Abstract
The energy cost of contractions in skeletal muscle involves activation of both actomyosin and sarcoplasmic reticulum (SR) Ca2+-pump (SERCA) ATPases, which together determine the overall ATP demand. During repetitive contractions leading to fatigue, the relaxation rate and Ca2+ pumping become slowed, possibly because of intracellular metabolite accumulation. The role of the energy cost of cross-bridge cycling during contractile activity on Ca2+-pumping properties has not been investigated. Therefore, we inhibited cross-bridge cycling by incubating isolated Xenopus single fibers with N-benzyl-p-toluene sulfonamide (BTS) to study the mechanisms by which SR Ca2+ pumping is impaired during fatiguing contractions. Fibers were stimulated in the absence (control) and presence of BTS and cytosolic calcium ([Ca2+]c) transients or intracellular pH (pHi) changes were measured. BTS treatment allowed normal [Ca2+]c transients during stimulation without cross-bridge activation. At the time point that tension was reduced to 50% in the control condition, the fall in the peak [Ca2+]c and the increase in basal [Ca2+]c did not occur with BTS incubation. The progressively slower Ca2+ pumping rate and the fall in pHi during repetitive contractions were reduced during BTS conditions. However, when mitochondrial ATP supply was blocked during contractions with BTS present (BTS + cyanide), there was no further slowing in SR Ca2+ pumping during contractions compared with the BTS-alone condition. Furthermore, the fall in pHi was significantly less during the BTS + cyanide condition than in the control conditions. These results demonstrate that factors related to the energetic cost of cross-bridge cycling, possibly the accumulation of metabolites, inhibit the Ca2+ pumping rate during fatiguing contractions.
Keywords: Ca2+ uptake rate, skeletal muscle, cross-bridge cycling, fatigue, glycolysis, oxidative phophorylation
during the transition from rest to exercise in skeletal muscle, the demand for ATP increases several hundredfold, primarily because of the activation of both actomyosin (i.e., cross-bridge cycling) and sarcoplasmic reticulum (SR) Ca2+ ATPase (SERCA) pumps (6). After membrane depolarization, cytosolic Ca2+ concentration is transiently increased, thereby elevating actomyosin and SERCA ATPase activities. Although the Na+-K+-ATPase activity has a high contribution in the energy cost at rest [∼40% of total; (27)], it is quite small during contractions [∼1.5–7%; (6)]. Conversely, during contractions, actomyosin ATPase and SERCA account for ∼60% and 40% of the total ATP demand, respectively (5, 6, 22, 30). However, the energy cost of Ca2+ handling by SERCA during contractions may be as high as 80% of the total energy cost in type IIB fibers (40). Interestingly, although the high cost of SR Ca2+ pumping during exercise is often not appreciated, SERCA dysfunction (i.e., slowing in the Ca2+ uptake after contractions) has been implicated in many pathological states, including chronic obstructive pulmonary disease (13), muscular dystrophy (11), and heart failure (19). Because SERCA dysfunction may be an important factor associated with the decreased locomotor activity in these diseases (13), the study of the factors that influence SERCA activity during fatigue is very relevant.
The increased ATP demand during exercise is met by three energetic sources: phosphocreatine breakdown, anaerobic glycolysis, and mitochondrial oxidative phosphorylation (2). The contribution of each source is time- and fiber-type-dependent and is regulated by the total metabolic energy cost of the contractile work (2, 14). Also, the contribution of each energetic source during exercise has been shown to regulate the development of muscle fatigue. During high-intensity exercise, skeletal muscle fatigue develops rapidly, particularly in fast-twitch, glycolytic fibers (2). Fatigue development is highly dependent on intracellular product accumulation (e.g., Pi and H+), as well as substrate depletion, which may interfere directly with the energy supply metabolism and with the excitation-contraction coupling in skeletal muscle (2, 10). One hallmark of fatigue is the slowing of relaxation, which has often been associated with a decrease in SR Ca2+ pumping rate due to SERCA dysfunction (2, 28). The reduced ATP hydrolysis rate of SERCA at fatigue along with a reduced SR Ca2+ release may directly affect the total energy cost during contractions (26). Although the impairment in Ca2+ uptake at fatigue has been well described, the importance of the ATP demand on SERCA regulation during repetitive contractions leading to fatigue is controversial (2, 28). Furthermore, little is known concerning whether the ATP cost of Ca2+ handling during muscle activation is sufficient to regulate the SR Ca2+ uptake kinetics and function during fatiguing contractile work.
Several studies have assessed SR Ca2+ uptake energy cost by blocking the activation of cross-bridge cycling using the compound N-benzyl-p-toluene sulfonamide (BTS) (5, 7, 22, 30, 40). This compound inhibits the affinity between myosin and actin (23) but does not affect Ca2+ release and uptake in single muscle fibers (9, 30, 39). Interestingly, the role of SERCA energy requirements during repetitive contractions on real-time measures of intracellular pH (pHi) and SR Ca2+ pumping function has not been investigated. The aim of the present investigation was to use BTS as a tool to test the hypothesis that the SR Ca2+ pumping dysfunction that occurs during repetitive contractions leading to fatigue is dependent on the accumulation of intracellular metabolites that arise from the energy cost of cross-bridge cycling.
METHODS
Animal care and single fiber isolation.
All procedures were approved by the University of California, San Diego Institutional Animal Care and Use Committee and conformed to American Physiological Society guidelines. Female adult Xenopus laevis were anesthetized (pentobarbital sodium: 120 mg/kg), double pithed, and decapitated, and the lumbrical muscles (II–IV) were removed. Single skeletal muscle fibers (n = 29) were isolated using a pair of scissors and forceps under dark-field illumination. Dissection and experiments were performed in Ringer solution (116.5 mM NaCl, 2 mM KCl, 1.9 mM CaCl2, 2 mM NaH2PO4, and 0.1 mM EGTA, at pH 7.0) at room temperature (20–22°C).
Intracellular Ca2+ and pH detection.
Cytosolic calcium concentration ([Ca2+]c) and pHi changes were obtained by fluorescence spectroscopy using a Photon Technology International illumination and detection system (DeltaScan model), integrated with a Nikon inverted microscope with a 40× Fluor objective. After dissection and isolation, each fiber was pressure injected using a micropipette with either the ratiometric compounds Fura-2 (12 mM) for [Ca2+]c detection, or 2′,7′-bis-(2-carboxyethyl)-5-(and -6)carboxyfluorescein (BCECF; 10 mM) for pHi detection (both compounds were diluted in 150 mM KCl and 10 mM HEPES at pH 7.0). After injection, the myofiber rested for 1 h. Both indicators were obtained from Invitrogen (Carlsbad, CA).
For detection of [Ca2+]c during contractions, the fibers were illuminated with two rapidly alternating (200 Hz) excitation wavelengths of 340 nm and 380 nm, and the resulting fluorescence emissions at 510 nm were divided (340 nm/380 nm) to obtain the Ca2+-dependent signal. Fluorescence excitation ratio (340/380 nm; R) was converted to [Ca2+]c, according to Eq. 1.
| (1) |
From Eq. 1, KD, the dissociation constant for Ca2+-Fura-2, was set to 224 nM (33). β is the fluorescence ratio between high and no [Ca2+]c at 380 nm, and Rmin and Rmax are the fluorescence ratios at no cytosolic calcium and high calcium, respectively. Rmin and Rmax were determined using an internal in vivo calibration described by Kabbara and Allen (16) with small modifications. To determine Rmin, fibers were incubated for 30 min with a no-calcium Ringer-EGTA solution (116.5 mM NaCl, 2 mM KCl, 2 mM NaH2PO4, 10 mM EGTA, at pH 7.3) supplied with 2 mM caffeine. Then, 100 μM BAPTA-AM, solubilized in DMSO and then in Ringer-EGTA (DMSO final concentration was 1%), was incubated for 30 min followed by washout with Ringer-EGTA solution for an additional 20 min. Rmin was determined as the smallest R value observed (0.30 ± 0.04; n = 4; means ± SE). For Rmax, another group of fibers were exposed to a high-calcium Ringer-MG solution (116.5 mM N-methyl-d-glucamine, 2 mM KCl, 20 mM CaCl2, 0.1 mM EGTA, pH 7.0) for 120 min followed by a 2 mM 4-chloro-m-cresol to obtain Rmax (6.97 ± 0.56; n = 4; means ± SE). β was determined (4.05 ± 0.36; n = 6 fibers, means ± SE) as described previously (3, 4). The contraction-induced fluorescence ratio was calculated by averaging the fluorescent signal in the final 100 ms of stimulation and was then used to determine peak [Ca2+]c. The intracellular cytosolic Ca2+ before each contraction (i.e., basal [Ca2+]c) was calculated by averaging the signal in the 100 ms before each stimulation.
When the pHi changes were monitored during contractions, fibers were illuminated at 440 nm and 490 nm, and the resulting fluorescence emissions at 535 nm were divided (490 nm/440 nm) to obtain the pH-dependent signal. An in vivo calibration was performed in a different group of fibers (n = 5), according to Westerblad and Allen (32). Fibers were injected with BCECF, mounted in the experimental chamber, and incubated for 20 min in each of two different pH buffered solutions (175 mM KCl, 1.2 mM KH2PO4, 0.5 mM MgCl2, 10 mM HEPES, 10 μM nigericin), previously titrated with KOH to pH 5.0 and 9.0, as the fluorescence signal was detected. Fluorescence excitation ratio (440/490 nm; F) was then converted to pHi according to Eq. 2.
| (2) |
From Eq. 2, pKA, the logarithmic dissociation constant, was set to 7.15 (32). Sb and Sa are the fluorescence signals from 440 nm excitation at pH 9.0 and pH 5.0, respectively, and the fluorescence ratio of Sb/Sa was determined as 0.73 ± 0.06 (n = 5 fibers). Fa and Fb are the fluorescence ratios at low (acid) and high (basic) pH, respectively, and were determined as 0.59 ± 0.01 for Fa and 1.82 ± 0.12 for Fb (n = 5 fibers; means ± SE).
Determination of [Ca2+]c pumping rate by the sarcoplasmic reticulum.
High-affinity Ca2+ probes (e.g., Fura-2 and Indo-1) have been used to investigate the kinetics of Ca2+ uptake by the SR during contractions (36). To explore the effects of fatigue development in the presence and absence of cross-bridge cycling on Ca2+ uptake kinetics, the poststimulation period (i.e., relaxation) of selected contractions was used to determine either the fast-phase rate of Ca2+ uptake and the function of SR Ca2+ pumping. Different time points of the contractile periods, such as the first contraction, contractions at 60, 120, 240, 360, 480, and 600 s, as well as the last contraction, were selected, and [Ca2+]c kinetics were determined as follows.
To determine the fast-phase rate constant of [Ca2+]c decay (λ1), the poststimulation [Ca2+]c signal was fitted using a double-exponential equation, as shown in 3.
| (3) |
From Eq. 3, A and B represent that fraction of [Ca2+]c decay for each exponential phase, λ1 and λ2 represent the rate of Ca2+ decay for the fast and slow phases, respectively, and t is the time of Ca2+ decay.
To measure the function of SR Ca2+ pumping during the contractile bouts, we used a method described by Klein et al. (17), which was used in several reports by the group of Dr. Westerblad (34, 35). This method assumes that the elevated long tail of intracellular [Ca2+]c decline (from 200 to 2.5 s after the stimulation period), represents a state which Ca2+ pumping by SERCA is proportional to the rate of [Ca2+]c decline (d[Ca2+]c/dt). Therefore, SERCA Ca2+ pumping curve was obtained by plotting -d[Ca2+]c/dt during the tail of [Ca2+]c decay vs. [Ca2+]c (Eq. 4).
| (4) |
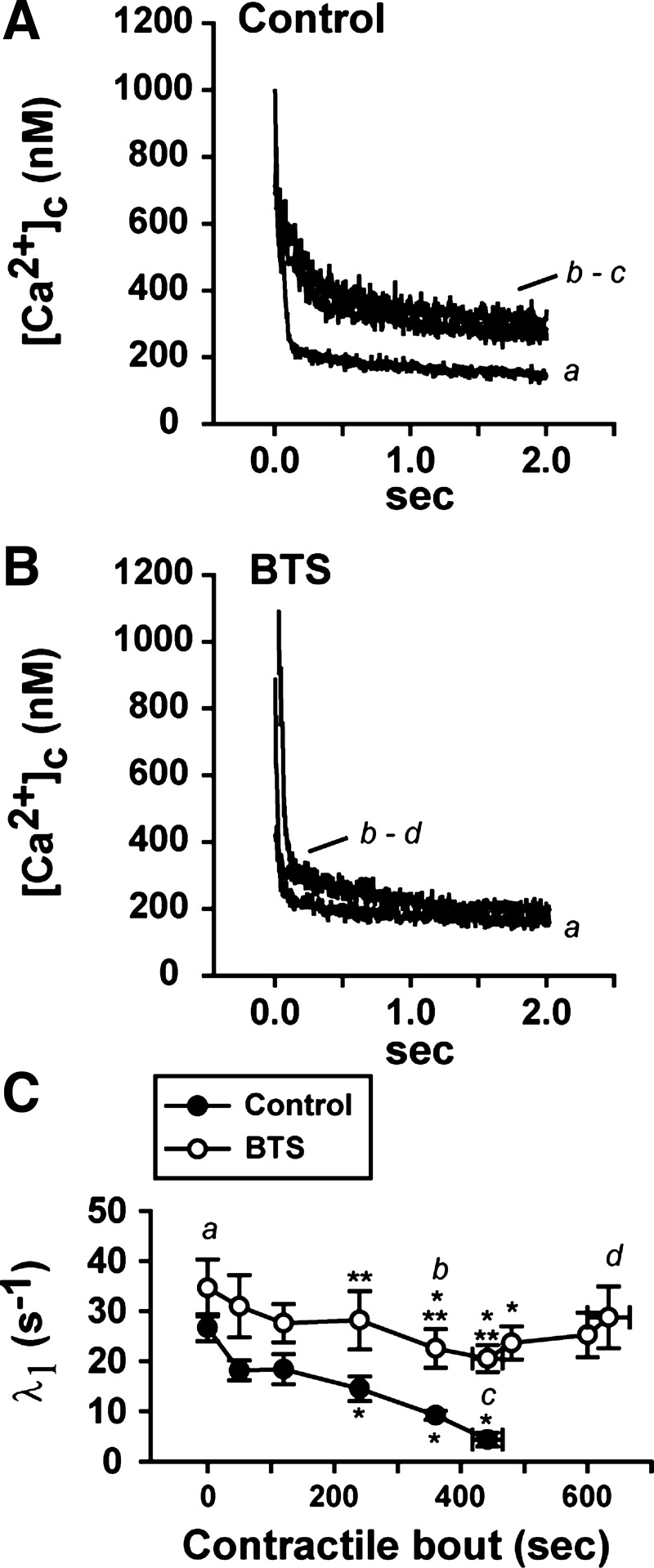
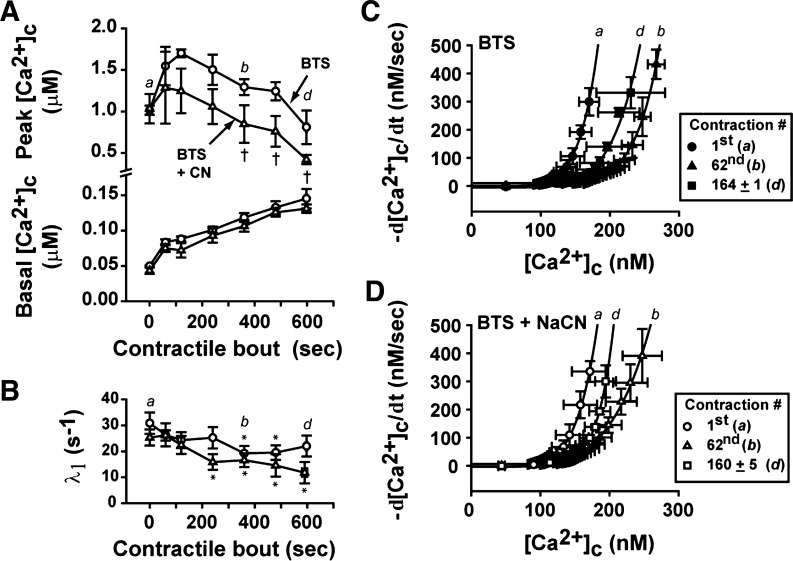
From Eq. 4, -d[Ca2+]c/dt is the rate of intracellular [Ca2+]c decline, and A, N, and L are three adjustable parameters representing the rate of SR Ca2+ pumping (in μMN−1/s), the power function, and the SR Ca2+ leak, respectively. To compare directly the SR Ca2+ pumping function (in μM−3/s) between contractions [first contraction: 0.15–2.5 s; 62nd contraction: 0.35–2.5 s; fatigue time point in the control (442 ± 23 s): 0.5–2.5 s; or last contraction with BTS: 0.2–2.5 s], the curves were fitted setting N = 4 and L = 5 nM/s, with a maximum increase in least-square error of 15% (34). The prolonged tail of [Ca2+]c decay after the stimulation period is dependent on the time course of the fast phase of [Ca2+]c decay (λ1), which was determined by Eq. 3. The λ1 was slowed during the time course of the contractile bout under control conditions, as shown in Fig. 3C; therefore, the beginning of the slow phase of [Ca2+]c decay was delayed between the first, the 62nd, and the fatigue time point. Moreover, the fast phase of [Ca2+]c decay with BTS present was not significantly slowed (Fig. 3C), so the beginning of the tail of [Ca2+]c decay was not modified from the first contraction.
Fig. 3.
Kinetics of the initial fast-phase rate of [Ca2+]c decay (λ1) at different time points of a repetitive contractile bout in the presence and absence of BTS. A and B: trace recordings of [Ca2+]c decay after stimulation period (starting at 0 s) at different time points during the contractile bouts in the absence (control; A) and the presence of BTS (BTS; B). In A, the first contraction is shown (a), then the 62nd contraction (360 s; b), and the fatigue time point (c). In B, the first contraction is shown (a), then the 62nd contraction (360 s; b), and the last contraction (d). C: fast-phase rate of [Ca2+]c decay (λ1) in the absence (●) and the presence of BTS (○). n = 5 fibers. *P < 0.05 vs. initial contraction, one-way ANOVA, repeated measures; **P < 0.05 control vs. BTS, two-way repeated-measures ANOVA. Values are expressed as means ± SE. The time points shown in A and B are indicated in C as a, b, c, and d.
Experimental protocol.
Following the fiber injection and subsequent rest period, platinum clips were attached to the tendons, and each fiber was mounted in a single muscle strip myograph system [model 920CS, Danish Myo Technology (DMT), Atlanta, GA] and was placed on the stage of the inverted microscope. After mounting, the fiber length that promotes the maximal isometric tension (L0) was determined by stretching the fiber until maximal tetanic tension (70 Hz) was developed. After setting L0, the fiber was allowed to rest for 30 min. Tetanic contractions (70 Hz, 250-ms train duration, 2-ms monophasic pulses, 8 V) were evoked by direct stimulation, using a Grass S48 stimulator (Quincy, MA). Tension development was measured with a force transducer system (model KG4, 0–50 mN, DMT, Atlanta, GA) that was previously calibrated with weights (20 mg to 1 g) and then converted to millinewtons. A Biopac Systems MP100WSW (Santa Barbara, CA) A-D converter was used to convert the analog to digital data, and AcqKnowledgeIII 3.2.6 software (Biopac Systems) was used to analyze the data. Tension development (in millinewtons) was normalized to the cross-sectional area (in millimeters squared) determined from the diameter of the fiber (in micrometers).
Fatigue-inducing contraction bouts were utilized in the present study in the presence or absence of 12.5 μM BTS. To avoid an order effect, the fibers performed two consecutive contractile periods with and without BTS in random order separated by 60 min of recovery. It has been previously shown that this rest period allows complete recovery of the myofiber and should not interfere with the time to fatigue on a second contractile bout (24). When BTS was present during the fatigue run, maximal tension (70 Hz) was previously tested to ensure the same tension development between contractile periods, and fibers were preincubated with BTS for 10 min, which produced a strong inhibition of peak isometric tension developed at 70-Hz stimulation (∼95%) (30). The effect of BTS on fiber contractility was transient, and when this compound was washed out, the normal contractile characteristics of the fiber were restored after ∼10 min. The fatigue-inducing contraction bout consisted of a series of repeated contractions with decreases in the rest period between contraction, using increments in train frequency (one contraction each at 8, 6, 4, 3, 2 and 1 s) for 2 min each. This protocol was performed only with fast-fatiguing fibers [time to reach the fatigue time point in control conditions of less than 600 s (25)]. The bout was terminated (i.e., fatigue time point) when the initial isometric tension was reduced by 50% (control condition; when BTS was absent) or the initial Fura-2 fluorescence ratio decreased to ∼50–60% of the initial (when BTS was present).
In a separate group of fibers, after the first contractile period in the presence of BTS, a second contractile bout was carried out in the presence of BTS plus 2 mM NaCN to inhibit mitochondrial respiration and, thereby, aerobic ATP supply. In these cases, the fibers were exposed to NaCN for 2 min before the beginning of the work period and during the whole contractile period. These contractile bouts were followed by extensively washing out BTS and CN with normal Ringer solution, and a third contractile bout (control) was performed to ensure fiber viability starting 60 min after the second contractile bout.
Data analysis.
The experimental results are presented as means ± SE. For comparisons between multiple groups, one-way ANOVA followed by the Tukey test or two-way ANOVA was used, as indicated. Analyses were carried out using PRISM 4 software and P < 0.05 was considered to represent significant difference.
RESULTS
Steady-state isometric tension and [Ca2+]c responses.
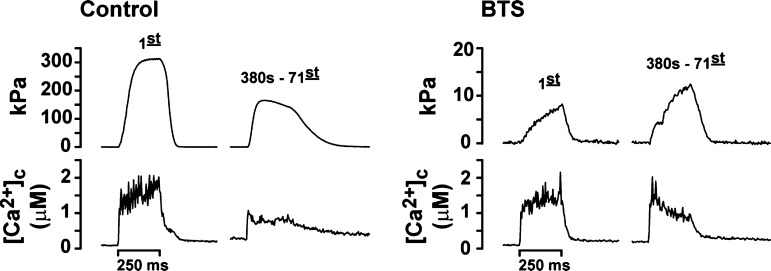
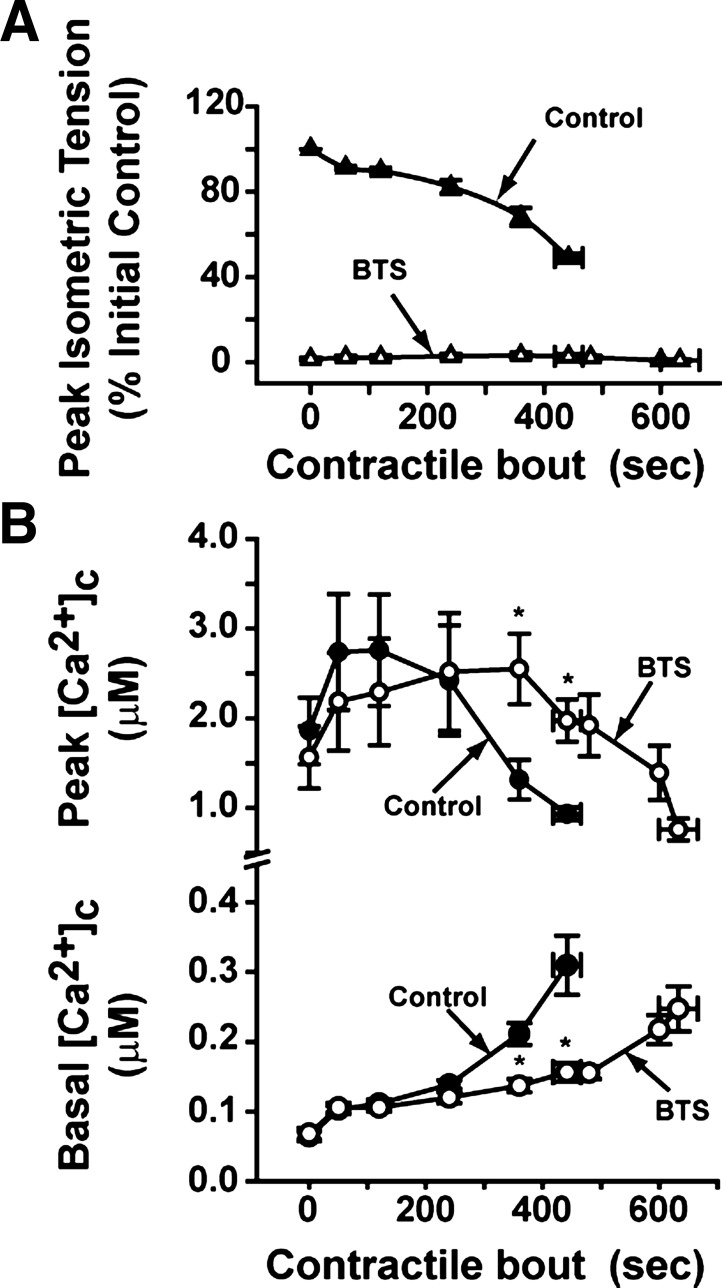
As illustrated in Fig. 1, incubating fibers with BTS (12.5 μM) inhibited tension development (70-Hz stimulation, which produces maximal tetanic tension development), by ∼95%, while cytosolic calcium ([Ca2+]c) transients were not affected at the beginning of the contractile period. When tension development and [Ca2+]c were analyzed during the contractile bouts under control conditions (no BTS), time to fatigue was 442 ± 23 s (Fig. 2A). Also, the changes in tension were accompanied by fall in peak [Ca2+]c and calcium accumulated in the cytosol (i.e., increased basal [Ca2+]c; Fig. 2B, closed symbols). However, at the same time points that fibers developed fatigue under control conditions, basal [Ca2+]c in the BTS treatment was less (47 ± 5%, Fig. 2B; P < 0.05), and peak [Ca2+]c was higher (120 ± 40%, Fig. 2B; P < 0.05). At 630 ± 33 s of contractions during the BTS treatment, both Ca2+ accumulation and fall in peak [Ca2+]c reached similar values compared with the control fatigue time point (Fig. 2B).
Fig. 1.
Representative trace recordings for isometric tension (top) and [Ca2+]c (bottom) from an intact fiber evoked by a 70-Hz stimulation. Note scale differences. The first contraction and the contraction after 380 s of repetitive stimulations are shown before (control) and after benzyl-p-toluene sulfonamide treatment (BTS). For this particular fiber, after 380 s, tension had decreased 50% from the initial contraction under control conditions. Bar indicates the period of electrical stimulation.
Fig. 2.
Intracellular Ca2+ transient responses during the repetitive contraction protocol in the absence of cross-bridge cycling. Peak isometric tension development at 70 Hz (A), basal [Ca2+]c, and peak [Ca2+]c responses (B) in the absence (closed symbols) and presence of BTS (open symbols); n = 5 fibers. *P < 0.05 BTS vs. control, two-way repeated-measures ANOVA; Values are expressed as means ± SE.
Ca2+ uptake kinetics and SR pumping function with and without cross-bridge cycling.
Figure 3 illustrates the time course of [Ca2+]c decay after the end of the stimulation period for selected contractions and at the fatigue time point, for control conditions (Fig. 3A), or at the last contractions, for BTS conditions (Fig. 3B).
The [Ca2+]c decay period (from 0 to 2.5 s after the end of stimulation) was fitted by using a double-exponential equation (see methods). This was performed to determine the fast-phase of intracellular Ca2+ decay (λ1), which is a sum of several factors, such as Ca2+ uptake rate by SERCA, Ca2+ dissociation off-kinetics from troponin C, and Ca2+ loading rate by parvalbumin (35). At control, λ1 was gradually slowed up to the fatigue time point, as demonstrated by the gradual decrease in λ1 during the contractile bout (83 ± 6% decrease at the fatigue time point, Fig. 3C; P < 0.05). In the presence of BTS, the highest decrease in λ1 was obtained by 480 s of contractions (30 ± 6% decrease, Fig. 3C; P < 0.05). However, the changes in λ1 in the BTS conditions were significantly smaller compared with the control conditions starting at 240 s of contractions (P < 0.05; Fig. 3C).
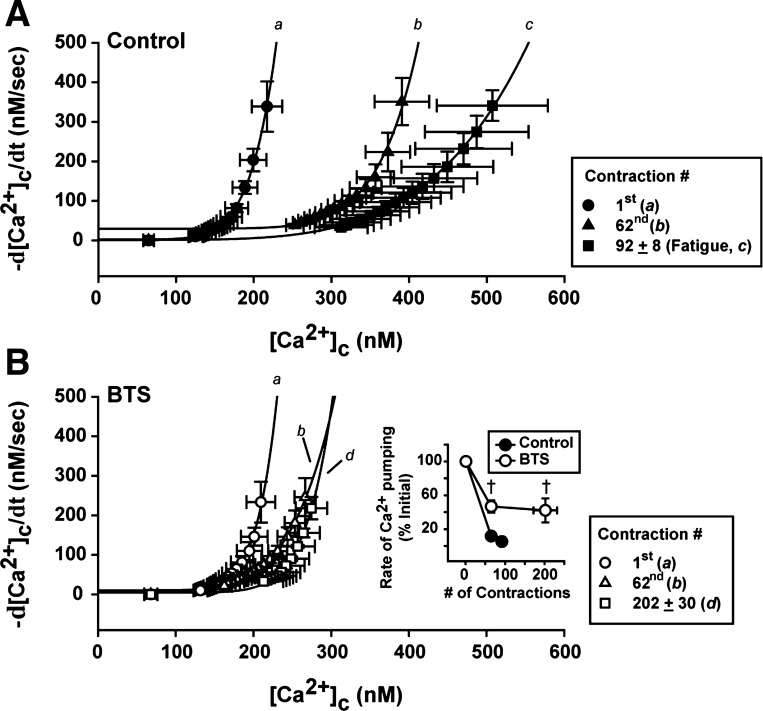
To follow the changes in SR Ca2+ pumping function during the contractile period in the presence and absence of BTS, the tail of elevated [Ca2+]c after the stimulation period was used. The tail [Ca2+]c measurements were fitted to a double-exponential function, and this function was used to generate the rate of [Ca2+]c decay during the tail (−d[Ca2+]c/dt; see methods for details). The plots of the mean data for tail [Ca2+]c with the mean data for rate of [Ca2+]c decay are shown in Fig. 4A (for control condition) and in Fig. 4B (for BTS condition).
Fig. 4.
Analysis of SR Ca2+ pumping at different time points of the contractile bout in presence (control; A) and absence of cross-bridge cycling (BTS; B). The tail of [Ca2+]c decay trace recordings (as shown in Fig. 3, A and B) were fitted with a sum of two exponentials, plotted against the rate of [Ca2+]c decay (−d[Ca2+]c/dt) at the same periods of time, and the SR Ca2+ pumping rate was determined from Eq. 4, assuming the power function (N) and the SR Ca2+ leak (L) as 4 and 5, respectively (as described in methods). Mean data from five individual fibers obtained from the first contraction (a), the 62nd contraction (b), and at the fatigue time-point (c; control), or at the last contraction with BTS (d). The curves from the mean data were fitted with Eq. 4, but N and L were not fixed. Inset: individual rates of SR Ca2+ pumping were normalized by the first contraction and compared between control and BTS conditions. †P < 0.05 control vs. BTS, two-way repeated-measures ANOVA; n = 5 fibers. Values are expressed as means ± SE.
At the control conditions, the rate of SR Ca2+ pumping (the parameter A in Eq. 4) was significantly reduced during the time course of the fatiguing contractile bout (in μM−3/s: 135 ± 42, 13 ± 3, and 7 ± 3, for first contraction, 62nd contraction, and at the fatigue time point, respectively, P < 0.01 vs. first contraction, one-way repeated-measures ANOVA). With BTS present, there was also a decrease in slowing in the rate of SR Ca2+ pumping (in μM−3/s: 114 ± 30, 48 ± 8, and 38 ± 13, for first contraction, 62nd contraction, and last contraction, 202 ± 30 contractions, respectively, P < 0.01 vs. first contraction, one-way repeated-measures ANOVA). However, comparing the changes in rate of SR Ca2+ pumping between the two conditions, the presence of BTS significantly attenuated the slowing in the rate of SR Ca2+ pumping (∼40% smaller slowing, Fig. 4B, inset; P < 0.05, vs. control, two-way repeated-measures ANOVA).
Ca2+ handling and decay kinetics in the presence of NaCN.
To evaluate whether the delayed changes in [Ca2+]c when BTS was present were due to smaller accumulation of anaerobic metabolites, through a sufficient contribution of aerobic ATP supply when cross-bridge cycling was absent, mitochondrial respiration was inhibited by 2 mM NaCN (Fig. 5A). Peak [Ca2+]c fell faster when NaCN + BTS were present compared with the BTS-alone condition (P < 0.05; Fig. 5A). However, basal [Ca2+]c was not significantly modified when NaCN + BTS were present compared with BTS alone.
Fig. 5.
[Ca2+]c transients, [Ca2+]c decay kinetics, and SR Ca2+ pumping rate during contractions when mitochondrial respiration was blocked with NaCN. A: basal and peak [Ca2+]c responses in the presence of BTS (○) and in the presence of BTS + NaCN (△). †P < 0.05 BTS + NaCN vs. BTS, two-way repeated-measures ANOVA. B: fast-phase rate of [Ca2+]c decay (λ1) in the presence of BTS (○) or BTS + NaCN (△). *P < 0.05 vs. initial contraction, one-way repeated-measures ANOVA. C: mean data of [Ca2+]c dependence of the rate of [Ca2+]c decline at different time points of the contractile bout in the presence of BTS (closed symbols) or BTS + NaCN (open symbols). n = 4 fibers. Values are expressed as means ± SE. Time points selected were the same as in Fig. 4 (indicated as a, b, and d). Rate of SR Ca2+ pumping was determined as described in methods and in Fig. 4. The curves from the mean data were fitted with Eq. 4, but N and L were not fixed.
The differences in the fast-phase [Ca2+]c decay rate (λ1) with BTS during the contractile bout were also analyzed in the presence of NaCN + BTS. Figure 5B shows that λ1 was progressively slowed when NaCN + BTS were present (Fig. 5B; P < 0.05 vs. initial contraction).
SR Ca2+ pumping function was also analyzed during the contractile periods in presence of BTS or BTS + NaCN (Fig. 5, C and D, respectively). As occurred in the first group of fibers with BTS (Fig. 4B), the SR Ca2+ pumping was slowed in the beginning of the contractile period (i.e., 62nd contraction), but no further slowing was detected in the SR Ca2+ pumping in the following contractions (Fig. 5C). Interestingly with BTS + NaCN, there was also no additional slowing in SR Ca2+ pumping after the 62nd contraction, compared with the BTS alone condition (Fig. 5D).
pHi changes.
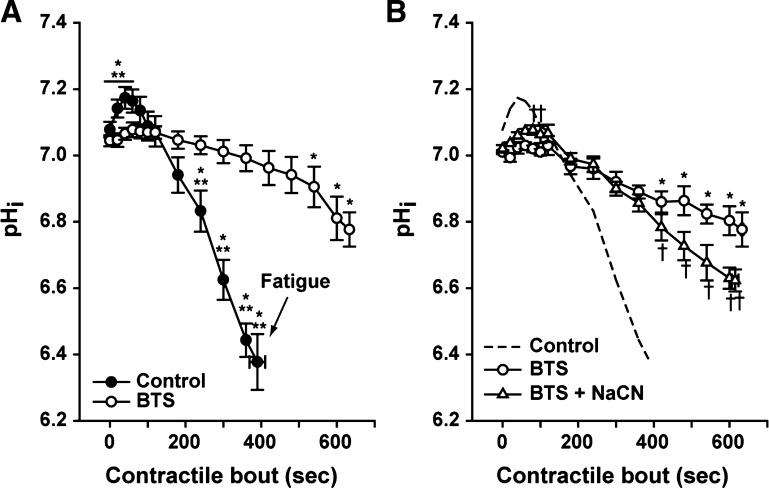
The changes in pHi were monitored during the contractile periods. As shown in Fig. 6, all control fibers showed the expected immediate alkalosis and subsequent acidosis induced by the work period (31). At the fatigue time point (390 ± 21 s), pHi was significantly decreased to 6.37 ± 0.08 (Fig. 6A; P < 0.05). In the presence of BTS, no significant alkalosis was detected, and the pHi was only statistically smaller from resting values by 540 s of contractions (P < 0.05). At the end of the contractile period with BTS (at 633 ± 3 s), pHi was significantly decreased to 6.78 ± 0.05 compared with resting values.
Fig. 6.
Intracellular pH (pHi) changes during repetitive contractions. A: pHi was measured during the work periods in the presence (○) and the absence of BTS (●). *P < 0.05 vs. initial contraction, one-way ANOVA, repeated measures. **P < 0.05 BTS vs. control, two-way repeated-measures ANOVA; n = 5 fibers. B: pHi responses in the presence of BTS (○) and BTS + NaCN (△). *P <0.05 vs. initial contraction, one-way repeated-measures ANOVA. †P < 0.05 BTS vs. BTS + NaCN, two-way repeated-measures ANOVA; n = 4 fibers. Dashed line represents the mean data of pHi measurements under control conditions (no BTS and no NaCN) shown in A. Values are expressed as means ± SE.
To investigate whether the Ca2+ handling work during contractions was supplied by mitochondrial ATP turnover, mitochondrial respiration was blocked with NaCN. When both BTS + NaCN were present during the contractile protocol, there was a significant early alkalosis (P < 0.05), compared with the BTS condition alone (Fig. 6B). The presence of BTS + NaCN during contractions produced a significant reduction in pHi compared with BTS alone by 420 s (Fig. 3B; P < 0.05) but significantly smaller than the control conditions (e.g., pHi was decreased to 6.63 ± 0.03 after 600 s with BTS + NaCN compared with 6.38 ± 0.08 after 390 s under control, open triangles vs. dashed line, respectively, P < 0.05).
DISCUSSION
Our results demonstrate that inhibition of cross-bridge cycling during repetitive contractions results in enhanced maintenance of the SR Ca2+ pump function, possibly due to a reduced intracellular accumulation of H+. Specifically, BTS administration in contracting myocytes: 1) inhibited the contraction-induced slowing in both fast-phase [Ca2+]c decay kinetics and SR Ca2+ pumping function (Figs. 3 and 4); and 2) abolished the initial intracellular alkalosis and diminished the delayed intracellular acidification (Fig. 6).
Intracellular Ca2+ responses during contractions.
The peak [Ca2+]c transient and basal [Ca2+]c during contractions are determined by the interplay of SR Ca2+ release and uptake and leak (2). At the contractile fatigue time point, the decrease in SR Ca2+ release has been shown to play a large role in decreasing peak [Ca2+]c (1) and may be impaired by the accumulation of some metabolites [e.g., free Mg2+, ADP, IMP, ROS, Pi; (1, 20–22)]. The production rate of these metabolites is controlled by the energy requirements of the contractions, as well as the ability to supply ATP aerobically (20). In our current experiments, peak and basal [Ca2+]c was 2.1 ± 0.3 × higher and 34 ± 5% smaller during BTS treatment, respectively, compared with control at 360 s of the contractile period (Fig. 2A). These changes have been reported in intact single mouse fibers (8) and were correlated to a diminished metabolite accumulation as a consequence of a smaller metabolic demand during contractions (8). In the present study, the basal [Ca2+]c in the last contraction with BTS was very similar to the basal [Ca2+]c obtained at the fatigue time point in the control condition. This result is different from the results obtained in single mouse fibers (8); however, single mouse fibers do not develop significant acidosis during repetitive contractions (32), and experimental protocols between the two studies were quite different (8). Although we cannot speculate which metabolites were diminished during the BTS condition, the data obtained from the [Ca2+]c decay periods (discussed below in details) suggest that accumulation of free Mg2+ and ADP were likely reduced in the cytosol when the metabolic demand was diminished because of BTS. However, the intracellular accumulation of Pi would produce Ca-P precipitates within the SR, decreasing SR Ca2+ release and increasing SR Ca2+ leak (28). Therefore, it is reasonable to suggest that the decrease in energy cost of contractions with BTS would delay intracellular Pi accumulation, thereby delaying the changes in [Ca2+]c with contractions.
Ca2+ pump function in intact single fibers.
It has been previously shown by our group and by others that blocking cross-bridge cycling during contractions produces a ∼60% decrease in total energy utilization, thereby implying that almost 40% of the total energy cost of contractions is due to SERCA Ca2+ pumping (5, 6, 22, 30). Although BTS inhibits actin-myosin interaction (23), it did not affect SERCA Ca2+ pumping function in the first contraction (see Fig. 4). Thus, this makes BTS treatment an excellent tool to study the kinetics of either SERCA or SR Ca2+ uptake function, particularly during fatigue development. During fatigue, the slowing in the relaxation period has been associated with either a reduction in cross-bridge dissociation rate or a decrease in SR Ca2+ pumping rate or both (for review, see Refs. 2 and 28). The present study evaluated the [Ca2+]c decay rate without the confounding factors involved in cross-bridge cycling, such as the actin-myosin dissociation and the cross-bridge ATP demand. We demonstrated in the current study that the absence of cross-bridge cycling during contractions blunted the slowing in [Ca2+]c decay (during the relaxation cycle of a contraction) detected in the control condition. Although this phenomenon has been reported in intact single mouse fibers (8), it has not been examined in a systematic fashion.
The initial [Ca2+]c decay rate (λ1) accounts for ∼90% and ∼20% of the relaxation time period during the unfatigued state and at the fatigue time point, respectively. Any changes in λ1 have significant implications for the overall muscle relaxation. The λ1 is strongly regulated by the parvalbumin-Mg2+ off-rate, and the increase in [Mg2+]free slows this phase (15). BTS did not modify λ1 during the unfatigued state, which suggests that parvalbumin-cation binding properties were not affected. During a repetitive contractile bout, λ1 was progressively reduced under control conditions, but not with BTS present. Although [Mg2+]free was not measured, the slowing in λ1 when mitochondrial respiration and cross-bridge cycling were blocked together, suggests that [Mg2+]free was either smaller or not increased in the absence of cross-bridge cycling due to sufficient ATP generation by mitochondrial respiration. Therefore, actomyosin ATP cost during repetitive contractions is fundamental for slowing the initial phase of relaxation.
The SR Ca2+ pumping function was measured during the prolonged elevated tail of [Ca2+]c decay after the stimulation period (35). As expected, contractions after the onset of fatigue produced a progressive slowing in the SR Ca2+ pumping rate during control conditions, which was ∼40% less slowed by the last contraction with BTS (Fig. 4). Interestingly, SR Ca2+ pumping during contractions when BTS + NaCN were present was similarly slowed compared with BTS alone (Fig. 5, C and D).
The data from the present investigation show that the primary condition in which SERCA function was significantly inhibited during contractions was when intracellular acidification and cross-bridge cycling were present. There is a large body of literature, particularly performed in mechanically skinned single fibers (18, 28), on the negative effects of metabolite accumulation (e.g., high [ADP], [H+], [Pi], and [Mg2+]free) on SERCA ATPase activity, SR Ca2+ leak, and SR Ca2+ uptake rate. Our data suggest that the ATP breakdown of cross-bridge cycling (together with the ATP cost of SR Ca2+ uptake) plays an important role in regulating intracellular metabolite accumulation, particularly H+, and these factors may ultimately modulate SERCA function during contractions.
The inhibition of SERCA function may also be due to nonmetabolic effects of fatigue. Several reports have shown, by using in vitro isolated SR vesicles and muscle homogenates to measure SERCA function under “nonfatiguing” conditions, that SR Ca2+ uptake rate and SERCA ATPase activity are persistently diminished (for hours) after fatiguing exercise (12, 29), suggesting potential protein structural changes in SERCA. However, the data from the present investigation show that SERCA function was restored rapidly between contractile periods (1-h rest periods), thereby suggesting that it was likely that the changes in SERCA function were due to the acute metabolic perturbations occurring with contractions, which were diminished in the absence of cross-bridge cycling.
Changes in intracellular H+ during repetitive contractions.
Intact myofibers, as whole muscle, develop marked intracellular pH (pHi) changes during repetitive fatiguing contractions (31). Alkalosis occurs early during contractions and is then followed by acidosis (31), being driven by creatine kinase (CK) activity (H+ buffering) and activation of anaerobic glycolysis, respectively (31). The changes in pHi detected in myofibers are the result of an equilibrium between H+ production by ATP hydrolysis and anaerobic glycolysis, intracellular H+ buffering, and the H+ extrusion from the cell (32).
The absence of cross-bridge cycling blocked the early alkalosis that occurred during the first 60 s of contractions in control conditions (Fig. 6A), indicating that the CK activity was reduced. These results suggest that the SERCA Ca2+-handling ATP requirements were not high enough to fully activate CK in the beginning of the contractile period. Reducing the energy cost of contractions by blocking cross-bridge cycling produced a smaller contraction-induced acidosis (Fig. 6A), possibly because of a smaller activation of anaerobic glycolysis (22). Comparing the changes in pHi (Fig. 6A) with SERCA function (Fig. 4), SR Ca2+ pumping rate was significantly reduced after 360 s of contractions (62nd contraction), which was associated with ∼0.7 units of pHi acidification. It is important to note that the acidification detected in the control condition (i.e., ∼pH 6.4–6.3) has been reported to inhibit SERCA activity in vitro (26, 38) and in vivo (34), as well as decreased relaxation rate in mammalian single fibers at low (i.e., 22°C) and high (i.e., 32°C) temperatures (37). As discussed above, the SR Ca2+ pumping rate was significantly less inhibited with BTS compared with the control, and pHi was not changed. Furthermore, at the end of the contractile protocol with BTS (633 ± 3 s), there was a small, but statistically significant, acidification (∼0.26 units of pH), yet the SR Ca2+ pumping rate was not changed further. Interestingly, when NaCN + BTS were present, contraction-induced acidosis was significantly increased compared with BTS alone (∼0.4 units of pH decrease), but the SR Ca2+ pumping rate was not slowed compared with BTS alone. This suggests that there is possibly a threshold of intracellular acidification that produces SERCA Ca2+ pumping dysfunction, and in the current study, this was not achieved when cross-bridge cycling was inhibited. Therefore, reducing the energy cost of contractions by ∼60% avoided the contraction-induced acidification that is required to inhibit SERCA.
Perspectives and Significance
The results of this study demonstrate that the ATP cost of cross-bridge cycling during contractions is an important determinant of metabolite accumulation, particularly H+ in the cytosol and possibly Pi within the SR, during repetitive contractions and that these regulate peak [Ca2+]c responses and SR Ca2+ pumping function. These changes will ultimately inhibit SERCA function during contractions, thereby slowing the relaxation period. These results provide significant information concerning the relationship between the energy cost of cross-bridge cycling and SR Ca2+ uptake with intracellular metabolite accumulation during repetitive contractions, as well as correlate the energy cost of contractions with the changes in SERCA function in skeletal muscle during fatigue development.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.N. and M.C.H. conception and design of research; L.N. and A.A.S. performed experiments; L.N. analyzed data; L.N., P.G.G., and M.C.H. interpreted results of experiments; L.N. prepared figures; L.N. and M.C.H. drafted manuscript; L.N., A.A.S., P.G.G., and M.C.H. edited and revised manuscript; L.N., A.A.S., P.G.G., and M.C.H. approved final version of manuscript.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health Grant AR-40155. A.A.S. was a recipient of the American Physiological Society's Undergraduate Summer Research Fellowship Program.
REFERENCES
- 1. Allen DG, Lamb GD, Westerblad H. Impaired calcium release during fatigue. J Appl Physiol 104: 296–305, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509: 565–575, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bakker AJ, Head SI, Williams DA, Stephenson DG. Ca2+ levels in myotubes grown from the skeletal muscle of dystrophic (mdx) and normal mice. J Physiol 460: 1–13, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barclay CJ, Lichtwark GA, Curtin NA. The energetic cost of activation in mouse fast-twitch muscle is the same whether measured using reduced filament overlap or N-benzyl-p-toluenesulphonamide. Acta Physiol (Oxf) 193: 381–391, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Barclay CJ, Woledge RC, Curtin NA. Energy turnover for Ca2+ cycling in skeletal muscle. J Muscle Res Cell Motil 28: 259–274, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Brault JJ, Pizzimenti NM, Dentel JN, Wiseman RW. Selective inhibition of ATPase activity during contraction alters the activation of p38 MAP kinase isoforms in skeletal muscle. J Cell Biochem 114: 1445–1455, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruton J, Pinniger GJ, Lannergren J, Westerblad H. The effects of the myosin-II inhibitor N-benzyl-p-toluene sulphonamide on fatigue in mouse single intact toe muscle fibres. Acta Physiol (Oxf) 186: 59–66, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Cheung A, Dantzig JA, Hollingworth S, Baylor SM, Goldman YE, Mitchison TJ, Straight AF. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol 4: 83–88, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev 74: 49–94, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Goonasekera SA, Lam CK, Millay DP, Sargent MA, Hajjar RJ, Kranias EG, Molkentin JD. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J Clin Invest 121: 1044–1052, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Green HJ. Cation pumps in skeletal muscle: potential role in muscle fatigue. Acta Physiol Scand 162: 201–213, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Green HJ, Burnett M, Duhamel TA, D'Arsigny C, O'Donnell DE, Webb KA, Ouyang J. Abnormal sarcoplasmic reticulum Ca2+-sequestering properties in skeletal muscle in chronic obstructive pulmonary disease. Am J Physiol Cell Physiol 295: C350–C357, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Hogan MC, Ingham E, Kurdak SS. Contraction duration affects metabolic energy cost and fatigue in skeletal muscle. Am J Physiol Endocrinol Metab 274: E397–E402, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Hou TT, Johnson JD, Rall JA. Effect of temperature on relaxation rate and Ca2+, Mg2+ dissociation rates from parvalbumin of frog muscle fibres. J Physiol 449: 399–410, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kabbara AA, Allen DG. Measurement of sarcoplasmic reticulum Ca2+ content in intact amphibian skeletal muscle fibres with 4-chloro-m-cresol. Cell Calcium 25: 227–235, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Klein MG, Kovacs L, Simon BJ, Schneider MF. Decline of myoplasmic Ca2+, recovery of calcium release and sarcoplasmic Ca2+ pump properties in frog skeletal muscle. J Physiol 441: 639–671, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lamb GD. Excitation-contraction coupling and fatigue mechanisms in skeletal muscle: studies with mechanically skinned fibres. J Muscle Res Cell Motil 23: 81–91, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Middlekauff HR, Vigna C, Verity MA, Fonarow GC, Horwich TB, Hamilton MA, Shieh P, Tupling AR. Abnormalities of calcium handling proteins in skeletal muscle mirror those of the heart in humans with heart failure: a shared mechanism? J Card Fail 18: 724–733, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagesser AS, Van der Laarse WJ, Elzinga G. ATP formation and ATP hydrolysis during fatiguing, intermittent stimulation of different types of single muscle fibres from Xenopus laevis. J Muscle Res Cell Motil 14: 608–618, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Nagesser AS, van der Laarse WJ, Elzinga G. Metabolic changes with fatigue in different types of single muscle fibres of Xenopus laevis. J Physiol 448: 511–523, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ortenblad N, Macdonald WA, Sahlin K. Glycolysis in contracting rat skeletal muscle is controlled by factors related to energy state. Biochem J 420: 161–168, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Shaw MA, Ostap EM, Goldman YE. Mechanism of inhibition of skeletal muscle actomyosin by N-benzyl-p-toluenesulfonamide. Biochemistry 42: 6128–6135, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Stary CM, Hogan MC. Intracellular pH during sequential, fatiguing contractile periods in isolated single Xenopus skeletal muscle fibers. J Appl Physiol 99: 308–312, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Stary CM, Mathieu-Costello O, Hogan MC. Resistance to fatigue of individual Xenopus single skeletal muscle fibres is correlated with mitochondrial volume density. Exp Physiol 89: 617–621, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Stienen GJ, Papp Z, Zaremba R. Influence of inorganic phosphate and pH on sarcoplasmic reticular ATPase in skinned muscle fibres of Xenopus laevis. J Physiol 518: 735–744, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swaminathan R, Chan EL, Sin LY, King NS, Fun NS, Chan AY. The effect of ouabain on metabolic rate in guinea-pigs: estimation of energy cost of sodium pump activity. Br J Nutr 61: 467–473, 1989 [DOI] [PubMed] [Google Scholar]
- 28. Tupling AR. The sarcoplasmic reticulum in muscle fatigue and disease: role of the sarco(endo)plasmic reticulum Ca2+-ATPase. Can J Appl Physiol 29: 308–329, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Tupling R, Green H, Grant S, Burnett M, Ranney D. Postcontractile force depression in humans is associated with an impairment in SR Ca2+ pump function. Am J Physiol Regul Integr Comp Physiol 278: R87–R94, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Walsh B, Howlett RA, Stary CM, Kindig CA, Hogan MC. Measurement of activation energy and oxidative phosphorylation onset kinetics in isolated muscle fibers in the absence of cross-bridge cycling. Am J Physiol Regul Integr Comp Physiol 290: R1707–R1713, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Walsh B, Stary CM, Howlett RA, Kelley KM, Hogan MC. Glycolytic activation at the onset of contractions in isolated Xenopus laevis single myofibres. Exp Physiol 93: 1076–1084, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Westerblad H, Allen DG. Changes of intracellular pH due to repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol 449: 49–71, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Westerblad H, Allen DG. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol 98: 615–635, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Westerblad H, Allen DG. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J Physiol 466: 611–628, 1993 [PMC free article] [PubMed] [Google Scholar]
- 35. Westerblad H, Allen DG. Mechanisms underlying changes of tetanic [Ca2+]i and force in skeletal muscle. Acta Physiol Scand 156: 407–416, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Westerblad H, Allen DG. Slowing of relaxation and [Ca2+]i during prolonged tetanic stimulation of single fibres from Xenopus skeletal muscle. J Physiol 492: 723–736, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Westerblad H, Bruton JD, Lannergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol 500: 193–204, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolosker H, Rocha JB, Engelender S, Panizzutti R, De Miranda J, de Meis L. Sarco/endoplasmic reticulum Ca2+-ATPase isoforms: diverse responses to acidosis. Biochem J 321: 545–550, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Young IS, Harwood CL, Rome LC. Cross-bridge blocker BTS permits direct measurement of SR Ca2+ pump ATP utilization in toadfish swimbladder muscle fibers. Am J Physiol Cell Physiol 285: C781–C787, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Zhang SJ, Andersson DC, Sandstrom ME, Westerblad H, Katz A. Cross bridges account for only 20% of total ATP consumption during submaximal isometric contraction in mouse fast-twitch skeletal muscle. Am J Physiol Cell Physiol 291: C147–C154, 2006 [DOI] [PubMed] [Google Scholar]