Abstract
The human cervix is a tissue target of sex steroid hormones as estradiol (E2) which exerts its action through of the estrogen receptors alpha and beta (ER-α and ER-β). In this study we investigated the expression of ER-α and ER-β in human invasive cervical carcinomas using immunohistochemistry and RT-PCR analyses and compared with that observed in the corresponding normal tissue. The results show nuclear expression of ER-α mainly in the first third of normal cervical epithelium, however, decreased or absent expression were present in invasive cervical carcinoma, indicating that expression of ER-α is lost in cervical cancer. Nevertheless, by RT-PCR we were able to demonstrate mRNA expression of ER-α in invasive cervical tissues. These results suggest that loss of ER-α could be due to a mechanism of post-transcriptional and/or post-translational regulation of its gene during the progression to invasive carcinoma. On the other hand, ER-β was expressed in normal cervix with an expression pattern similar to ER-α. In addition to its nuclear localization, cytoplasmic immunoreaction of ER-β was present in the epithelium of invasive cervical carcinomas, suggesting an association between cytoplasmic ER-β expression and invasive phenotype in the cervical tumors. In summary, the results show that the cervical malignant cells tend to loss the ER-α but maintain the ER-β actively expressed. Loss of expression of ER-α in neoplastic tissue suggests that the estrogenic effects could be conducted through the ER-β in human neoplastic cervical tissue. More detailed studies are needed to confirm this suggestion and to determine the role of ER-β in cervical cancer.
Keywords: ER-α, ER-β, cervical cancer, HPV
Introduction
Epidemiological studies have implicated the HPV infection and estrogen exposure in cervical carcinogenesis, and it has been suggested that HPV and estrogen may play synergistic roles in cervical tumorigenesis [1]. The human uterine cervix is a target tissue for sex steroid hormones, particularly progesterone (P4) and estradiol (E2), and these hormones exert their action through of the specific receptors to progesterone (PR) and estradiol (ER), respectively. Upon binding to E2, ER dimerizes and binds to DNA sequences denominate estrogen response element (ERE) located in the promoter of various genes target to regulate its transcriptional activity [2].
In the mid 90’s, a second receptor was discovered and characterized, which was called estrogen receptor beta (ER-β) [3] and the classic ER now is known as estrogen receptor alpha or ER-α. Both receptors are highly homologous in the amino acid sequence of its DNA binding domain (96%) and to a lesser extent in the ligand binding domain (about to 55%) [4]. The identification of the ER-β subtype, has led a reevaluation of physiology and signaling pathways of estrogens. With the development of antibodies and RNA probes for each ER subtypes, it has been analyzed their expression and localization in different tissues, including male and female reproductive organs [5].
The ER-α receptor has been localized in the epithelial and muscle cells of the uterus and vagina, in the epithelial and stromal cells of mammary gland, in the theca cells and germinal epithelium of the ovary, and testicular Leydig cells [5], whereas ER-β have been detected in follicles and granulose cells of the ovary, epithelial and stromal uterine muscle cells, epithelial and stromal cells of mammary gland, testicular Sertoli and Leydig cells, in the outflow ducts, and the basal, stromal, and secretory cells of the prostate [6,7]; indicating the cell-specific expression of ER-α and ER-β in reproductive human tissues.
So far the information about the role of steroid hormones and their receptors in human cervical epithelium has been performed analyzing the classical ER-α, and it has been suggested that its expression decreases or vanishes in the cervical cancer (CC), however, it had not conclusive.
On the other hand, limited information exists about the ER-β subtype expression in the cervical epithelium [8,9]. In our knowledge there are not reports on the expression and function of ER-β in CC. Thus, in the present study the expression and localization of ER-α and ER-β in the invasive cervical carcinoma were analyzed.
Material and methods
Tissues samples
A group of fifty formalin-fixed, paraffin-embedded tissue samples composed by sixteen healthy cervical tissues and 34 invasive squamous cell carcinomas were obtained from the Department of Pathology, Oncology Hospital, National Medical Center Century XXI, IMSS. Additionally, 9 fresh normal cervix tissues from women who had been subjected to radical hysterectomy due to uterine myomatosis and with a negative Pap smear, and 9 invasive squamous cervical carcinomas were collected from the Oncology Service at Oncology Hospital, National Medical Center Century XXI, IMSS. Immediately after surgery, the tissues were snap frozen in liquid nitrogen and stored at -70°C until RNA extraction. A section of each tissue was used for the HPV detection and typing. All tissues samples were obtained from patients according to the procedures approved by the institutional review boards.
DNA extraction and PCR
Genomic DNA was extracted using the DNAzol® Reagent (Life Technologies Corporation, Carlsbad, CA, USA) and 100 nanograms were used as template in a PCR reaction consisting of 50 mM KCl, 1.5 mM MgCl2, 10 mM of Tris (pH 8.3), 200 mM of each dNTPs (dATP, dCTP, dGTP and dTTP), 1U Taq DNA polymerase (Promega, Madison, WI, USA) and 50 pmol each of the HPV MY09/MY11 consensus primers [10] in a final reaction volume of 50 μl with the following cycling conditions: an initial denaturation for 10 min at 94°C, followed by 40 cycles of 30 seconds at 94°C (denaturation), 30 seconds at 55°C (alignment), and 45 seconds at 72°C (extension), and by a final extension of 7 min at 72°C in a Perkin Elmer 480 Thermocycler. SiHa DNA (HPV16) and H2O were included as positive and negative control, respectively. The PCR products were resolved on 1.5% agarose gels stained with ethidium bromide, visualized under ultraviolet light (Eagle Eye II, Stratagene, La Jolla, CA, USA) and purified (Qiaex II, Qiagen Valencia, CA, USA). Typing of HPV was performed by direct labeling (Big Dye Terminator, Perkin Elmer Inc., Wellesley, MA, US) and sequencing, and by comparing with reported HPV sequences in the BLAST database (www.ncbi.nlm.nih.gov).
Immunohistochemistry analysis
Sections of paraffin-embedded tissue of 4 μm were mounted on glass slides coated with poly-L-lysine, deparaffinized and rehydrated in serial alcohols (100, 90, 70 and 30%) until water. The sections were heated in microwave antigen retrieval solution (Vector Laboratories, Burlingame, CA, USA), rinsed in 1x PBS pH 7.4 and incubated for 30 min on 3% H2O2 in metanol to inactivate endogenous peroxidase, and subsequently blocked with 10% BSA in 1x PBS for 30 min. Tissues were then incubated with primary anti-ER-α (ER-α, sc-8002) or anti-ER-β (ER-β, sc-8974) antibodies, both from Santa Cruz Biotechnology (Santa Cruz, CA, USA) to 4°C overnight. Sections were washed in PBS, incubated at room temperature for 2 hours with the Mouse/Rabbit Immunodetector HRP/DAB (Bio SB Inc. CA, USA) and washed with 1x PBS. The peroxidase reaction was developed with diaminobenzidine and H2O2 generating a brown precipitate. Finally, slides were counterstained with hematoxylin, dehydrated and mounted with synthetic resin. Mammary gland tissues were used as positive control for both receptors and negative controls consisted of BSA in PBS instead of primary antibody.
RNA extraction and RT-PCR
Total RNA was extracted from normal and malignant frozen tissues using the TRIzol reagent (Life Technologies Corporation, Carlsbad, CA, USA) according to the manufacturer’s conditions, and its quality was evaluated by visualization of the 28S and 18S ribosomal bands on agarose gel. The RNA was quantified in a spectrophotometer (NanoDrop® ND-1000, Thermo Fisher Scientific, Waltham, MA, USA), and the DNA contamination was removed by digestion with the RNase-free DNase (TURBO DNA-free Kit, Ambion Co., Austin, TX, USA). The purified RNA was reverse transcribed by using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) and the cDNA was amplified in a PCR reaction by using the AccessQuickTM Master Mix, (Promega, Madison, WI, USA) following the suggested protocol with specific oligonucleotides: ER-β sense (5’-GTCCATCGCCAGTTATCACATC-3’) and ER-β antisense (5’-GCCTTACATCCTTCACACGA-3’) to ER-β gene; ER-α sense (5’-TGTGCAATGACTATGCTTCA-3’) and ER-α antisense (5’-GCTCTTCCTCCTTGTTTTTA-3’) to ER-α gene, according to Leygue et al [11] in a Perkin Elmer 480 Thermocycler. The amplification reactions were performed with the next conditions: an initial denaturation step at 95°C for 2 min, followed by 30 cycles of amplification at 95°C for 30 sec (denaturation), 55°C to ER-α or 57°C to ER-β for 1 min (alignment of primers), and 72°C for 1 min (elongation), and a final elongation cycle of 7 min at 72°C. The amplicons size was 148 pb and 242 bp for ER-α and ER-β respectively. The amplification products were resolved on 1.5% agarose gel stained with ethidium bromide and visualized under UV light (Eagle Eye II, Stratagene, La Jolla, CA, USA). RNA from human uterus and H2O were included as positive and negative controls, respectively. The β-actin gene was included as an internal amplification control.
Statistical analysis
The statistical analysis was performed using the software SPSS v15. The Fisher’s exact or X 2 tests were conducted and considered of statistical significance with a level of p<0.05.
Results
HPV typing in cervical tissues
All normal cervical tissue (16/16) were negative for the presence of HPV viral sequences, whereas in invasive tissues, 29 of 34 cases were positive for HPV16 (85.3%), 2 cases HPV18 (5.9%), 2 cases HPV58 (5.9%) and 1 case HPV59 (2.9%) (Table 1).
Table 1.
HPV types in cervical tissues
| Tissue | n | HPV positives | Viral type (cases) |
|---|---|---|---|
| Normal | 16 | 0 | |
| Cancer | 34 | 34 | HPV16 (29) |
| HPV18 (2) | |||
| HPV58 (2) | |||
| HPV59 (1) |
ER-α and ER-β expression in normal cervix
We focused in determinate the expression of the both ER subtypes in normal cervix. Nuclear immunoreaction of ER-α was detected predominantly in the parabasal and basal cells in 15/16 (93.7%) of the normal tissues, as well as in stromal cells whereas apical epithelial cells were negative for this protein (Table 2, Figure 1A). On the other hand, nuclear and cytoplasmic immunoreaction of ER-β was observed in the epithelial basal and parabasal cells in 14/16 (87.5%) normal tissues (Table 2, Figure 1C). Some normal tissues showed nuclear and cytoplasmic immunoreaction in cells throughout the all squamous epithelium (data not shown). Few stromal cells showed exclusively nuclear immunoreaction for ER-β. To confirm the results obtained by immunohistochemistry, analyses by RT-PCR were made in normal tissues chosen randomly. We detected mRNA expression of ER-α and ER-β genes in 7/9 (77.8%) and 9/9 (100%) normal tissues, respectively (Figure 2). These results demonstrate that ER-α and ER-β are expressed in the epithelium of adult cervical tissue and suggest specific roles in the human cervical biology.
Table 2.
Expression of ER subtypes in human cervical tissues
| Cancer | Normal | Total | p-value | ||
|---|---|---|---|---|---|
| ER-α | |||||
| Negative | 34 | 1 | 35 | ||
| Positive | 0 | 15 | 15 | 0.0001* | |
| ER-β | |||||
| Negative | 10 | 2 | 12 | ||
| Positive | 24 | 14 | 38 | 0.29 |
Data analyses were performed with Fisher’s exact test.
Significant association.
Figure 1.
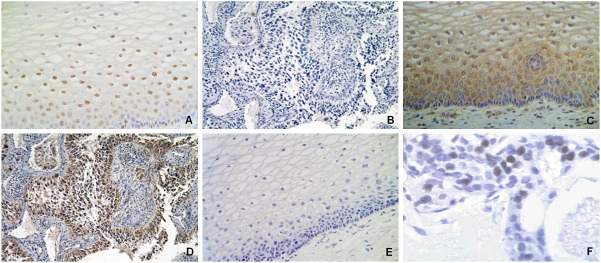
Immunostaining of ER-α and ER-β receptors in human cervical tissues. A. Immunopositivity of ER-α in the normal cervical epithelium showing nuclear reaction mainly in the parabasal layers (X40). B. Immunonegativity of ER-α in invasive carcinomas (X10). C. Immunopositivity of ER-β in normal cervical epithelium showing nuclear and cytoplasmic reaction in the cells of basal and parabasal layers (X40). D. Immunopositivity of ER-β in cervical invasive carcinoma with nuclear and cytoplasmic reaction in epithelial invasion zones (X10). E. A negative control in normal cervical tissue consisted of the omission of the primary antibody (X40). F. Positive control of ER-α in normal breast tissue (X100).
Figure 2.
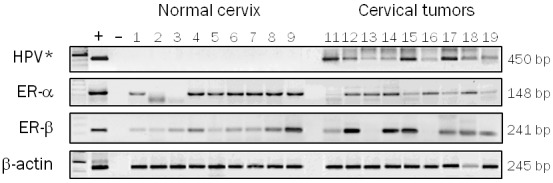
ER-α and ER-β analyses expression in human cervical tissues by RT-PCR. Invasive carcinomas and normal tissues were chosen randomly. (+) = positive control (RNA from uterus), (-) = negative control (H2O), 1-9 = normal cervix, 11-19 = invasive carcinomas. Amplification for β-actin mRNA was used as an internal control. *RNA from SiHa and H2O were included as positive and negative controls respectively.
Expression of ER-β in invasive cervical carcinoma
According to our results, ER-α expression was not detected in any of the analyzed invasive cervical carcinomas (Table 2, Figure 1B). These results show a statistically significant correlation between the specific loss of expression of ER-α subtype and the neoplastic cervical phenotype (p≤0.0001) (Table 2). To confirm our results obtained by immunohistochemistry, analyses by RT-PCR were made in invasive carcinomas chosen randomly. We detected mRNA transcripts of ER-α in 8 of 9 (90%) invasive carcinomas (Figure 2) but not its protein (Figure 1B). These results suggest that a regulation mechanism of the ER-α gene could be involved in cervical cancer. Interestingly, immunopositivity to ER-β was observed in 24/34 (70.6%) invasive cervical carcinomas (Table 2). Figure 1D shows an invasive cervical carcinoma where can be observed nuclear and cytoplasmic immunoreaction for ER-β in neoplastic cells, besides, ER-β mRNA transcripts were found in 7/9 invasive cervical tumors, a similar proportion of positive cases (77.6%) (Figure 2). We found not a statistically significant correlation between the ER-β expression and the neoplastic phenotype (p=0.29), however, these results demonstrate that ER-β is expressed in the cervical neoplastic cells and could be an important role in cervical carcinogenesis.
Discussion
The discovery of a second estrogen receptor, ERβ, has led to a reevaluation of estrogen action on their target tissues and its possible role in human carcinogenesis. In this study we analyzed the expression of ER-α and ER-β in normal and neoplastic human cervical tissues in order to determine their potential roles in cervical cancer development. Our results show strong nuclear expression of ER-α in the cervical squamous epithelium parabasal cells mainly, and to a lesser extent in the basal cells and in some cells of the intermediate layers. These results agree with previous data that reported nuclear expression of ER mainly in the parabasal cells of the normal cervical squamous epithelium [12,13]. It has been reported the expression of ER between 9% [13,14] and 18% [12] of invasive cervical carcinomas, however, we did not detect immunopositivity of ER-α in the neoplastic cells on practically any of invasive carcinomas analyzed in this study, according with other groups [13,15,16].
The inconsistency between our results and those could be due to the preparation and handling of tissues or to the primary antibody used; we used antibodies specific for the human ER-α and ER-β which fails to cross between them, however other groups could have used antibodies that identify more than one type of ER. Zhai et al [17] have suggested that this loss of ER-α has a major role in the invasion and progression of cervical cancer, as demonstrated by in vitro assays in cell lines derived from cervical cancer where the inhibition of ER-α with short hairpin RNA increased the cellular invasiveness, while restoring of ER-α in ER-α negative cell lines reduced its invasiveness. Additionally, it has been suggested that ER-α plays an important role in the early stages of cervical carcinogenesis in transgenic mice K14E7/ERα-/- [18].
The invasive cervical carcinoma included in our work were negative for the ER-α protein, and positive for HPV infection, suggesting an association between loss of expression specifically of ER-α and the presence of high-risk HPV, according to previous reports [13,14,16]. Interestingly, we were able to detect significant levels of mRNA ER-α by RT-PCR in invasive cervical carcinomas. These results contrast with those found by immunohistochemistry, but a similar inconsistency between mRNA and protein ER expression have also been reported in mammary gland carcinomas [19] where differences in handling, preservation and preparation time of tissues, as well as in the detection sensitivity of the methods may have influenced the results [20,21].
Previous data indicate that ER-α is probably regulated by multiple mechanisms, including transcriptional level, post-transcriptional and post-translational in breast cancer [22] and during the progression of cervical cancer [17]. ER-α have typical CpG islands within its promoter and first exon, and though bisulfite sequence analysis demonstrated promoter methylation in different cervical cancer cell lines, ER-α is not frequently methylated in primary tumors of cervical cancer [23]. Thus, although ER-α methylation is associated with a reduced or absent expression of ER-α in some cell lines of cervical cancer, it is probably not involved in the negative status of ER-α in the cervical cancer tissues without protein expression [17]. According to these reports, in our study the presence of the messenger RNA of ER-α suggests that a regulatory mechanism at the level of ER-α promoter methylation would not be involved; so our findings suggest that the down regulation of ER-α in CC could be due to a mechanism of post-transcriptional regulation, as has been suggested for the ER-β subtype [24,25]. Additionally, the loss of ER-α could be due to the dedifferentiation progressive of the cancer cells caused by the ubiquitination and proteolysis processes or by a mechanism of post-translational regulation as has been suggested previously in breast cancer [25]. Thus, down regulation of ER expression may be the first alteration to take place in normal epithelium during the development of cervical dysplasia in women infected with high-risk HPV [26].
On the other hand, there is limited and contradictory information about the expression of ER-β in the human uterine cervix [8,9,18] and its role in human cancer is not completely understood. Beside the presence of ER-α, we identified nuclear expression of ER-β in the normal cervical tissue with an epithelial expression pattern similar to the ER-α, and it has been speculated that the biology effect of estrogens might be governed by the relative expression of both estrogen receptors on the same tissue [27], so, the simultaneous expression of ER-α and ER-β in the normal cervix suggest that each subtype has specific physiological functions in the human cervical epithelium. Furthermore, ER-β can alter gene expression profiles in the presence of estradiol [28] and regulate the expression of genes associated with cell cycle and apoptosis [28], whereby ER-β could constitute a tumor suppressor gene with antiproliferative capacities [29].
We found immunopositivity for the ER-β protein in the most (70.6%) of invasive cervical carcinoma in our study. These results demonstrate that cervical neoplastic cells express ER-β and suggest that estrogens could be regulating some cervical tumors functions via the ER-β. Furthermore, a high expression of ER-β in endometrial cancer samples with severe myometrium invasion has been reported [30]. Some of the proteins involved in invasion and metastasis as metalloproteinase MMP2 and MMP9, are activated by the estrogen effects [31], over-regulated in cervical cancer [32] and maybe activated in these neoplastic cervical cells through ER-β [33].
Previous results published by our group have shown overexpression of MMP9, 10, 11 and 12 in invasive cervical carcinomas [34], so that some of the mechanisms of invasion in cervical cancer could be also governed in part by the activation of MMPs via the ER-β subtype. Additionally, ER-β might have a role in transcriptional activation of MMP9 in the invasive cervical carcinomas by the no canonical (genomic) way through to the activation of theirs sites AP-1 [35].
We detected significant levels of ER-β in the invasive cervical carcinomas, and interestingly, almost exclusively cytoplasmic staining was found in the neoplastic cells in these tumors. This pattern of cytoplasmic expression of ER-β has also been reported in vulvar squamous cell carcinoma [36], breast cancer [37] and colorectal cancer [38].
Although the ER-β is a transcription factor, changing ER-β nuclear expression in healthy tissue to cytoplasmic expression in neoplastic cells was an interesting finding; the presence of ER-β in the cytoplasm of the neoplastic cells may indicate well-defined roles on the gene activation. The presence of ER-β in cytoplasm could be due to the transcriptional activation of ER-β that shows preferentially by the non-genomic way rather than the classic way [39], modulating the transcriptional activity of AP-1 sites by the non-genomic way. Additionally, the cytoplasmic immunoreaction in the neoplastic cervical cells may be due in part to the specific expression of some isoforms of ER-β which can present nuclear or cytoplasmic expression that has been associated with different prognoses in human cancer depending on the isoform involved and its subcellular distribution [22,40,41], and apparently could be related to neoplastic progression with a high incidence of carcinoma in situ/invasive compared with healthy tissue [42].
The specific cytoplasmic expression of ER-β2 and ER-β5 isoforms has been associated with an overall survival poor and metastasis in breast and prostate cancers [40,43]. In our study, the neoplastic cells presented cytoplasmic staining of ER-β in most of the cervical tumors, but we were unable to discriminate any specific receptor isoform since our primary antibody recognizes the N-terminal domain present in the ER-β1, β2 and β3, but absent in the ER-β4 and β5 isoforms, therefore, we could not discern which ER-β isoforms are expressed in invasive carcinomas, and if these could play a role in the cervical neoplastic tissue. We are currently investigating the specific expression of ER-β isoforms in invasive tumors and precancerous lesions of the uterine cervix and its association with clinical variables in an attempt to determine if the expression of any isoform may be relevant as a diagnostic marker and/or important to the prognosis in cancer patients cervical or as a target for the development of new therapies in this neoplasm.
In conclusion, our results demonstrate that ER-α and ER-β are expressed in the normal cervical epithelium, but only ER-β is expressed en the invasive cervical tumors. The loss of ER-α could be due to mechanisms of post-transcriptional and/or post-translational regulation in the ER-α gene. The loss of ER-α is likely one of the factors involved in the onset of carcinogenesis process while that ER-β expression might be involved in the subsequent stages in this malignancy. Furthermore, our findings suggest that the loss of expression specifically of ER-α is that associated with infection with high-risk HPV, mainly with the viral types HPV16 and 18. Our results are not conclusive, but suggest that cytoplasmic expression of ER-β can be important in cervical cancer. Since tumor cells do not express ER-α, any effect of estrogens on these cells could be mediated through ER-β in cervical cancer.
Acknowledgments
This work was partially supported by grants from CONACyT-Mexico No. 87244 and IMSS-FIS/478.
Disclosure of conflict of interest
We have no conflict of interest in association with this work.
References
- 1.Nair HB, Luthra R, Kirma N, Liu YG, Flowers L, Evans D, Tekmal RR. Induction of aromatase expression in cervical carcinomas: effects of endogenous estrogen on cervical cancer cell proliferation. Cancer Res. 2005 Dec 1;65:11164–73. doi: 10.1158/0008-5472.CAN-05-1087. [DOI] [PubMed] [Google Scholar]
- 2.Harris HA, Bapat AR, Gonder DS, Frail DE. The ligand binding profiles of estrogen receptors alpha and beta are species dependent. Steroids. 2002 Apr;67:379–84. doi: 10.1016/s0039-128x(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 3.Mosselman S, Polman J, Dijkema R. ERβ: Identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 4.Kuiper G, Gustafsson A. The novel estrogen receptor-b subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogens. FEBS Lett. 1997;410:87–90. doi: 10.1016/s0014-5793(97)00413-4. [DOI] [PubMed] [Google Scholar]
- 5.Pelletier G, El-Alfy M. Immunocytochemical localization of estrogen receptors α and β in the human reproductives organs. J Clin Endocrinol Metab. 2000;85:4835–4840. doi: 10.1210/jcem.85.12.7029. [DOI] [PubMed] [Google Scholar]
- 6.Younes M, Honma N. Estrogen receptor β. Arch Pathol Lab Med. 2011 Jan;135:63–6. doi: 10.5858/2010-0448-RAR.1. [DOI] [PubMed] [Google Scholar]
- 7.Cavaillès V. Estrogens and receptors: an evolving concept. Climacteric. 2002 Jun;5(Suppl 2):20–6. [PubMed] [Google Scholar]
- 8.Stygar D, Wang H, Vladic YS, Ekman G, Eriksson H, Sahlin L. Co-localization of oestrogen recptor β and leukocyte markers in the human cervix. Mol Hum Reprod. 2001;7:881–886. doi: 10.1093/molehr/7.9.881. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Stjernholm Y, Ekman G, Eriksson H, Sahlin L. Different regulation of oestrogen receptors alpha and beta in the human cervix at term pregnancy. Mol Hum Reprod. 2001 Mar;7:293–300. doi: 10.1093/molehr/7.3.293. [DOI] [PubMed] [Google Scholar]
- 10.Manos MM, Ting Y, Wright DK. Use of Polymerase Chain Reaction amplification for the detection of genital Human Papillomaviruses. In: Furth M, Greaves M, editors. Cancer cells 7/Molecular Diagnostics of Human Cancer. U.S.A.: Cold Spring Harbor Lab Press; 1989. pp. 209–214. [Google Scholar]
- 11.Leygue E, Dotzlaw H, Watson PH, Murphy LC. Altered estrogen receptor α and β Messenger RNA expresión during human breast tumorigenesis. Cancer Res. 1998;58:3197–3201. [PubMed] [Google Scholar]
- 12.Kanai M, Shiozawa T, Xin L, Nikaido T, Fujii S. Immunohistochemical detection of sex sterpoid receptor, cyclins, and cyclin-dependent kinases in the normal snd neoplastic squamous epithelia of the uterini cervix. Cancer. 1998;82:1709–1719. doi: 10.1002/(sici)1097-0142(19980501)82:9<1709::aid-cncr18>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Nonogaki H, Fujii S, Konishi I, Nanbu Y, Ozaki S, Ishikawa Y, Mori T. Estrogen receptor localization in normal and neoplastic epithelium of the uterine cervix. Cancer. 1990;66:2620–2627. doi: 10.1002/1097-0142(19901215)66:12<2620::aid-cncr2820661226>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Konishi I, Fujii S, Nonogaki H, Nanbu Y, Iwai T, Mori T. Immnunohistochemical analisys of estrogen receptor, progesterone receptors, Ki-67 antigen, and normal papillomavirus DNA in normal and neoplastic epithelium of the uterine cervix. Cancer. 1991;68:1340–1350. doi: 10.1002/1097-0142(19910915)68:6<1340::aid-cncr2820680626>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Mosny DS, Herholz J, Degen W, Bender HG. Immunohistochemical investigations of steroid receptors in normal and neoplastic squamous epithelium of the uterine cervix. Gynecol Oncol. 1989 Dec;35:373–7. doi: 10.1016/0090-8258(89)90082-6. [DOI] [PubMed] [Google Scholar]
- 16.Monsonego J, Magdelenat H, Catalan F, Coscas Y, Sastre X. Estrogen and progesterone receptors in cervical human papillomavirus related lesions. Int J Cancer. 1991;48:533–539. doi: 10.1002/ijc.2910480410. [DOI] [PubMed] [Google Scholar]
- 17.Zhai Y, Bommer GT, Feng Y, Wiese AB, Fearon ER, Cho KR. Loss of estrogen receptor 1 enhances cervical cancer invasion. Am J Pathol. 2010 Aug;177:884–95. doi: 10.2353/ajpath.2010.091166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung SH, Wiedmeyer K, Shai A, Korach KS, Lambert PF. Requirement for estrogen receptor alpha in a mouse model for human papillomavirus-associated cervical cancer. Cancer Res. 2008 Dec 1;68:9928–34. doi: 10.1158/0008-5472.CAN-08-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham DM, Jin L, Lloyd RV. Detection of estrogen receptor in paraffin-embedded sections of breast carcinoma by immunocytochemistry and in situ hibridization. Am J Surg Pathol. 1991;15:475–485. doi: 10.1097/00000478-199105000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Subramaniam M, Negron V, Cicek M, Reynolds C, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC, Hawse JR. Development, characterization, and applications of a novel estrogen receptor beta monoclonal antibody. J Cell Biochem. 2012 Feb;113:711–23. doi: 10.1002/jcb.23443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carder PJ, Murphy CE, Dervan P, Kennedy M, McCann A, Saunders PT, Shaaban AM, Foster CS, Witton CJ, Bartlett JM, Walker RA, Speirs V. A multi-centre investigation towards reaching a consensus on the immunohistochemical detection of erbeta in archival formalinfixed paraffin embedded human breast tissue. Breast Cancer Res Treat. 2005;92:287–293. doi: 10.1007/s10549-004-4262-8. [DOI] [PubMed] [Google Scholar]
- 22.Parl FF. Multiple mechanisms of estrogen receptor gene repression contribute to ER-negative breast cancer. Pharmacogenomics J. 2003;3:251–253. doi: 10.1038/sj.tpj.6500201. [DOI] [PubMed] [Google Scholar]
- 23.Wentzensen N, Sherman ME, Schiffman M, Wang SS. Utility of methylation markers in cervical cancer early detection: appraisal of the state-of-the-science. Gynecol Oncol. 2009;112:293–299. doi: 10.1016/j.ygyno.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarzabek K, Koda M, Kozlowski L, Mittre H, Sulkowski S, Kottler ML, Wolczynski S. Distinct mRNA, protein expression patterns and distribution of oestrogen receptors α and β in human primary breast cancer: Correlation with proliferation marker Ki-67 and clinicopathological factors. Eur J Cancer. 2005;41:2924–2934. doi: 10.1016/j.ejca.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Smith L, Coleman LJ, Cummings M, Satheesha S, Shaw SO, Speirs V, Hughes TA. Expression of oestrogen receptor beta isoforms is regulated by transcriptional and post-transcriptional mechanisms. Biochem J. 2010 Jul 15;429:283–90. doi: 10.1042/BJ20100373. [DOI] [PubMed] [Google Scholar]
- 26.Bekkers RL, van der Avoort IA, Melchers WJ, Bulten J, de Wilde PC, Massuger LF. Down regulation of estrogen receptor expression is an early event in human papillomavirus infected cervical dysplasia. Eur J Gynaecol Oncol. 2005;26:376–82. [PubMed] [Google Scholar]
- 27.Bottner M, Thelen P, Jarry H. Estrogen receptor beta: Tissue distribution and the still largely enigmatic physiological function. J Steroid Biochem Mol Biol. 2013 Mar 20; doi: 10.1016/j.jsbmb.2013.03.003. pii: S0960-0760(13)00052-6. [DOI] [PubMed] [Google Scholar]
- 28.Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. 2006 Oct;147:4831–42. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 29.Morani A, Warner M, Gustafsson JA. Biological functions and clinical implications of oestrogen receptors alfa and beta in epithelial tissues. J Intern Med. 2008 Aug;264:128–42. doi: 10.1111/j.1365-2796.2008.01976.x. [DOI] [PubMed] [Google Scholar]
- 30.Takama F, Kanuma T, Wang D, Kagami I, Mizunuma H. Oestrogen receptor β expression and depth of myometrial invasion in human endometrial cancer. Br J Cancer. 2001;84:545–549. doi: 10.1054/bjoc.2000.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merlo S, Sortino MA. Estrogen activates matrix metalloproteinases-2 and -9 to increase beta amyloid degradation. Mol Cell Neurosci. 2012 Apr;49:423–9. doi: 10.1016/j.mcn.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Rouyer N, Wolf C, Chenard MP, Rio MC, Chambon P, Bellocq JP, Basset P. Stromelysin-3 gene expression in human cancer: an overview. Invasion Metastasis. 1994-1995;14:269–75. [PubMed] [Google Scholar]
- 33.Lu T, Achari Y, Rattner JB, Hart DA. Evidence that estrogen receptor beta enhances MMP-13 promoter activity in HIG-82 cells and that this enhancement can be influenced by ligands and involves specific promoter sites. Biochem Cell Biol. 2007 Jun;85:326–36. doi: 10.1139/o07-016. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez-Ortiz G, Pina-Sanchez P, Vazquez K, Duenas A, Taja L, Mendoza P, Garcia JA, Salcedo M. Overexpression of cathepsin F, matrix metalloproteinases 11 and 12 in cervical cancer. BMC Cancer. 2005 Jun 30;5:68. doi: 10.1186/1471-2407-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshizaki T, Sato H, Murono S, Pagano JS, Furukawa M. Matrix metalloproteinase 9 is induced by the Epstein-Barr virus BZLF1 transactivator. Clin Exp Metastasis. 1999 Jul;17:431–6. doi: 10.1023/a:1006699003525. [DOI] [PubMed] [Google Scholar]
- 36.Stefano I, Vizzielli G, Tortorella L, Fagotti A, Scambia G, Gallo D. Cytoplasmic expression of oestrogen receptor beta (ERβ) as a prognostic factor in vulvar squamous cell carcinoma in elderly women. Histopathology. 2011 Nov;59:909–17. doi: 10.1111/j.1365-2559.2011.04029.x. [DOI] [PubMed] [Google Scholar]
- 37.Fuqua SA, Schiff R, Parra I, Moore JT, Mohsin SK, Osborne CK, Clark GM, Allred DC. Estrogen receptor beta protein in human breast cancer: correlation with clinical tumor parameters. Cancer Res. 2003 May 15;63:2434–9. [PMC free article] [PubMed] [Google Scholar]
- 38.Xie LQ, Yu JP, Luo HS. Expression of estrogen receptor beta in human colorectal cancer. World J Gastroenterol. 2004 Jan 15;10:214–7. doi: 10.3748/wjg.v10.i2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Björnström L, Sjöberg M. Estrogen receptor-dependent activation of AP-1 via non-genomic signalling. Nucl Recept. 2004 Jun 14;2:3. doi: 10.1186/1478-1336-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaaban AM, Green AR, Karthik S, Alizadeh Y, Hughes TA, Harkins L, Ellis IO, Robertson JF, Paish EC, Saunders PT, Groome NP, Speirs V. Nuclear and Cytoplasmic Expression of ERB1, ERB2, and ERB5 Identifies Distinct Prognostic Outcome for Breast Cancer Patients. Clin Cancer Res. 2008 Aug 15;14:5228–35. doi: 10.1158/1078-0432.CCR-07-4528. [DOI] [PubMed] [Google Scholar]
- 41.Shaaban AM, O’Neill PA, Davies MP, Sibson R, West CR, Smith PH, Foster CS. Declining estrogen receptor-h expression defines malignant progression of human breast neoplasia. Am J Surg Pathol. 2003;27:1502–12. doi: 10.1097/00000478-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Shaaban AM, Jarvis C, Moore F. Prognostic significance of estrogen receptor h in epithelial hyperplasia of usual type with known outcome. Am J Surg Pathol. 2005;29:1593–9. doi: 10.1097/01.pas.0000184807.38037.75. [DOI] [PubMed] [Google Scholar]
- 43.Leung YK, Lam HM, Wu S, Song D, Levin L, Cheng L, Wu CL, Ho SM. Estrogen receptor β2 and β5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocr Relat Cancer. 2010 Sep;17:675–689. doi: 10.1677/ERC-09-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]


