Abstract
The contribution of metabotropic glutamate receptors (mGluR) and opioid receptors to inhibition of bladder overactivity by tibial nerve stimulation (TNS) was investigated in cats under α-chloralose anesthesia using LY341495 (a group II mGluR antagonist) and naloxone (an opioid receptor antagonist). Slow infusion cystometry was used to measure the volume threshold (i.e., bladder capacity) for inducing a large bladder contraction. After measuring the bladder capacity during saline infusion, 0.25% acetic acid (AA) was infused to irritate the bladder, activate the nociceptive C-fiber bladder afferents, and induce bladder overactivity. AA significantly (P < 0.0001) reduced bladder capacity to 26.6 ± 4.7% of saline control capacity. TNS (5 Hz, 0.2 ms) at 2 and 4 times the threshold (T) intensity for inducing an observable toe movement significantly increased bladder capacity to 62.2 ± 8.3% at 2T (P < 0.01) and 80.8 ± 9.2% at 4T (P = 0.0001) of saline control capacity. LY341495 (0.1–5 mg/kg iv) did not change bladder overactivity, but completely suppressed the inhibition induced by TNS at a low stimulus intensity (2T) and partially suppressed the inhibition at high intensity (4T). Following administration of LY341495, naloxone (0.01 mg/kg iv) completely eliminated the high-intensity TNS-induced inhibition. However, without LY341495 treatment a 10 times higher dose (0.1 mg/kg) of naloxone was required to completely block TNS inhibition. These results indicate that interactions between group II mGluR and opioid receptor mechanisms contribute to TNS inhibition of AA-induced bladder overactivity. Understanding neurotransmitter mechanisms underlying TNS inhibition of bladder overactivity is important for the development of new treatments for bladder disorders.
Keywords: neurotransmitter, neuromodulation, bladder, cat
glutamatergic neurotransmission involves activation of both ionotropic glutamate receptors (iGluR) and metabotropic glutamate receptors (mGluR) that consist of eight subtypes classified into group I (mGluR 1 and 5), group II (mGluR 2 and 3), and group III (mGluR 4, 6, 7, and 8). An interaction between iGluR and opioid receptor mechanisms is believed to be important in pain pathways, because block of ionotropic N-methyl-d-aspartate (NMDA) receptors enhances the antinociceptive effect of morphine (an opioid receptor agonist) (14, 28, 36, 37). Recently, the interaction between mGluR and opioid receptors has also been identified in studies of opioid-induced antinociception (15, 16, 29, 45). Opioid-induced antinociceptive effects were enhanced by antagonists (15, 16, 45) and agonists (29) of group II mGluR in different types of somatic nociception. Currently, it is unknown whether this interaction between group II mGluR and opioid receptors occurs in visceral nociceptive mechanisms.
Recent results from this laboratory (38) revealed that tibial nerve stimulation (TNS) inhibits bladder overactivity elicited by intravesical infusion of acetic acid (AA), which stimulates nociceptive bladder afferent nerves. This TNS-induced antinociceptive effect is completely eliminated by naloxone (an opioid receptor antagonist), indicating a role for opioid receptor activation in TNS-induced antinociception (39). Tramadol, which produces an active metabolite with opioid receptor agonist activity, enhances the TNS-induced inhibition of AA-induced bladder overactivity (47).
The current study examined the contribution of group II mGluR and the possible interaction between these receptors and opioid receptor mechanisms in TNS-induced inhibition of AA-induced bladder overactivity in cats anesthetized with α-chloralose. Intravesical infusion of dilute AA (0.25%) was used to irritate the bladder and activate nociceptive C-fiber bladder afferents. LY341495 (a group II mGluR antagonist) and naloxone were administered to evaluate the role of glutamatergic and opioid receptors. The effects of these agents on TNS inhibition may provide insights into the mechanisms underlying the clinical efficacy of tibial neuromodulation for the treatment of overactive bladder (OAB) symptoms (30, 31).
MATERIALS AND METHODS
The Animal Care and Use Committee at the University of Pittsburgh approved all protocols involving the use of animals in this study.
Experimental setup.
Experiments were conducted in 18 cats (10 male, 8 female, 2.7–3.9 kg, 6- to 12-mo-old domestic shorthairs; Liberty Research, Waverly, NY) anesthetized initially with isoflurane (2–5% in oxygen) and maintained with α-chloralose (65 mg/kg iv with supplementation as necessary). Heart rate and blood oxygen level were monitored by a pulse oximeter (9847 V; NONIN Medical, Plymouth, MN) with the sensor attached to the tongue. Systemic blood pressure was monitored via a catheter in the right carotid artery. Drug and fluid were administered via the right cephalic vein, and airway access was secured with a tracheotomy tube.
The ureters were isolated via an abdominal incision, cut, and drained externally using penrose drainage tubing. The bladder was cannulated through the urethra with a double-lumen catheter. One lumen was used to infuse saline or 0.25% AA at a rate of 0.5–2 ml/min, and the other lumen was attached to a pressure transducer to record the bladder pressure. A ligature was tied around the proximal urethra ∼2 cm from the bladder to prevent leakage. The tibial nerve was exposed on the medial side of right hindlimb above the ankle. A tripolar cuff electrode (NC223pt; MicroProbe, Gaithersburg, MD) was applied around the nerve and connected to a stimulator (S88; Grass Medical Instruments, Quincy, MA).
Stimulation protocol and drug administration.
Uniphasic rectangular pulses (5 Hz frequency, 0.2-ms pulse width) were delivered to the tibial nerve via the cuff electrode. The intensity threshold (T) for inducing toe movement was determined by gradually increasing the stimulation intensity. Because our previous study indicated that a 2T stimulus intensity was required to inhibit reflex bladder contractions (40), intensities of 2T or 4T were used in this study to suppress nociceptive bladder overactivity induced by 0.25% AA irritation.
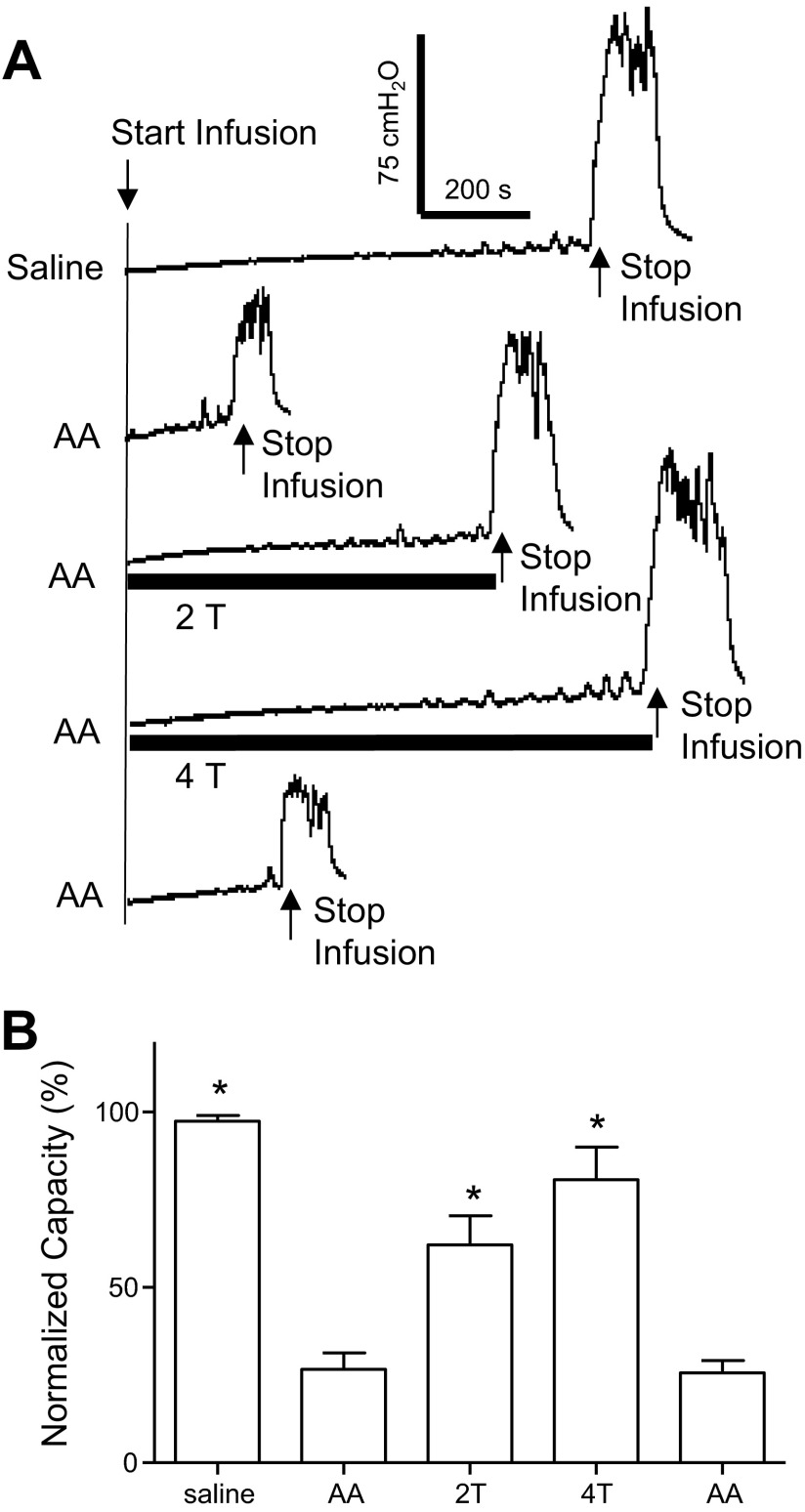
A cystometrogram (CMG) was performed with saline infusion to measure bladder capacity, which was defined as the bladder volume threshold to induce a large amplitude (>30 cmH2O) and long duration (>20 sec) bladder contraction. Then, multiple (3–5) saline CMG were repeated to evaluate the reproducibility in a 30- to 90-min period. Once the bladder capacity was determined during saline infusion, 0.25% AA was infused into the bladder during repeated CMG to activate nociceptive C-fiber bladder afferents and induce an OAB reflex. After 15–40 min of AA infusion for bladder capacity to be stabilized, four CMG were performed during AA infusion: 1) control without TNS; 2) during 2T TNS; 3) during 4T TNS; and 4) control without TNS (Fig. 1A). Then, pharmacological studies were performed.
Fig. 1.
Inhibition of bladder overactivity by tibial nerve stimulation (TNS). A: repeated cystometrogram (CMG) during saline or 0.25% acetic acid (AA) infusion with and without TNS. T indicates threshold intensity of stimulation (5 Hz, 0.2 ms; T = 1.0 V). Arrows indicate the start and stop of the infusion. Infusion rate = 1 ml/min. B: summarized results of TNS inhibitory effect on bladder capacity during AA infusion (n = 10 cats). Infusion rate = 0.5–2 ml/min. *Significantly different from AA control (one-way ANOVA).
Cumulative doses (0.1, 0.3, 1, 3, and 5 mg/kg iv) of LY341495 {(2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprp-1-yl]-3-(xanth-9-yl) propanoic acid; Abcam, Cambridge, MA} were given in 10 cats. LY341495 is an antagonist for both mGluR2 and mGluR3 receptors that are classified as group II mGluR. Starting 15 min after administering each dose of LY341495, four CMG were performed during AA infusion: 1) control without TNS; 2) during 2T TNS; 3) during 4T TNS; and 4) control without TNS. In 9 cats following the testing of LY341495, cumulative doses (0.001, 0.01, and 0.1 mg/kg iv) of naloxone (Sigma-Aldrich, St. Louis, MO) were injected to block opioid receptors prior to repeated testing of TNS. Naloxone is a nonselective antagonist for μ, κ, and δ opioid receptors. Starting 5 min after administering each dose of naloxone, four CMG were performed during AA infusion: 1) control without TNS; 2) during 2T TNS; 3) during 4T TNS; and 4) control without TNS. A 5-min rest period was inserted between the repeated CMG to allow the bladder to recover from previous contractions.
In another experimental group (n = 8 cats), the animals were not treated with LY341495, but the same protocol for repeated CMG tests (control, 2T, 4T, and control) was also used during AA infusion. Naloxone alone was administered in cumulative doses (0.001, 0.01, and 0.1 mg/kg iv) to evaluate the effect of varying levels of opioid receptor blockade on TNS-induced inhibition in the absence of LY341495.
Data analysis.
For the repeated CMG recordings, bladder capacities were measured and normalized to the measurement of the first saline control CMG or the AA control CMG before the naloxone test in the same animal so that the results from different animals could be compared. Repeated measurements in the same animal under the same experimental conditions were averaged. The results from different animals are reported as means ± SE. Statistical significance (P < 0.05) was detected by one-way ANOVA followed by Dunnett's multiple comparison, or two-way ANOVA followed by Bonferroni multiple comparison.
RESULTS
TNS inhibition of bladder overactivity induced by AA irritation.
Intravesical infusion of 0.25% AA irritated the bladder, activated nociceptive C-fiber bladder afferents, and significantly (P < 0.0001) reduced bladder capacity to 26.6 ± 4.7% of the saline control capacity (8.5 ± 1.3 ml, n = 10 cats) (Fig. 1). TNS at 2T and 4T intensity suppressed AA-induced bladder overactivity and significantly increased bladder capacity to 62.2 ± 8.3% (P < 0.01) and 80.8 ± 9.2% (P = 0.0001) of the saline control capacity, respectively (Fig. 1B). After the 2T and 4T TNS, bladder capacity returned to 26.7 ± 5.7% of the saline control capacity (Fig. 1B).
Dose-dependent effect of LY341495 on TNS inhibition of bladder overactivity.
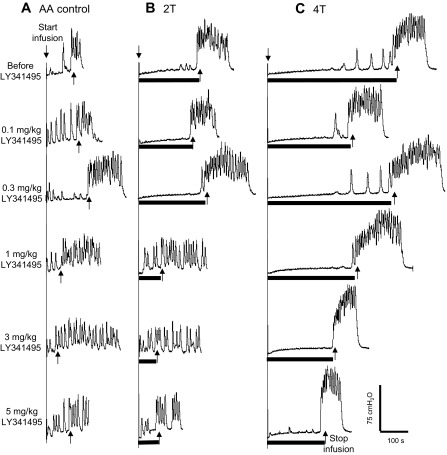
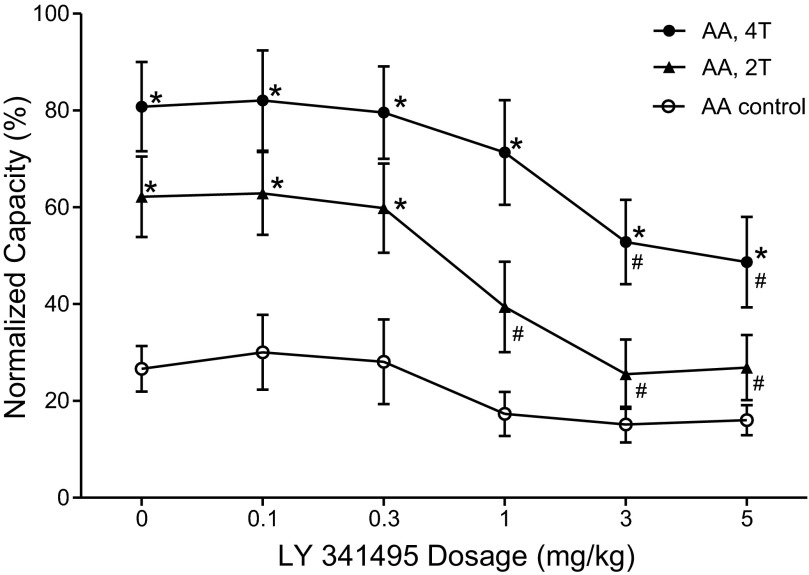
The effect of LY341495 on TNS inhibition of bladder overactivity was dependent on drug dosage and TNS intensity (Fig. 2 and Fig. 3). Administering cumulative doses of LY341495 (0.1, 0.3, 1, 3 and 5 mg/kg iv) did not significantly (P > 0.05) change the control bladder capacity in the absence of stimulation (Fig. 2A and Fig. 3). However, LY341495 completely blocked the inhibition induced by low-intensity (2T) TNS at doses of 1–5 mg/kg (Fig. 2, A and B; Fig. 3), and significantly (P < 0.001) reduced the inhibition induced by high-intensity (4T) TNS at doses of 3–5 mg/kg (Fig. 2C; Fig. 3). Compared with AA control capacity, high-intensity (4T) TNS could still significantly increase the bladder capacity at doses of 3–5 mg/kg. In these experiments following 2T and 4T TNS, bladder capacity returned to the control level prior to stimulation (i.e., a poststimulation effect did not occur). Poststimulation inhibition following TNS has been observed during saline CMG (40) but not during AA irritation of the bladder (38).
Fig. 2.
Dose-dependent effect of LY341495 on tibial inhibition of bladder overactivity induced by 0.25% AA. CMG at each dose of LY341495 was performed in sequence from left to right in A–C. A: AA CMG without stimulation. B: AA CMG during 2T stimulation. C: AA CMG during 4T stimulation. Black bars under pressure trace indicate stimulation duration. Stimulation: 5 Hz, 0.2 ms, intensity threshold T = 0.7 V. Arrows indicate start and stop of bladder infusion. Infusion rate = 1 ml/min.
Fig. 3.
Dose-dependent effect of LY341495 on TNS inhibition of bladder overactivity induced by 0.25% AA (n = 10 cats). *Significantly different from AA control at each dosage (two-way ANOVA). #Significantly different from bladder capacity measured during TNS before LY341495 treatment (i.e., at 0 mg/kg of LY341495) (one-way ANOVA). Bladder capacity was normalized to the capacity during saline CMG prior to infusion of AA. Stimulation: 5 Hz, 0.2 ms, T = 0.35–1.2 V.
The number of small, uninhibited detrusor contractions that occurred before the micturition contraction was not changed significantly (P > 0.05) by LY341495 treatment or stimulation. On average from the 10 cat experiments, the number of uninhibited detrusor contractions was 1.8 ± 0.5 under AA control conditions before LY341495 treatment, and 2.3 ± 0.5 after the last dose (5 mg/kg) of LY341495 treatment. There were 1.7 ± 0.8 uninhibited detrusor contractions during 4T TNS before LY341495 treatment and 1.4 ± 0.5 contractions after the last dose of LY341495 treatment.
Dose-dependent effect of naloxone on TNS inhibition of bladder overactivity.
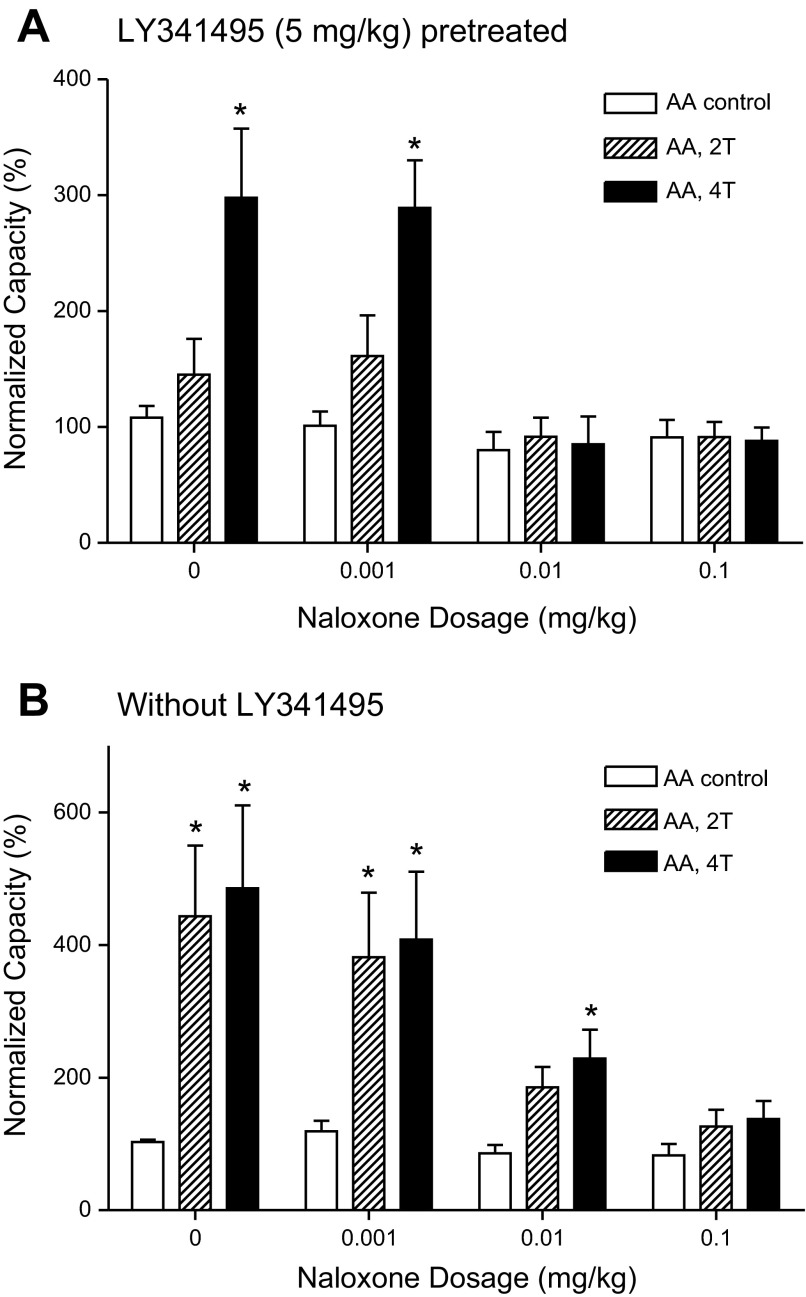
After the last dose (5 mg/kg) of LY341495 was administered, the effect of cumulative doses of naloxone (0.001–0.1 mg/kg iv) on residual TNS inhibition elicited by 4T stimulation was examined in 9 cats. Naloxone did not change the AA control bladder capacity in the absence of TNS, but a dose of 0.01 mg/kg completely blocked residual TNS inhibition (Fig. 4A and Fig. 5A).
Fig. 4.
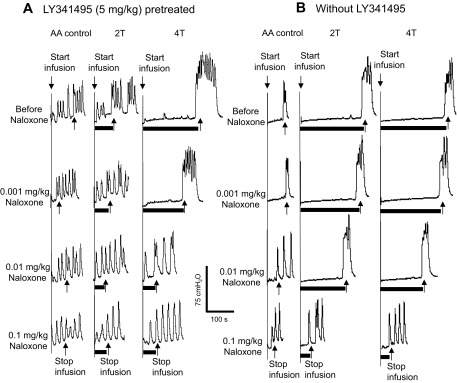
Effect of naloxone on TNS inhibition of bladder overactivity induced by 0.25% AA. A: CMG during 0.25% AA infusion after 5 mg/kg LY341495 pretreatment. B: CMG during 0.25% AA infusion without LY341495 pretreatment. Black bars under bladder pressure traces indicate stimulation duration. T, threshold intensity of stimulation (5 Hz, 0.2 ms, T = 0.7 V in A; T = 1 V in B). Infusion rate = 1 ml/min.
Fig. 5.
LY341495 pretreatment significantly reduces the naloxone dosage required to eliminate TNS inhibition of bladder overactivity induced by 0.25% AA infusion. A: naloxone effect in LY341495 (5 mg/kg) pretreated animals (n = 9 cats). B: naloxone effect without LY341495 pretreatment (n = 8 cats). Bladder capacities were normalized to the capacity measured during AA control CMG before naloxone treatment (i.e., at 0 mg/kg naloxone). Stimulation: 5 Hz, 0.2 ms, T = 0.35–2.4 V. *Significantly different from control capacity at each dosage (two-way ANOVA).
In another group of experiments (n = 8 cats) without LY341495 pretreatment, cumulative doses of naloxone (0.001–0.1 mg/kg iv) also did not change the AA control bladder capacity prior to TNS (Fig. 4B and Fig. 5B) but dose dependently reduced the magnitude of TNS inhibition. However, compared with the results in animals pretreated with LY341495 (5 mg/kg), a 10 times higher naloxone dose (0.1 mg/kg) was needed to completely block the 4T TNS inhibition (Fig. 4 and Fig. 5). In these animals the 0.01 mg/kg naloxone dose eliminated the inhibition induced by 2T TNS (Fig. 5B). Inhibition at this intensity was also eliminated by 3 mg/kg and 5 mg/kg doses of LY341495 (Fig. 3 and Fig. 5A).
DISCUSSION
This study revealed that LY341495 (a group II mGluR antagonist) administered intravenously in a range of doses did not change the bladder overactivity induced by AA irritation, but partially suppressed in a dose-dependent manner TNS inhibition of bladder overactivity (Fig. 2 and Fig. 3). After the maximal dose of LY341495 (5 mg/kg), naloxone (an opioid receptor antagonist) completely eliminated the remaining TNS inhibition (Fig. 4 and Fig. 5) at a dose (0.01 mg/kg) 10 times smaller than the dose (0.1 mg/kg) required to completely block the inhibition in animals that were not treated with LY341495. These results indicate that neurotransmitter mechanisms involving group II mGluR and opioid receptors are essential for TNS inhibition of reflex bladder overactivity and that there may be a significant synergistic interaction between these two mechanisms.
Various studies have implicated group II mGluR in peripheral and central nociceptive mechanisms. Group II mGluR have been identified with immunohistochemical methods in the spinal cord and brain, and in small to medium size dorsal root ganglia neurons, and in cutaneous sensory nerves that coexpress TRPV1 receptors (5, 7, 27). Group II mGluR antagonists enhance nociceptive behavior and primary afferent firing induced by intraplantar injection of capsaicin; and a group II mGluR agonist suppressed this enhancement (9). Group II mGluR agonists are also believed to act in the spinal cord to produce analgesia (13, 34) by suppressing the release of glycine, GABA, or glutamate (35, 48), whereas knockdown of group II mGluR enhances nociceptive responses (46). These results indicate that somatic nociceptive pathways are controlled by tonically active group II mGluR inhibitory mechanisms. However, our studies did not detect similar tonically active inhibitory mechanisms in the control of nociceptive bladder reflexes. Bladder overactivity was not altered after administration of LY341495, which induces hyperalgesia and enhances nociceptive behavior in somatic pain models (9, 34). However, the drug did suppress TNS-induced inhibition of bladder overactivity. This suggests that the group II mGluR inhibitory mechanism in bladder reflex pathways was not effective except during TNS.
Pharmacological studies of somatic nociceptive mechanisms have provided evidence for a linkage between group II mGluR and opioid receptors. Group II mGluR antagonists (15, 16, 45) enhance the inhibitory effect of opioid receptor agonists on somatic nociceptive responses induced by thermal stimuli or formalin injection. On the other hand, a group II mGluR agonist also enhances the opioid-induced antinociceptive effect on neuropathic pain (29). These results indicate that mGluR-opioid interaction could be complex depending on different types of nociception. Our study showing that LY341495 enhances the potency of naloxone in blocking TNS inhibition (Figs. 4 and 5) raises the possibility that group II mGluR also facilitate endogenous opioid inhibitory mechanisms in the TNS inhibitory pathway and that removal of the facilitation by LY341495 reduces the opioid inhibition and makes it more susceptible to blockade by naloxone.
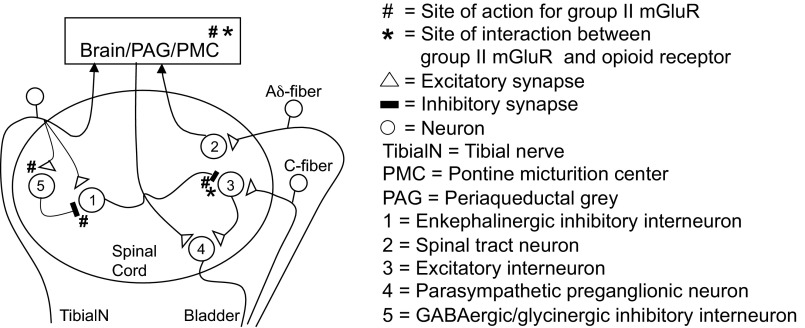
The inhibition of reflex bladder activity by TNS is believed to occur by modulation of the micturition reflex pathway at a site in the central nervous system. As shown in Fig. 6, both spinal and supraspinal pathways can mediate reflex bladder activity. Therefore, TNS inhibition could occur in the brain or spinal cord. Because naloxone and LY341495 were both administered systemically, it is impossible to determine their site of action. However, it is likely that these drugs interact at least in part at synapses in the lumbosacral spinal cord on the basis of the following observations: 1) group II mGluR and opioid receptors are expressed in the spinal dorsal horn (6, 8, 21, 22, 32, 41); 2) intrathecal administration of group II mGluR agonists suppress nociceptive behavior (13, 23, 24, 34); 3) AA-induced bladder overactivity is mediated by a reflex pathway organized in the spinal cord (17); 4) our previous study (39) suggested that TNS inhibition of AA-induced bladder overactivity could be mediated by suppression of transmission at a spinal interneuronal synapse prior to the sacral parasympathetic preganglionic neurons; and 5) spinal opioid receptors have a prominent inhibitory effect on micturition (12, 20, 42, 43).
Fig. 6.
Putative opioid receptor and group II mGluR mechanisms underlying the inhibitory effect of TNS on overactive bladder reflexes induced by nociceptive stimulation of the bladder with AA. Reflex pathways (right) show spinal and supraspinal circuits mediating excitatory input to the bladder activated by C-fiber and Aδ bladder afferents, respectively; an interneuronal pathway (left) mediating TNS-induced inhibition of the bladder reflexes. Interneuron-1 activated by tibial afferents releases opioid peptides and glutamate, which inhibit interneuron-3 on the spinal micturition reflex pathway by activating opioid receptors (*) and group II mGluR (#), respectively. Activation of interneuron-5 by the tibial afferent modulates TNS inhibition by eliciting GABAergic/glycinergic inhibition of interneuron-1. Presynaptic group II mGluR at sites (#) on the interneuronal-5 pathway suppress transmitter release. Block of these receptors with LY341495 enhances the inhibitory modulation of interneuron-1 and reduces TNS inhibition of the micturition reflex. Opioid receptor (*) and group II mGluR (#) mechanisms in micturition centers (PAG/PMC) in the brain stem may also contribute to TNS inhibition. The spinal mechanism for TNS enkephalinergic inhibition of the supraspinal micturition reflex pathway is not included in this figure.
Figure 6 shows a hypothetical spinal mechanism for TNS inhibition, which is intended to facilitate the discussion about possible sites of interaction between naloxone and LY341495. Alternative sites of action in the brain are also indicated but detailed pathways are not shown. In the diagram, TNS inhibition mediated by inhibitory interneuron-1 targets excitatory interneuron-3 on the spinal micturition pathway interposed between the bladder C-fiber afferent and a bladder preganglionic neuron (neuron-4). Electrophysiological evidence for this type of interneuronal inhibition by activation of somatic afferent nerves has been obtained in the cat spinal cord (11). Because TNS inhibition is blocked by naloxone, we propose that inhibitory interneuron-1 is enkephalinergic and releases an opioid peptide. Opioid peptides may be released as cotransmitters with glutamic acid because they are contained in subpopulations of glutamatergic neurons in the spinal dorsal horn (26, 44). Thus these two transmitters may interact to elicit a synergistic postsynaptic inhibitory effect at the synapse between interneuron-1 and interneuron-3. Group II mGluR and opioid receptors share a common intracellular signaling mechanism that inhibits adenylate cyclase activity and reduces cAMP levels (4, 10). This may account for the synergism and for the enhancement of opioid-induced analgesia by exogenously administered group II mGluR agonists. In addition, activation of group II mGluR can inhibit voltage-gated Ca2+ channels, activate potassium channels to decrease neuronal excitability, and suppress synaptic transmission (4, 10, 18, 49). Elimination of the synergism following block of group II mGluR with LY341495 may explain the reduction in TNS inhibition after LY341495 and the increased potency of naloxone to block TNS inhibition.
Group II mGluR mechanisms could also indirectly affect TNS opioid inhibitory mechanisms via changes in NMDA iGluR. Activation of group II mGluR inhibits NMDA receptor mechanisms (1, 25, 33), and inhibition of NMDA receptors enhances opioid-induce antinociception (14, 28, 36, 37). Therefore, it is possible in our experiments that LY341495 reduced a tonic inhibition of NMDA receptors mediated by group II mGluR, thereby weakening an opioid antinociceptive effect and partially suppressing the TNS inhibition. This could be tested in future experiments by evaluating the sensitivity of TNS inhibition to other drugs that modulate NMDA receptors. Additionally, nonspecific effects of the tested drugs could also play a role in the drug interaction such as changes in drug metabolism, which could alter the potency of the drug.
An alternate hypothesis for the effect of LY341495 on TNS inhibition is that the drug acts at more proximal sites on the TNS reflex pathway to downregulate the firing of inhibitory interneuron-1. This mechanism would require a collateral inhibitory pathway that modulates TNS inhibition (interneuron-5; Fig. 6). It is known that group II mGluR are located presynaptically on glutamatergic (10, 33) and GABAergic/glycinergic nerve terminals (35, 48) where they suppress transmitter release. Block of group II mGluR with LY341495 would increase the release of the transmitters, enhance transmission in the collateral inhibitory pathway, and therefore decrease the firing of inhibitory interneuron-1.
Perspectives and Significance
Our recent studies showing that tramadol, which produces an active metabolite with opioid receptor agonist activity, can significantly enhance TNS inhibition of AA-induced bladder overactivity (47) have provided additional support for the involvement of opioid receptor mechanisms in TNS inhibition of bladder nociception. Our current results raise the possibility that group II mGluR agonists might interact with tramadol to further enhance TNS inhibition of bladder nociception. However, whether these results obtained in anesthetized animals can be translated into clinical applications in human subjects still needs to be validated. Understanding neurotransmitter mechanisms underlying TNS inhibition of nociceptive bladder overactivity is important for development of new therapies to treat OAB or painful bladder syndrome/interstitial cystitis (2, 3, 19) and reduce adverse drug effects by combining drug therapy with neuromodulation.
GRANTS
This study is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-068566, DK-090006, and DK-091253.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.M., A.D.M., F.Z., B.S., J.W., J.R.R., W.C.d.G., and C.T. conception and design of research; Y.M., A.D.M., F.Z., B.S., J.W., J.R.R., W.C.d.G., and C.T. performed experiments; Y.M., A.D.M., F.Z., B.S., J.W., J.R.R., W.C.d.G., and C.T. analyzed data; Y.M., A.D.M., F.Z., B.S., J.W., J.R.R., W.C.d.G., and C.T. interpreted results of experiments; Y.M., A.D.M., F.Z., B.S., J.W., J.R.R., W.C.d.G., and C.T. prepared figures; Y.M., A.D.M., F.Z., B.S., J.W., J.R.R., W.C.d.G., and C.T. drafted manuscript; Y.M., A.D.M., F.Z., B.S., J.W., J.R.R., W.C.d.G., and C.T. edited and revised manuscript; Y.M., A.D.M., F.Z., B.S., J.W., J.R.R., W.C.d.G., and C.T. approved final version of manuscript.
REFERENCES
- 1. Ambrosini A, Bresciani L, Fracchia S, Brunello N, Racagni G. Metabotropic glutamate receptors negatively coupled to adenylate cyclase inhibit N-methyl-D-aspartate receptor activity and prevent neurotoxicity in mesencephalic neurons in vitro. Mol Pharmacol 47: 1057– 1064, 1995 [PubMed] [Google Scholar]
- 2. Andersson KE. New pharmacologic targets for the treatment of the overactive bladder: an update. Urology 63, Suppl 1: 32–41, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatment of urinary incontinence. Pharmacol Rev 56: 581– 631, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Rev 29: 83– 120, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Aronica E, Catania MV, Geurts J, Yankaya B, Troost D. Immunohistochemical localization of group I and II metabotropic glutamate receptors in control and amyotrophic lateral sclerosis human spinal cord: upregulation in reactive astrocytes. Neuroscience 105: 509– 520, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Budai D, Fields HL. Endogenous opioid peptides acting at mu opioid receptors in the dorsal horn contribute to midbrain modulation of spinal nociceptive neurons. J Neurophysiol 79: 677– 687, 1988 [DOI] [PubMed] [Google Scholar]
- 7. Carlton SM, Du J, Zhou S. Group II metabotropic glutamate receptor activation on peripheral nociceptors modulates TRPV1 function. Brain Res 1248: 86– 95, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carlton SM, Hargett GL, Coggeshall RE. Localization of metabotropic glutamate receptors 2/3 on primary afferent axons in the rat. Neuroscience 105: 957– 969, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Carlton SM, Zhou S, Govea R, Du J. Group II/III metabotropic glutamate receptors exert endogenous activity-dependent modulation of TRPV1 receptors on peripheral nociceptors. J Neurosci 31: 12727– 12737, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37: 205– 237, 1997 [DOI] [PubMed] [Google Scholar]
- 11. de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst 3: 135– 160, 1981 [DOI] [PubMed] [Google Scholar]
- 12. Dray A, Metsch A. Inhibition of urinary bladder contractions by a spinal action of morphine and other opioids. J Pharmacol Exp Ther 231: 254– 260, 1984 [PubMed] [Google Scholar]
- 13. Dolan S, Nolan AM. Behavioural evidence supporting a differential role for group I and II metabotropic glutamate receptors in spinal nociceptive transmission. Neuropharmacology 39: 1132– 1138, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Fischer BD, Carrigan KA, Dykstra LA. Effects of N-methyl-D-aspartate receptor antagonists on acute morphine-induced and l-methadone-induced antinociception in mice. J Pain 6: 425– 433, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Fischer BD, Miller LL, Henry FE, Picker MJ, Dykstra LA. Increased efficacy of micro-opioid agonist-induced antinociception by metabotropic glutamate receptor antagonists in C57BL/6 mice: comparison with (-)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid (LY235959). Psychopharmacology (Berl) 198: 271– 278, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Fischer BD, Zimmerman EI, Picker MJ, Dykstra LA. Morphine in combination with metabotropic glutamate receptor antagonists on schedule-controlled responding and thermal nociception. J Pharmacol Exp Ther 324: 732– 739, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453– 466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gerber G, Zhong J, Youn D, Randic M. Group II and group III metabotropic glutamate receptor agonists depress synaptic transmission in the rat spinal cord dorsal horn. Neuroscience 100: 393– 406, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Giannantoni A, Bini V, Dmochowski R, Hanno P, Nickel JC, Proietti S, Wyndaele JJ. Contemporary management of the painful bladder: a systematic review. Eur Urol 61: 29– 53, 2012 [DOI] [PubMed] [Google Scholar]
- 20. Hisamitsu T, de Groat WC. The inhibitory effect of opioid peptides and morphine applied intrathecally and intracerebroventricularly on the micturition reflex in the cat. Brain Res 298: 51– 65, 1984 [DOI] [PubMed] [Google Scholar]
- 21. Huang J, Wang Y, Wang W, Wei Y, Li Y, Wu S. Preproenkephalin mRNA is expressed in a subpopulation of GABAergic neurons in the spinal dorsal horn of the GAD67-GFP knock-in mouse. Anat Rec 291: 1334– 1341, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Jia H, Rustioni A, Valtschanoff JG. Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. J Comp Neurol 410: 627– 642, 1999 [PubMed] [Google Scholar]
- 23. Kumar N, Laferriere A, Yu JS, Poon T, Coderre TJ. Metabotropic glutamate receptors (mGluRs) regulate noxious stimulus-induced glutamate release in the spinal cord dorsal horn of rats with neuropathic and inflammatory pain. J Neurochem 114: 281– 290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee HG, Park SK, Yoon MH. Potentiation of morphine antiallodynic efficacy by ACPT-III, a group III metabotropic glutamate receptor agonist, in rat spinal nerve ligation-induced neuropathic pain. Pharmacol Biochem Behav 96: 108– 113, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Martin G, Nie Z, Siggins GR. Metabotropic glutamate receptors regulate N-methyl-D-aspartate-mediated synaptic transmission in nucleus accumbens. J Neurophysiol 78: 3028– 3038, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Marvizón JC, Chen W, Murphy N. Enkephalins, dynorphins, and beta-endorphin in the rat dorsal horn: an immunofluorescence colocalization study. J Comp Neurol 517: 51– 68, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mills CD, Fullwood SD, Hulsebosch CE. Changes in metabotropic glutamate receptor expression following spinal cord injury. Exp Neurol 170: 244– 257, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Nemmani KV, Grisel JE, Stowe JR, Smith-Carliss R, Mogil JS. Modulation of morphine analgesia by site-specific N-methyl-D-aspartate receptor antagonists: dependence on sex, site of antagonism, morphine dose, and time. Pain 109: 274– 283, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Osikowicz M, Mika J, Makuch W, Przewlocka B. Glutamate receptor ligands attenuate allodynia and hyperalgesia and potentiate morphine effects in a mouse model of neuropathic pain. Pain 139: 117– 126, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Peters KM, Carrico DJ, Perez-Marrero RA, Khan AU, Wooldridge LS, Davis GL, Macdiarmid SA. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 183: 1438– 1443, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Peters KM, Macdiarmid SA, Wooldridge LS, Leong FC, Shobeiri SA, Rovner ES, Siegel SW, Tate SB, Jarnagin BK, Rosenblatt PL, Feagins BA. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol 182: 1055– 1061, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience 71: 949– 976, 1996 [DOI] [PubMed] [Google Scholar]
- 33. Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology 34: 1– 26, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Ren BX, Gu XP, Zheng YG, Liu CL, Wang D, Sun YE, Ma ZL. Intrathecal injection of metabotropic glutamate receptor subtype 3 and 5 agonist/antagonist attenuates bone cancer pain by inhibition of spinal astrocyte activation in a mouse model. Anesthesiology 116: 122– 132, 2012 [DOI] [PubMed] [Google Scholar]
- 35. Romei C, Raiteri M, Raiteri L. Glycine release is regulated by metabotropic glutamate receptors sensitive to mGluR2/3 ligands and activated by N-acetylaspartylglutamate (NAAG). Neuropharmacology 66: 311– 316, 2013. [DOI] [PubMed] [Google Scholar]
- 36. Srivastava RK, Gombar KK, Kaur AH, Khosla P. Attenuation of morphine-induced antinociception by L-glutamic acid at the spinal site in rats. Can J Anaesth 42: 541– 546, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Suh HW, Song DK, Choi YS, Kim YH. Multiplicative interaction between intrathecally and intracerebroventricularly administered morphine for antinociception in the mouse: involvement of supraspinal NMDA but not non-NMDA receptors. Life Sci 56: PL181– PL185, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Tai C, Chen M, Shen B, Wang J, Roppolo JR, de Groat WC. Irritation induced bladder overactivity is suppressed by tibial nerve stimulation in cats. J Urol 186: 326– 330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tai C, Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC. Differential role of opioid receptor in tibial nerve inhibition of nociceptive and non-nociceptive bladder reflexes in cats. Am J Physiol Renal Physiol 302: F1090– F1097, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tai C, Shen B, Chen M, Wang J, Roppolo JR, de Groat WC. Prolonged post stimulation inhibition of bladder activity induced by tibial nerve stimulation in cats. Am J Physiol Renal Physiol 300: F385– F392, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang FR, Sim MK. Pre- and/or post-synaptic localisation of metabotropic glutamate receptor 1alpha (mGluR1alpha) and 2/3 (mGluR2/3) in the rat spinal cord. Neurosci Res 34: 73– 78, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Thor KB, Roppolo JR, deGroat WC. Naloxone induced micturition in unanesthetized paraplegic cats. J Urol 129: 202– 205, 1983 [DOI] [PubMed] [Google Scholar]
- 43. Thor KB, Roppolo JR, Kawatani M, Erdman S, de Groat WC. Plasticity in spinal opioid control of lower urinary tract function in paraplegic cats. Neuroreport 5: 1673– 1678, 1994 [DOI] [PubMed] [Google Scholar]
- 44. Todd AJ, Hughes DI, Polgár E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci 17: 13– 27, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Yoon MH, Choi J, Bae HB, Kim SJ, Chung ST, Jeong SW, Chung SS, Yoo KY, Jeong CY. Antinociceptive effects and synergistic interaction with morphine of intrathecal metabotropic glutamate receptor 2/3 antagonist in the formalin test of rats. Neurosci Lett 394: 222– 226, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Zammataro M, Chiechio S, Montana MC, Traficante A, Copani A, Nicoletti F, Gereau RW., 4th mGlu2 metabotropic glutamate receptors restrain inflammatory pain and mediate the analgesic activity of dual mGlu2/mGlu3 receptor agonists. Mol Pain 7: 6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang F, Mally AD, Ogagan PD, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Inhibition of bladder overactivity by a combination of tibial neuromodulation and tramadol treatment in cats. Am J Physiol Renal Physiol 302: F1576– F1582, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou HY, Chen SR, Chen H, Pan HL. Functional plasticity of group II metabotropic glutamate receptors in regulating spinal excitatory and inhibitory synaptic input in neuropathic pain. J Pharmacol Exp Ther 336: 254– 264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou HY, Zhang HM, Chen SR, Pan HL. Increased nociceptive input rapidly modulates spinal GABAergic transmission through endogenously released glutamate. J Neurophysiol 97: 871– 882, 2007 [DOI] [PubMed] [Google Scholar]