Abstract
Gelatinous bone marrow transformation (GMT) is a rare disorder characterized by the presence of fat cell atrophy, loss of hematopoietic cells, and deposition of extracellular gelatinous materials. GMT is not a specific disease, but is strongly associated with malnutrition and drugs. Albeit extremely rare, GMT has been reported in patients with myeloproliferative disorders. Herein, we report the second documented case of hypoplastic myelodysplastic syndrome (MDS) accompanying GMT. A 73-year-old Japanese male with excellent nutrition status and no history of alcohol or drug intake was detected with pancytopenia. The initial bone marrow aspirate specimen reveled hypocellular marrow without dysplastic signs in the myeloid cells. Bone marrow biopsy demonstrated hypocellular bone marrow with prominent GMT. He received blood transfusions, however, pancytopenia continued to progress. The second bone marrow aspirate specimen showed dysplastic changes, such as pseudo-Pelger-Huët cells, hypogranular or agranular granulocytes, and megakaryocytes with multiple small nuclei. Cytogenetic study demonstrated deletion of chromosome 7. Therefore, an ultimate diagnosis of hypoplastic MDS accompanying GMT was made. Only a limited number of cases of myeloproliferative disorders with GMT have been reported. Our analysis of these cases revealed that chromosome 7 abnormality is frequently observed in this condition. Moreover, findings from the current case suggested that myeloproliferative disorders including MDS must be included in the differential diagnostic considerations of GMT patients, who have no history of malnutrition or drugs, and careful examination of the bone marrow smear specimen and cytogenetic analysis are necessary for early detection of underlying myeloproliferative disorders.
Keywords: Gelatinous bone marrow transformation, myelodysplastic syndrome, bone marrow
Introduction
Gelatinous bone marrow transformation (GMT), also referred as gelatinous degeneration or serous atrophy of the bone marrow, is a rare disorder, characterized histopathologically by the presence of fat cell atrophy, loss of hematopoietic cells, and deposition of extracellular gelatinous substances, which have been histochemically identified as mucopolysaccharides [1,2]. Although the pathogenesis of GMT is still unknown, it is speculated that the deposition of extracellular gelatinous material leads to alterations in the bone marrow microenvironment, resulting in loss of hematopoietic cells [1]. GMT is not a specific disease, but like a symptom, and it is well known that this disorder is strongly associated with chronic debilitating diseases, such as severe malnutrition, anorexia nervosa, human immunodeficiency virus (HIV) infection, and administration of cytotoxic drugs [1,3,4]. In addition, albeit extremely rare, GMT has also been reported in patients with primary myeloproliferative disorders, such as myelodysplastic syndrome (MDS), acute myeloid leukemia, and myelofibrosis [5-8]. Herein, we report a case of GMT presenting prior to hypoplastic MDS and discuss the clinicopathological features of myeloproliferative disorders accompanying GMT.
Case report
A 73-year-old Japanese male with a past history of hypothyroidism was referred to our hospital because of pancytopenia. He had undergone total gastrectomy for gastric cancer at the age of 64, and then pancytopenia was detected 6 months earlier at a local clinic. Vitamin preparation was administrated under a clinical diagnosis of megaloblastic anemia; however, pancytopenia did not improve.
At first presentation, the peripheral blood showed pancytopenia (red blood cells 1.6 x 1012 /L, hemoglobin 6.0 g/dL, leukocytes 3.9 x 109 /L, and platelets 44 x 109 /L). His folic acid and vitamin B12 concentrations as well as liver and renal function tests were within normal ranges. Physical examination revealed no hepatosplenomegaly. His nutritional status was excellent (he was 155.2 cm tall and weighed 52.7 kg), and he did not use any alcohol or drugs. Bone marrow aspiration and biopsy were performed, and cytogenetic analysis was also done.
Under a clinical diagnosis of aplastic anemia, the patient received blood transfusions, however, pancytopenia worsened (red blood cells 2.2 x 1012 /L, leukocytes 1.5 x 109 /L, and platelets 9 x 109 /L). The patient was then admitted for administration of cyclosporine and anti-thymoglobulin treatment 6 months after his first visit. Subsequently, the second bone marrow aspiration was performed, and cytogenetic study was also done.
After the diagnosis, he received frequent blood transfusions, however, chemotherapy was not performed. He succumbed to pneumonia 10 months after his first visit.
The first bone marrow aspirate specimen demonstrated hypocellular bone marrow with decreases in erythroid and myeloid series and a relative increase in lymphocytes. Megakaryoctes were hardly detected in the smear. Although erythroid cells showed mild dysplastic changes (such as karyorrhexis), no dysplastic signs were detected in the myeloid cells. The first bone marrow biopsy specimen revealed hypocellular bone marrow with a decrease in trilineage cells (Figure 1A). Atrophy of fat cells and prominent deposition of extracellular slightly eosinophilic materials were observed (Figure 1A). These extracellular materials were positive for Alcian blue staining (Figure 1B) and digested by hyaluronidase (Figure 1C). No chromosomal abnormality was detected by the cytogenetic analysis.
Figure 1.
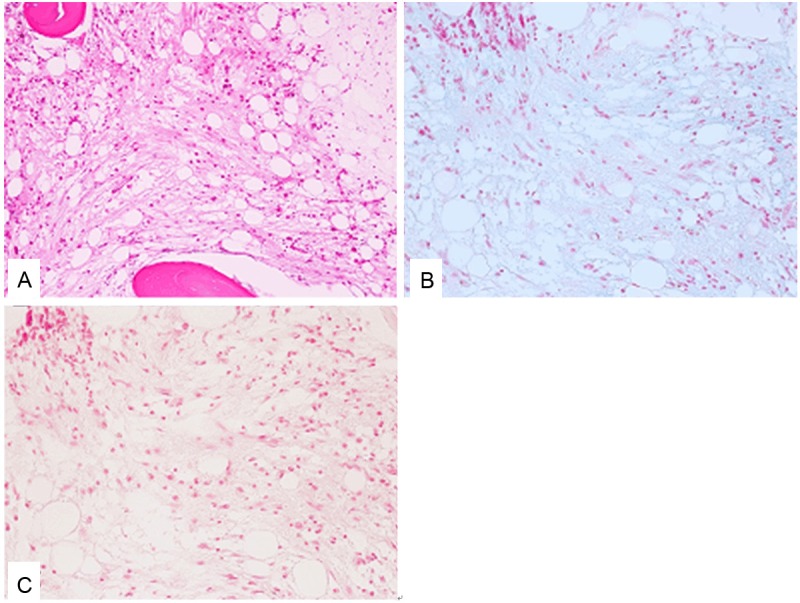
Histopathological features of the bone marrow of the first biopsy specimen. A: Fat cell atrophy, deposition of extracellular gelatinous materials, and decrease in the trilineage hematopoietic cells are observed. HE, x 200. B: The extracellular gelatinous materials are positive for Alcian blue staining. Alcian blue staining, x 200. C: The extracellular gelatinous materials are digested by hyaluronidase. Alcian blue staining with digestion by hyaluronidase, x 200.
The second bone marrow aspirate specimen showed hypocellular bone marrow with a decrease in myeloid cells and a relative increase in lymphocytes. Erythroid cells were hardly detected. Myeloblasts were slightly increased (2.5%). Pseudo-Pelger-Huët cells (9%), which are neutrophils with bilobed nucleus instead of the normal trilobed shape, and hypogranular or agranular granulocytes (10%) were observed (Figure 2A). Moreover, only five megakaryocytes were present in the smear, and all of them showed dysplastic changes (such as multiple small nuclei) (Figure 2B). These features were typical for hypoplastic MDS. The second bone marrow clot section revealed hypocellular bone marrow with a decrease in trilineage cells (Figure 3A). A few small-sized megakaryocytes were also present. Atrophy of fat cells and prominent deposition of extracellular materials were observed as in the first bone marrow specimen. These extracellular materials were positive for Alcian blue staining (Figure 3B) and digested by hyaluronidase (Figure 3C). Immunohistochemical analyses showed that p53 protein-positive cells were scattered in the bone marrow, and CD41-positive small-sized megakaryocytes were also present (Figure 3A, inset).
Figure 2.
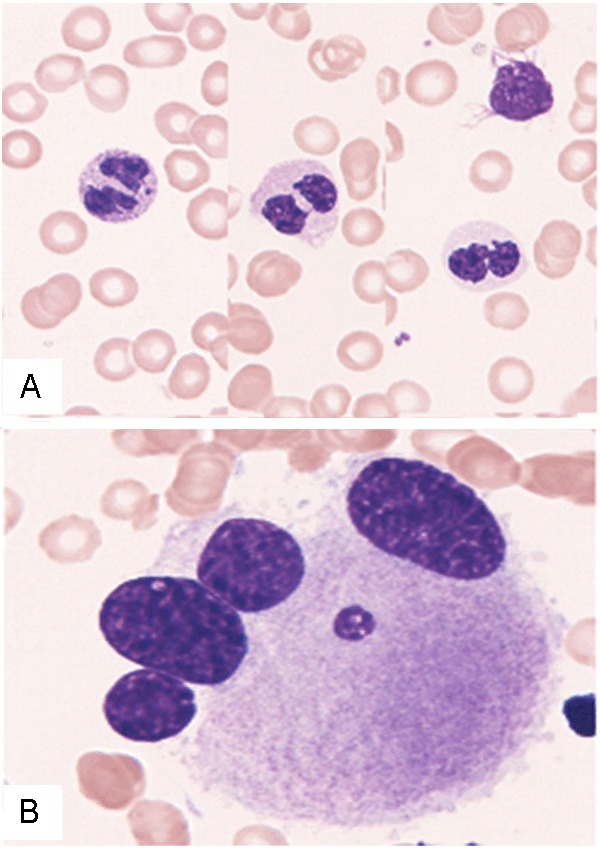
Bone marrow smear specimen of the second bone marrow aspirate. A: Pseudo-Pelger-Huët cell with bilobed nucleus and hypogranular granulocytes are observed. Giemsa stain, x 1,000. B: Dysplastic megakaryocytes with multiple small nuclei. Giemsa stain, x 1,000.
Figure 3.
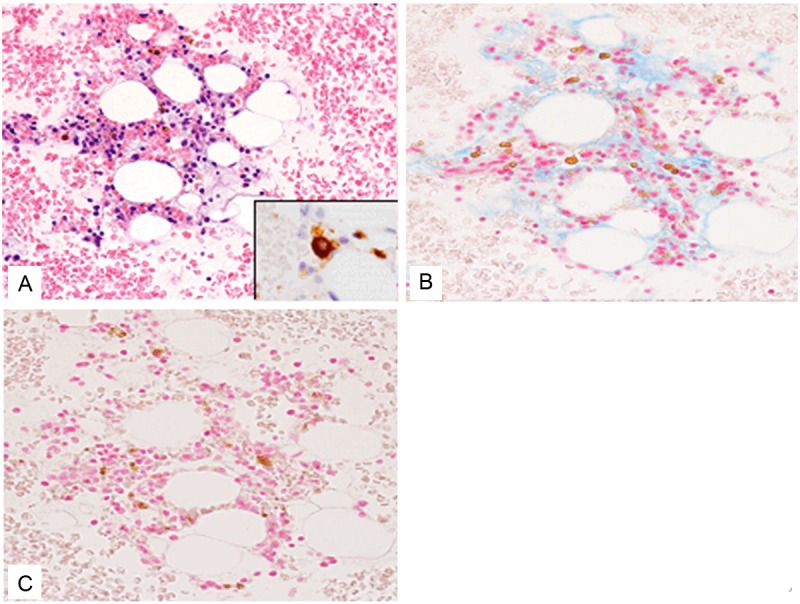
Histopathological features of the clot section of the second bone marrow aspirate. A: Fat cell atrophy,deposition of extracellular gelatinous materials, and decrease in the trilineage hematopoietic cells are observed. HE, x 200. CD41-positive small-sized megakaryocyteis noted. x 400. B: The extracellular gelatinousmaterials are positive for Alcian blue staining. Alcianblue staining, x 200. C: The extracellular gelatinous materials are digested by hyaluronidase. Alcian blue staining with digestion by hyaluronidase, x 200.
Fluorescence in situ hybridization analysis of the bone marrow revealed deletion of 7q31 in 20.2% of cells.
According to these results, an ultimate diagnosis of hypoplastic MDS preceding GMT was made.
Discussion
GMT is a rare phenomenon of the bone marrow characterized by fat cell atrophy, loss of hematopoietic cells, and deposition of extracellular gelatinous materials. The extracellular gelatinous materials in GMT are considered to consist of mucopolysaccharides, mainly hyaluronic acid, as seen in the present case, because these materials are positive for Alcian blue staining and digested by hyaluronidase [1,2]. It is well known that GMT is strongly associated with debilitating diseases, although the pathogenesis is still unknown. Böhm analyzed 158 cases of GMT among 80,000 bone marrow biopsy specimens and found that the incidence is approximately 0.2% [1]. In the series, the most common underlying disease of GMT is malignant tumor (37.5%), such as malignant lymphoma and carcinoma, followed by malnutrition (16.8%), infection (11.8%) such as AIDS, and maldigestion (10.1%) [1]. Most of the patients with malignant tumors in the series were in an advanced stage and had metastases, therefore, it was plausible that they were emaciated and may have been in poor nutritional condition, in addition to possible administration of drugs to these patients. Generalized infectious disease with GMT, such as HIV, is suspected to be under conditions of malnutrition and consumption. In addition, drugs can also cause GMT. The bone marrow after chemotherapy for acute myeloid leukemia and acute lymphoblastic leukemia showed severe GMT [1]. Therefore, these results suggest that the main causes of GMT are malnutrition and drugs [1].
Albeit extremely rare, a few cases of GMT with myeloproliferative disorders have been reported [1,5-8]. Table 1 summarizes the clinicopathological features of GMT with myeloproliferative disorders as well as the present case. GMT series by Böhm contained 5 cases of MDS, and two cases showed weight loss and one case suffered from alcoholism [1]. However, the detailed clinicopathological features were not available, therefore, these cases were not included in this analysis. This condition affects mainly elderly males, and monosomy or deletion of chromosome 7 was detected in three cases including the present case. Arranz et al. reported a case of GMT with MDS/acute myeloid leukemia [5]. In their case, the patient presented with pancytopenia and fever, and initial bone marrow biopsy revealed GMT, while initial bone marrow aspirate showed mild dysplastic change in the erythroid precursors. Ten months after the initial biopsy, the second bone marrow biopsy showed an increase in the amount of extracellular gelatinous materials and dysplastic changes in the trilineage hematopoietic cells. Twenty-eight months after the initial biopsy, blasts were present in both the peripheral blood and bone marrow, and cytogenetic analysis demonstrated monosomy of chromosome 7 [5]. The clinical course of that case was similar to that of the present case, and GMT was observed prior to a diagnosis of MDS in both cases. This is the second documented case of MDS preceding GMT. Both cases had no main cause of GMT, such as malnutrition or drugs. In addition, both of these cases had loss or deletion of chromosome 7 [5].
Table 1.
Clinicopathological features of gelatinous bone marrow transformation with myeloproliferative disorders
| Case No. | Age/Gender | Initial presentation | The relationship between GMT and myeloproliferative disorders | Cytogenetic analysis | Outcome | Reference |
|---|---|---|---|---|---|---|
| 1 | 48/Male | Pancytopenia and fever | GMT was present 28 months prior to MDS/AML | Monosomy 7 | Died of disease | 5 |
| 2 | 79/Male | Pancytopenia | GMT and acute leukemia simultaneously | Not performed | Died of brain hemorrhage | 6 |
| 3 | 65/Male | Not available | GMT occurred after treatment for MDS | Monosomy 7 | Died of bone marrow failure | 7 |
| 4 | 74/Female | Leukocytopenia | GMT and myelofibrosis simultaneously | Not performed | Died of acute cholecystitis | 8 |
| Present case | 73/Male | Pancytopenia | GMT was present 6 months prior to MDS | del (7q) | Died of pneumonia |
AML, acute myeloid leukemia; GMT, gelatinous bone marrow transformation; MDS, myelodysplastic syndrome.
The relationship between myeloproliferative disorders and GMT has not been resolved. Under physiological conditions, mucopolysaccharides are not present in the bone marrow [1,2,8]. It is speculated that the deposition of the extracellular gelatinous materials leads to an alteration in the hematopoietic microenvironment and the stroma of the bone marrow to inhibit the interactions between hematopoietic cells and cell-signaling molecules [1,7]. Although GMT was observed prior to a diagnosis of MDS in the present case and the reported case by Arranz et al., the possibility that deposition of the gelatinous materials in the bone marrow resulted in the development of MDS was unlikely [5]. Thus, underlying MDS may induce the deposition of gelatinous materials in the bone marrow by an unknown etiology.
Hypoplastic MDS accounts for approximately 10% of MDS, and defined as hematopoiesis that comprises less than 30% of the area of a total specimen [9,10]. This variant of MDS is characterized by a better prognosis, lower peripheral white blood cell counts, and lower bone marrow blast percentage as compared to normo-/hypercellular MDS [9]. A difference in chromosomal abnormalities between hypoplastic and normo-/hypercellular MDS has been reported [9-13]. In all MDS cases, the most common karyotype is normal (48.3%), followed by del (5q) (15.1%) and -7/del (7q)(10.7%), the latter of which is a characteristic chromosomal abnormality of MDS [11]. By contrast, in hypoplastic MDS cases, the incidence of -7/del (7q) is significantly low (3.8% of 71 cases of hypoplastic MDS) [9,10,12,13]. The incidence of -7/del (7q) is relatively high in cases of GMT with myeloproliferative disorders, therefore, a relationship between abnormality of chromosome 7 and GMT in myeloproliferative disorders may be present.
In conclusion, we describe the second documented case of MDS preceding GMT. GMT is a rare phenomenon of the bone marrow; however this is not a specific disease and may have some underlying conditions. This case suggests that myeloproliferative disorders including MDS must be included in the differential diagnostic considerations of patients with GMT, who have no history of malnutrition or drugs, and careful observation of the bone marrow smear specimen and cytogenetic analysis are necessary for early detection of underlying myeloproliferative disorders.
References
- 1.Böhm J. Gelatinous transformation of the bone marrow. The spectrum of underlying diseases. Am J Surg Pathol. 2000;24:56–65. doi: 10.1097/00000478-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Seaman JP, Kjeldsberg CR, Linker A. Gelatinous transformation of the bone marrow. Hum Pathol. 1978;9:685–692. doi: 10.1016/s0046-8177(78)80051-3. [DOI] [PubMed] [Google Scholar]
- 3.Mehta K, Gascon P, Robboy S. The gelatinous bone marrow (serous atrophy) in patients with acquired immunodeficiency syndrome. Evidence of excess sulfated glycosaminoglycan. Arch Pathol Lab Med. 1992;116:504–508. [PubMed] [Google Scholar]
- 4.Aisa Y, Mori T, Nakazato T, Yamazaki R, Ikeda Y, Okamoto S. Gelatinous degeneration in a recipient of allogeneic bone marrow transplantation. Int J Hematol. 2006;84:465–467. doi: 10.1532/IJH97.06136. [DOI] [PubMed] [Google Scholar]
- 5.Arranz R, Gil-Fernandez JJ, Acevedo A, Tomas JF, Alegre A, Fernandez-Ranada JM. Gelatinous degeneration presenting as a prelekuemic syndrome. J Clin Pathol. 1996;49:512–514. doi: 10.1136/jcp.49.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ifrah N, Saint-Andre JP, De Gentile L, Foussard C, Chevailler A, Flandrin G, Boasson M. Gelatinous transformation of the bone marrow: manifestation of an acute leukemia? Acta Haemat. 1989;82:165–168. doi: 10.1159/000205369. [DOI] [PubMed] [Google Scholar]
- 7.Niscola P, Maurillo L, Palombi M, Fratoni S, Perrotti AP, Piccioni D, Panetta P, Scaramucci L, Del Poeta G, De Fabritiis P. Gelatinous degeneration of the bone marrow: two case reports showing different hematological features and clinical outcomes. Acta Haematol. 2007;118:165–166. doi: 10.1159/000108766. [DOI] [PubMed] [Google Scholar]
- 8.Böhm J, Schmitt-Gräff A. Gelatinous bone marrow transformation in a case of idiopathic myelofibrosis: a morphological paradox. Pathol Res Pract. 2000;196:775–779. doi: 10.1016/S0344-0338(00)80111-9. [DOI] [PubMed] [Google Scholar]
- 9.Huang TC, Ko BS, Tang JL, Hsu C, Chen CY, Tsay W, Huang SY, Yao M, Chen YC, Shen MC, Wang CH, Tien HF. Comparison of hypoplastic myelodysplastic syndrome (MDS) with normo-/hypercellular MDS by International Prognostic Scoring System, cytogenetic and genetic studies. Leukemia. 2008;22:544–550. doi: 10.1038/sj.leu.2405076. [DOI] [PubMed] [Google Scholar]
- 10.Maschek H, Kaloutsi V, Rodriguez-Kaiser M, Werner M, Choritz H, Mainzer K, Dietzfelbinger M, Georgii A. Hypoplastic myelodysplastic syndrome: incidence, morphology, cytogenetics, and prognosis. Ann Hematol. 1993;66:117–122. doi: 10.1007/BF01697619. [DOI] [PubMed] [Google Scholar]
- 11.Haase D, Germing U, Schanz J, Pfeilstöcker M, Nösslinger T, Hidebrandt B, Kundgen A, Lübbert M, Kunzmann R, Giagounidis AA, Aul C, Trümper L, Krieger O, Stauder R, Müller TH, Wimazal F, Valent P, Fonatsch C, Steidl C. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110:4385–4395. doi: 10.1182/blood-2007-03-082404. [DOI] [PubMed] [Google Scholar]
- 12.Nand S, Godwin JE. Hypoplastic myelodysplastic syndrome. Cancer. 1988;62:958–964. doi: 10.1002/1097-0142(19880901)62:5<958::aid-cncr2820620519>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Tuzuner N, Cox C, Rowe JM, Watrous D, Bennett JM. Hypocellular myelodysplastic syndrome (MDS): new proposals. Br J Haematol. 1995;91:612–617. doi: 10.1111/j.1365-2141.1995.tb05356.x. [DOI] [PubMed] [Google Scholar]


