Abstract
Sarcomatoid carcinoma of the urinary bladder is an uncommon neoplasm characterized histopathologically by the presence of malignant spindle cell and epithelial components. Albeit extremely rare, sarcomatoid carcinoma with small cell carcinoma has been reported. Herein, we describe an additional case of sarcomatoid carcinoma with small cell carcinoma and squamous cell carcinoma of the urinary bladder and review the clinicopathological features of this type of tumor. An 82-year-old Japanese male presented with hematuria. Computed tomography demonstrated a large tumor in the urinary bladder. Histopathological study of the resected urinary bladder tumor showed that approximately 80% of the tumor was comprised of small cell carcinoma, and the remaining components were spindle cell proliferation (approximately 15%) and squamous cell carcinoma (5%). Both the spindle cell and squamous cell carcinoma components were intermingled with nests of the small cell carcinoma. This is the fifth documented case of sarcomatoid carcinoma with small cell carcinoma of the urinary bladder. Our review of the clinicopathological features of this type of tumor revealed that: i) elderly males are mainly affected, ii) the most common chief complaint is hematuria, iii) the epithelial component may include urothelial carcinoma, adenocarcinoma, and/or squamous cell carcinoma, and iv) the sarcomatous component is composed of spindle cell proliferation. The histogenesis of this type of tumor remains a matter of controversy. However, recent molecular analyses demonstrated a monoclonal origin of both components. This theory can account for the various types of carcinomatous components in this tumor as seen in the present case.
Keywords: Carcinosarcoma, small cell carcinoma, urinary bladder
Introduction
Sarcomatoid carcinoma, also referred as carcinosarcoma, of the urinary bladder is an uncommon neoplasm, accounting for 0.1 to 0.3% of all bladder malignancies [1,2]. This carcinoma is characterized histopathologically by the presence of malignant spindle cell and epithelial components [3]. The malignant spindle cell component is usually a high-grade sarcomatous component, resembling undifferentiated high-grade sarcoma. Heterologous differentiation in the form of rhabdomyosarcoma, chondrosarcoma, and osteosarcoma may be present [3]. The malignant epithelial component is usually urothelial carcinoma (in situ or invasive), but adenocarcinoma and/or squamous cell carcinoma may be occasionally present as well [1-3].
Small cell carcinoma of the urinary bladder is also rare and is a highly aggressive neoplasm with a mean frequency of 0.7% of all bladder malignancies [4]. This type of tumor is morphologically identical to small cell carcinoma of the lung, and admixed with a component of urothelial carcinoma, squamous cell carcinoma, and adenocarcinoma in approximately 40 to 80% of all cases [4,5]. Albeit extremely rare, sarcomatoid carcinoma with small cell carcinoma as an epithelial component has been reported [1,6-8]. Herein, we describe an additional case of sarcomatoid carcinoma with small cell carcinoma and squamous cell carcinoma of the urinary bladder and review the clinicopathological features of this type of tumor.
Case report
An 82-year-old Japanese male presented with gross hematuria. Computed tomography demonstrated a large irregular-shaped tumor involving the entire layer of the urinary bladder (Figure 1). Cytological examination of the urine revealed small cell carcinoma, but no spindle cell, conventional urothelial carcinoma or squamous cell carcinoma components were noted. Trans-urethral resection of the urinary bladder tumor also showed small cell carcinoma, and then subsequent radical cystoprostatectomy with lymph node dissection was performed.
Figure 1.
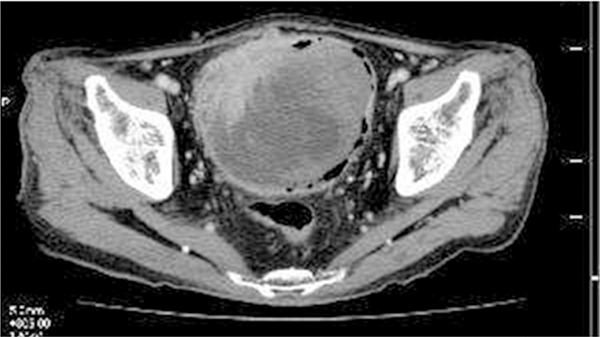
Computed tomography shows an irregular-shaped tumor involving the entire layer of the urinary bladder in the right anterior wall.
Histopathological study of the resected urinary bladder tissue revealed an infiltrative neoplastic growth forming sheets or irregular nests that accompanied occasional geographic necrosis which involved the entire urinary bladder wall (Figure 2A). The neoplastic growth consisted of small round cells containing scant cytoplasm and round to oval nuclei with coarse chromatin and inconspicuous nucleoli (Figure 2A, inset). Mitotic figures were frequently observed (27/10 high-power fields) (Figure 2A, inset), and many apoptotic bodies were also noted. Approximately 80% of the tumor was composed of the above-mentioned component, and the remaining components were spindle cell proliferation (approximately 15%) and squamous cell carcinoma (approximately 5%). Proliferation of spindle cells with large round to oval nuclei and slightly eosinophilic cytoplasm was observed, and these cells were intermingled with nests of the small round cells (Figure 2B), while bizarre giant cells and multinucleated atypical cells were scattered (Figure 2B). Mitotic figures were also frequently observed (19/10 high power fields). No striation and chondromatous or osseous component were noted. Moreover, atypical squamous cell nests composed of atypical epithelial cells with rich eosinophilic cytoplasm and centrally located large nuclei were scattered within the small round cell nests, and keratinization was also observed (Figure 2C). Neither conventional urothelial carcinoma (invasive or in situ) nor adenocarcinoma component was observed.
Figure 2.
Histopathological features of the urinary bladder tumor. A: Proliferation of small round cells invading into the muscular layer of the urinary bladder. The neoplastic cells have scant cytoplasm and round to oval nuclei without conspicuous nucleoli. Mitotic figures are scattered (arrows, inset). HE, x40, x400 (inset). B: Proliferation of atypical spindle cells adjacent to the small round cells. Multinucleated giant cells and bizarre cells are observed. HE, x100. C: A squamous cell carcinoma component is present adjacent to small round cell nest. HE, x200.
Neither prostate invasion nor lymph node metastasis was noted.
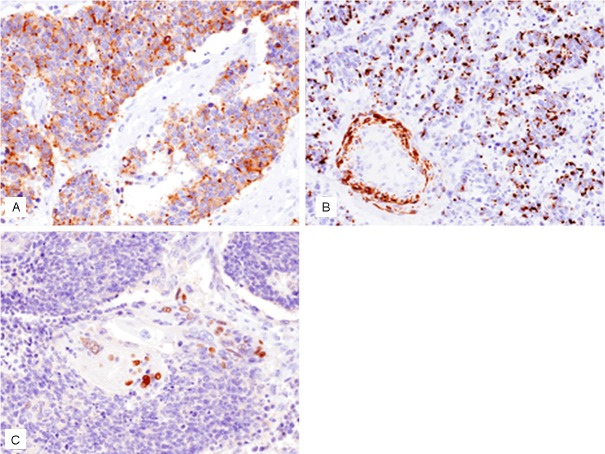
Table 1 summarizes the immunohistochemical results of the present case. Small round cells were diffusely positive for synaptophysin and CD56 (Figure 3A). The spindle cell component was diffusely positive for vimentin and focally positive for alpha-smooth muscle actin, but negative for cytokeratin. The squamous cell carcinoma component was positive for p63 (Figure 3B). A peculiar finding was dot-like positive immunoreactivity for desmin in the small round cells (Figure 3C).
Table 1.
Summary of immunohistochemical results
| Antibody | Source | SCC component | Sarcomatous component | SqCC component |
|---|---|---|---|---|
| Cytokeratin (AE/AE3) | DAKO | + | - | + |
| Chromogranin A | DAKO | - | - | - |
| Synaptophysin | Novocastra | + | - | - |
| CD56 | Novocastra | + | - | - |
| Alpha-smooth muscle actin | Novocastra | - | Focally (+) | - |
| Desmin | Novocastra | Focally + (dot-like) | - | - |
| Vimentin | Novocastra | - | + | - |
| p63 | Novocastra | - | - | + |
| S-100 protein | Nichirei | - | - | - |
SCC, small cell carcinoma; SqCC, squamous cell carcinoma.
Figure 3.
Immunohistochemical features of the urinary bladder tumor. A: Synaptophysin is expressed in the small round cells, x200. B: p63 is expressed in the squamous cell carcinoma, x200. C: Desmin is expressed in the small round cells (note: vascular wall is also positive for desmin), x200.
According to these results, an ultimate diagnosis of sarcomatoid carcinoma with small cell carcinoma and squamous cell carcinoma of the urinary bladder was made (pT3b N0 M0, Stage III).
Discussion
In this report, we described the fifth documented case of sarcomatoid carcinoma with small cell carcinoma component. Sarcomatoid carcinoma usually has urothelial carcinoma (in situ or invasive) as an epithelial component, but squamous cell carcinoma and/or adenocarcinoma may be occasionally present as well. Ikegami et al. reported 14 cases of sarcomatoid carcinoma of the urinary bladder, and twelve of these cases had urothelial carcinoma alone and the remaining 2 cases had squamous cell carcinoma as well as urothelial carcinoma as epithelial components [2]. Torenbeek et al. also described the clinicopathological features of this type of tumor. Three of their 18 cases had squamous cell carcinoma and urothelial carcinoma, one case had adenocarcinoma, and one case had both adenocarcinoma and squamous cell carcinoma as epithelial components [1]. Moreover, only one case of sarcomatoid carcinoma with all three urothelial carcinoma, adenocarcinoma, and small cell carcinoma components was documented in their series [1].
Table 2 summarizes the clinicopathological features of the previously reported cases of sarcomatoid carcinoma with small cell carcinoma as well as the present case. Although Young and Eble showed an image of sarcomatoid carcinoma with small cell carcinoma in their review article regarding unusual carcinoma of the urinary bladder [8], the clinicopathological features of their case were not available; therefore, that case was not included in the present report. This type of tumor mainly affects elderly males (male: female 4:1; average age is 73.8 years, range from 64 to 82 years). The most common chief complaint is hematuria. The epithelial components of sarcomatoid carcinoma other than small cell carcinoma can be urothelial carcinoma (invasive or in situ), squamous cell carcinoma, and/or adenocarcinoma. The sarcomatous component is composed of spindle cell proliferation in all cases. No osteosarcomatous or chondrosarcomatous component has been noted. Moreover, Li et al. recently reported a case of sarcomatoid carcinoma (carcinosarcoma) with a large cell neuroendocrine carcinoma component [9]. These results suggest that sarcomatoid carcinoma can have a variety of histopathological subtypes of carcinoma components.
Table 2.
Clinicopathological features of sarcomatoid carcinoma with small cell carcinoma of the urinary bladder
| Case No. | Age/Gender | Chief complaint | Epithelial components | Sarcomatous component | Reference |
|---|---|---|---|---|---|
| 1 | 82/Male | Obstruction | SCC, UC, SqCC, and Adeno | Spindle cell | 5 |
| 2 | 64/Female | Hematuria | SCC and CIS | Spindle cell | 4 |
| 3 | 66/Male | Hematuria | SCC and CIS | Spindle cell | 4 |
| 4 | 75/Male | Hematuria, incontinence | SCC, UC, and Adeno | Spindle cell | 1 |
| Present Case | 82/Male | Hematuria | SCC and SqCC | Spindle cell |
Adeno, adenocarcinoma; CIS, urothelial carcinoma in situ; SCC, small cell carcinoma; SqCC, squamous cell carcinoma; UC, urothelial carcinoma.
In the present case, the urine cytological examination revealed only the small cell carcinoma, but no sarcomatoid component was detected. Arita et al. reported cytological features of three cases of the sarcomatoid variant of urothelial carcinoma of the urinary bladder [10]. They found that while all six urine cytological specimens from the 3 patients (these cases had conventional high-grade urothelial carcinoma component as an epithelial component) included the conventional high-grade urothelial carcinoma component, however, only one specimen demonstrated a sarcomatous component [10]. In the present case, only the small cell carcinoma component was present in the urine cytological specimen. This reflects the fact that the sarcomatoid component is usually present in the deeper portion of the tumor, therefore, detection of the sarcomatoid component is low.
A peculiar finding of the present case is dot-like positive immunoreactivity for desmin in the small cell carcinoma component. Eusebi et al. reported a case of small cell carcinoma with urothelial carcinoma in situ of the urinary bladder, in which the small cell carcinoma showed positive immunoreactivity for desmin [11]. These results suggest that small cell carcinoma rarely shows a muscular phenotype.
The histogenesis of sarcomatoid carcinoma remains a matter of controversy. Two main theories have been proposed. Some investigators advocate that this type of tumor represents a collision tumor comprised of two independent but simultaneously occurring epithelial and mesenchymal monoclonal neoplasms, whereas others suggest that sarcomatoid carcinoma has a common clonal origin with divergent differentiation into both components [12]. Some recent molecular analyses have demonstrated that both the carcinomatous and sarcomatous components are of monoclonal origin, and suggested a model of carcinogenesis of sarcomatoid carcinoma [12]. Sarcomatoid carcinoma may progress through multistep carcinogenesis with the accumulation of genetic alterations, genetic instability, and generation of multiple subclones, and followed by secondary transdifferentiation from an epithelial to a mesenchymal phenotype induced by the stromal microenvironment [12]. This theory can account for the presence of various subtypes of carcinomatous components including small cell carcinoma and squamous cell carcinoma as seen in the present case.
The prognosis of sarcomatoid carcinoma of the urinary bladder is very poor. The median survival is 14 months overall; 21 months for patients with localized disease, and 2 months for those with distant metastases. Overall survival rates are 54% at 1 year and 28% at 5 years [12]. Therefore, early detection and correct diagnosis of this type of tumor is important.
References
- 1.Torenbeek R, Blomjous CE, de Bruin PC, Newling DW, Meijer CL. Sarcomatoid carcinoma of the urinary bladder. Clinicopathologic analysis of 18 cases with immunohistochemical and electron microscopic findings. Am J Surg Pathol. 1994;18:241–249. [PubMed] [Google Scholar]
- 2.Ikegami H, Iwasaki H, Ohjimi Y, Takeuchi T, Ariyoshi A, Kikuchi M. Sarcomatoid carcinoma of the urinary bladder: A clinicopathologic and immunohistochemical analysis of 14 patients. Hum Pathol. 2000;31:332–340. doi: 10.1016/s0046-8177(00)80247-6. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Beltran A, Sauter G, Gasser T, Hartmann A, Schmitz-Drager BJ, Helpap B, Ayala AG, Tamboli P, Knowles MA, Sidransky D, Cordon-Cardo C, Jones PA, Cairns P, Simon R, Amin MB, Tyczynski JE. Infiltrating urothelial carcinoma. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. pp. 93–109. [Google Scholar]
- 4.Ismaili N. A rare bladder cancer-small cell carcinoma: review and update. Orphanet J of Rare Dis. 2011;6:75. doi: 10.1186/1750-1172-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrahams NA, Moran C, Reyes AO, Siefker-Radtke A, Ayala AG. Small cell carcinoma of the bladder: a contemporary clinicopathological study of 51 cases. Histopathology. 2005;46:57–63. doi: 10.1111/j.1365-2559.2004.01980.x. [DOI] [PubMed] [Google Scholar]
- 6.Mazzucchelli L, Kraft R, Gerber H, Egger C, Studer UE, Zimmermann A. Carcinosarcoma of the urinary bladder: a distinct variant characterized by small cell undifferentiated carcinoma with neuroendocrine features. Virchows Arch A Pathol Anat Histopathol. 1992;421:477–83. doi: 10.1007/BF01606876. [DOI] [PubMed] [Google Scholar]
- 7.Mills SE, Wolf JT III, Weiss MA, Swanson PE, Wick MR, Fowler JE Jr, Young RH. Small cell undifferentiated carcinoma of the urinary bladder: a light-microscopic, immunocytochemical, and ultrastructural study of 12 cases. Am J Surg Pathol. 1987;11:606–617. doi: 10.1097/00000478-198708000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Young RH, Eble JN. Unusual forms of carcinoma of the urinary bladder. Hum Pathol. 1991;22:948–965. doi: 10.1016/0046-8177(91)90003-8. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Outman JE, Mathur SC. Carcinosarcoma with a large cell neuroendocrine epithelial component: first report of an unusual biphasic tumor of the urinary bladder. J Clin Pathol. 2004;57:318–320. doi: 10.1136/jcp.2003.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arita N, Ishida M, Yoshida K, Kagotani A, Iwamoto N, Iwai M, Okabe H. Sarcomatoid variant of urothelial carcinoma: Cytological analysis of three cases. Oncol Lett. 2012;5:49–52. doi: 10.3892/ol.2012.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eusebi V, Damiani S, Pasquinelli G, Lorenzini P, Reuter VE, Rosai J. Small cell neuroendocrine carcinoma with skeletal muscle differentiation. Report of three cases. Am J Surg Pathol. 2000;24:223–30. doi: 10.1097/00000478-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Cheng L, Zhang S, Alexander R, MacLennan GT, Hodges KB, Harrison BT, Lopez-Beltran A, Montironi R. Sarcomatoid carcinoma of the urinary bladder: the final common pathway of urothelial carcinoma dedifferentiation. Am J Surg Pathol. 2011;35:e34–46. doi: 10.1097/PAS.0b013e3182159dec. [DOI] [PubMed] [Google Scholar]