Abstract
Metastatic malignant melanoma has a wide spectrum of histopathologic patterns and often lacks melanin pigment. Without a known primary tumor, the diagnosis of metastatic malignant melanoma relies on a combination of morphology and immunohistochemical profile. Infrequently, commonly used markers for melanoma (S100, HMB45, Melan-A and Tyrosinase A) are negative. These cases pose critical diagnostic challenges. Recent studies show that Microphthalmia Transcription Factor (MITF) has high sensitivity (88-100%) and specificity for metastatic melanoma. We are reporting here three cases of high grade tumors that were studied by a comprehensive immunohistochemical panel including cytokeratins, S100, HMB-45, Melan A, Tyrosinase, and MITF. All three tumors were also analyzed for the presence of BRAF mutations. All three metastatic tumors were negative for S100, Melan A, HMB-45 and Tyrosinase but positive for MITF. Subsequent to the diagnoses, previously existing or concurrent primary melanomas were identified in 2 of the 3 cases. Interestingly, S100, Melan A, and HMB-45 were positive in the primary tumors. No BRAF (V600E) mutations were identified in the three metastatic melanomas and CD 117 (c-kit) was positive in one of the cases. In summary, our experience shows that MITF can be a valuable adjunct in the diagnosis of metastatic tumors that are suspicious for melanoma but negative for other melanoma markers.
Keywords: Melanoma, immunohistochemistry, Microphthalmia Transcription Factor
Introduction
Cutaneous and extracutaneous malignant melanoma is one of the most lethal skin cancers and accounts for approximately seventy-five percent of skin cancer related death. The overall survival rate with advanced stage melanoma is still extremely low in spite of currently available therapeutic modalities: less than fifty percent with regional metastasis and fifteen percent with distant metastasis. Early diagnosis and effective treatment are critical for improved outcome and long term survival [1,2]. Histopathologically, metastatic melanoma has a wide spectrum of morphological patterns and often lacks melanin pigment, which makes immunohistochemical characterization necessary. In some cases, the commonly used markers for melanoma (S100, HMB45, Melan-A and Tyrosinase A) are negative in these tumors and makes diagnosis challenging. However, correct diagnosis is critical [3-6]. Many studies have shown that Microphthalmia Transcription Factor (MITF) has high sensitivity (88-100%) and specificity for metastatic melanoma in addition to the traditional markers mentioned above [7-9]. Here we reported three cases of high-grade tumor with MITF as the only markering point to a diagnosis of metastatic malignant melanoma.Subsequent to the diagnoses, previously existing or concurrent primary melanomas were identified in 2 of the 3 cases.
Case report
Case 1
The patient was an 85 year old male and presented with an enlarged parotid gland. A fine needle aspiration was performed and scattered highly atypical cells were identified. A subsequent parotidectomy was performed on the patient along with partial neck lymph node dissection. A 1.5 cm round mass was found within the parotid gland. Histopathologically, the tumor cells are moderate to large in size with epithelioid and pleomorphic cytological features. Mitotic activity was brisk with many atypical mitotic figures. Some tumor cells had rhabdoid features and peripheral halo. Necrosis was extensive and centrally located. Mucoid substances were present in the interstitia of the tumor. No pigmentation was found in the lesion (Figure 1A and 1B). The tumor apparently invaded into the normal parotid parenchyma. A battery of immunohistochemical stains were performed, including S100, Pan-melanoma cocktail (including HMB-45, Melan-A, and Tyrosinase) (Figure 1C and 1D), neuroendocrine markers, pan-cytokeratin (AE1/AE3), cytokeratin 7, cytokeratin 20, cytokeratin 5/6, desmin, smooth muscle actin, Thyroid transcription factor 1 (TTF1), napsin A, polyclonal carcinoembryonic antigen (CEA), which were all negative in tumor cells. Epithelial membrane antigen (EMA) and P63 were positive in small number of tumor cells, and CD117 was weakly positive in some tumor cells. A MITF stain was requested later and the result showed strong nuclear staining in most tumor cells (Figure 1E). A diagnosis of metastatic melanoma was suggested given other MITF expressing malignant tumors were ruled out.
Figure 1.
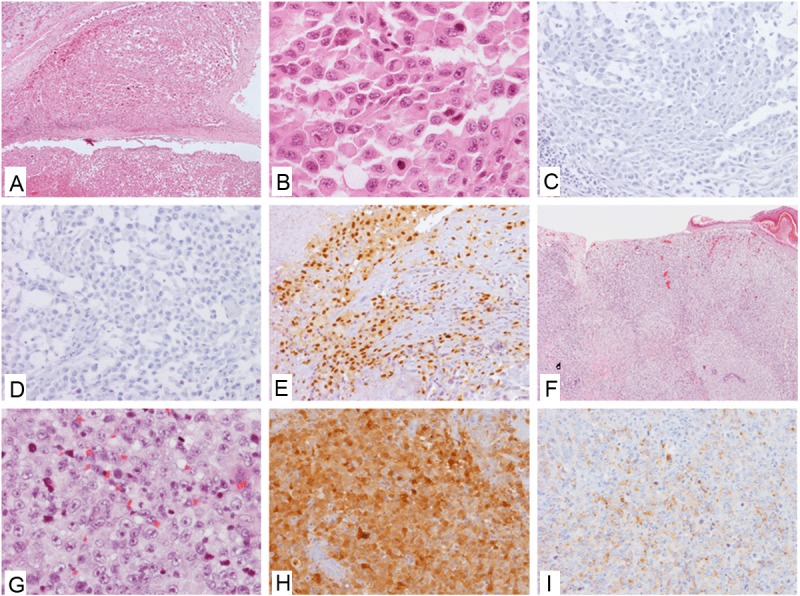
Characteristics of Case 1. A, B: H&E of the metastatic parotid lesion, low (A) and high (B) power views; C, D: Negative IHC stains of S100 (C) and Pan-Melanoma cocktail (D) in the metastatic parotid lesion; E: Positive nuclear IHC staining of MITF in the metastatic parotid lesion; F, G: H&E of the primary temporal lesion, low (F) and high (G) views; H, I: Positive IHC stains of S100 (H) and Pan-Melanoma cocktail (I) in the metastatic parotid lesion.
Simultaneously, a thorough physical examination was done for the patient and a pigmented lesion was identified on the right temple scalp, which was masked by the patient’s hair previously. A biopsy was done in an outside facility and diagnosed as “malignant melanoma”. The patient was referred back to us for a wide excision of the skin lesion. Histopathologically, the excisional biopsy was consistent with a cutaneous melanoma, nodular type with subcutaneous invasion. The cytological features were similar to the parotid tumor described above. Melanin pigmentation was absent as well (Figure 1F and 1G). However, immunohistochemical stains showed strong positivity for S100 and Pan-Melanoma cocktail (Figure 1H and 1I). At the same time, BRAF gene mutation study was performed on the primary skin lesion and no mutation was detected (including V600E, K601E and K601Q).
After comprehensive evaluation of the case together with other clinical information, we determined that the parotid lesion was mostly likely a metastatic lesion from the primary melanoma of the temple skin. The loss of S100, HMB-45, Melan-A and tyrosinase expression during the process of metastasis was rare and intriguing mechanistically.
Case 2
The patient was a 70 year old male with a recent diagnosis of primary cutaneous melanoma of forehead from outside facility. It was reported that the Breslow thickness was 0.55 mm. The tumor cells were epithelioid cytologically. S100 and Melan-A were also reported to be unequivocally positive in tumor cells (slides not available). In addition, he was also recently diagnosed of plasmacytoma from a bone marrow biopsy.
Seven months later, the patient was found to have a small right cheek nodule and a right auricular nodule. A fine needle aspiration was performed to the auricular nodule in our hospital and pleomorphic tumor cells with macronucleoli and occasional intracytoplasmic pigment were identified. A tentative diagnosis of metastatic melanoma was rendered. Immunohistochemical stain was not performed because of paucity of cellularity in the cytological material.
At the same time, a core biopsy of the right cheek nodule was also done. Histologically, the nodule had no epidermal connection and surrounded by fibroconnective tissue. The tumor cells were small to moderate in size and pleomorphic. The nuclei were large with prominent nucleoli and high nuclear/cytoplasm ratio. No melanin pigmentation was identified (Figure 2A and 2B). Hemorrhagic change was prominent. Immunohistochemical stains with S100, pan-melanoma cocktail (Figure 2C), Pan-cytokeratin, cytokeratin 5/6 and battery of hematological markers were performed, but all of them were negative. BRAF mutation and C-Kit mutation were also done and no mutation was detected. A MITF stain was subsequently requested and showed moderate positive nuclear staining in most tumor cells (Figure 2D). A diagnosis of metastatic melanoma from the forehead primary was suggested. Similar to the previous case, it also represented an example of metastatic melanoma with loss of some melanocytic markers during the process of metastasis.
Figure 2.
Characteristics of Case 2. A, B: H&E of the metastatic cheek lesion, low (A) and high (B) power views; C: Negative IHC stains of S100 (C1) and Pan-Melanoma cocktail (C2) in the metastatic cheek lesion, scattered cells with non-specific staining present; D: Positive nuclear IHC staining of MITF in the metastatic cheek lesion.
Case 3
The patient was a 58 year old male with a history of prostate adenocarcinoma status post radical prostatectomy three and half years ago. A 5.5 cm right forearm subcutaneous tumor was recently found and resected in outside facility. According to the report, the tumor was composed of spindle cells and epithelioid cells, with high grade pleomorphic nuclei. Prominent nucleoli and brisk atypical mitosis were present. No melanin pigmentation was observed. Immunohistochemical stains were patchy positive for S100, but negative for HMB-45, Melan-A, EMA, pan-cytokeratin, cytokeratin 7, desmin and CD34. The lesion was considered to be a possible metastatic malignant neoplasm. The main differential diagnoses included malignant peripheral nerve sheath tumor (MPNST) and melanoma with unusual marker expression pattern. However, the patient did not have NF1 gene defect and associated nerve trunk was not identified in the lesion, therefore the diagnosis of MPNST was in doubt. It was also very unlikely a metastatic prostate adenocarcinoma. The patient was thoroughly evaluated, and no other lesion was found on the skin or mucosal sites.
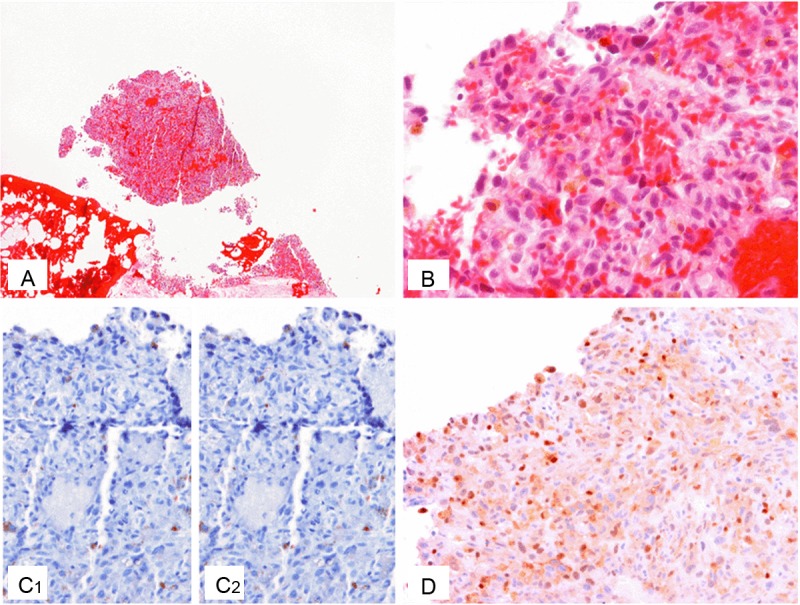
One month after the resection, a 1.8 cm recurrent nodule was found in the previous excision site. A re-excision was performed in our hospital. Pathological examination revealed a tumor with similar histomorphology as the previous excisional specimen (Figure 3A-B), and further immunohistochemical workup was done. In the new panel, pan-Melanoma cocktail, more keratin markers, blood vessel markers, muscle markers, CD117 were added, which were however still negative (Figure 3C1). Interestingly, S100 staining was negative in this recurrent lesion in contrast to the outside results (Figure 3C2). An INI1 staining showed nuclear positivity. MITF stain was subsequently performed which showed moderate nuclear staining pattern in tumor cells (Figure 3D). Based on the above findings, a diagnosis of metastatic melanoma with unknown primary was given, and this tumor also had the unusual pattern of negative staining for the other melanocytic markers including HMB-45, Melan-A and Tyrosinase. BRAF gene mutations were not detected.
Figure 3.

Characteristics of Case 3. A, B: H&E of the re-excised metastatic subcutaneous tumor, low (A) and high (B) power views; C: Negative IHC stains of Pan-Melanoma cocktail (C1) and S100 in the tumor (C2); D: Positive nuclear IHC staining of MITF in the tumor.
Six months later, the same tumor was found in an axillary lymph node in spite of the previous complete re-excision. It should be noted that the diagnosis of clear cell sarcoma of soft part was not completely ruled out, although the histopathologic features along with their presence in lymph nodes still favored the diagnosis of melanoma.
Discussion
The transcription factor MITF is essential in normal melanocytes development and controls the expression of pigment cell phenotypes. When malignant melanoma develops, MITF appears to be critical in tumor cell survival [10]. The application of MITF as a marker in diagnosis of melanoma has been extensively studied. Although there are variable results, it has been consistently shown that MITF has high sensitivity and specificity in identification of melanoma, which is comparable to other widely used markers, including S100, HMB-45 and Melan-A [4,7-9]. In addition, the clean nuclear staining pattern of MITF in the application of immunohistochemistry makes it a superior marker over the others for the ease of interpretation. Despite the high specificity of MITF in melanocytic lesions, it has been shown that MITF can be positive in considerable portion of perivascular epithelioid cell tumors, as well as in rare cases of lymphoid neoplasms, breast carcinomas, renal cell carcinomas, histiocytes, fibroblasts, Schwann cells, smooth muscle cells and mast cells [9,11-15]. Therefore, comprehensive evaluation with morphology and clinical information is required in utilizing MITF appropriately for diagnosis. It should be also noted that MITF does not appear to be very sensitive in desmoplastic melanomas [14,16].
In our cases, the challenge existed in that no clear medical history was available at the time of biopsy. The tumors were all amelanotic and highly atypical without specific patterns. Because of the location of these tumors as discrete deposits in the subcutaneous tissue, the possibility of metastatic lesions was highly considered for all three cases. Based on the H&E features, the differential diagnoses included high-grade carcinoma, high-grade sarcoma, metastatic melanoma and some unusual hematological malignancies. Batteries of immunohistochemical stains were performed on all three cases, including S100, melanoma marker cocktail (tyrosinase, HMB-45 and Melan-A), multiple markers for mesenchymal differentiation and cytokeratins, as well as some hematological markers. The results were either negative or weakly focally positive, and none were diagnostic or highly suggestive of certain lesion. Patchy weak positivity of S100 was observed in one case, but diagnosis of melanoma could not be made solely based on this finding. Later on a MITF stain was added to all three cases, which were all unequivocally positive with clean nuclear staining pattern. We therefore suggested the primary physicians further investigate the patients for the possibility of previous or concurrent history of melanoma, as well as requested BRAF gene mutation analysis [17,18]. It was later found that one of the patients did have a history of melanoma 7 months ago, and another patient had a concurrent scalp melanoma hidden under the hair. No clear history of primary melanoma was revealed in the third patient. BRAF mutation was not detected in any case. To further investigate the possible target for treatment, we did CD117/C-Kit immunohistochemical stain [19], which was positive in one case.
With the help of the clinicians, we excluded other possible tumors that may have positive MITF staining and concluded that these cases were all metastatic melanomas with unusual patterns of marker expression. There have been several studies that compared the existing melanoma markers, and the result indicated that a combination of S100, HMB-45 and Melan-A should be able to detect more than 99% of melanomas statistically [7-9]. There was one study that showed MITF was positive in 9 out of 14 cases HMB-45 negative melanoma. However, these cases were still variably positive for S100, Melan-A and tyrosinase [4]. Only one case of metastatic melanoma with triple negative markers was reported, in which case the MITF stain was also positive similar to our cases [20]. Our current findings further confirmedwith this larger scale study that MITF has a unique value in unrevealing the unusual nature of the melanomas with other negative markers. It will therefore be ideal to include MITF in a routine immunohistochemical stain panel when dealing with a high-grade tumor of unknown etiology, so that an unusual melanoma would not be missed.
One case of S100, HMB-45 and Melan-A negative primary melanoma was reported with its metastatic counterpart positive for these markers [6]. Interestingly, we also obtained information of the marker studies on two cases with subsequently identified primary melanoma, either through outside pathology report or our own work. The staining of S100, HMB-45, Melan-A and Tyrosinase was unequivocally positive in both primary melanomas. Therefore our observations are opposite to the case reported above. It is hence intriguing that the metastatic lesions had their marker expression pattern completely changed. This phenomenon may reflect an unknown mechanism underlying the process of the metastasis of these cases and would be worth further academic investigation.
Disclosure of conflict of interest
There is no conflict of interest to declare.
References
- 1.Kyrgidis A, Tzellos TG, Triaridis S. Melanoma: Stem cells, sun exposure and hallmarks for carcinogenesis, molecular concepts and future clinical implications. J Carcinog. 2010;9:3. doi: 10.4103/1477-3163.62141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Sheffield MV, Yee H, Dorvault CC, Weilbaecher KN, Eltoum IA, Siegal GP, Fisher DE, Chhieng DC. Comparison of five antibodies as markers in the diagnosis of melanoma in cytologic preparations. Am J Clin Pathol. 2002;118:930–936. doi: 10.1309/EWK9-LUPR-6BC5-1GXV. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Chu AY, Pasha TL, Elder DE, Zhang PJ. Immunoprofile of MITF, tyrosinase, melan-A, and MAGE-1 in HMB45-negative melanomas. Am J Surg Pathol. 2002;26:82–87. doi: 10.1097/00000478-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237–239. doi: 10.5858/arpa.2011-0405-LE. [DOI] [PubMed] [Google Scholar]
- 6.Sheth A, Arsenovic N. Melanoma markers-negative primary melanoma with melanoma markers-positive metastasis. Int J Surg Pathol. 2011;19:127–130. doi: 10.1177/1066896910382004. [DOI] [PubMed] [Google Scholar]
- 7.Prieto VG, Shea CR. Immunohistochemistry of melanocytic proliferations. Arch Pathol Lab Med. 2011;135:853–859. doi: 10.5858/2009-0717-RAR.1. [DOI] [PubMed] [Google Scholar]
- 8.Chang KL, Folpe AL. Diagnostic utility of microphthalmia transcription factor in malignant melanoma and other tumors. Adv Anat Pathol. 2001;8:273–275. doi: 10.1097/00125480-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Ohsie SJ, Sarantopoulos GP, Cochran AJ, Binder SW. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433–444. doi: 10.1111/j.1600-0560.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 10.Vachtenheim J, Borovansky J. Microphthalmia transcription factor: a specific marker for malignant melanoma. Prague Med Rep. 2004;105:318–24. [PubMed] [Google Scholar]
- 11.Miettinen M, Fernandez M, Franssila K, Gatalica Z, Lasota J, Sarlomo-Rikala M. Microphthalmia transcription factor in the immunohistochemical diagnosis of metastatic melanoma: comparison with four other melanoma markers. Am J Surg Pathol. 2001;25:205–211. doi: 10.1097/00000478-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Granter SR, Weilbaecher KN, Quigley C, Fisher DE. Role for microphthalmia transcription factor in the diagnosis of metastatic malignant melanoma. Appl Immunohistochem Mol Morphol. 2002;10:47–51. doi: 10.1097/00129039-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Makhlouf HR, Ishak KG, Shekar R, Sesterhenn IA, Young DY, Fanburg-Smith JC. Melanoma markers in angiomyolipoma of the liver and kidney: a comparative study. Arch Pathol Lab Med. 2002;126:49–55. doi: 10.5858/2002-126-0049-MMIAOT. [DOI] [PubMed] [Google Scholar]
- 14.King R, Googe PB, Weilbaecher KN, Mihm MC Jr, Fisher DE. Microphthalmia transcription factor expression in cutaneous benign, malignant melanocytic, and nonmelanocytic tumors. Am J Surg Pathol. 2001;25:51–57. doi: 10.1097/00000478-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Busam KJ, Iversen K, Coplan KC, Jungbluth AA. Analysis of microphthalmia transcription factor expression in normal tissues and tumors, and comparison of its expression with S-100 protein, gp100, and tyrosinase in desmoplastic malignant melanoma. Am J Surg Pathol. 2001;25:197–204. doi: 10.1097/00000478-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Koch MB, Shih IM, Weiss SW, Folpe AL. Microphthalmia transcription factor and melanoma cell adhesion molecule expression distinguish desmoplastic/spindle cell melanoma from morphologic mimics. Am J Surg Pathol. 2001;25:58–64. doi: 10.1097/00000478-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346–356. doi: 10.1097/PAT.0b013e328360b61d. [DOI] [PubMed] [Google Scholar]
- 18.Kudchadkar RR, Smalley KS, Glass LF, Trimble JS, Sondak VK. Targeted therapy in melanoma. Clin Dermatol. 2013;31:200–208. doi: 10.1016/j.clindermatol.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Flaherty KT, Hodi FS, Bastian BC. Mutation-driven drug development in melanoma. Curr Opin Oncol. 2010;22:178–183. doi: 10.1097/cco.0b013e32833888ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JF, Sarma DP, Ulmer P. Diagnostic dilemma: HMB-45 and Melan-A negative tumor, can it be still a melanoma?: MITF (Microphthalmia-associated transcription factor) stain may confirm the diagnosis. Internet Journal of Dermatology. 2007;5 [Google Scholar]


