Abstract
Anaplastic large cell lymphoma (ALCL) is a rare T-cell lymphoma composed of CD30-positive lymphoid cells. ALCL arising in the dura matter of the brain is even more infrequent, in which only one case has been reported worldwide so far. We report a case of a 30-year-old immunocompetent male with a dura-based mass, radiographically consistent with meningioma. However, the excised mass via a left parieto-occipital craniotomy was composed of large, pleomorphic lymphoid cells to be immunopositive for CD3, CD30, anaplastic lymphoma kinase protein-1 (ALK-1) and epithelial membrane antigen (EMA), and immunonegative for CD20, CD15 and CD68. Multiple ALK gene fusion signals in the ALK locus were detected by fluorescence in situ hybridization (FISH) analysis. The patient was treated with CHOP chemotherapy and intrathecal methotrexate along with brain radiation therapy, which resulted in a complete remission. In an analysis of 25 previously reported primary CNS ALCLs, ALK-1 positivity was shown to be prevalent in younger age, as ALCL occurs outside the brain. Patient less than 23 years, ALK-1 positivity and unifocal tumor may be associated with a better prognosis. However, sex, dural or leptomeningeal involvement, immune status, and tumor necrosis do not appear to have any influence on survival.
Keywords: Anaplastic large cell lymphoma, ALK-positive, dura, prognosis, primary tumor
Introduction
Primary central nervous system (CNS) lymphomas are mostly non-Hodgkin’s lymphomas of B cell origin accounting for 0.5% to 1.5% of all intracranial tumors [1]. Primary CNS lymphomas of T-cell origin are very rare and comprise 1.0–3.6% of all primary CNS lymphomas [2]. ALCL, first described by Stein in 1985 [3], is defined as a T-cell lymphoma composed of large pleomorphic lymphoid cells with an expression of CD30 [4].
The most frequent genetic alteration in ALCL is a translocation between the anaplastic lymphoma kinase (ALK) gene on 2p23 and the nucleophosmin (NPM) gene on 5p35, resulting in a hybrid gene encoding an 80-kDa chimeric protein termed NPM-ALK [4]. Although the pathogenesis of ALCL is not fully understood, the up-regulation of ALK is known to induce mitogenic activity and thus is likely to be involved in the neoplastic transformation process [5]. The ALK protein is easily detected by immunohistochemistry and absent from all postnatal normal human tissues except rare cells in the brain. Therefore, the expression of ALK protein is a reliable molecular test for the diagnosis of ALK-positive ALCL [4].
The vast majority of ALCLs are present as nodal diseases, involving the skin, bone, soft tissue, lung and liver as common extranodal sites [4]. However, ALCL rarely occurs in CNS, and is even more infrequent in the dura of the brain. A thorough review of the literature on primary CNS ALCL reveals that this case is the second report on primary ALCL arising in the dura and the 26th documented case overall (Table 1) [1,6-26].
Table 1.
Summary of all documented primary CNS ALCLs in the literature
| Case | Age (yr)/Sex | Location | Clinical presentation | Immune status | Pathological features | Treatment | Outcome (post-diagnosis) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Hallmark cells | Positive T-cell smarkers | Positive B-cell markersi | Lineage | EMA | Necrosis | |||||||
| ALK-1 positive cases | ||||||||||||
| 1. Havlioglu et al. 1995 [6] | 4/F | Multifocal lobes, brain stem, spinal cord | Headaches, neck stiffness | Normal | Y | N | N | Null | Pos | N | Ex, R, C | NED at 73 months |
| 2. Buxton et al. 1998 [7] | 10/F | Rt. parietal lobe, falx | Left hemiparesis | Normal | ND | CD3, CD45RO | ND | T | ND | Y | Ex, R, C | Dead at 6 months from post chemo sepsis |
| 3. Abdulkader et al. 1999 [8]y | 13/M | Rt. fontal and parietal lobe (meningeal involvement) | As mycobacterial CNS infection | Normal | Y | CD45RO | N | T | Pos | Y | C | Dead shortly after chemotherap |
| 4. Ponzoni et al. 2002 [9] | 29/M | Lt. fontal and temporal lobes | Fever, headache, generalized seizures | ND | Few | CD3, CD45RO | N | T | Pos | Y | R, C | NED at 19 months |
| 5. George et al. 2003 [10] | 17/M | Rt. parietal dura | ND | Normal | ND | CD3, CD43, CD45RO | N | T | ND | Y | Ex, R | NED at 57 months |
| 6. George et al. 2003 [10] | 18/F | Lt. temporal lobe (dural involvement) | ND | Normal | ND | CD45RO | N | T | ND | N | Ex, R, C | NED at 62 months |
| 7. Rupani et al. 2005 [11] | 17/M | Rt. fronto-parietal lobe | Headache, left partial seizures | Normal | Y | CD43 | ND | T | Pos | N | Ex, R, C | Dead at 4 months |
| 8. Cooper et al. 2006 [12] | 39/M | Rt. parieto-occipital lobe | Headaches, seizures | Normal | Few | CD3, CD43 | N | T | Pos | ND | Ex, R, C | NED at 9 months |
| 9. Carmichael et al. 2007 [13] | 38/M | Rt. parieto-occipital lobe | Seizure, left hemiparesis | Normal | ND | CD45 | ND | T | ND | ND | R, C | NED at 15 months |
| 10. Karikari et al. 2007 [14] | 4/ M | Frontal and parietal lobe (leptomeningeal involvement),pineal region | Generalized tonic clonic seizures | ND | ND | CD3, CD4, CD7 | N | T | ND | ND | R, C | ED at 1 month |
| 11. Merlin et al. 2008 [15] | 13/M | Leptomeninges in the Rt. frontal lobe | Headache, diplopia | Normal | ND | CD3, CD8 | N | T | ND | ND | R, C | Dead at 27 months from 2nd relapse and MOF |
| 12. Shah et al. 2010 [16] | 2/M | Rt. parieto-occipital lobe (leptomeningeal and dural involvement) | Left hemiparesis, complex partial seizures | Normal | ND | CD43 | N | T | Neg | Y | C | NED at 108 months |
| 13. Present cases | 30/M | Lt. parieto-occipital dura | Headache | Normal | Few | CD3 | N | T | Pos | N | Ex, R, C | NED at 16 month |
| ALK-1 negative cases | ||||||||||||
| 14. Paulus et al. 1994 [17] | 63/M | Multifocal lobes(dural involvement) | NA | Normal | Y | CD3, CD45RO | NAs | T | NA | NA | Ex, R | Dead at 11 week |
| 15. Nuckols et al. 1994 [18] | 66/F | Rt. temporal lobe | NA | SLE, CRF, thymoma | NA | CD3 | NA | T | NA | NA | Ex, Supportive | Dead at 4 days |
| 16. Chuang et al. 2001 [19] | 46/F | Lt. occipital lobe(dural involvement) | Headache, weaknessof her right extremity, limited eye movement | EBV infection | Y | CD43, TiA-1, Granzyme B | N | T | Neg | ND | Ex, R | NED at 25 mont |
| 17. Tajima et al. 2003 [20] | 52/F | Rt. frontal lobe | Right hemiparesis | Essential thrombo-cythemia | ND | N | N | Null | Neg | Y | R, C | ED |
| 18. George et al. 2003 [10] | 22/F | Multifocal lobes, cerebellum | ND | Normal | ND | CD3, CD8 | N | T | ND | Y | Ex, Supportive | Dead at 11 days |
| 19. George et al. 2003 [10] | 50/F | Multifocal lobes (dural involvement) | ND | Normal | ND | N | N | Null | ND | Y | Ex, R | Dead at 2 months |
| 20. Rowsell et al. 2004 [21] | 46/M | Rt. occipital lobe | Ataxia, inability to ambulate | HIV infection, Crohn disease | Y | .CD2, CD43, CD45 | N | T | Pos | N | Ex, R, Conformalproton therapy | Dead at 2 months from aspiration fungalpneumonia with infection of Aspergillus fumigatus. |
| 21. Kodama et al. 2009 [22] | 79/M | Lt. parieto-occipital lobe | Dementia-like symptoms | Normal | ND | CD3, CD5, CD45RO, GranzymeB | CD79a | T | Pos | Y | Ex, Supportive | Dead at 4 months from local recurrence |
| 22. Colen et al. 2010 [23] | 65/ M | Lt. fronto-temporal lobe | Headaches, blurry vision | Atypical meningio-ma | ND | CD3 | ND | T | Neg | ND | C | NED |
| 23. Sugino et al. 2012 [24] | 75/ M | Rt. temporal lobe | Memory loss (dementia) | Normal | Y | CD43 | N | T | Neg | N | R | Dead at 8 months |
| Cases ALK-1 not reported | ||||||||||||
| 24. Bergmann et al. 1991 [25] | 12/F | Lt. occipital lobe | NA | NA | NA | NA | NA | NA | NA | NA | Ex, R, C | Dead at 4 months |
| 25. Feldges et al. 1992 [26] | 20/M | Lt. parietal lobe | Generalized convulsiveseizures, right hemiparesis | ND | Y | CD3, CD45RO | N | T | Pos | ND | Ex, R, C | NED at 24 months |
| 26. Goldbrunner et al. 1996 [1] | 63/M | Right frontal and parietallobes | Focal motor seizure, left hemiparesis | Normal | Y | CD3, CD45RO | ND | T | ND | Y | Ex, R | Dead at 3 months |
YR, years; F, female; M, male; Rt, right; Lt, left; CNS, central nervous system; ND, Not described; NA, Not full text available for free; SLE, systemic lupus erythematosus; CRF, chronic renal failure; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; Y, yes; N, no; TiA-1, T-cell intracellular antigen-1; EMA, epithelial membrane antigen; Pos, positive; Neg, negative; Ex, excision; R, radiotherapy; C, chemotherapy; NED, no evidence of disease; ED, evidence of disease; MOF, multiple organ failure.
In this article, we present a case of primary ALK-positive ALCL occurring in the left parieto-occipital dura without evidence of systemic disease, mimicking a meningioma radiographically. We also suggest clinical and pathologic features that affect prognosis thought a review of the available literature on primary CNS ALCL.
Case report
A 30-year-old immunocompetent man with no previous medical history had suffered from a progressive headache on left parietal area for 6 weeks. On admission, the patient had alert mental status with no neurologic deficit. There was no sign of fever, recent illness, weight loss, night sweats, fatigue, or enlargement of lymphoid organs suggestive of systemic lymphoma. Laboratory examination including lactate dehydrogenase (LDH) was within normal limits. Subsequent MRI of the brain revealed a 5 mm-sized, well enhancing mass in the left parieto-occipital dura, accompanied with edema of adjacent parenchyma. The mass was hypointense on T1-weighted imaging, hyperintense on T2-weighted imaging, and homogeneously enhanced by gadolinium-diethylenetriamine penta-acetic acid. A dural tail sign was also observed for this tumor (Figure 1). The radiological differential diagnosis included atypical meningioma and dura-based lymphoma. Extensive microbiological serology, including testing for HIV, was unremarkable. Cerebro-spinal fluid (CSF) showed negative for malignancy. The patient underwent a left parieto-occipital craniotomy and total resection of the tumor.
Figure 1.
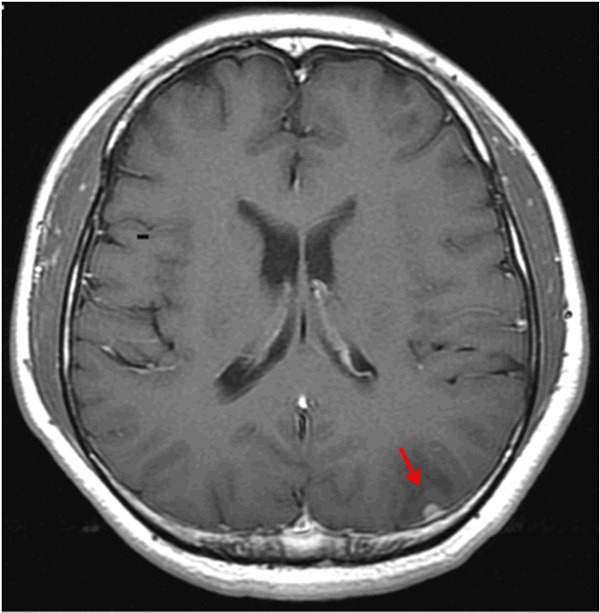
MRI scan of the brain. Axial T1-weighted imaging with contrast medium reveals a 5 mm-sized, well-enhancing mass with a dural tail sign in the left parieto-occipital region (T1-weighted with contrast medium).
Microscopic examination revealed a pleomorphic neoplasm consisting of large lymphoid cells with a moderate amount of amphophilic cytoplasm and bizarre nuclei. The nuclei had finely dispersed chromatin and prominent nucleoli. The malignant cells were admixed with reactive non-neoplastic cells, including lymphocytes and histiocytes. Brain parenchyma with gliosis was also observed. A few cells with multiple nuclei resembling Reed-Sternberg cell were seen (Figure 2A and 2D). The large atypical cells were neither cohesive nor present in the vascular lumina. Necrosis was absent. Prominent mitotic activity was noted. Ki-67 index was 75%.
Figure 2.
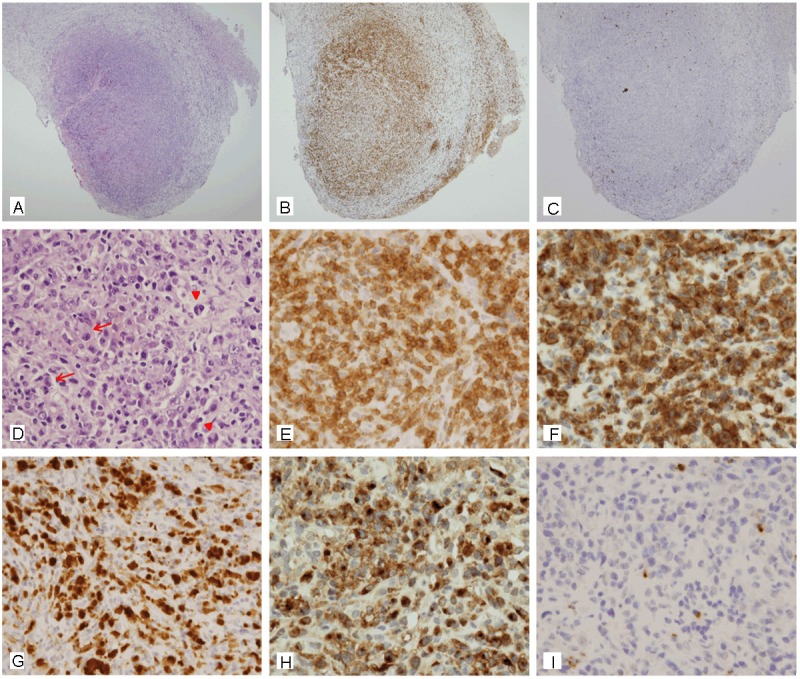
Microscopic features of the dural lymphoma. A: At low power field, the lymphoma is located in the dura matter composed of a thick, dense, fibrous connective tissue (hematoxylin and eosin stain, x 40). B: The lymphoma is composed predominantly of CD3-positive cells (CD3, x 40). C: CD20, the most widely used pan B-cell marker, is negative (CD20, x 40). D: At high power field, the lymphoma is composed of large anaplastic cells admixed with lymphocytes and histiocytes. The malignant cells have eccentric bizarre nuclei with prominent nucleoli (red arrow). Multinucleated cells resembling RS cell are seen (red arrow head) (hematoxylin and eosin stain, x 400). E: All malignant cells show strong membranous and cytoplasmic staining for CD3 (CD3, x 400). F: The malignant cells show membranous, cytoplasmic and paranuclear dot-like staining for CD30 (CD30, x 400). G: Anaplastic large cells show cytoplasmic and nuclear staining for ALK (ALK, x 400). H: The staining pattern for epithelial membrane antigen is similar to that seen with CD30 (EMA, x 400). I: Malignant cells are negative for CD15 (CD15, x 400).
By immunohistochemistry, the tumor cells were diffusely and strongly positive for pan-T cell marker (CD3) (Figure 2B and 2E) but negative for pan-B cell marker (CD20) (Figure 2C). They were also positive for CD30 (Figure 2F), ALK-1 (Figure 2G), EMA (Figure 2H), and leukocyte common antigen (LCA). They were negative for CD15 (Figure 2I), monocyte-macrophage marker (CD68), glial fibrillary acidic protein (GFAP), and S100 protein.
FISH analysis for the t(2;5) ALK gene translocation was performed with chromosome-specific probes (LSI ALK Dual Color break apart rearrangement probe, Vysis Inc, Downers Grove, Ill) to the ALK gene in chromosome 2 (2p23). Tumor proportion of the tested tissues was 90%. At least 60 tumor cells were counted. Splitting of the red and green signals and isolated red signals which were associated with ALK rearrangement were detected in tumor cells (Figure 3).
Figure 3.
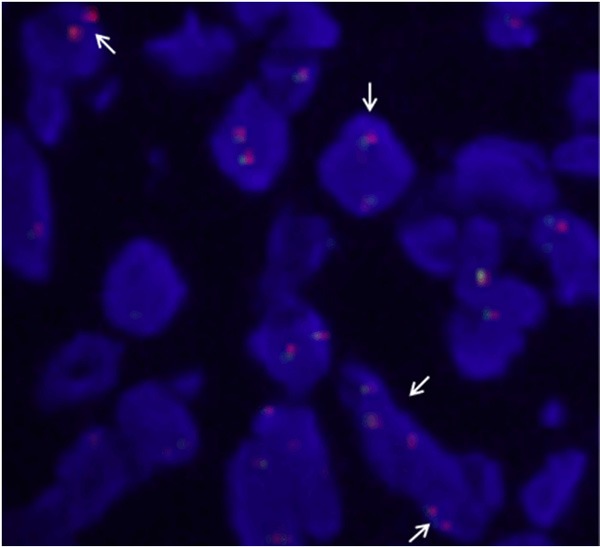
Break-apart anaplastic lymphoma kinase (ALK) fluorescence in situ hybridization (FISH) assay. Splitting of the red and green signals and isolated red signals (arrows) are associated with ALK rearrangement.
Extensive evaluation including chest, neck and abdominal computed tomography (CT) scans, thoracic and lumbar spine MRI, whole-body positron emission tomography and bone marrow biopsy revealed no evidence of systemic disease, confirming lymphoma of dural origin. The patient was treated with CHOP (C: cyclophosphamide, H: doxorubicin, O: vincristine, P: prednisone) chemotherapy and intrathecal methotrexate along with brain radiation therapy. The patient remains free of disease at 16 months post-surgery.
Statistical analysis
χ2 test and Fisher’s exact test were used to analyze its association with various clinicopathological factors and the survival rate in primary CNS ALCL. A value of P < 0.05 was considered significant. Statistical analysis was done with the software SPSS 12.0 (SPSS Inc., Chicago, Ill., USA).
Discussion
CNS involvement of Non-Hodgkin’s lymphomas falls into one of three categories [27]; primary CNS lymphomas, disseminated lymphomas with CNS involvement, and primary dural lymphomas. Primary CNS lymphomas are found in the brain parenchyma and other CNS structures [28]. Disseminated lymphomas typically involve the brain parenchyma or meninges, but do not occur within the dura matter [29]. Primary dural lymphomas typically do not involve the brain parenchyma and usually localize at areas rich in meningothelial cells, which lead to localized masses or plaque-like thickening of the dura matter, resembling meningioma radiologically [30,31]. Primary CNS lymphomas and disseminated lymphomas with CNS involvement are mostly high-grade diffuse large B-cell lymphomas, but primary dural lymphomas are usually low-grade mucosa-associated lymphoid tissue (MALT) type B-cell lymphomas [30]. Nonetheless, the ALCL type is rare kind of primary dural lymphomas. A review of all documented primary CNS ALCLs reveals only one piece of evidence of dural origin out of the 25 previously reported cases (case No. 5). Table 1 summarizes the clinical and pathological features of the available literature on primary ALCL in the brain [1,6-26].
Analysis of primary CNS ALCLs in the literature indicates that 12 cases are ALK-1 positive, 10 cases are ALK-1 negative, and 3 cases have no data on ALK-1 positivity. The age of all 25 patients ranges from 2 to 79 years (mean 34.5 years). Their age distribution is bimodal: 13 patients are < 30 years of age and 10 are > 46 years of age. The disease is found to be more frequent in males than in females. As with systemic ALCL, ALK-1 positivity is associated with younger age. Most ALK-1 positive ALCLs occur in the first three decades of life, and show a male predominance (M:F = 9:3). However, most ALK-1 negative ALCLs occur in older age, and there is no consistent gender predominance (M:F = 5:5) (Figure 4).
Figure 4.
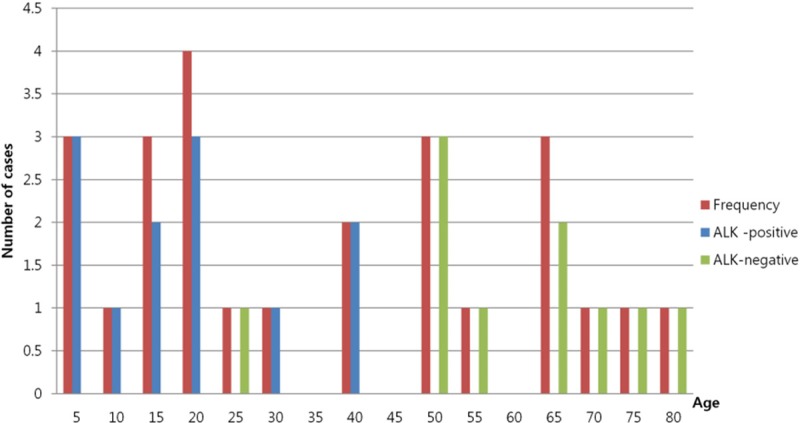
Distribution of primary CNS ALCLs by age. This bar diagram shows the cumulative frequency of primary CNS ALCLs by different ages in all documented reports. A standard bimodal distribution is observed for age. Most ALK-positive ALCL patients are < 30 years of age, but most ALK-negative ALCL patients are > 50 years of age.
The majority of primary CNS ALCLs are supratentorial tumors, except for 2 cases with an additional infratentorial lesion. 16 of all 25 cases have a unifocal tumor, and 9 have multifocal tumors (case No. 1, 2, 3, 4, 10, 14, 18, 19 and 26). Dural or leptomeningeal involvement by primary CNS ALCLs is not rare, being present in 5 of 12 ALK-positive tumors (case No. 3, 6, 10, 11 and 12) and in 3 of 10 ALK-negative tumors (case No. 14, 16 and 19). The clinical presentations of primary CNS ALCLs include headache, hemiparesis, seizure and dementia. Most patients are immunocompetent except for five cases: Case No. 15 has a history of systemic lupus erythematosus, chronic renal failure and thymoma resection in the past. Case No. 16 shows Epstein-Barr virus positivity. Case No. 17 has a seventeen-year history of essential thrombocythemia. Case No. 20 shows human immunodeficiency virus positivity. Case No. 22 has a history of near-total resection of atypical meningioma and radiation therapy.
Most of primary CNS ALCLs are of T cell lineage. However, a few “null cell” phenotype are observed in 1 of 12 ALK-positive tumors (case No. 1), and in 2 of 10 ALK-negative tumors (case No. 17 and 19). EMA positivity is higher in ALK-positive tumors; it is observed in 5 of 12 ALK-positive tumors (41.7%) and 2 of 10 ALK-negative tumors (20.0%). However, the relationship between EMA positivity and ALK positivity in ALCL is undetermined, since 12 cases have no data on EMA immunoreactivity. Tumor necrosis is present in 10 of all 25 tumors, absent in 5 tumors, and undetermined in 10 tumors due to limited availability of the literature.
Due to its relative rarity, standard treatment of primary CNS ALCL has not been established. Our review of previous reports indicates that treatment varies from supportive care alone to aggressive combinations of radiation and chemotherapy, with or without surgical resection. Chemotherapy along with cranial irradiation is provided to 9 out of 12 patients with ALK-1 positive ALCL, and 1 out of 10 ALK-1 negative ALCL. An ALK-1 positive (case No. 11) and an ALK-1 negative (case No. 21) tumors out of all 25 tumors had relapsed, and both resulted in fatal outcomes.
Tumor-associated mortality may be lower in younger age, ALK-1 positivity and unifocal tumors, but all of them were not statistically significant (Table 2). 6 of 13 dead patients were younger than 23 years and 7 dead were older than 45 years. Tumor-associated death occurred in 4 of 12 patients with ALK-1 positive ALCL (case No. 2, 3, 7 and 11), and in 7 of 10 ALK-1 negative ALCL (case No. 14, 15, 18, 19, 20, 21 and 23). 7 of 16 patients with a unifocal tumor, and 6 of 9 patients with multifocal tumors expired.
Table 2.
Correlation between clinicopathologic factors and prognosis in primary ALCL of the brain
| Clinicopathologic factor | Alive cases/total cases | P value |
|---|---|---|
| Age | 0.226 | |
| ≤ 40 | 9/15 (60.0%) | |
| > 40 | 3/10 (30.0%) | |
| Sex | 1.000 | |
| Male | 8/16 (50.0%) | |
| Female | 4/9 (44.4%) | |
| ALK | 0.198 | |
| Positive | 8/12 (66.7%) | |
| Negative | 3/10 (30.0%) | |
| Number of tumor | 0.411 | |
| Unifocal | 9/16 (56.3%) | |
| Multifocal | 3/9 (33.3%) | |
| Involvement of dural or leptomeninges | 0.688 | |
| Uninvolved | 9/16 (43.8%) | |
| Involved | 4/9 (44.4%) | |
| Immune status | 0.611 | |
| Immunocompetent | 6/16 (37.5%) | |
| Immunocompromised | 3/5 (60.0%) | |
| Tumor necrosis | 1.000 | |
| Absent | 2/5 (40.0%) | |
| Present | 4/10 (40.0%) |
P values were calculated using χ2 test.
Patient sex, dural or leptomeningeal involvement, immune status and tumor necrosis do not appear to have any influence on survival (Table 2). 8 of 16 males and 5 of 9 females died of the tumor. Tumor-associated death occurred in 4 of 8 patients with dural or leptomeningeal involvement and 9 of 16 patients without such involvement. 10 of 16 immunocompetent patients and 2 of 5 immunocompromised patients died of the tumor. 6 of 10 tumors with necrosis and 3 of 5 tumors without necrosis gave rise to fatal outcomes.
In summary, ALCL is a very rare T-cell lymphoma composed of CD30-positive lymphoid cells. To the best of our knowledge, the present article is the second report on primary dural ALCL in the brain. A review of previous reports on primary CNS ALCL reveals that some clinicopathological features and prognostic indicators are similar to systemic ALCL. Patients less than 23 years, ALK-1 positivity and unifocal tumor may be favorable prognostic indicators for primary CNS ALCL. Sex, dural or leptomeningeal involvement, immune status and tumor necrosis may not be associated with prognosis. Therefore, our patient is expected to show a better prognosis. However, because recurrence of the tumor could be fatal, close follow-up is crucial.
Disclosure of conflict of interest
The author has no conflict of interest.
References
- 1.Goldbrunner R, Warmuth-Metz M, Tonn JC, Vince GH, Roosen K. Primary Ki-1-positive T-cell lymphoma of the brain--an aggressive subtype of lymphoma: case report and review of the literature. Surg Neurol. 1996;46:37–41. doi: 10.1016/0090-3019(96)00033-x. [DOI] [PubMed] [Google Scholar]
- 2.Gijtenbeek JM, Rosenblum MK, DeAngelis LM. Primary central nervous system T-cell lymphoma. Neurology. 2001;57:716–8. doi: 10.1212/wnl.57.4.716. [DOI] [PubMed] [Google Scholar]
- 3.Stein H, Mason DY, Gerdes J, O’Connor N, Wainscoat J, Pallesen G, Gatter K, Falini B, Delsol G, Lemke H, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–58. [PubMed] [Google Scholar]
- 4.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. World Health Organization Classification of Tumors of Haematopoietic and Lymphoid Tissues. 4th Edition. Lyon: IARC Press; 2008. pp. 312–316. [Google Scholar]
- 5.Nakamura S, Shiota M, Nakagawa A, Yatabe Y, Kojima M, Motoori T, Suzuki R, Kagami Y, Ogura M, Morishima Y, Mizoguchi Y, Okamoto M, Seto M, Koshikawa T, Mori S, Suchi T. Anaplastic large cell lymphoma: a distinct molecular pathologic entity: a reappraisal with special reference to p80 (NPM/ALK) expression. Am J Surg Pathol. 1997;21:1420–32. doi: 10.1097/00000478-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Havlioglu N, Manepalli A, Galindo L, Sotelo-Avila C, Grosso L. Primary Ki-1 (anaplastic large cell) lymphoma of the brain and spinal cord. Am J Clin Pathol. 1995;103:496–9. doi: 10.1093/ajcp/103.4.496. [DOI] [PubMed] [Google Scholar]
- 7.Buxton N, Punt J, Hewitt M. Primary Ki-1-positive T-cell lymphoma of the brain in a child. Pediatr Neurosurg. 1998;29:250–2. doi: 10.1159/000028731. [DOI] [PubMed] [Google Scholar]
- 8.Abdulkader I, Cameselle-Teijeiro J, Fraga M, Rodriguez-Nunez A, Allut AG, Forteza J. Primary anaplastic large cell lymphoma of the central nervous system. Hum Pathol. 1999;30:978–81. doi: 10.1016/s0046-8177(99)90253-8. [DOI] [PubMed] [Google Scholar]
- 9.Ponzoni M, Terreni MR, Ciceri F, Ferreri AJ, Gerevini S, Anzalone N, Valle M, Pizzolito S, Arrigoni G. Primary brain CD30+ ALK1+ anaplastic large cell lymphoma (‘ALKoma’): the first case with a combination of ‘not common’ variants. Ann Oncol. 2002;13:1827–32. doi: 10.1093/annonc/mdf300. [DOI] [PubMed] [Google Scholar]
- 10.George DH, Scheithauer BW, Aker FV, Kurtin PJ, Burger PC, Cameselle-Teijeiro J, McLendon RE, Parisi JE, Paulus W, Roggendorf W, Sotelo C. Primary anaplastic large cell lymphoma of the central nervous system: prognostic effect of ALK-1 expression. Am J Surg Pathol. 2003;27:487–93. doi: 10.1097/00000478-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Rupani A, Modi C, Desai S, Rege J. Primary anaplastic large cell lymphoma of central nervous system--a case report. J Postgrad Med. 2005;51:326–7. [PubMed] [Google Scholar]
- 12.Cooper PB, Auerbach A, Aguilera NS, Adair C, Moores L, Geyer D, Rushing EJ. Rare primary CNS anaplastic large cell lymphoma in an immunocompetent adult: a clinical-pathologic case report and review case of the literature. Clin Neuropathol. 2006;25:232–6. [PubMed] [Google Scholar]
- 13.Carmichael MG. Central nervous system anaplastic large cell lymphoma in an adult: successful treatment with a combination of radiation and chemotherapy. Mil Med. 2007;172:673–5. doi: 10.7205/milmed.172.6.673. [DOI] [PubMed] [Google Scholar]
- 14.Karikari IO, Thomas KK, Lagoo A, Cummings TJ, George TM. Primary cerebral ALK-1-positive anaplastic large cell lymphoma in a child. Case report and literature review. Pediatr Neurosurg. 2007;43:516–21. doi: 10.1159/000108799. [DOI] [PubMed] [Google Scholar]
- 15.Merlin E, Chabrier S, Verkarre V, Cramer E, Delabesse E, Stephan JL. Primary leptomeningeal ALK+ lymphoma in a 13-year-old child. J Pediatr Hematol Oncol. 2008;30:963–7. doi: 10.1097/MPH.0b013e31818a959a. [DOI] [PubMed] [Google Scholar]
- 16.Shah AC, Kelly DR, Nabors LB, Oakes WJ, Hilliard LM, Reddy AT. Treatment of primary CNS lymphoma with high-dose methotrexate in immunocompetent pediatric patients. Pediatr Blood Cancer. 2010;55:1227–30. doi: 10.1002/pbc.22752. [DOI] [PubMed] [Google Scholar]
- 17.Paulus W, Ott MM, Strik H, Keil V, Muller-Hermelink HK. Large cell anaplastic (KI-1) brain lymphoma of T-cell genotype. Hum Pathol. 1994;25:1253–6. doi: 10.1016/0046-8177(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 18.Nuckols JD, Liu K, Burchette JL, McLendon RE, Traweek ST. Primary central nervous system lymphomas: a 30-year experience at a single institution. Mod Pathol. 1999;12:1167–73. [PubMed] [Google Scholar]
- 19.Chuang SS, Huang W, Lin CN, Chio CC, Tsai TC, Li CY, Shen CH. Primary cerebral anaplastic large cell lymphoma containing abundant reactive histiocytes and eosinophils. A case report and literature review. Pathol Res Pract. 2001;197:647–52. doi: 10.1078/0344-0338-00140. [DOI] [PubMed] [Google Scholar]
- 20.Tajima Y, Miyazaki Y, Higashi T, Kishimoto R, Sudoh K, Matsumoto A, Kikuchi S, Tashiro K. Primary CD30/Ki-1 positive anaplastic large cell lymphoma of the central nervous system occurring in a patient with a seventeen-year history of essential thrombocythemia. Leuk Lymphoma. 2003;44:1243–5. doi: 10.1080/1042819031000077106. [DOI] [PubMed] [Google Scholar]
- 21.Rowsell EH, Zekry N, Liwnicz BH, Cao JD, Huang Q, Wang J. Primary anaplastic lymphoma kinase-negative anaplastic large cell lymphoma of the brain in a patient with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 2004;128:324–7. doi: 10.5858/2004-128-324-PALKAL. [DOI] [PubMed] [Google Scholar]
- 22.Kodama K, Hokama M, Kawaguchi K, Tanaka Y, Hongo K. Primary ALK-1-negative anaplastic large cell lymphoma of the brain: case report and review of the literature. Neuropathology. 2009;29:166–71. doi: 10.1111/j.1440-1789.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- 23.Colen CB, Rayes M, Kupsky WJ, Guthikonda M. Synchronous meningioma and anaplastic large cell lymphoma. Neuropathology. 2010;30:260–6. doi: 10.1111/j.1440-1789.2009.01054.x. [DOI] [PubMed] [Google Scholar]
- 24.Sugino T, Mikami T, Akiyama Y, Wanibuchi M, Hasegawa T, Mikuni N. Primary central nervous system anaplastic large-cell lymphoma mimicking lymphomatosis cerebri. Brain Tumor Pathol. 2013;30:61–5. doi: 10.1007/s10014-012-0094-0. doi: 10.1007/s.10014.2012.0094.0. [DOI] [PubMed] [Google Scholar]
- 25.Bergmann M, Edel G. Primary intracerebral non-Hodgkin’s lymphoma. Pathologe. 1991;12:246–53. [PubMed] [Google Scholar]
- 26.Feldges A, Gerhard L, Reinhardt V, Budach V. Primary cerebral anaplastic T-cell-lymphoma (type Ki-1): review and case report. Clin Neuropathol. 1992;11:55–9. [PubMed] [Google Scholar]
- 27.Matmati K, Matmati N, Hannun YA, Rumboldt Z, Patel S, Lazarchick J, Stuart R, Giglio P. Dural MALT lymphoma with disseminated disease. Hematol Rep. 2010;2:e10. doi: 10.4081/hr.2010.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Commins DL. Pathology of primary central nervous system lymphoma. Neurosurg Focus. 2006;21:E2. doi: 10.3171/foc.2006.21.5.3. [DOI] [PubMed] [Google Scholar]
- 29.Low I, Allen J. Low-grade follicular lymphoma in the dura: rare mimic of meningioma. Neuropathology. 2006;26:564–8. doi: 10.1111/j.1440-1789.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 30.Iwamoto FM, Abrey LE. Primary dural lymphomas: a review. Neurosurg Focus. 2006;21:E5. doi: 10.3171/foc.2006.21.5.6. [DOI] [PubMed] [Google Scholar]
- 31.Venkataraman G, Rizzo KA, Chavez JJ, Streubel B, Raffeld M, Jaffe ES, Pittaluga S. Marginal zone lymphomas involving meningeal dura: possible link to IgG4-related diseases. Mod Pathol. 2011;24:355–66. doi: 10.1038/modpathol.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]


