Abstract
Stress exacerbates symptoms of functional lower urinary tract disorders including interstitial cystitis (IC)/bladder pain syndrome (BPS) and overactive bladder (OAB) in humans, but mechanisms contributing to symptom worsening are unknown. These studies address stress-induced changes in the structure and function of the micturition reflex using an animal model of stress in male rats. Rats were exposed to 7 days of repeated variate stress (RVS). Target organ (urinary bladder, thymus, adrenal gland) tissues were collected and weighed following RVS. Evans blue (EB) concentration and histamine, myeloperoxidase (MPO), nerve growth factor (NGF), brain-derived neurotropic factor (BDNF), and CXCL12 protein content (ELISA) were measured in the urinary bladder, and somatic sensitivity of the hindpaw and pelvic regions was determined following RVS. Bladder function was evaluated using continuous, open outlet intravesical infusion of saline in conscious rats. Increases in body weight gain were significantly (P ≤ 0.01) attenuated by day 5 of RVS, and adrenal weight was significantly (P ≤ 0.05) increased. Histamine, MPO, NGF, and CXCL12 protein expression was significantly (P ≤ 0.01) increased in the urinary bladder after RVS. Somatic sensitivity of the hindpaw and pelvic regions was significantly (P ≤ 0.01) increased at all monofilament forces tested (0.1–4 g) after RVS. Intercontraction interval, infused volume, and void volume were significantly (P ≤ 0.01) decreased after RVS. These studies demonstrate increased voiding frequency, histamine, MPO, NGF, and CXCL12 bladder content and somatic sensitivity after RVS suggesting an inflammatory component to stress-induced changes in bladder function and somatic sensitivity.
Keywords: micturition, stress, nerve growth factor, ELISA, somatic sensitivity
stress contributes to symptom exacerbation in many disease states, including functional disorders of the urinary bladder such as overactive bladder (OAB) and interstitial cystitis (IC)/bladder pain syndrome (BPS) (3, 38, 58, 72). Urinary frequency is a common symptom among patients with OAB or IC/BPS, although the end result of frequent voiding may differ [reduce incontinence episodes (OAB) vs. reduce pain with bladder filling (IC/BPS)]. Patients with IC/BPS report symptom worsening during stress, as do patients with other disorders associated with IC/BPS including rheumatoid arthritis, psoriasis, and irritable bowel syndrome (47, 72). Symptom worsening during stress may be due, in part, to disruption of the hypothalamic-pituitary-adrenal (HPA) axis. Cortisol, through feedback on the HPA axis, normally acts to attenuate inflammation (47); however, abnormalities in the feedback may cause dysregulation of the inflammatory response. Therefore, patients diagnosed with IC/BPS and other functional urinary tract disorders may have abnormalities in the HPA axis, and stress could contribute to bladder symptoms, including frequency, urgency, and/or pain, reported by these patient populations (47). The pathophysiology underlying stress-induced effects on micturition reflex function remain undetermined.
The most well-established animal models of stress used to examine effects on urinary bladder structure and function include the resident-intruder model (15, 22, 74), immobilization stress (10, 11), water avoidance stress (WAS) (14, 54, 55, 62), and electrical footshock (7, 55). Although these stress paradigms produce bladder dysfunction and target tissue (i.e., urinary bladder) abnormalities similar to conditions like IC/BPS, the data in some cases are conflicting, and the stress models used in these studies may not be relevant to the variety of life stressors experienced by humans on a daily basis. In the present study, a repeated variate stress (RVS) protocol (30, 63) that lacks habituation was used, in which a different stressor was presented every day for 7 days (see Table 1).
Table 1.
Outline of stressor exposure for each of 7 days of repeated variate stress
| Day | Stressor | Duration |
|---|---|---|
| 1 | Oscillation | 30 min |
| 2 | Swim | 5 min |
| 3 | Footshock | 5 s (x2) |
| 4 | Restraint | 60 min |
| 5 | Pedestal | 30 min |
| 6 | Swim | 5 min |
| 7 | Footshock | 5 s (x2) |
Exposure to each of 5 different stressors, as described in the materials and methods section, and the duration of each stressor, are listed for each day they are administered. Swim and footshock stressors are repeated on the last two days of repeated variate stress (RVS). s, seconds; min, minutes.
When compared with other animal models of stress (e.g., resident intruder, immobilization, WAS, and electrical footshock) where the same stressors are presented daily, the RVS paradigm is unique in that various stressors are presented throughout the 7-day protocol, which may be more relevant to human daily life stressors. Other advantages of the RVS paradigm include: 1) novel stressor exposure on a daily basis with lack of habituation; and 2) reproducible and robust responses with rats exposed to RVS exhibiting a 10% decrease in weight gain during the stress exposure. Furthermore, RVS or chronic variate stress paradigms are commonly and widely used to characterize many aspects of the chronic stress response including 1) central nervous system (CNS) and peripheral nervous system responses to chronic stress; 2) neurochemical plasticity of CNS to chronic stress exposure; 3) comorbidity of stress-related disorders; and 4) the role of the limbic system and neuroendocrine cascade in chronic stress (30, 63). In this study, we extend the use of the RVS protocol to characterizing the effects on the autonomic nervous system and somatic sensitivity.
The goal of these studies was to use RVS exposure in male rats and to characterize urinary bladder function and somatic sensitivity at the end of the 7-day stress protocol in the absence of a direct urinary bladder stimulation. Bladder function was assessed using continuous, intravesical infusion of saline in conscious, unrestrained male rats with an open outlet. Biomarkers of stress including adrenal, thymus, bladder, and body weights were measured. We also evaluated urinary bladder nerve growth factor (NGF) protein content and somatic sensitivity (e.g., hindpaw and pelvic region) after RVS, as both are observed in animal models of urinary bladder inflammation as well as in functional urinary tract disorders. Finally, barrier function of the urothelium following RVS was assessed by Evans blue (EB) administration and determination of plasma protein extravasation.
MATERIALS AND METHODS
Animals
Adult male Wistar rats (300–400 g) were purchased from Charles River Laboratories (Wilmington, MA) and housed singly and maintained in standard laboratory conditions with free access to food and water. The University of Vermont Institutional Animal Care and Use Committee approved all animal use procedures (protocol 08-085). Animal experimentation was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Repeated Variate Stress
Rats were assigned, based on body weight, to control or RVS groups. Rats in the RVS group were exposed to 7 days of stress (Table 1) with rats exposed to a single stressor on each day as described previously (30). All rats were weighed 1 day before the first day of stress (baseline; day 0) and every subsequent day immediately before stress (days 1–7). Control rats remained in home cages in the animal facility following weight measurement and received no handling aside from weighing.
Oscillation stress.
Rats were placed inside a plastic chamber 28 × 17 ×13 cm (L ×W × H), which was secured to a clinical rotator (Fisher Scientific, Morris Plains, NJ), and oscillated at low to medium speed for 30 min.
Forced swim.
Rats were placed in a cylindrical container 29 × 37 cm (D × H) that was filled with room temperature water to a depth that prevented the rat's tail from touching the bottom of the container. After 5 min of monitored swimming, rats were placed in a holding chamber for 30 min before being returned to their home cage.
Electrical footshock.
Rats were placed inside a Plexiglas conditioning chamber (Med Associates, St. Albans, VT) 30 × 25 × 35 cm (L × W × H). After a 5-min acclimation period, two 1.0 mA 5 second scrambled footshocks were delivered through the grid floor with a 1-min intertrial interval.
Restraint.
Rats were placed in a cylindrical restraining device 9 × 15 cm (D × H) for 60 min.
Pedestal.
Rats were placed on an elevated (60 cm from floor) platform 20 × 20 cm (L × W) for 30 min.
Tissue Harvest for Organ Weights and Preparation of Bladder Tissue for ELISAs
Rats from both experimental groups (RVS and control) were euthanized immediately following the last stressor with isoflurane (4%), followed with a thoracotomy. The urinary bladder, thymus, and adrenal glands were subsequently harvested and blotted dry and weights were recorded. Individual bladders were immediately weighed and solubilized in tissue protein extraction reagent (1 g tissue/20 ml) and treated with complete protease inhibitor cocktail tablets. Tissue was homogenized and centrifuged (10,000 rpm for 10 min). The resulting supernatant was used for nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and histamine protein quantification. NGF and BDNF (R&D Systems, Minneapolis, MN) and histamine (GenWay Biotech, San Diego, CA) were quantified using standard 96-well ELISA plates according to the manufacturer's specifications.
EB Administration to Assess Plasma Protein Extravasation in the Urinary Bladder
Plasma protein extravasation in the urinary bladder was measured in control and RVS groups by an EB dye leakage technique as described previously (35, 60) 24 h after the last stressor exposure. As an additional comparison, 4 h cyclophosphamide (CYP; 150 mg/kg ip)-injected rats were anesthetized (2%), and the femoral vein was exposed. With the use of a butterfly catheter, EB (50 mg/kg) was injected into the femoral vein. Once all the EB had been injected, the rats remained anesthetized. After 15 min, the urinary bladder was harvested, transferred to a weigh boat, and placed in an oven at 50°C for 24 h. After 24 h, bladders were removed from the oven and dry weights were collected. Bladders were then placed in tubes with 1 ml formamide and stored at −4°C in total darkness for 72 h (35, 60).
The formamide-EB solution was gently aspirated without disturbing the tissue at the bottom of the tube and transferred to a 1-ml cell. The content of dye in the formamide solution was measured in duplicate using a microplate reader set at 620 nm wavelength with pure formamide as the reference. The duplicate measures were averaged, and the mean was converted to micrograms of EB by reporting the results on a standard curve of the refractive index versus dilution of EB. Results are expressed as micrograms of EB per dry weight of the bladder.
Measurement of Urinary Bladder NGF, BDNF, and Histamine Protein Content by ELISAs
Mictrotiter plates (R&D Systems and GenWay Biotech) were coated with a mouse anti-rat NGF antibody, a mouse anti-rat BDNF antibody (R&D Systems), or anti-rabbit histamine antibody (GenWay Biotech). Sample and standard solutions were run in duplicate. A horseradish peroxidase-streptavidin conjugate was used to detect the antibody complex. Tetramethyl benzidine was the substrate, and the enzyme activity was measured by the change in optical density. The NGF and BDNF standards provided with these protocols generated a linear standard curve from 15 to 1,000 pg/ml (R2 = 0.998, P ≤ 0.0001) for bladder samples. The provided histamine standards generated a linear standard curve from 0.5 to 125 pg/ml (R2 = 0.997, P ≤ 0.0001). The absorbance values of standards and samples were corrected by subtraction of the background absorbance due to nonspecific binding. No samples fell below the minimum detection limits of the assay and no samples were diluted before use.
Myeloperoxidase Assay
Inflammation of the urinary bladder was also assessed with an assay for myeloperoxidase (MPO). Polymorphonuclear (PMN) cell infiltration is a characteristic of inflammation, and MPO is a naturally occurring enzyme contained in the primary granules of the PMN cells. Greater MPO activity in a tissue represents increased PMN cell infiltration in inflamed tissue (12). Thus an MPO assay was performed on freshly harvested urinary bladder tissue obtained from isoflurane-anesthetized animals. The MPO assay was performed as described by Bradley et al. (12). Briefly, MPO was extracted from homogenized bladder tissue by suspending the material in 0.5% hexadecyltrimethylammonium bromide (Sigma) in 50 mM potassium phosphate buffer (pH 6.0) before sonication in an ice bath for 30 s. The specimens were freeze thawed three times, and the sonication was repeated. Suspensions were then centrifuged (40,000 g) for 15 min, and the supernatant was assayed spectrophotometrically. Supernatant (0.1 ml) was mixed with 2.9 ml of potassium phosphate buffer containing 0.167 ml/ml of o-dianisidine dihydrochloride (Sigma) and 0.0005% hydrogen peroxide. The change in absorbency was measured at 460 nm. One unit of MPO activity was defined as that degrading 1 μmol of peroxide per minute at 25°C.
Mechanical Sensitivity Testing
Mechanical sensitivity testing was performed in separate groups (hindpaw, pelvic region) of rats not used for bladder function determination or biochemical assays. Referred (secondary) hyperalgesia and tactile allodynia were tested using calibrated von Frey hairs with forces of 0.1–4 g applied to the hindpaw or pelvic region. Rats were tested in individual Plexiglas chambers with a stainless steel wire grid floor. Rats were acclimated to the chambers for a period of 2 h (61). The von Frey hairs were applied in an up-down method for 1–3 s with an interstimulus interval of 15 s. For pelvic region stimulation, stimulation was confined to the lower abdominal area overlying the urinary bladder. Testing of the plantar region of the hindpaw and lower abdominal area was performed by perpendicular application of von Frey hairs to the indicated areas until the hair bent slightly. The following behaviors were considered positive responses to pelvic region stimulation: sharp retraction of the abdomen, jumping, or immediate licking or scratching of the pelvic area (59). A positive response to hindpaw stimulation was sharp withdrawal of the paw or licking of the tested hindpaw (67). All somatic testing was performed in a blinded manner with respect to treatment. The groups were decoded after data analysis.
Intravesical Catheter Implant
On day 8, 24 h after presentation of the last stressor, a lower midline abdominal incision was made under general anesthesia with 2–3% isoflurane using aseptic techniques (16, 33, 40, 68). One end of polyethylene tubing (PE-50; Clay Adams, Parsippany, NJ) was flared with a flame and inserted in the dome of the bladder and secured in place with a 6-0 nylon purse-string suture (16, 33, 40, 68). The distal end of the tubing was tunneled subcutaneously to the back of the neck where it was buried in an incision in the back of the neck, out of the animal's reach (16, 33, 40, 68). Rats received buprenorphine (0.05 mg/kg sc) starting at the time of surgery and then every 8–12 h postoperatively for a total of four doses. Animals were maintained for 72 h after surgery before conscious cystometry was initiated to ensure complete recovery.
Open Voiding Cystometry in Conscious, Unrestrained Rats
The effects of RVS on bladder function were evaluated using conscious cystometry and continuous infusion of intravesical saline. During cystometry, unrestrained and conscious rats were placed in a recording cage over a scale and pan to collect and measure voided urine. To elicit repetitive bladder contractions, room temperature saline was infused at a constant rate (10 ml/h). After an initial stabilization period (25–30 min), at least six reproducible micturition cycles were recorded. Intravesical pressure changes were recorded using a small Animal Cystometry System (Med Associates) (16, 39). Baseline resting pressure, pressure threshold for voiding, maximal voiding pressure, and intercontraction interval were measured. The bladder pressure measurements are not corrected for pressure drop along the infusion catheter as we do not expect the pressure drop to be biased by treatment. Furthermore, the pressure drop is consistent across groups. Nonvoiding bladder contractions (NVCs), defined as rhythmic intravesical pressure increases 7 cmH2O above baseline without the release of fluid from the urethra, and high-frequency oscillations (HFOs) present during voiding (phase 2) (65, 66) were also determined per voiding cycle. Bladder capacity was measured as the volume of saline infused in the bladder at the time when micturition commenced (6, 32).
Exclusion Criteria
In the present study, no rats were excluded from the study or from analysis; all rats completed the protocol. Behavioral movements such as grooming, standing, walking, and defecation rendered bladder pressure recordings during these events unusable.
Statistical Analyses
All values represent means ± SE. Data were compared with analysis of variance (ANOVA). Percentage data from weight data were arcsin transformed to meet the requirements of this statistical test. Maximal bladder pressures recorded from the highest HFO during voiding (phase 2) were compared using an unpaired t-test. Animals, processed and analyzed on the same day, were tested as a block in the ANOVA. When F ratios exceeded the critical value (P ≤ 0.05), the Newman-Keuls post hoc test was used to compare group means.
RESULTS
Organ and Body Weights in the Rat After RVS
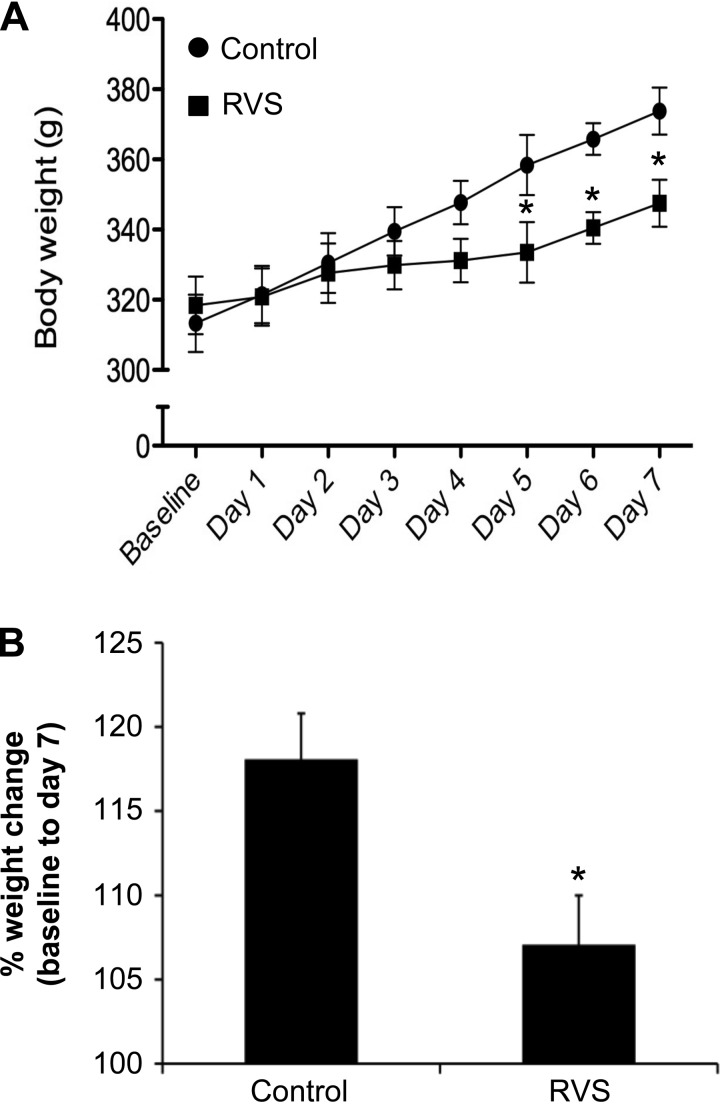
No change in bladder weight (% of body weight) was observed in male rats exposed to RVS and control groups (5.9 × 10−2 ± 8.3 × 10−3 vs. 5.7 × 10−2 ± 9.8 × 10−3, P = 0.85). Similarly, no change in thymus weight (% of body weight) was observed between RVS and control groups (0.12 ± 1.6 × 10−2 vs. 0.12 ± 2.8 × 10−3, P = 0.94). Adrenal weight (% of body weight) increased significantly (1.2-fold) after RVS compared with controls (0.03 ± 1.2 × 10−3 vs. 0.02 ± 1.5 × 10−3, P ≤ 0.02). As shown previously (56), increases in body weight were significantly (P ≤ 0.01) attenuated during the RVS protocol (Fig. 1). Animals in the RVS group gained significantly (P ≤ 0.01) less weight compared with controls on days 5–7 of RVS (Fig. 1A). Animals in the RVS group exhibited a significantly (P ≤ 0.01) smaller weight change (expressed as a % on day 7 of RVS compared with baseline) throughout the 7-day RVS paradigm compared with control animals (Fig. 1B).
Fig. 1.
Changes in body weight of rats during 7 days of repeated variate stress (RVS). A: both control rats and rats exposed to 7 days of RVS exhibit body weight gain at a similar rate for the first 4 days of RVS. On days 5–7 of stressor exposure, rats exposed to 7 days of RVS exhibit significant weight gain attenuation compared with controls rats. Body weights were significantly (P ≤ 0.01) decreased in the RVS group on days 5–7 of stress compared with control rats. B: percentage weight change from baseline body weight to body weight on day 7 of stressor exposure was significantly (P ≤ 0.01) decreased in rats exposed to 7 days of RVS compared with control rats. Values are means ± SE. Sample sizes are n of 14; *P ≤ 0.01.
Plasma Extravasation in the Rat Urinary Bladder After RVS and Acute (4 h) CYP Administration
After EB infusion into the femoral vein, a significantly (7.7-fold) increased concentration of EB in the urinary bladder was demonstrated in 4 h CYP-treated rats compared with controls (2.18 ± 0.25 vs. 0.28 ± 0.03, P ≤ 0.0001), but no change was observed in the RVS group compared with controls (0.27 ± 0.02 vs. 0.28 ± 0.03, P = 0.68).
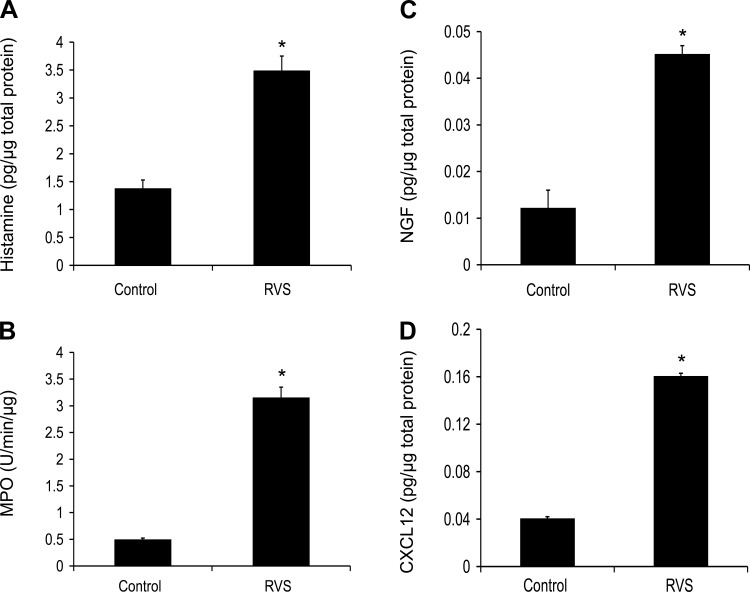
Histamine, MPO, NGF, BDNF, and CXCL12 Protein Expression in Rat Urinary Bladder After RVS
After a 7-day RVS protocol, urinary bladder expression of histamine (Fig. 2A) and MPO (Fig. 2B) was significantly (P ≤ 0.001) increased (2.4–7.8-fold) compared with control rats.
Fig. 2.
Increased histamine, myeloperoxidas (MPO), nerve growth factor (NGF), and CXCL12 protein expression in urinary bladder following RVS. Histamine (A), MPO (B), NGF (C), and CXCL12 (D) protein content were significantly (P ≤ 0.001) increased in the urinary bladder after 7 days of RVS compared with control rats. Values are means ± SE. Sample sizes are n of 6–8; *P ≤ 0.001.
NGF protein expression in the whole urinary bladder increased significantly (P ≤ 0.001) following RVS (4.0-fold; Fig. 2C) compared with controls as determined with ELISAs. No significant differences in BDNF protein expression, determined with ELISAs, were seen after RVS compared with control rats (data not shown). The chemokine, CXCL12, protein expression was significantly (P ≤ 0.001) increased (4.0-fold) in rat urinary bladder after RVS (Fig. 2D).
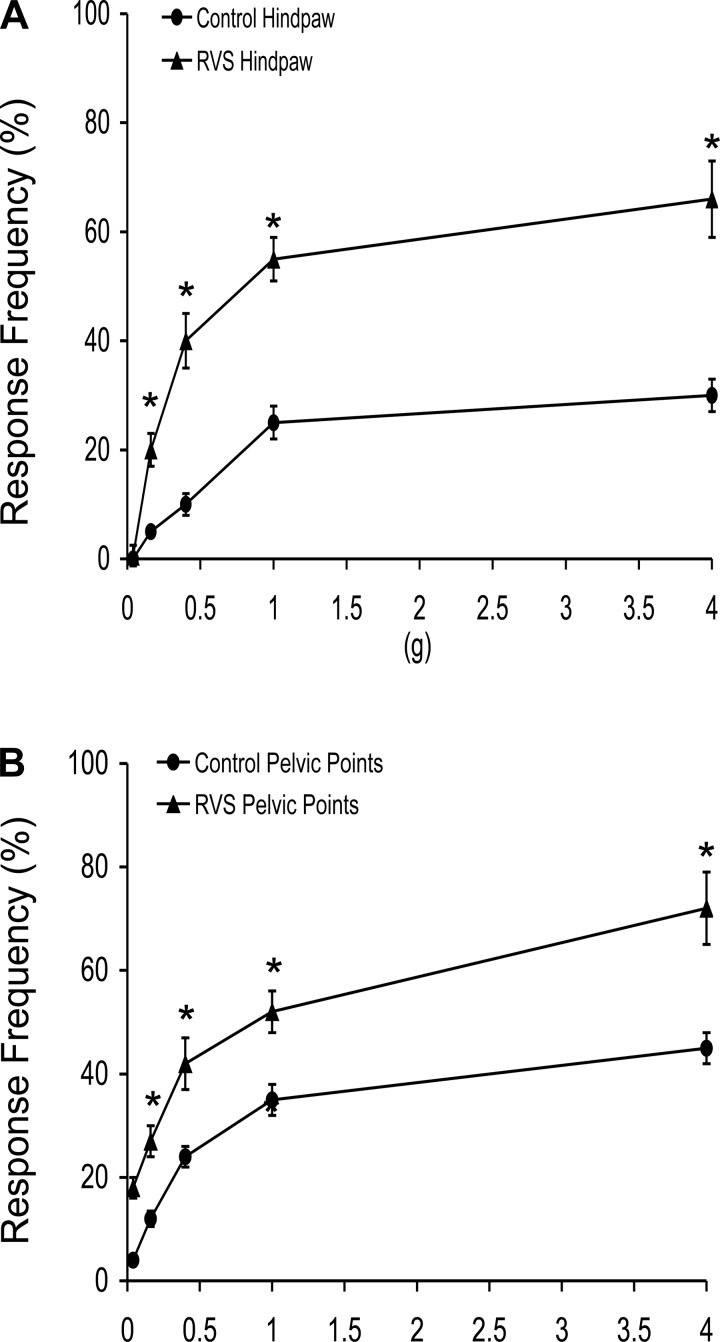
Hindpaw and Pelvic Somatic Sensitivity After RVS
Somatic sensitivity in the hindpaw, as determined with von Frey monofilaments, was significantly (P ≤ 0.01) increased following RVS among the monofilament forces tested (0.1–4 g) compared with control rats (Fig. 3A). Similarly, pelvic somatic sensitivity was also significantly (P ≤ 0.01) increased after RVS at all monofilament forces evaluated (0.1–4 g) (Fig. 3B).
Fig. 3.
Somatic sensitivity testing of the hindpaw and pelvic regions with von Frey hairs in control (circles) rats and rats exposed to 7 days of RVS (triangles). The von Frey hairs were applied in an up-down method for 1–3 s with an interstimulus interval of 15 s. For pelvic region stimulation, stimulation was confined to the lower abdominal area overlying the urinary bladder. The following behaviors were considered positive responses to pelvic region stimulation: sharp retraction of the abdomen, jumping, or immediate licking or scratching of the pelvic area. A positive response to hindpaw stimulation was sharp withdrawal of the paw or licking of the tested hindpaw. Rats exposed to 7 days of RVS had a significantly (*P ≤ 0.01) increased hindpaw response frequency (A) and pelvic response frequency (B) with all von Frey hairs (0.1–4 g) tested compared with control rats (no RVS). Hindpaw or pelvic sensitivity testing with calibrated von Frey hairs was determined in separate groups of rats. All somatic testing was performed in a blinded manner with respect to treatment. Values are means ± SE. Sample sizes are n of 8; *P ≤ 0.01.
Conscious Cystometry in Rats After RVS
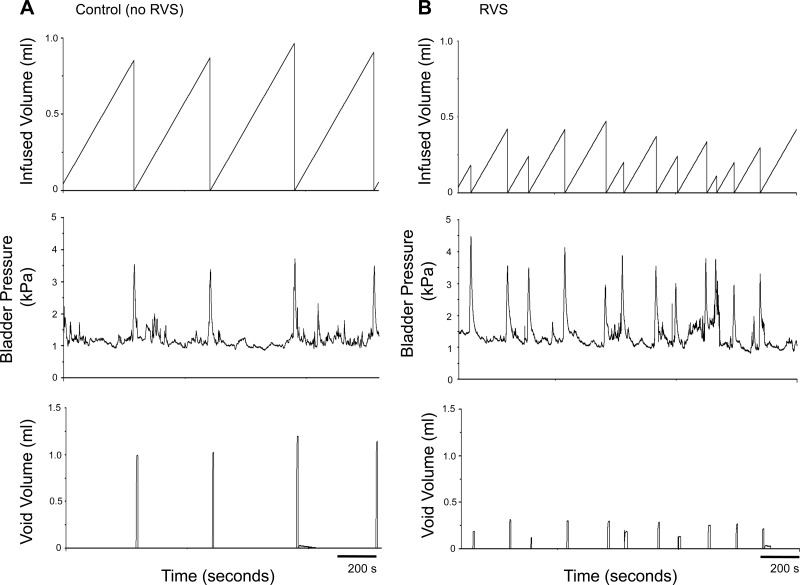
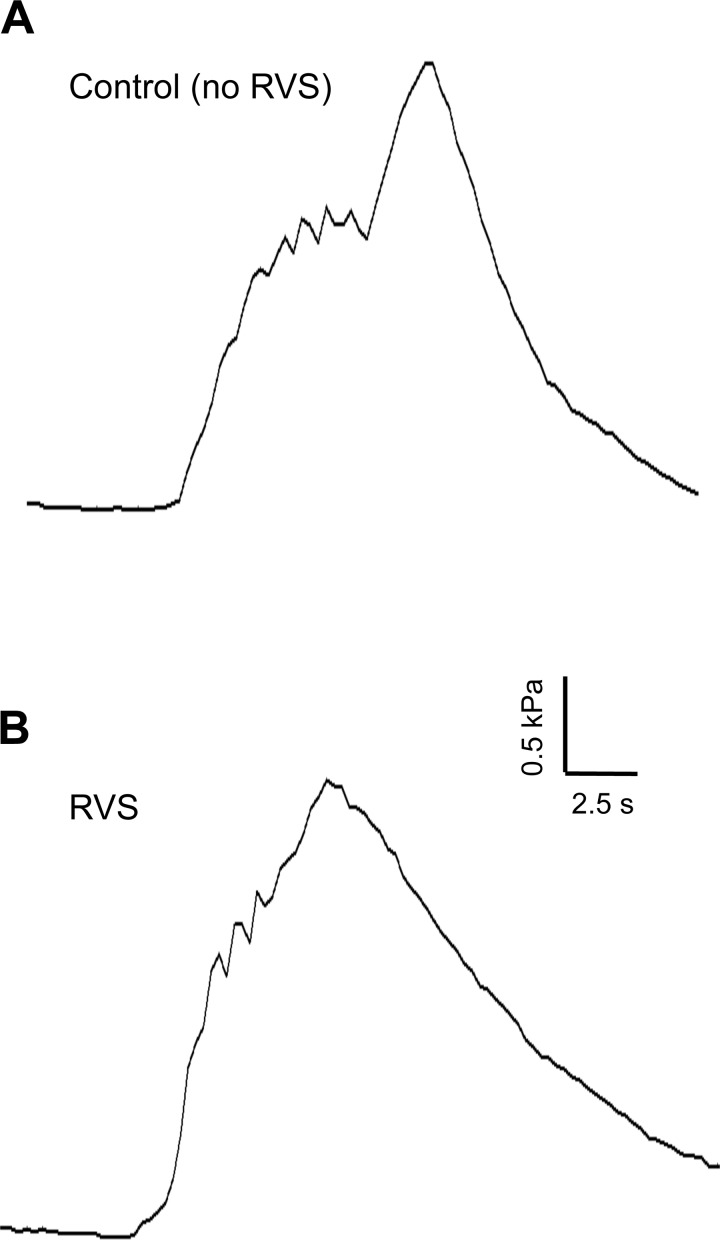
To determine whether a 7-day RVS protocol in rats resulted in changes in urinary bladder function, we performed a series of urodynamic studies on rats subjected to RVS as well as control rats. Figure 4, A and B, shows representative open voiding cystometrograms in conscious, unrestrained rats, in which voiding reflexes were measured in response to a continuous infusion of saline with an open outlet. In contrast to controls, in which normal micturition contractions were observed (Fig. 4A), the rats subjected to RVS had increased voiding frequency (Fig. 4B) with significantly reduced infused volumes (2.4-fold), void volumes (2.3-fold), and intercontraction intervals (2.4-fold) compared with control rats (P ≤ 0.01, Table 2). The reductions in void volume, infused volume, and intercontraction interval were present in both male and female rats with a similar magnitude of change (Table 2). No changes in baseline resting pressure, micturition threshold pressure, or maximal voiding pressure were observed between control and RVS male rats (Table 2). NVCs were not observed in either control rats or in rats after a 7-day RVS protocol (data not shown). Residual volumes were not affected by RVS and were comparable (<10 μl) in both control and RVS groups. From the RVS and control groups, micturition events were examined for the presence of HFOs during voiding (phase 2) (65, 66). There was no significant difference in the proportion of micturition events with HFOs between groups (Fig. 5). There was no significant difference in HFO maximal bladder pressure between RVS and control groups (Table 2).
Fig. 4.
Representative cystometrogram recordings using continuous intravesical infusion of saline in conscious rats with an open outlet from a control (no RVS) rat and a rat exposed to 7 days of RVS. A: bladder function in a control rat that has not been exposed to 7 days of stress; B: bladder function in a rat that has been exposed to RVS with continuous intravesical infusion of saline. Bladder function recordings are from one control rat (A) and one rat exposed to RVS (B). Infused volume (ml, top), bladder pressure (kPa, middle), and void volume (ml, bottom) in a control (A) and RVS-treated (B) rat are shown.
Table 2.
Mean bladder pressures and urodynamic parameters during conscious cystometry in control rats and those exposed to repeated variate stress
| HFO Max, kPa | Threshold Pressure, kPa | Micturition Pressure, cmH2O | Baseline Pressure, cmH2O | Intercontraction Interval, s | Infused Volume, ml | Void Volume, ml | |
|---|---|---|---|---|---|---|---|
| Control | 2.3 ± 0.1 | 1.1 ± 0.1 | 3.1 ± 0.3 | 0.9 ± 0.1 | 480.8 ± 63.2 | 1.3 ± 0.2 | 1.3 ± 0.2 |
| RVS | 2.3 ± 0.1 | 1.3 ± 0.1 | 2.9 ± 0.3 | 1.1 ± 0.1 | 203.9 ± 34.5* | 0.6 ± 0.1* | 0.6 ± 0.1* |
Mean bladder pressures and urodynamic parameters during conscious cystometry in male rats with or without RVS exposure. Values are means ± SE; n = 4/group. Threshold, micturition, baseline, and maximum high-frequency oscillation (HFO) pressures (kPa) during conscious cystometry for control and RVS-treated (7 days) rats are shown. Urodynamic parameters including intercontraction interval, infused volume, and void volume are also shown.
P ≤ 0.01 compared with controls.
Fig. 5.
Representative intravesical pressure traces of high-frequency oscillations (HFOs) during voiding from a control (A) and RVS-exposed (B) rat. HFOs can be clearly seen in phase 2 of micturition during voiding. HFOs, indicative of efficient voiding in the rat, are present in similar proportions of micturition events in both control (A) rats and rats that have been exposed to 7 days of RVS (B). kPa, kilopascals; s, seconds.
DISCUSSION
These studies demonstrate several findings with respect to stress-induced changes in micturition reflexes in rats, particularly regarding NGF protein expression, somatic sensitivity, and bladder function. In the present studies, we demonstrate that RVS 1) produces biological markers of stress (body and adrenal weights) similar to previous findings (30, 31, 36, 57) including a robust and reproducible reduction (10%) in body weight gain during RVS exposure; 2) increases NGF protein expression and other inflammatory markers (e.g., MPO, histamine, chemokines) in the urinary bladder; 3) increases somatic sensitivity in the hindpaw and pelvic regions; and 4) decreases bladder capacity, void volume, and intercontraction interval during conscious cystometry. Previous studies using this stress model have focused on its role in anxiety-like behavior demonstrating neurochemical plasticity [i.e., pituitary adenylate cyclase-activating polypeptide (PACAP) and its cognate receptor PAC1] in the bed nucleus of the stria terminalis (BNST), a critical structure in the brain in mediating fear- and anxiety-like behavior in humans and animals (30, 31). Furthermore, excitotoxic lesions to the BNST have been shown to attenuate the effects of RVS on weight gain by the last day of stressor exposure (56). The current studies extend the use of the RVS model to the study of micturition reflex function and demonstrate RVS-induced changes in urinary bladder function, somatic sensitivity, and in the inflammatory milieu of the urinary bladder.
Urothelial Barrier is Intact After RVS
The integrity of the urothelium after RVS, determined by EB plasma extravasation, was intact. Bladder function testing using conscious cystometry demonstrated increased voiding frequency following RVS. A disrupted barrier function of the urothelium could underlie increased voiding frequency via activation of the suburothelial nerve plexus (2, 5, 21). Animal models of cystitis produce dramatic increases in EB levels in the urinary bladder, indicative of a leaky urothelial barrier (35, 60). It is well known that EB dye extravasation can be considered an early marker of inflammation. In the present study, we did not observe an increase in EB levels 7 days following RVS as we demonstrated in bladders from rats following CYP injection (150 mg/kg). This suggests that the protective urothelial barrier remains intact following RVS, unlike in the CYP model, and that altered barrier function of the urothelium does not contribute to altered voiding function following RVS. In the clinical syndrome IC/BPS, a defect in the urothelial barrier has been suggested as either a causative or contributing factor to symptom onset and/or maintenance (52). A number of other causative factors have also been suggested (e.g., altered sensory processing, mast cell involvement, neurogenic inflammation), but the underlying etiology of the disease syndrome is unknown (52). Rats exposed to RVS exhibit increased voiding frequency in the absence of a defect in the urothelial barrier, suggesting that such a defect does not contribute to increased voiding frequency at least in the time frame of the RVS exposure (7 days).
Changes in the Inflammatory Milieu of the Urinary Bladder After RVS
Although differences in plasma extravasation at the level of the urinary bladder were not detected with RVS, increased NGF protein expression in the urinary bladder after 7 days of RVS was demonstrated. Neurotrophins, particularly NGF, are thought to play a role in bladder function (18, 20, 24, 25, 29, 34, 77), and particularly in bladder diseases including IC/BPS (44, 50), OAB (37, 42) and bladder outlet obstruction (43). NGF exerts pleiotropic effects in the peripheral and central nervous system, regulating sensory and sympathetic neuronal development and maintenance (25, 48, 61). NGF plays well-established roles in urinary bladder inflammation (61, 71), most likely contributing to increased voiding frequency (18, 20, 24, 28, 33, 49, 77). Administration of NGF intravesically (24), intrathecally (76), intramuscularly (77), or via adenovirus-mediated delivery to the urinary bladder (41) induces increased voiding frequency in the urinary bladder as well as afferent neuronal hyperexcitability in rodents. Additionally, sequestration of NGF or its receptor TrkA, as well as administration of Trk inhibitors, reduces urinary frequency in rodent models of urinary bladder inflammation (24, 33, 40). Increased levels of NGF have also been detected in the urine and the urothelium of individuals with IC/BPS or other painful bladder conditions (44, 50). The finding of increased NGF protein expression in urinary bladders from rats exposed to 7 days of RVS in the present study is consistent with previous research demonstrating NGF effects on bladder function. Given the established contribution of NGF to bladder function (18, 20, 24, 25, 29, 34, 77), we hypothesized that increased voiding frequency after RVS would be associated with increased bladder NGF content. NGF is often associated with numerous inflammatory conditions and is being considered as a potential biomarker for inflammation in individuals with numerous urinary bladder disorders/dysfunction including IC/BPS, OAB, diabetic cystopathy, and bladder outlet obstruction (37, 43, 50). In contrast, BDNF bladder protein expression was not altered after RVS, although BDNF has also been implicated in increasing voiding frequency (49, 64, 71). In this study, we also evaluated other markers of inflammation including chemokines (e.g., CXCL12), MPO (for PMN), and histamine (mast cells). Increased CXCL12 and histamine expression as well as MPO activity significantly increased in the urinary bladder after RVS. Collectively, these studies suggest that the inflammatory milieu of the urinary bladder may contribute to urinary bladder dysfunction following RVS.
Increased Somatic Sensitivity After RVS
We have also demonstrated in the present study that somatic sensitivity in the hindpaw and pelvic region is increased following RVS similar to CYP-induced cystitis in rodents. In addition to the role of NGF in bladder function, it has also been identified as a key molecule in the signaling of pain during inflammation (1, 17, 23, 24, 26, 61). Visceral inflammation is often accompanied by an increase in sensitivity of somatic structures to noxious stimuli, commonly called referred hyperalgesia (9, 75). Since the direct measurement of visceral pain is difficult, referred hyperalgesia is often used as a method to exemplify visceral pain. Increased sensitivity to pain and somatic stimuli is frequently observed in patients with cystitis (28). For example, patients with IC/BPS report increased sensitivity to application of pressure to deep (muscular) tissues (8, 69). Similarly, increases in peripheral sensitivity to mechanical stimuli using von Frey filament application to the hindpaw are seen following CYP-induced cystitis in rodents (73). Therefore, increases in hindpaw and pelvic referred somatic sensitivity in the present study are similar to results seen in animal models of IC/BPS (45, 61, 67).
NGF produced in the bladder has been suggested to modulate peripheral mechanical nociception in the presence of cystitis (29), and studies involving NGF have implicated its importance in the development of visceral hyperalgesia and inflammatory pain (41, 51, 53, 78). Intravesical administration of NGF decreases the threshold of the hindpaw to mechanical stimulation similar to CYP (28). Furthermore, immunoneutralization of NGF by administration of NGF antiserum, as well as administration of k252a, a Trk receptor antagonist, block the development of visceral hyperalgesia (28, 41). NGF protein levels are increased in the urinary bladder following 7 days of RVS, consistent with a role for NGF in increased somatic sensitivity of the hindpaw and pelvic regions seen in our model. However, other inflammatory mediators may also contribute to the observed changes in bladder function and somatic sensitivity. Therefore, it is not known whether NGF is sufficient to induce peripheral sensitization (41) in the RVS model. Further studies should address other potential modulators of increased voiding frequency following RVS, including cytokines/chemokines (2), neuropeptides (e.g., PACAP) (13, 27, 70), and transient receptor potential (TRP) channels whose expression can be regulated by growth factor expression (19).
RVS Induces Changes in Urinary Bladder Function
The present study demonstrates that 7 days of RVS decreases bladder capacity, void volume, and intercontraction interval. Minimal residual volumes together with no changes in the presence or amplitude of high-frequency oscillations during voiding in rats exposed to RVS suggest that voiding efficiency was not altered. However, urethral tone and the activity of the external urethral sphincter were not examined in these studies given our use of conscious rats. Thus future studies should be pursued in anesthetized rats where potential effects of the outlet including the urethra and EUS can be evaluated after RVS. RVS-induced increases in voiding frequency are similar to other reports regarding several other animal models of stress including social defeat (4, 15, 46, 74), nontraumatic immobilization/restraint (11, 74), and WAS (14, 54, 62). We suggest that the 7-day RVS model has several advantages compared with other stress models. The RVS paradigm is unique in that various stressors are presented throughout the 7-day protocol, which may be more relevant to daily life stressors experienced by humans. Other advantages of the RVS paradigm include: 1) novel stressor exposure on a daily basis with lack of habituation; and 2) reproducible and robust responses with rats exposed to RVS exhibiting a 10% decrease in weight gain over the course of the stress exposure. It is clear that various stress protocols including RVS can affect autonomic function including changes in urinary bladder function and that specific changes in bladder function are dependent on the stress paradigm used, suggesting context-dependent effects.
Perspectives and Significance
Many disorders of the urinary bladder, including IC/BPS and OAB, exhibit symptom (i.e., urinary frequency) exacerbation with stress. These studies characterize the effects of RVS on the inflammatory milieu of the urinary bladder as well as on bladder function with the long-term goal of identifying potential targets for therapeutic intervention. RVS produces altered function of the urinary bladder characterized by increased urinary frequency with reduced bladder capacity and void volume. Increased expression of histamine, MPO, NGF, and CXCL12 in the urinary bladder and increased somatic sensitivity of the hindpaw and pelvic regions were also detected following RVS, suggesting an inflammatory component to bladder function changes. Future studies will explore underlying mechanisms of RVS-induced changes in urinary bladder structure and function by determining the contributions of inflammatory mediators (e.g., NGF), neurochemicals (e.g., PACAP) (13, 27, 70) and ion channels (e.g., transient receptor potential channels) (19) modulated by growth factors to RVS-induced changes in urinary bladder function and somatic sensitivity.
GRANTS
This work was funded by National Institutes of Health (NIH) Grants DK-051369, DK-060481, and DK-065989. NIH Grant P20 RR-16435 from the COBRE Program of the National Center also provided resource support for the project.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.M., S.E.M., and M.A.V. conception and design of research; L.M. and S.E.M. performed experiments; L.M. and M.A.V. analyzed data; L.M., S.E.M., and M.A.V. interpreted results of experiments; L.M., S.E.M., and M.A.V. prepared figures; L.M. drafted manuscript; L.M., S.E.M., and M.A.V. edited and revised manuscript; L.M., S.E.M., and M.A.V. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Sayamwong (Jom) Hammack and Dr. Victor May for advice and guidance with the RVS model and for numerous discussions related to this work.
REFERENCES
- 1. Aloe L, Tuveri MA, Levi-Montalcini R. Studies on carrageenan-induced arthritis in adult rats: presence of nerve growth factor and role of sympathetic innervation. Rheumatol Int 12: 213– 216, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Arms L, Girard BM, Vizzard MA. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 298: F589– F600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bazi T, Hajj-Hussein IA, Awwad J, Shams A, Hijaz M, Jurjus A. A modulating effect of epigallocatechin gallate (EGCG), a tea catechin, on the bladder of rats exposed to water avoidance stress. Neurourol Urodyn 32: 287– 292, 2012 [DOI] [PubMed] [Google Scholar]
- 4. Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol 18: 13– 24, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol 4: 46– 54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856– 860, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Black LV, Ness TJ, Robbins MT. Effects of oxytocin and prolactin on stress-induced bladder hypersensitivity in female rats. J Pain 10: 1065– 1072, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bohm-Starke N, Hilliges M, Brodda-Jansen G, Rylander E, Torebjork E. Psychophysical evidence of nociceptor sensitization in vulvar vestibulitis syndrome. Pain 94: 177– 183, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Bon K, Lichtensteiger CA, Wilson SG, Mogil J. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. J Urol 170: 1008– 1012, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Boucher W, Kempuraj D, Michaelian M, Theoharides TC. Corticotropin-releasing hormone-receptor 2 is required for acute stress-induced bladder vascular permeability and release of vascular endothelial growth factor. BJU Int 106: 1394– 1399, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Boucher WS, Letourneau R, Huang M, Kempuraj D, Green M, Sant GR, Theoharides TC. Intravesical sodium hyaluronate inhibits the rat urinary mast cell mediator increase triggered by acute immobilization stress. J Urol 167: 380– 384, 2002 [PubMed] [Google Scholar]
- 12. Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78: 206– 209, 1982 [DOI] [PubMed] [Google Scholar]
- 13. Calupca MA, Vizzard MA, Parsons RL. Origin of pituitary adenylate cyclase-activating polypeptide (PACAP)-immunoreactive fibers innervating guinea pig parasympathetic cardiac ganglia. J Comp Neurol 423: 26– 39, 2000 [PubMed] [Google Scholar]
- 14. Cetinel S, Ercan F, Cikler E, Contuk G, Sener G. Protective effect of melatonin on water avoidance stress induced degeneration of the bladder. J Urol 173: 267– 270, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Chang A, Butler S, Sliwoski J, Valentino R, Canning D, Zderic S. Social stress in mice induces voiding dysfunction and bladder wall remodeling. Am J Physiol Renal Physiol 297: F1101– F1108, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheppudira BP, Girard BM, Malley SE, Dattilio A, Schutz KC, May V, Vizzard MA. Involvement of JAK-STAT signaling/function after cyclophosphamide-induced bladder inflammation in female rats. Am J Physiol Renal Physiol 297: F1038– F1044, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chien CC, Fu WM, Huang HI, Lai YH, Tsai YF, Guo SL, Wu TJ, Ling QD. Expression of neurotrophic factors in neonatal rats after peripheral inflammation. J Pain 8: 161– 167, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol 165: 975– 979, 2001 [PubMed] [Google Scholar]
- 19. Ciobanu C, Reid G, Babes A. Acute and chronic effects of neurotrophic factors BDNF and GDNF on responses mediated by thermo-sensitive TRP channels in cultured rat dorsal root ganglion neurons. Brain Res 1284: 54– 67, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Clemow DB, Steers WD, McCarty R, Tuttle JB. Altered regulation of bladder nerve growth factor and neurally mediated hyperactive voiding. Am J Physiol Regul Integr Comp Physiol 275: R1279– R1286, 1998 [DOI] [PubMed] [Google Scholar]
- 21. de Groat WC. The urothelium in overactive bladder: passive bystander or active participant? Urology 64: 7– 11, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science 182: 939– 941, 1973 [DOI] [PubMed] [Google Scholar]
- 23. Dmitrieva N, McMahon SB. Sensitisation of visceral afferents by nerve growth factor in the adult rat. Pain 66: 87– 97, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Dmitrieva N, Shelton D, Rice AS, McMahon SB. The role of nerve growth factor in a model of visceral inflammation. Neuroscience 78: 449– 459, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Frias B, Lopes T, Pinto R, Cruz F, Cruz CD. Neurotrophins in the lower urinary tract: becoming of age. Curr Neuropharmacol 9: 553– 558, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Friess H, Zhu ZW, di Mola FF, Kulli C, Graber HU, Andren-Sandberg A, Zimmermann A, Korc M, Reinshagen M, Buchler MW. Nerve growth factor and its high-affinity receptor in chronic pancreatitis. Ann Surg 230: 615– 624, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Girard BM, Wolf-Johnston A, Braas KM, Birder LA, May V, Vizzard MA. PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci 36: 310– 320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guerios SD, Wang ZY, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett 392: 193– 197, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol 295: R111– R122, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology 34: 833– 843, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, May V. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J Mol Neurosci 42: 327– 340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol 551: 893– 903, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol 173: 1016– 1021, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Jaggar SI, Scott HC, Rice AS. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth 83: 442– 448, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Jasmin L, Janni G, Manz HJ, Rabkin SD. Activation of CNS circuits producing a neurogenic cystitis: evidence for centrally induced peripheral inflammation. J Neurosci 18: 10016– 10029, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jorgensen A, Maigaard K, Wortwein G, Hageman I, Henriksen T, Weimann A, Moller P, Loft S, Hau J, Poulsen HE, Jorgensen MB. Chronic restraint stress in rats causes sustained increase in urinary corticosterone excretion without affecting cerebral or systemic oxidatively generated DNA/RNA damage. Prog Neuropsychopharmacol Biol Psychiatry 40C: 30– 37, 2012 [DOI] [PubMed] [Google Scholar]
- 37. Kim JC, Park EY, Seo SI, Park YH, Hwang TK. Nerve growth factor and prostaglandins in the urine of female patients with overactive bladder. J Urol 175: 1773– 1776; discussion 1776, 2006. [DOI] [PubMed] [Google Scholar]
- 38. Klausner AP, Steers WD. Corticotropin releasing factor: a mediator of emotional influences on bladder function. J Urol 172: 2570– 2573, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Klinger MB, Girard B, Vizzard MA. p75NTR expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. J Comp Neurol 507: 1379– 1392, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F1778– F1789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lamb K, Gebhart GF, Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain 5: 150– 156, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur Urol 56: 700– 706, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Liu HT, Kuo HC. Urinary nerve growth factor levels are increased in patients with bladder outlet obstruction with overactive bladder symptoms and reduced after successful medical treatment. Urology 72: 104– 108; discussion 108, 2008. [DOI] [PubMed] [Google Scholar]
- 44. Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol 79: 572– 577, 1997 [DOI] [PubMed] [Google Scholar]
- 45. May V, Vizzard MA. Bladder dysfunction and altered somatic sensitivity in PACAP−/− mice. J Urol 183: 772– 779, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 60: 253– 259, 1979 [DOI] [PubMed] [Google Scholar]
- 47. Nazif O, Teichman JM, Gebhart GF. Neural upregulation in interstitial cystitis. Urology 69: 24– 33, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Ochodnicky P, Cruz CD, Yoshimura N, Cruz F. Neurotrophins as regulators of urinary bladder function. Nat Rev Urol 9: 628– 637, 2012 [DOI] [PubMed] [Google Scholar]
- 49. Oddiah D, Anand P, McMahon SB, Rattray M. Rapid increase of NGF, BDNF and NT-3 mRNAs in inflamed bladder. Neuroreport 9: 1455– 1458, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol 161: 438–441; discussion 441–432, 1999. [PubMed] [Google Scholar]
- 51. Ramer MS, Kawaja MD, Henderson JT, Roder JC, Bisby MA. Glial overexpression of NGF enhances neuropathic pain and adrenergic sprouting into DRG following chronic sciatic constriction in mice. Neurosci Lett 251: 53– 56, 1998 [DOI] [PubMed] [Google Scholar]
- 52. Ratliff TL, Klutke CG, McDougall EM. The etiology of interstitial cystitis. Urol Clin North Am 21: 21– 30, 1994 [PubMed] [Google Scholar]
- 53. Ro LS, Chen ST, Tang LM, Jacobs JM. Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Pain 79: 265– 274, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Robbins MT, DeBerry J, Ness TJ. Chronic psychological stress enhances nociceptive processing in the urinary bladder in high-anxiety rats. Physiol Behav 91: 544– 550, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Robbins MT, Ness TJ. Footshock-induced urinary bladder hypersensitivity: role of spinal corticotropin-releasing factor receptors. J Pain 9: 991– 998, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Roman CW, Lezak KR, Kocho-Schellenberg M, Garret MA, Braas K, May V, Hammack SE. Excitotoxic lesions of the bed nucleus of the stria terminalis (BNST) attenuate the effects of repeated stress on weight gain: evidence for the recruitment of BNST activity by repeated, but not acute, stress. Behav Brain Res 227: 300– 304, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rostamkhani F, Zardooz H, Zahediasl S, Farrokhi B. Comparison of the effects of acute and chronic psychological stress on metabolic features in rats. J Zhejiang Univ Sci B 13: 904– 912, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rothrock NE, Lutgendorf SK, Kreder KJ, Ratliff T, Zimmerman B. Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology 57: 422– 427, 2001 [DOI] [PubMed] [Google Scholar]
- 59. Rudick CN, Chen MC, Mongiu AK, Klumpp DJ. Organ cross talk modulates pelvic pain. Am J Physiol Regul Integr Comp Physiol 293: R1191– R1198, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Saitoh C, Yokoyama H, Chancellor MB, de Groat WC, Yoshimura N. Comparison of voiding function and nociceptive behavior in two rat models of cystitis induced by cyclophosphamide or acetone. Neurourol Urodyn 29: 501– 505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298: R534– R547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Smith AL, Leung J, Kun S, Zhang R, Karagiannides I, Raz S, Lee U, Glovatscka V, Pothoulakis C, Bradesi S, Mayer EA, Rodriguez LV. The effects of acute and chronic psychological stress on bladder function in a rodent model. Urology 78: 967 e961– e967, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Solomon MB, Jones K, Packard BA, Herman JP. The medial amygdala modulates body weight but not neuroendocrine responses to chronic stress. J Neuroendocrinol 22: 13– 23, 2010 [DOI] [PubMed] [Google Scholar]
- 64. Steers WD, Kolbeck S, Creedon D, Tuttle JB. Nerve growth factor in the urinary bladder of the adult regulates neuronal form and function. J Clin Invest 88: 1709– 1715, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Streng T, Santti R, Andersson KE, Talo A. The role of the rhabdosphincter in female rat voiding. BJU Int 94: 138– 142, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Streng T, Santti R, Talo A. Similarities and differences in female and male rat voiding. Neurourol Urodyn 21: 136– 141, 2002 [DOI] [PubMed] [Google Scholar]
- 67. Studeny S, Cheppudira BP, Meyers S, Balestreire EM, Apodaca G, Birder LA, Braas KM, Waschek JA, May V, Vizzard MA. Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)-/- mice. J Mol Neurosci 36: 175– 187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Uvin P, Everaerts W, Pinto S, Alpizar YA, Boudes M, Gevaert T, Voets T, Nilius B, Talavera K, De Ridder D. The use of cystometry in small rodents: a study of bladder chemosensation. J Vis Exp 66: e3869. 10.3791/3869, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain 93: 7– 14, 2001 [DOI] [PubMed] [Google Scholar]
- 70. Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 21: 125– 138, 2001 [DOI] [PubMed] [Google Scholar]
- 71. Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161: 273– 284, 2000 [DOI] [PubMed] [Google Scholar]
- 72. Westropp JL, Buffington CA. In vivo models of interstitial cystitis. J Urol 167: 694– 702, 2002 [DOI] [PubMed] [Google Scholar]
- 73. Wilson SG, Mogil JS. Measuring pain in the (knockout) mouse: big challenges in a small mammal. Behav Brain Res 125: 65– 73, 2001 [DOI] [PubMed] [Google Scholar]
- 74. Wood SK, Baez MA, Bhatnagar S, Valentino RJ. Social stress-induced bladder dysfunction: potential role of corticotropin-releasing factor. Am J Physiol Regul Integr Comp Physiol 296: R1671– R1678, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xu GY, Winston JH, Shenoy M, Yin H, Pendyala S, Pasricha PJ. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology 133: 1282– 1292, 2007 [DOI] [PubMed] [Google Scholar]
- 76. Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci 26: 10847– 10855, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol 7: 9, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zwick M, Molliver DC, Lindsay J, Fairbanks CA, Sengoku T, Albers KM, Davis BM. Transgenic mice possessing increased numbers of nociceptors do not exhibit increased behavioral sensitivity in models of inflammatory and neuropathic pain. Pain 106: 491– 500, 2003 [DOI] [PubMed] [Google Scholar]