in rodents exposed to chronic restraint stress in combination with feeding a high-fat diet [20]. Leptin is an adipokine which has been shown to promote the progression of NAFLD in experimental NASH models [21].
The aim of this study was to analyze the effect of chronic psychosocial stress exposure on the progression of NAFLD in mice. To induce chronic psychosocial stress in male mice they were exposed to chronic subordinate colony housing (CSC), a validated paradigm relevant for human affective and somatic disorders [22]. CSC exposure was combined with feeding a high-fat, NASH-inducing diet, which has been shown to induce similar pathological changes as observed in NASH-patients [23,24].
Materials and methods
Animals
Male C57BL/6 mice (Charles River, Sulzfeld, Germany) with an age of 36 - 45 days and a body weight of 19 - 22 g (experimental mice) were individually housed in standard polycarbonate mouse cages (16 x 22 x 14 cm) for one week before the subordinate colony housing (CSC) paradigm started (days 6 to day 1). The male offspring (weighing 30 - 35 g) of high anxiety-related behavior female mice (kindly provided by Dr. R. Landgraf, Max Planck Institute of Psychiatry in Munich) and C57BL/6 male mice (Charles River, Sulzfeld, Germany) were used as resident/dominant animals showing appropriate aggression and dominance. All mice were kept under standard laboratory conditions (12 h light/dark cycle, lights on at 0600 h, 22°C, 60% humidity) and had free access to tap water and either standard or a NASH inducing mouse diet. All experimental protocols were approved by the Committee on Animal Health and Care of the local government, and conformed to international guidelines on the ethical use of animals.
Experimental procedures
Two sets of experimental mice were either exposed to the CSC paradigm for 20 days as described previously [22] or were single-housed (single housed controls, SHC). Briefly, four CSC mice were housed together with a larger dominant male in a polycarbonate observation cage (38 x 22 x 35 cm) for 19 d consecutively. Prior to the CSC exposure, all potential male dominant mice were tested for their aggressive behavior. Males that started to bite and injure their opponents were not used for the CSC procedure. To avoid habituation, each dominant male was replaced by a novel dominant male on day 8 and day 15.
One set of CSC and SHC mice was fed with standard chow. The second set was fed with a NASH inducing mouse diet [24,25] from day 6 until day 20 of CSC.
In the morning of day 20 between 0800 and 1000 h mice of both sets were killed by decapitation and trunk blood was collected. Afterwards, the liver was removed and treated according to the respective readout parameter as described below.
Determination of serum parameters
Serum aspartate transaminase (AST) and alanine transaminase (ALT) and cholesterol derivatives levels were measured applying standard procedures as described [26].
Expression analysis
Isolation of total cellular RNA from murine liver tissue and reverse transcription were performed as described previously [27]. Quantitative real time-PCR was performed with specific sets of primers for TNFα, MCP-1 and HMOX (Qiagen, Hilden, Germany) using LightCycler technology (Roche, Mannheim, Germany) [27].
Histological analysis
For histological analysis murine liver tissue specimens were fixed for 24 h in 4% formalin at room temperature, dehydrated by graded ethanol and embedded in paraffin. Tissue sections (thickness 5 μm) were deparaffinized with xylene and stained with eosin/hematoxylin (H&E) [28].
Analysis of hepatic triglyceride and cholesterol content
Total triglycerides were extracted using the method of Bligh and Dyer with slight modifications [23,29,30] and quantified by the triglyceride determination kit (GPO) (Sigma, Deisenhofen, Germany) as described [31]. Hepatic cholesterol levels were measured using the cholesterol/cholesteryl ester quantifi cation kit (BioVision, Mountain View, CA, USA) according to the manufacturer’s instructions.
Statistical analysis
Results are expressed as mean ± standard error (range) of the mean (SEM) or percentage. Comparison between groups was made using the Student’s unpaired t-test. A p value <0.05 was considered statistically significant. All calculations were performed by using the GraphPad Prism Software (GraphPad Software, Inc., San Diego, USA).
Results
Effect of chronic psychosocial stress on hepatic lipid levels and pro-inflammatory gene expression
First, we analyzed the effect of chronic stressor exposure on hepatic lipid content. CSC exposure did not significantly alter hepatic cholesterol levels (data not shown). In contrast, levels of triglycerides, the main storage form of hepatic lipids, were significantly lower in the liver of CSC mice (Figure 1A). Triacylglycerol and cholesterol that is not required by the liver for synthesis of bile acids are secreted into the plasma in form of very-low-density lipoproteins (VLDL). In the bloodstream VLDL are degraded to intermediate-density lipoprotein (IDL) and either taken up by the LDL receptor on the liver cell surface, or continue to lose triacylglycerols in the bloodstream until they form low-density lipoprotein (LDL) molecules [32,33]. This is in line with recently published data showing higher serum levels of LDL-lipoproteins in CSC compared with SHC mice [26]. Together these findings suggest that higher export of lipids may account for slightly lowered hepatic triglyceride levels in CSC mice.
Figure 1.
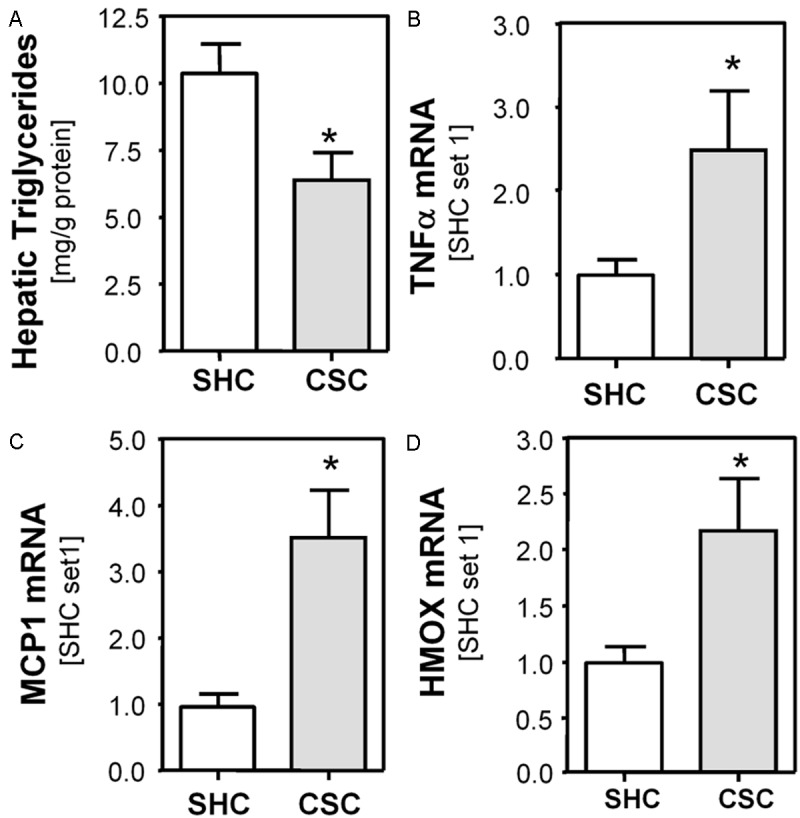
Effect of chronic psychosocial stress on hepatic lipid levels and pro-inflammatory gene expression in mice. A: Hepatic triglyceride content in mice exposed to 19 days of chronic subordinate colony housing (CSC; n=8) compared to single-housed control mice (SHC; n=8). Hepatic mRNA levels of (B) TNFα, (C) MCP-1 and (D) HMOX measured with quantitative RT-PCR in CSC and SHC mice. (*p<0.05 compared to SHC).
Psychosocial stress has also been suggested to influence the course of hepatic inflammation by inducing tumor necrosis factor alpha (TNFα) production in rats [34]. In accordance, we observed increased hepatic TNFα expression in CSC mice (Figure 1B). Furthermore, we observed increased hepatic monocyte chemotactic protein-1 (MCP-1) mRNA expression in CSC compared with SHC control mice (Figure 1C). Both TNFα and MCP-1 play a critical role in the development of progression of chronic liver disease including NAFLD [35,36]. Furthermore, we analyzed expression of heme oxygenase (decycling) 1 (HMOX). HMOX cleaves heme to form biliverdin and is increased via induction of reactive oxygen species (ROS) [37,38]. Thus, higher hepatic HMOX expression levels in CSC compared with SHC mice indicated enhanced hepatic ROS formation in livers of chronically-stressed mice (Figure 1D). Still, serum transaminase levels (AST, ALT) did not significantly differ between CSC und SHC mice and also histological analysis did not show any pathology (data not shown). Together, these results indicated that chronic psychosocial stress caused oxidative stress and an overall proinflammatory millieu in the liver of CSC mice without however causing notable hepatocellular injury.
Effect of chronic psychosocial stress on development of non-alcoholic steatohepatitis (NASH) in mice
Next, we analyzed the effect of chronic stress on the development of non-alcoholic steatohepatitis (NASH) in mice. Here, we combined CSC exposure with a NASH-inducing high-fat diet (HFD). We and others have previously shown that HFD feeding induces significant hepatic steatosis and inflammation which closely mimics the liver pathology observed in NASH patients [24,25,39]. CSC did not affect the increased weight gain during the 3-week HFD feeding period (Figure 2A). Also, glucose, triglyceride and adiponectin serum levels did not significantly differ between HFD-fed CSC and SHC mice (data not shown). Adiponectin is an adipokine produced by adipose tissue, and its expression has been shown to affect NASH-development and progression [40]. Histological analysis revealed similar hepatic steatosis and inflammation in HFD-fed mice with and without chronic stress exposure (Figure 2B). Still, hepatic triglyceride levels were slightly lower in CSC compared with SHC mice, both fed with HFD, (Figure 2C), while hepatic cholesterol levels were similar in both groups (data not shown). LDL serum levels did not significantly differ between CSC and SHC mice fed with HFD (Figure 2D).
Figure 2.
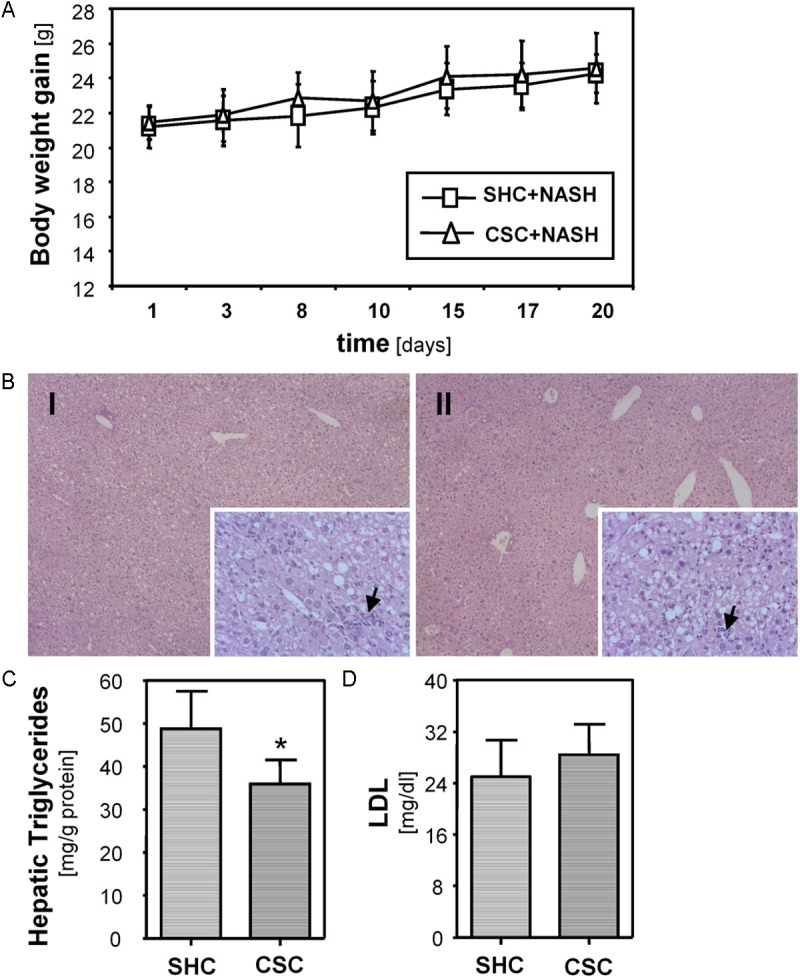
Effect of chronic psychosocial stress on development of non-alcoholic steatohepatitis (NASH) in mice. A: Body weight gain of mice exposed to 19 days of chronic subordinate colony housing (CSC; n=7) and single-housed control (SHC; n=6) mice, both fed with a NASH-inducing high fat diet. B: Histological images of H&E stained livers of CSC (I) and SHC (II) mice fed with a NASH-inducing diet showing similar mixed micro- and macro-vesicular steatosis. Both groups showed similar hepatic infiltration with immune cells (black arrows; magnification 40x and 200x). C: Hepatic triglyceride content of CSC and SHC mice fed with a NASH inducing diet. D: Serum LDL levels of CSC and SHC mice fed with a NASH inducing diet.
Surprisingly, and different from mice fed with standard chow (Figure 1C, 1D), expression of MCP-1 and TNFα did not differ significantly between CSC and SHC mice fed with the NASH inducing HFD (Figure 3A, 3B). Also, HMOX expression was similar in both groups (Figure 3C). In line with this, hepatic carnitine palmitoyltransferase I (CPT-1) and peroxisomal acyl-coenzyme A oxidase 1 (ACOX) expression did not significantly differ between CSC and SHC mice fed with HFD (data not shown). CPT1 is associated with the outer mitochondrial membrane and mediates the transport of long-chain fatty acids across the membrane by binding them to carnitine. ACOX is the first enzyme of the fatty acid beta-oxidation pathway. Both enzymes have been shown to critically contribute to ROS formation in response to hepatocellular lipid accumulation [41,42]. However, AST and ALT serum levels were significantly lower in CSC compared with SHC mice fed with the same NASH-inducing high-fat diet (Figure 3D, 3E).
Figure 3.
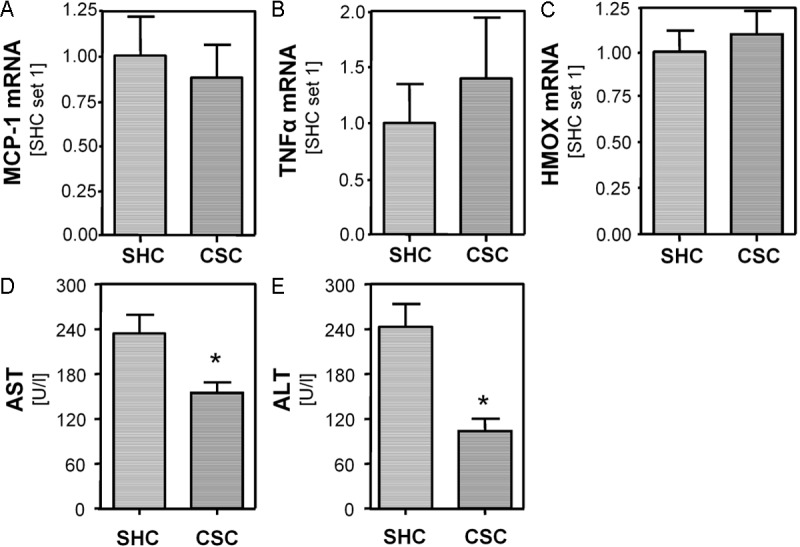
Effect of chronic stress in combination with a NASH-inducing high fat diet on hepatic pro-inflammatory gene expression and injury in mice. Expression of MCP-1 (A), TNFα (B) and HMOX (C) mRNA in mice exposed to 19 days of chronic subordinate colony housing (CSC; n=7) and single-housed control (SHC; n=6) mice, both fed with a NASH inducing diet. Serum levels of aspartate transaminase AST (D) and alanine transaminase ALT (E) in CSC and SHC mice, both fed with a NASH inducing diet. (*p<0.05 compared to SHC).
Discussion
Non-alcoholic fatty liver disease is mainly caused by a sedentary, food-abundant lifestyle, in Western countries often coinciding with high levels of perceived life stress. Therefore, we aimed to analyze the effect of chronic stress on the development and progression of NAFLD. Chronic subordinate colony (CSC) housing was applied to induce chronic psychosocial stress in mice. This model has been shown to be a validated paradigm relevant for human psychiatric and somatic disorders [15]. We and others have previously found altered LDL serum levels upon exposed to various stressors [43,44] and confirmed this finding also in this study (data not shown). In contrast, we did not observe that CSC exposure affects serum triglyceride levels. However, we found decreased hepatic triglyceride levels in stressed mice, while hepatic cholesterol content was comparable to SHC mice. So far, to our knowledge, alterations in hepatic lipid content solely in response to chronic psychosocial stressor exposure have not been described. Still, Fromenty and colleagues showed reduced body weight gain and hepatic triglycerides in mice after chronic alcohol consumption [45]. In contrast, aggravation of the obese phenotype could be observed in rats treated with a HFD in combination with a series of different stressors [46], and Fu and colleagues obtained similar results in rats treated with a HFD in combination with a chronic random electronic foot shock stress paradigm [47]. In our study, we did not observe a significant effect of chronic stress, induced by 19 day-exposure to the CSC paradigm, on body weight gain in HFD-fed mice. Also, hepatic triglyceride levels were only slightly reduced in CSC compared with SHC mice in the HFD-fed group. Furthermore, serum levels of glucose, triglycerides and lipoproteins were similar in HFD-fed mice with and without stressor exposure. Similarly, Paternain et al. observed no significant effects of chronic stress on weight gain, blood glucose concentrations and serum lipid profile in rats fed with a so called cafeteria diet, which was similar to our NASH inducing diet [48]. In a study performed by Manting et al. chronic stress accompanying HFD did not significantly affect hepatic triglyceride content in rats [46]. Variant species, strains, and stress models may be one reason that chronic stress differently affected body weight, hepatic lipid composition and serum lipid and lipoprotein levels in these studies. Also, differences in the exact composition of the high-fat diets, gender and age may play a role. However, it appears mostly consistent that chronic stress causes higher circulating LDL levels under normal feeding conditions. In our study, this was accompanied by lower hepatic triglyceride levels while these stress effects were less prominent or totally absent upon feeding a HFD. Enhanced LDL serum levels in CSC mice fed with standard chow [26] might reflect an increased hepatic LDL secretion due to a higher demand of nutrients or a reduced cellular calorie uptake upon chronic stressor exposure. In contrast, a high calorie diet may abolish these effects.
In addition to hepatic steatosis, NASH is characterized by hepatocellular injury and inflammation [21,49]. Already under standard feeding conditions, CSC exposure induced the expression of pro-inflammatory genes and HMOX, indicative for increased levels of oxidative stress, in the liver of mice. Therefore, one might have expected that additional feeding of a HFD would have promoted the development of NASH in this model. Surprisingly, the analyzed parameters of hepatic inflammation and oxidative stress were similar in CSC and SHC mice fed with NASH-inducing HFD. Even more surprisingly, CSC mice revealed significantly lower serum transaminase levels than SHC mice fed with the same NASH inducing diet.
Together, these findings indicate that in our experimental setting chronic psychosocial stressor exposure ameliorates NASH-induced hepatic injury. Noteworthy, this outcome appears to be independent of a direct CSC effect on hepatic steatosis and inflammation. Currently, we can only speculate on the molecular mechanisms by which this protective effect is mediated. For instance, stress may positively affect extrahepatic pathomechanism as visceral fat abundance or adipokine secretion which are known to promote NASH pathology [1]. However, in our NASH-CSC study chronic stress did not affect adiponectin serum levels or body weight indicative for similar adipose tissue composition. Further studies are necessary to reveal how chronic stress ameliorated NASH development in mice and to verify these factors in patients. Potentially, these findings may lead to novel prognostic parameters or therapeutic targets for NAFLD progression.
Acknowledgements
We are indebted to Heidi Gschwendtner, Monika Artinger, Dominik Langgartner and Nicole Grunwald for excellent technical assistance. This work was supported by grants from the German Research Association (DFG) to S.O.R. and C.H.
Disclosure of conflict of interest
None to declare.
References
- 1.Bosserhoff A, Hellerbrand C. Obesity and fatty liver are ‘grease’ for the machinery of hepatic fibrosis. Dig Dis. 2011;29:377–383. doi: 10.1159/000329800. [DOI] [PubMed] [Google Scholar]
- 2.Hellerbrand C. Pathophysiological similarities and synergisms in alcoholic and non-alcoholic steatohepatitis. Dig Dis. 2010;28:783–791. doi: 10.1159/000324286. [DOI] [PubMed] [Google Scholar]
- 3.Straub BK, Schirmacher P. Pathology and biopsy assessment of non-alcoholic fatty liver disease. Dig Dis. 2010;28:197–202. doi: 10.1159/000282086. [DOI] [PubMed] [Google Scholar]
- 4.Scott KA, Melhorn SJ, Sakai RR. Effects of Chronic Social Stress on Obesity. Curr Obes Rep. 2012;1:16–25. doi: 10.1007/s13679-011-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kouvonen A, Stafford M, De VR, Shipley MJ, Marmot MG, Cox T, Vahtera J, Vaananen A, Heponiemi T, Singh-Manoux A, Kivimaki M. Negative aspects of close relationships as a predictor of increased body mass index and waist circumference: the Whitehall II study. Am J Public Health. 2011;101:1474–1480. doi: 10.2105/AJPH.2010.300115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner EJ, Hemingway H, Walker BR, Page M, Clarke P, Juneja M, Shipley MJ, Kumari M, Andrew R, Seckl JR, Papadopoulos A, Checkley S, Rumley A, Lowe GD, Stansfeld SA, Marmot MG. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002;106:2659–2665. doi: 10.1161/01.cir.0000038364.26310.bd. [DOI] [PubMed] [Google Scholar]
- 7.Steptoe A, Feldman PJ, Kunz S, Owen N, Willemsen G, Marmot M. Stress responsivity and socioeconomic status: a mechanism for increased cardiovascular disease risk? Eur Heart J. 2002;23:1757–1763. doi: 10.1053/euhj.2001.3233. [DOI] [PubMed] [Google Scholar]
- 8.Puustinen PJ, Koponen H, Kautiainen H, Mantyselka P, Vanhala M. Psychological distress predicts the development of the metabolic syndrome: a prospective population-based study. Psychosom Med. 2011;73:158–165. doi: 10.1097/PSY.0b013e3182037315. [DOI] [PubMed] [Google Scholar]
- 9.Tamashiro KL, Sakai RR, Shively CA, Karatsoreos IN, Reagan LP. Chronic stress, metabolism, and metabolic syndrome. Stress. 2011;14:468–474. doi: 10.3109/10253890.2011.606341. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwenhuizen AG, Rutters F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol Behav. 2008;94:169–177. doi: 10.1016/j.physbeh.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Minami I, Tateno T, Yoshimoto T, Doi M, Izumiyama H, Akashi T, Hirata Y. Subclinical Cushings disease with amelioration of metabolic comorbidities after removal of pituitary tumor. Intern Med. 2006;45:1231–1235. doi: 10.2169/internalmedicine.45.1809. [DOI] [PubMed] [Google Scholar]
- 12.Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology. 2010;151:2117–2127. doi: 10.1210/en.2009-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathews EH, Liebenberg L. A practical quantification of blood glucose production due to high-level chronic stress. Stress Health. 2012;28:327–332. doi: 10.1002/smi.2415. [DOI] [PubMed] [Google Scholar]
- 14.Nirupama R, Devaki M, Yajurvedi HN. Chronic stress and carbohydrate metabolism: persistent changes and slow return to normalcy in male albino rats. Stress. 2012;15:262–271. doi: 10.3109/10253890.2011.619604. [DOI] [PubMed] [Google Scholar]
- 15.Chuang JC, Cui H, Mason BL, Mahgoub M, Bookout AL, Yu HG, Perello M, Elmquist JK, Repa JJ, Zigman JM, Lutter M. Chronic social defeat stress disrupts regulation of lipid synthesis. J Lipid Res. 2010;51:1344–1353. doi: 10.1194/jlr.M002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagano J, Nagase S, Sudo N, Kubo C. Psychosocial stress, personality, and the severity of chronic hepatitis C. Psychosomatics. 2004;45:100–106. doi: 10.1176/appi.psy.45.2.100. [DOI] [PubMed] [Google Scholar]
- 17.Chida Y, Sudo N, Kubo C. Does stress exacerbate liver diseases? J Gastroenterol Hepatol. 2006;21:202–208. doi: 10.1111/j.1440-1746.2006.04110.x. [DOI] [PubMed] [Google Scholar]
- 18.Swain MG. I. Stress and hepatic inflammation. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1135–G1138. doi: 10.1152/ajpgi.2000.279.6.G1135. [DOI] [PubMed] [Google Scholar]
- 19.Vere CC, Streba CT, Streba LM, Ionescu AG, Sima F. Psychosocial stress and liver disease status. World J Gastroenterol. 2009;15:2980–2986. doi: 10.3748/wjg.15.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macedo IC, Medeiros LF, Oliveira C, Oliveira CM, Rozisky JR, Scarabelot VL, Souza A, Silva FR, Santos VS, Cioato SG, Caumo W, Torres IL. Cafeteria diet-induced obesity plus chronic stress alter serum leptin levels. Peptides. 2012;38:189–196. doi: 10.1016/j.peptides.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 22.Reber SO, Birkeneder L, Veenema AH, Obermeier F, Falk W, Straub RH, Neumann ID. Adrenal insufficiency and colonic inflammation after a novel chronic psycho-social stress paradigm in mice: implications and mechanisms. Endocrinology. 2007;148:670–682. doi: 10.1210/en.2006-0983. [DOI] [PubMed] [Google Scholar]
- 23.Dorn C, Riener MO, Kirovski G, Saugspier M, Steib K, Weiss TS, Gabele E, Kristiansen G, Hartmann A, Hellerbrand C. Expression of fatty acid synthase in nonalcoholic fatty liver disease. Int J Clin Exp Pathol. 2010;3:505–514. [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, Ando H, Yokoyama M, Honda M, Zen Y, Nakanuma Y, Miyamoto K, Kaneko S. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–1403. doi: 10.1002/hep.21874. [DOI] [PubMed] [Google Scholar]
- 25.Dorn C, Kraus B, Motyl M, Weiss TS, Gehrig M, Scholmerich J, Heilmann J, Hellerbrand C. Xanthohumol, a chalcon derived from hops, inhibits hepatic inflammation and fibrosis. Mol Nutr Food Res. 2010;54(Suppl 2):S205–S213. doi: 10.1002/mnfr.200900314. [DOI] [PubMed] [Google Scholar]
- 26.Fuchsl AM, Uschold-Schmidt N, Reber SO. Chronic psychosocial stress in male mice causes an up-regulation of scavenger receptor class B type 1 protein in the adrenal glands. Stress. 2013;16:461–8. doi: 10.3109/10253890.2013.793303. [DOI] [PubMed] [Google Scholar]
- 27.Hellerbrand C, Amann T, Schlegel J, Wild P, Bataille F, Spruss T, Hartmann A, Bosserhoff AK. The novel gene MIA2 acts as a tumour suppressor in hepatocellular carcinoma. Gut. 2008;57:243–251. doi: 10.1136/gut.2007.129544. [DOI] [PubMed] [Google Scholar]
- 28.Gabele E, Muhlbauer M, Dorn C, Weiss TS, Froh M, Schnabl B, Wiest R, Scholmerich J, Obermeier F, Hellerbrand C. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem Biophys Res Commun. 2008;376:271–276. doi: 10.1016/j.bbrc.2008.08.096. [DOI] [PubMed] [Google Scholar]
- 29.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 30.Buettner R, Newgard CB, Rhodes CJ, O’Doherty RM. Correction of diet-induced hyperglycemia, hyperinsulinemia, and skeletal muscle insulin resistance by moderate hyperleptinemia. Am J Physiol Endocrinol Metab. 2000;278:E563–E569. doi: 10.1152/ajpendo.2000.278.3.E563. [DOI] [PubMed] [Google Scholar]
- 31.Wobser H, Dorn C, Weiss TS, Amann T, Bollheimer C, Buttner R, Scholmerich J, Hellerbrand C. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res. 2009;19:996–1005. doi: 10.1038/cr.2009.73. [DOI] [PubMed] [Google Scholar]
- 32.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 33.Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab. 2011;22:353–363. doi: 10.1016/j.tem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tjandra K, Sharkey KA, Swain MG. Progressive development of a Th1-type hepatic cytokine profile in rats with experimental cholangitis. Hepatology. 2000;31:280–290. doi: 10.1002/hep.510310204. [DOI] [PubMed] [Google Scholar]
- 35.Gabele E, Dostert K, Hofmann C, Wiest R, Scholmerich J, Hellerbrand C, Obermeier F. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J Hepatol. 2011;55:1391–1399. doi: 10.1016/j.jhep.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 36.Barsic N, Lerotic I, Smircic-Duvnjak L, Tomasic V, Duvnjak M. Overview and developments in noninvasive diagnosis of nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:3945–3954. doi: 10.3748/wjg.v18.i30.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26(Suppl 1):173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka Y, Maher JM, Chen C, Klaassen CD. Hepatic ischemia-reperfusion induces renal heme oxygenase-1 via NF-E2-related factor 2 in rats and mice. Mol Pharmacol. 2007;71:817–825. doi: 10.1124/mol.106.029033. [DOI] [PubMed] [Google Scholar]
- 39.Thomas A, Stevens AP, Klein MS, Hellerbrand C, Dettmer K, Gronwald W, Oefner PJ, Reinders J. Early changes in the liver-soluble proteome from mice fed a nonalcoholic steatohepatitis inducing diet. Proteomics. 2012;12:1437–1451. doi: 10.1002/pmic.201100628. [DOI] [PubMed] [Google Scholar]
- 40.Zhou D, Lin Z, Kong L, Li J. Serum Adiponectin Levels and Hepatic Diseases. Hepatol Res. 2013 doi: 10.1111/hepr.12114. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Shimoda H, Tanaka J, Kikuchi M, Fukuda T, Ito H, Hatano T, Yoshida T. Effect of polyphenol-rich extract from walnut on diet-induced hypertriglyceridemia in mice via enhancement of fatty acid oxidation in the liver. J Agric Food Chem. 2009;57:1786–1792. doi: 10.1021/jf803441c. [DOI] [PubMed] [Google Scholar]
- 42.Vial G, Dubouchaud H, Couturier K, Cottet-Rousselle C, Taleux N, Athias A, Galinier A, Casteilla L, Leverve XM. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. J Hepatol. 2011;54:348–356. doi: 10.1016/j.jhep.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 43.Zeeni N, Daher C, Fromentin G, Tome D, Darcel N, Chaumontet C. A cafeteria diet modifies the response to chronic variable stress in rats. Stress. 2013;16:211–219. doi: 10.3109/10253890.2012.708952. [DOI] [PubMed] [Google Scholar]
- 44.Neves VJ, Moura MJ, Tamascia ML, Ferreira R, Silva NS, Costa R, Montemor PL, Narvaes EA, Bernardes CF, Novaes PD, Marcondes FK. Proatherosclerotic effects of chronic stress in male rats: altered phenylephrine sensitivity and nitric oxide synthase activity of aorta and circulating lipids. Stress. 2009;12:320–327. doi: 10.1080/10253890802437779. [DOI] [PubMed] [Google Scholar]
- 45.Fromenty B, Vadrot N, Massart J, Turlin B, Barri-Ova N, Letteron P, Fautrel A, Robin MA. Chronic ethanol consumption lessens the gain of body weight, liver triglycerides, and diabetes in obese ob/ob mice. J Pharmacol Exp Ther. 2009;331:23–34. doi: 10.1124/jpet.109.155168. [DOI] [PubMed] [Google Scholar]
- 46.Manting L, Haihong Z, Jing L, Shaodong C, Yihua L. The model of rat lipid metabolism disorder induced by chronic stress accompanying high-fat-diet. Lipids Health Dis. 2011;10:153. doi: 10.1186/1476-511X-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu JH, Sun HS, Wang Y, Zheng WQ, Shi ZY, Wang QJ. The effects of a fat- and sugar-enriched diet and chronic stress on nonalcoholic fatty liver disease in male Wistar rats. Dig Dis Sci. 2010;55:2227–2236. doi: 10.1007/s10620-009-1019-6. [DOI] [PubMed] [Google Scholar]
- 48.Paternain L, Garcia-Diaz DF, Milagro FI, Gonzalez-Muniesa P, Martinez JA, Campion J. Regulation by chronic-mild stress of glucocorticoids, monocyte chemoattractant protein-1 and adiposity in rats fed on a high-fat diet. Physiol Behav. 2011;103:173–180. doi: 10.1016/j.physbeh.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 49.Fujii H, Kawada N. Inflammation and fibrogenesis in steatohepatitis. J Gastroenterol. 2012;47:215–225. doi: 10.1007/s00535-012-0527-x. [DOI] [PubMed] [Google Scholar]


