Abstract
The cold-sensitive cation channel TRPM8 is a target for menthol, which is used routinely as a cough suppressant and as an additive to tobacco and food products. Given that cold temperatures and menthol activate neurons through gating of TRPM8, it is unclear how menthol actively suppresses cough. In this study we describe the antitussive effects of (−)-menthol in conscious and anesthetized guinea pigs. In anesthetized guinea pigs, cough evoked by citric acid applied topically to the tracheal mucosa was suppressed by menthol only when it was selectively administered as vapors to the upper airways. Menthol applied topically to the tracheal mucosa prior to and during citric acid application or administered continuously as vapors or as an aerosol to the lower airways was without effect on cough. These actions of upper airway menthol treatment were mimicked by cold air delivered to the upper airways but not by (+)-menthol, the inactive isomer of menthol, or by the TRPM8/TRPA1 agonist icilin administered directly to the trachea. Subsequent molecular analyses confirmed the expression of TRPM8 in a subset of nasal trigeminal afferent neurons that do not coincidently express TRPA1 or TRPV1. We conclude that menthol suppresses cough evoked in the lower airways primarily through a reflex initiated from the nose.
Keywords: cough, turbinate, vagal
cold temperatures are sensed by subsets of cold-sensitive mammalian primary afferent neurons through activation of the cation channel TRPM8 (1, 20, 28). TRPM8-expressing neurons constitute a functionally distinct population of neurons specifically dedicated to innocuous cold sensing. Menthol also activates TRPM8, an effect that likely accounts for the cooling sensations associated with topical menthol application. Due in large part to its cooling and soothing effects on mucosal surfaces, menthol has been employed for centuries in medical applications and in consumer products. One of the most common uses of menthol is for the symptomatic treatment of upper and lower airway diseases with cough as a chief complaint (11, 48).
In addition to its therapeutic applications, menthol is also used to enhance the flavor of tobacco. The soothing sensation of menthol in cigarettes has been scrutinized in recent years, because it may disguise the irritant effects of the tobacco smoke, facilitating and rewarding the behavior and thus rendering smoking cessation efforts more difficult (14, 49, 50, 52). Indeed, Wise and colleagues described a counterirritant effect of menthol in the human nose (52), whereas Willis and co-workers (49) recently observed that menthol acts as a counterirritant in mice through activation of TRPM8, providing objective evidence that the pharmacological action of inhaled menthol vapor may reduce the perception of cigarette smoke–induced irritation.
Cough is triggered by airway irritation due to cigarette smoke but may also be suppressed by menthol. The mechanisms of menthol antitussive action are unknown but may include effects on respiratory tract secretion, increased mucosal blood flow, relief of bronchoconstriction, and/or direct or indirect effects on cough-initiating sensory neuronal pathways (29, 49, 51, 52). It should be noted, however, that antitussive effects of menthol are not a universal finding. Cough suppression has been reported in two studies performed by Morice and colleagues (22, 30), and there was perceived benefit in children with nocturnal cough receiving menthol containing vapor rub treatment (37), but Kenia et al. (18) documented no effect of inhaled (−)-menthol on the cough reflex in children, whereas Haidl and colleagues (13) observed no antitussive effects of a 1% (∼30 mM) menthol aerosol prior to bronchoscopy. Perhaps route of administration is critical to the antitussive actions of menthol.
The ability of menthol to activate sensory nerves is difficult to reconcile with cough suppression, at least with a mechanism directly targeting the sensory nerves implicated in cough. Rather, the data suggest an inhibitory effect resulting from the activation of a separate subset of sensory nerves. Excitatory and inhibitory effects of pulmonary and extrapulmonary sensory nerves on the cough reflex have been described, including excitatory effects of activating TRPA1- and/or TRPV1-expressing nasal afferent nerves in animals and in healthy human volunteers (5, 38, 39), and inhibitory effects induced by activation of pulmonary C-fibers (44, 45). In the present study we addressed the hypothesis that menthol suppresses cough secondary to activating subsets of afferent neurons expressing the cation channel TRPM8. We present molecular, physiological, and pharmacological evidence that the antitussive effects of menthol occur secondary to the activation of TRPM8+/TRPV1− nasal trigeminal afferent neurons.
METHODS
All experiments were performed on male Dunkin Hartley guinea pigs (250–350 g, pathogen free; Harlan, Boxmeer, the Netherlands), and all study protocols were first approved by local ethical and animal care and use committees, and conformed to animal welfare guidelines.
Citric acid–induced cough in awake guinea pigs.
Awake animals were placed in a double-chambered body plethysmograph (type 855; Hugo Sachs Electronik, Germany) and were exposed to 0.4 M citric acid aerosol (Fisher, Slovakia) for 10 min. This design allows for simultaneous recordings of cough and lung mechanics. The aerosol was generated via a jet nebulizer (Pariprovocation test I; Pari Starneberg, Germany) and delivered to the head part of the body plethysmograph. Aerosols (median particle size diameter 1.2 μm) were evacuated from the chamber with the same rate of aerosol delivery (5 liters/min) by vacuum.
Analog recordings (Multiscriptor Hellige 21; Germany) of respiratory reflexes and breathing pattern were monitored using a pneumotachograph (Godart, Germany) with a Fleish head connected to the head chamber. Cough was detected using a microphone placed in the roof of the head chamber and connected to a tape recorder. Cough was defined as a sudden enhancement of expiratory airflow accompanied by typical cough sound, with responses expressed as the total number of coughs induced during challenge. The number of elicited cough efforts was concurrently counted and finally analyzed by two independent and experienced researchers, with one of the evaluators blinded to the procedures performed. Cough responses were evaluated in each animal after exposure to room air or menthol vapors, with challenges performed 1 wk apart [using methods comparable to those described by Laude et al. (22)]. In separate experiments coughing evoked by citric acid (0.01–0.3 M) was studied before and 30 min after oral administration (gavage) of dissolved menthol (30–100 mg/kg) or an equivalent volume of the vehicle (10% ethanol in water) in an unpaired experimental design.
Cough challenge in anesthetized guinea pigs.
Male Dunkin Hartley guinea pigs (250–350 g, pathogen free; Harlan, Indianapolis, IN) were anesthetized with urethane (1.5 g/kg ip). The adequacy of the anesthesia was assessed by monitoring withdrawal responses to a sharp pinch of a hind limb or responses during surgery. When the experiments were completed, animals were euthanized by asphyxiation in a vessel filled with carbon dioxide.
Anesthetized guinea pigs were secured supine on a warming pad. A midline incision in the neck exposed the trachea, which was cannulated at its caudal-most end with a bent luer stub adaptor. The cannula, placed approximately equidistant from the larynx and carina (1–2 cm), was attached to a length of tubing that terminated inside a water-jacketed organ bath continuously filled with humidified and warmed air (this design serves as an artificial warming and humidifying nose). The innervation and vasculature of the trachea were carefully preserved throughout the dissection. A pressure transducer attached to a side port in the tracheal cannula monitored respiratory efforts that were recorded digitally (Biopac, Santa Barbara, California). Once the trachea was cannulated, the remaining rostral segment of the extrathoracic trachea was opened lengthwise. Polyethylene (PE) tubing was threaded through the upper airways via the larynx. Warmed, oxygenated Krebs buffer comprising (in mM) 118 NaCl, 5.4 KCl, 1 NaHPO4, 1.2 MgSO4, 1.9 CaCl2, 25 NaHCO3, and 11.1 dextrose pH 7.4, was superfused (3 ml/min) over the tracheal mucosa. The cyclooxygenase inhibitor indomethacin (3 μM) was added to the perfusate to limit formation of neuromodulatory prostanoids, although we have shown previously that cyclooxygenase inhibition does not modulate cough responses to citric acid in anesthetized animals (6) or coughing evoked by bradykinin in conscious animals (unpublished observations). The buffer was introduced into the tracheal lumen from the caudal-most exposed segment of the trachea and removed at the rostral end of the trachea by attaching the PE tubing threaded through the upper airways to a vacuum.
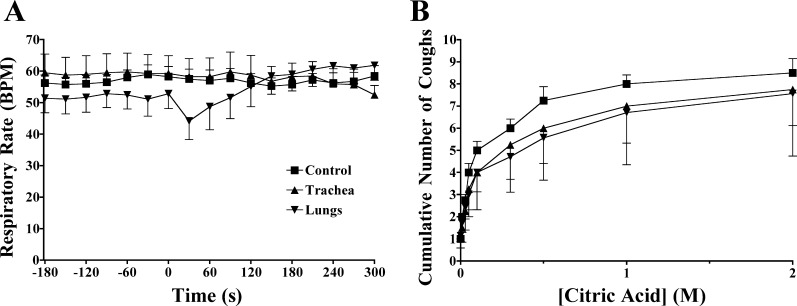
After a 5-min equilibration period, coughing was evoked by applying citric acid topically to the tracheal mucosa. Citric acid (0.001–2 M) was dissolved in water and applied in 100-μl aliquots directly into the Krebs buffer perfusing the trachea. Concentration response curves were constructed in an ascending fashion, with doses administered at 1-min intervals. Cough was defined on the basis of visual confirmation of a cough-like respiratory effort and a change in tracheal pressure that produced a >500% increase in peak expiratory pressure preceded by an enhanced inspiratory effort all in <1 s.
Coughing was evoked in control animals and in animals coincidentally challenged with menthol or cold air. Menthol vapors were delivered selectively to the upper airways through a cannula placed in the nostril and connected to an air pump (30°C air source) or directly to the lower airways via the tracheal cannula. A 130-ml chamber containing 150 mg of menthol crystals was connected in series with the air pump and nasal cannula (36, 42). Menthol vapors were administered to the lower airways using the same chamber connected to the air pump supplying fresh air to the artificial nose. Menthol (0.1 mM) was also delivered selectively to the trachea by addition to the tracheal perfusate. We also studied the effects of delivering frigid air (generated through 1 meter of tubing placed in an ice bath and connected to the air pump) to the upper or lower airways on breathing pattern and citric acid–evoked coughing. Icilin (10 μM) was administered by addition to the tracheal perfusate or by bolus administration to the nose. Changes in breathing pattern in response to these stimuli and the number of coughs evoked by citric acid during their administration was recorded using an unpaired experimental design.
Retrograde neuronal tracing and single-cell RT-PCR analysis.
In brief anesthesia, the fluorescent tracer DiI (dissolved in DMSO to 2% and subsequently diluted in saline to 0.05%; Invitrogen, Carlsbad, California) was injected into the nasal turbinate unilaterally at two sites according the methods described by Taylor-Clark et al. (46). In separate animals, neurons innervating the lower airways were labeled by DiI injection into the trachea or intrapulmonary airways. Seven to 14 days later the animals were euthanized by overdose and exsanguination, the trigeminal ganglia or jugular and nodose ganglia were harvested, and the ganglia were then incubated in enzyme buffer (2 mg/ml collagenase type 1A and 2 mg/ml dispase II in Ca2+-, Mg2+-free Hanks' balanced salt solution) for 30 min at 37°C. Neurons were then dissociated by trituration with three glass Pasteur pipettes of decreasing tip pore size, washed, suspended in l-15 medium containing 10% fetal bovine serum (l-15/FBS) and transferred onto Poly-d-Lysine/Laminin-coated coverslips. After the suspended neurons had adhered to the coverslips for 2 h, DiI-labeled neurons identified via fluorescent microscopy were individually harvested into a glass pipette (tip 50–150 μm) pulled with a micropipette puller (P-87, Sutter) by applying negative pressure. Single-cell RT-PCR studies were performed on labeled neurons as described previously (21, 33, 34).
Statistical analyses.
Results are presented as a mean ± SE of n experiments, where n refers to a single animal. The majority of the experiments were performed using an unpaired experimental design. Differences in group means were compared by t-test ANOVA and compared post hoc using Scheffé's test for unplanned comparisons. P < 0.05 was considered statistically significant.
Reagents.
Citric acid and urethane were purchased from Sigma (St. Louis, MO). Icilin was purchased from Tocris (Minneapolis, MN). Menthol (+) and (−) were purchased from MP Biochemicals (Aurora, OH).
RESULTS
Menthol inhibits cough in awake guinea pigs.
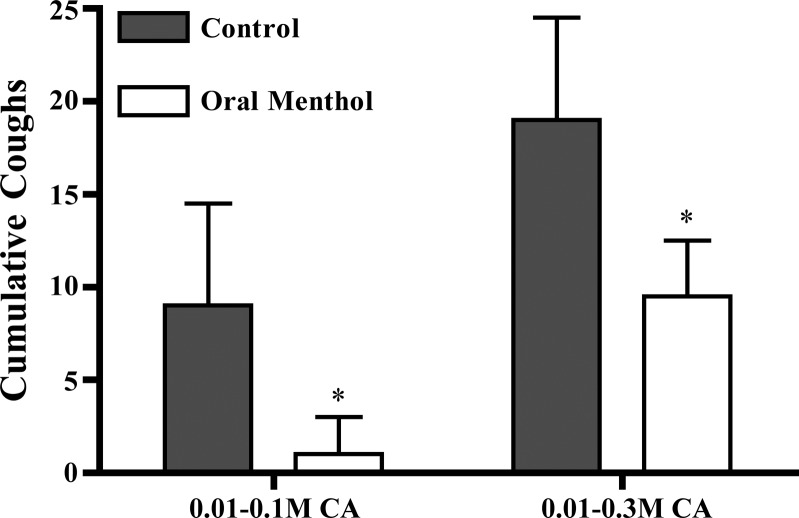
Orally administered menthol (100 mg/kg) significantly inhibited cough evoked by citric acid. Coughing evoked by low doses of citric acid (0.01–0.1 M) and the cumulative coughs evoked by all doses of citric acid (0.01–0.3 M) were inhibited. Overall, citric acid evoked 19 ± 2 coughs in control animals and 10 ± 2 coughs following orally administered menthol (n = 10–18; Fig. 1; P < 0.05). A lower dose of menthol (30 mg/kg) administered orally was without effect on cough (17 ± 4 coughs; n = 4; P > 0.1).
Fig. 1.
Menthol (100 mg/kg, p.o.) inhibited coughing evoked by citric acid (CA). Control animals received the vehicle for menthol (water). Each dose of CA (0.01–0.3 M) was administered by aerosol for 5 min, with 5-min intervals in between doses. Results are presented as mean ± SE cumulative coughs evoked by the lower concentrations of CA studied (0.01–0.1 M) and mean ± SE cumulative coughs evoked by all concentrations of CA (0.01–0.3 M). *Indicates that menthol significantly inhibited the coughing evoked by CA (P < 0.05; n = 10–18).
We also studied the effects of menthol vapor on 0.4 M citric acid–evoked coughing using our two-chamber plethysmograph design. Under these conditions, responses were variable and thus the menthol vapor fell just short of producing a statistically significant inhibition of citric acid–evoked coughing (13 ± 1 vs. 8 ± 2 coughs at baseline and during menthol challenge, respectively; n = 10; P = 0.05).
Effect of cold air, menthol, and icilin on breathing pattern and cough.
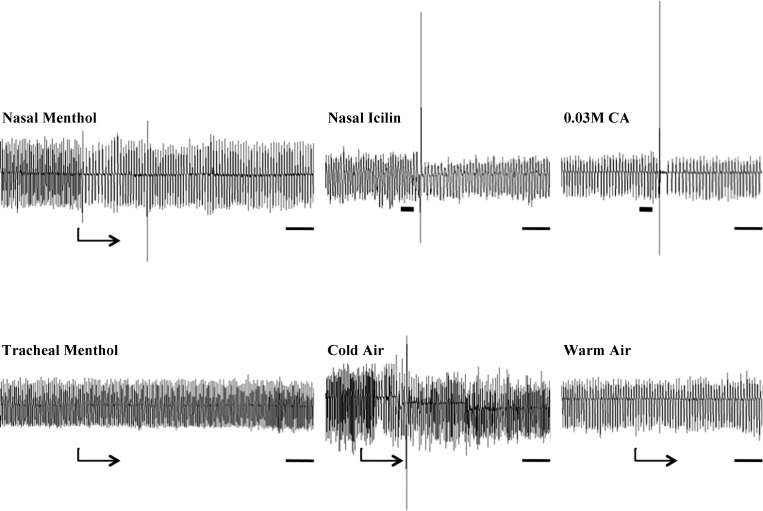
Cold (∼10°C) or warm (30°C) air was delivered to the nasal cavity of anesthetized guinea pigs through a nasal cannula. Flows were generated by an air pump at a rate of ∼30 ml/min, which is within the physiological range for guinea pigs. Control animals had the nasal cannula (without airflow) introduced into one nostril. Cold air significantly, acutely, and persistently decreased breathing rate (∼20% drop). Warm air (30°C) produced only modest (<10%), variable, and thus statistically insignificant effects on respiratory rate (Figs. 2 and 3). Adding (−)-menthol vapors to the warm air challenges produced a marked slowing of respiratory rate, comparable to the effects of cold air. Vapors of the inactive isomer, (+)-menthol, had little or no effect on respiratory rate (Figs. 2 and 4).
Fig. 2.
Representative traces of the respiratory reflex effects evoked by menthol, cold air, icilin, and citric acid (CA) administered to anesthetized guinea pigs. Menthol was delivered selectively to the nasal passages as vapors in warm air or administered to the trachea by addition to the tracheal perfusate (0.1 mM). Icilin (30 μM) was administered in 100-μl aliquots dissolved in saline directly and unilaterally into the nose. Note the respiratory slowing and augmented breaths evoked by nasal challenges with menthol, icilin, and cold air, but the modest or absent responses associated with warm air challenges to the nasal passages and tracheal mucosa. The onset of continuous challenges is denoted by the arrows under each trace, whereas the time of bolus challenges to the nose (icilin) or trachea (CA) are denoted by thick, horizontal bars. These stimuli were delivered prior and throughout subsequent CA challenges, which was applied topically to the tracheal mucosa, evoking cough. The cough-like respiratory reflex associated with nasal icilin challenges may be due to TRPA1 activation coincident with TRPM8 activation, rendering this stimulus of limited utility for hypothesis testing. The horizontal bars at the beginning of each trace denote 5 and 10 s in the upper and lower traces, respectively.
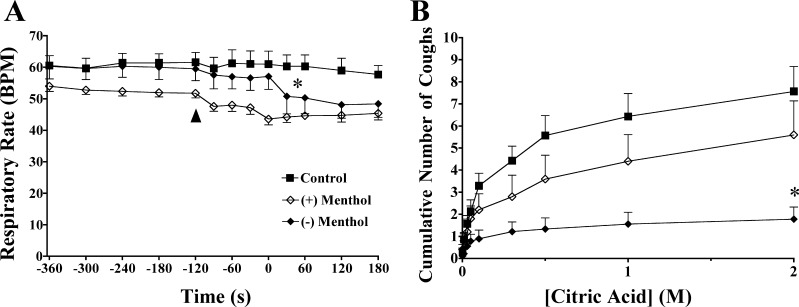
Fig. 3.
Nasal (−)-menthol vapor evoked respiratory slowing (A) and markedly inhibited citric acid–evoked coughing (B) in anesthetized guinea pigs, whereas nasal (+)-menthol challenges were without marked effects. The menthol vapors (carried by warm air) were delivered via a nasal cannula that was inserted into a nostril 2 min prior to (as indicated by the arrow) initiation of the challenges (which began at time 0). *Indicates a statistically significant decrease in respiratory rate and a statistically significant inhibition of citric acid–evoked coughing. Each data point represents the mean ± SE of 4–9 experiments.
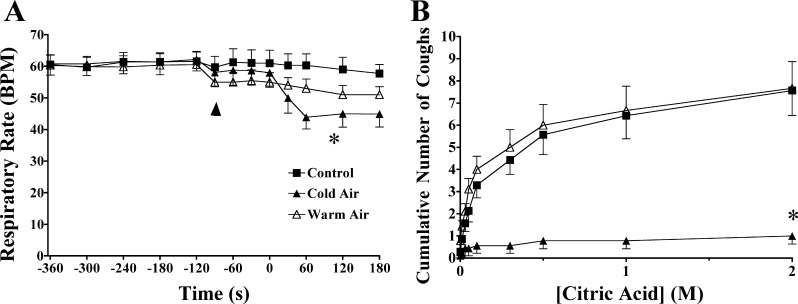
Fig. 4.
Nasal cold air challenge evoked respiratory slowing (A) and markedly inhibited citric acid–evoked coughing (B) in anesthetized guinea pigs, whereas warm air was without marked effects. The air was delivered via a nasal cannula that was inserted into a nostril 2 min prior to (as indicated by the arrow) initiation of the challenges (which began at time 0). *Indicates a statistically significant decrease in respiratory rate and a statistically significant inhibition of citric acid–evoked coughing. Each data point represents the mean ± SE of 7–9 experiments.
Cold air flow or (−)-menthol vapors delivered selectively to the nose nearly abolished coughing evoked by citric acid applied topically to the tracheal mucosa (Figs. 3 and 4). No significant effects of warm air or the less active isomer (+)-menthol were apparent, with citric acid evoking 8 ± 1, 7 ± 1, and 6 ± 1 coughs in control, warm air, and nasal (+)-menthol treated animals, respectively (P > 0.1).
The route of menthol delivery profoundly influenced its effects on respiratory rate and cough. Thus, in contrast to the inhibitory effects of nasal cold air and nasal (−)-menthol vapors on breathing rate, menthol delivered directly to the tracheal mucosa produced no consistent changes in breathing pattern (Fig. 2). If anything, menthol delivered to the intrapulmonary airways as vapors or as an aerosol increased respiratory rate while producing audible sounds of wheezing and evidence of increased airway secretions in some animals. Neither of these routes of menthol delivery inhibited citric acid–evoked coughing (Fig. 5).
Fig. 5.
Menthol failed to evoke respiratory slowing and was without effect on citric acid–evoked coughing when administered directly to the tracheal mucosa (0.1 mM) or when delivered as an aerosol to the intrapulmonary airways. Control animals in these figures received the vehicle for menthol delivered via the tracheal perfusate. Each data point represents the mean ± SE of 4–7 experiments.
Icilin, which like menthol activates TRPM8 (but also TRPA1), failed to alter breathing pattern when delivered selectively to the tracheal mucosa and did not inhibit coughing induced by citric acid. On the contrary, tracheal icilin administration enhanced citric acid–evoked coughing (9 ± 2 and 16 ± 1 coughs following vehicle or 10 μM icilin administration to the tracheal perfusate, respectively; n = 5–7; P < 0.05). This effect of icilin may be attributable to TRPA1-dependent activation of the jugular C-fibers innervating the trachea, which we have previously shown to sensitize cough responses evoked by citric acid challenge in anesthetized guinea pigs (26). Nasal challenge with a 100-μl bolus of 30 μM icilin evoked coughing immediately upon administration in two of three guinea pigs studied followed by respiratory slowing comparable to that evoked by menthol (Fig. 2). Due to its nonselective actions on TRPA1 and TRPM8, the effects of icilin on citric acid–evoked coughing were not extensively studied.
TRPM8 gene expression in trigeminal and vagal afferent neurons.
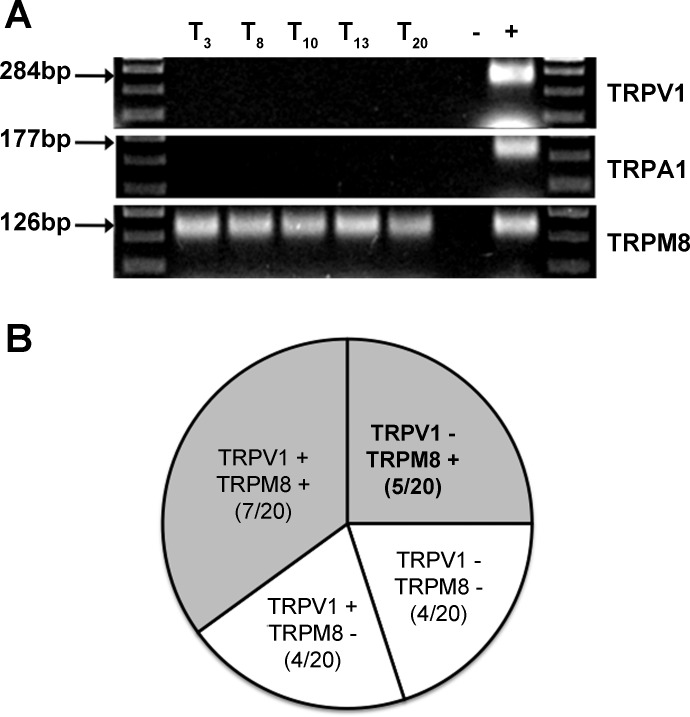
Using the single-cell RT-PCR technique we found that 60% (12/20) of labeled trigeminal nasal afferent neurons express mRNA for TRPM8. The TRPM8 gene was co-expressed with TRPV1 and TRPA1 mRNA in 7 of these 12 neurons, and they likely represent nasal nociceptors (35% of the overall labeled population). We have shown previously that either TRPV1 or TRPA1 activation in the nose enhances cough responsiveness in animals and in patients (5, 38, 39), thus it seems unlikely that these TRPM8-expressing nasal afferent neurons account for the inhibitory effects of nasal cold air or menthol challenges. The remaining five TRPM8+ neurons (25% of the overall population) did not express TRPV1 or TRPA1, and these neurons are likely the cold and menthol-sensitive nasal trigeminal afferents that initiate the inhibitory effects of menthol on cough (Fig. 6).
Fig. 6.
A majority (12/20) of trigeminal ganglia neurons retrogradely labeled from the nasal mucosa express mRNA for the menthol, icilin, and cold-sensitive cation channel TRPM8 as assessed by single-cell RT-PCR. Roughly half of these TRPM8+ neurons co-expressed mRNA for the capsaicin, heat, and proton-sensitive cation channel TRPV1. All TRPV1-expressing neurons (11/11) co-expressed the noxious cold-sensitive cation channel TRPA1 (data not shown). Because nasal TRPA1 or TRPV1 activation enhances cough responsiveness in animals and humans (5, 38, 39), we speculate that the inhibitory effects of nasal menthol and cold air challenge of the nasal mucosa depends on the activation of the TRPM8+/TRPV1-/TRPV1-subpopulation of neurons innervating the nasal mucosa.
Consistent with most previous studies (33, 54, 57) and consistent with the lack of effects of menthol on citric acid–evoked coughing when delivered to the lower airways, none of the retrogradely labeled TRPV1-nodose ganglia neurons (0/6) and few of the retrogradely labeled jugular ganglia neurons (2/5) innervating the trachea or intrapulmonary airways expressed TRPM8. Interestingly, a majority of the retrogradely labeled TRPV1+ nodose ganglia neurons (6/8) innervating the airways and lungs were found to express TRPM8.
DISCUSSION
Inhaled menthol exerts complex olfactory, sensory, and respiratory effects in patients and in animals (9, 35, 41). Menthol has also been reported to inhibit coughing. The goal of the present study was to determine the mechanism by which menthol prevents coughing in a relevant animal model.
We confirmed the results of Laude et al. (22), documenting the antitussive effects of orally administered (−)-menthol. Likely due to the variability of the citric acid challenge model, menthol vapor treatments of conscious animals failed to achieve statistical significance (P = 0.05, not < 0.05), but overall, our results confirm previous results by revealing an inhibitory effect of menthol on cough.
Guinea pigs are obligatory nasal breathers and so when they are exposed to vapors or aerosols, a considerable proportion of aerosolized substances are deposited onto the nasal mucosa (23). The nose is thus a likely target for menthol action when administered as an aerosol or vapor, and also perhaps when administered orally. Thus, nasal effects after ingestion of substances has been documented in gustatory rhinitis, which is characterized by watery rhinorrhea after eating pungent foods (e.g., wasabi, which contains the TRPA1 activator allylisothiocyanate). These responses most likely occur following stimulation of trigeminal sensory nerve endings in the upper aerodigestive tract (16).
Several lines of experimental evidence suggest that menthol prevents cough through activation of TRPM8. Thus, in addition to menthol, we observed that cold air inhalation also inhibited coughing. The less active isomer of menthol, (+)-menthol (10), was without effect on citric acid–evoked coughing when administered to the nose. Menthol could conceivably be antitussive through effects on TRPA1, but we found that menthol modulated cough in our anesthetized animals only in airways in which nerves consistently express TRPM8 (the upper airways). In these upper airways, the neurons expressing TRPA1 also express TRPV1, and we have shown previously that either TRPA1 or TRPV1 activation in the nose enhances subsequently evoked cough responses (5, 38, 39). It is difficult to envision a scenario in which activation of the same nerve subtypes could both enhance and inhibit subsequently evoked coughing. Thus, although we like other investigators (36, 42) are hampered in our ability to study multiple doses of menthol administered as vapors and by solubility limitations of menthol in our oral dosing studies, we believe the data argue in favor of a TRPM8-dependent antitussive effect of menthol on cough. Perhaps with the recent reports of potent and selective blockers of TRPM8 (2, 19, 25), which are at present not available commercially, this hypothesis may soon be addressed using pharmacological approaches.
Our results generated in anesthetized animals suggest that the antitussive effects of menthol occur through a reflex initiated from the nose. Neurophysiological, immunohistochemical, and molecular analyses have documented TRPM8 expression in nasal trigeminal afferents but minimal expression of TRPM8 by bronchopulmonary vagal afferents (11, 33, 54, 55). Consistent with these previous studies, we found that menthol and cold air delivered selectively to the nose inhibited coughing evoked by citric acid applied topically to the tracheal mucosa, whereas administering menthol directly to the trachea or delivering menthol to the intrathoracic airways had no effect on citric acid–evoked cough. Icilin applied topically to the tracheal mucosa also failed to inhibit cough.
As discussed above, menthol and icilin may also activate the polymodal irritant receptor and ion channel TRPA1, which may account for the burning sensations occasionally reported in studies of menthol challenge (16, 32, 52). Our molecular analyses identified at least two populations of TRPM8-expressing nasal trigeminal afferent neurons, with one population co-expressing TRPA1 and TRPV1. Such patterns of TRP channel expression have been noted previously (1, 3, 20, 43, 53, 56). We speculate that TRPM8-dependent inhibitory actions of these stimuli on cough depend upon the activation of the nasal trigeminal afferent neurons that do not co-express TRPA1 or TRPV1. We have shown previously in guinea pigs, cats, and human subjects that TRPA1 or TRPV1 stimulation in the nose enhances cough responsiveness (4, 5, 38–40); this argues against an antitussive effect of menthol transduced through TRPV1 and TRPM8-expressing neurons. In fact, we observed coughing in two of three animals challenged with nasal icilin.
Our studies do not rule out modulatory effects of menthol and cold air transduced through activation of vagal afferent nerves. If anything, icilin applied topically to the tracheal mucosa and menthol delivered selectively to intrathoracic airways tended to enhance cough responsiveness. The sensitizing effects of icilin on cough might be attributed to a TRPA1-dependent activation of the TRPV1/TRPA1 expressing jugular C-fibers (26, 31), which we show here may also express TRPM8. Adverse effects of menthol have been reported (8, 24, 49, 52).
We conclude that menthol suppresses cough by a reflex transduced through TRPM8-dependent activation of nasal trigeminal afferent neurons. These effects of menthol were apparent in conscious animals challenged with citric acid aerosols, a cough that likely depends on TRPV1-dependent activation of tachykinin-containing jugular C-fibers (7, 12, 31, 47), and in anesthetized guinea pigs, when coughing was evoked by citric acid applied in bolus challenges directly to the tracheal mucosa, which depends on TRPV1-independent activation of the acid-sensitive, mechanically sensitive cough receptors (6, 27). Menthol may thus be a broadly effective antitussive, preventing cough responses evoked by multiple mechanisms and pathways. These observations support the validity of upper airway menthol vapors in controlling cough, an approach that may be clinically effective in children (37). It is unclear whether comparable therapeutic benefits can be achieved in adults. Our results do not rule out additional actions of menthol and cold air transduced through effects on other airway or extrapulmonary afferent nerves, or through TRPM8-independent mechanisms. Such studies await the availability of more selective activators and blockers of TRPM8. The central inhibitory pathways connecting the upper airways to cough pattern generation also await further analysis (40, 41, 54, 55).
GRANTS
J. Plevkova is supported by VEGA Grant 1/0031/11 and by the Center of Experimental and Clinical Respirology (CEKR), which is funded by the European Union. This work was also supported by National Heart, Lung, and Blood Institute Grant HL083192 to B. J. Canning.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.P., M.K., I.P., M.T., and B.J.C. conception and design of research; J.P., M.B., L.S., and N.M. performed experiments; J.P., M.K., I.P., M.B., L.S., M.T., N.M., and B.J.C. analyzed data; J.P., M.K., I.P., M.B., L.S., M.T., and B.J.C. interpreted results of experiments; J.P., M.K., L.S., N.M., and B.J.C. prepared figures; J.P. and B.J.C. drafted manuscript; J.P. and B.J.C. edited and revised manuscript; J.P., M.K., I.P., M.B., L.S., M.T., N.M., and B.J.C. approved final version of manuscript.
REFERENCES
- 1. Abe J, Hosokawa H, Okazawa M, Kandachi M, Sawada Y, Yamanaka K, Matsumura K, Kobayashi S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res Mol Brain Res 136: 91–98, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, Garami A, Bautista D, Gavva NR, Romanovsky AA. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci 32: 2086–2099, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Brozmanova M, Plevkova J, Tatar M, Kollarik M. Cough reflex sensitivity is increased in the guinea pig model of allergic rhinitis. J Physiol Pharmacol 59, Suppl 6: 153–161, 2008 [PubMed] [Google Scholar]
- 5. Buday T, Brozmanova M, Biringerova Z, Gavliakova S, Poliacek I, Calkovsky V, Shetthalli MV, Plevkova J. Modulation of cough response by sensory inputs from the nose - role of trigeminal TRPA1 versus TRPM8 channels. Cough 8: 11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Canning BJ, Mori N, Farmer DG. Mechanistic studies of acid-evoked coughing in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 291: R454–R463, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Daoui S, Cognon C, Naline E, Emonds-Alt X, Advenier C. Involvement of tachykinin NK3 receptors in citric acid-induced cough and bronchial responses in guinea pigs. Am J Respir Crit Care Med 158: 42–48, 1998 [DOI] [PubMed] [Google Scholar]
- 8. dos Santos MA, Santos Galvão CE, Morato Castro F. Menthol-induced asthma: a case report. J Investig Allergol Clin Immunol 11: 56–58, 2001 [PubMed] [Google Scholar]
- 9. Eccles R. Menthol: effects on nasal sensation of airflow and the drive to breathe. Curr Allergy Asthma Rep 3: 210–214, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Eccles R, Griffiths DH, Newton CG, Tolley NS. The effects of D and L isomers of menthol upon nasal sensation of airflow. J Laryngol Otol 102: 506–508, 1988 [DOI] [PubMed] [Google Scholar]
- 11. Fisher JT. TRPM8 and dyspnea: from the frigid and fascinating past to the cool future? Curr Opin Pharmacol 11: 218–223, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Girard V, Naline E, Vilain P, Emonds-Alt X, Advenier C. Effect of the two tachykinin antagonists, SR 48968 and SR 140333, on cough induced by citric acid in the unanaesthetized guinea pig. Eur Respir J 8: 1110–1114, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Haidl P, Kemper P, Butnarasu SJ, Klauke M, Wehde H, Köhler D. Does the inhalation of a 1% L-menthol solution in the premedication of fiberoptic bronchoscopy affect coughing and the sensation of dyspnea? Pneumologie 55: 115–119, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Hoffman AC. The health effects of menthol cigarettes as compared to non-menthol cigarettes. Tob Induc Dis 9, Suppl 1: S7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hondoh A, Ishida Y, Ugawa S, Ueda T, Shibata Y, Yamada T, Shikano M, Murakami S, Shimada S. Distinct expression of cold receptors (TRPM8 and TRPA1) in the rat nodose-petrosal ganglion complex. Brain Res 1319: 60–69, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Jovancevic L, Georgalas C, Savovic S, Janjevic D. Gustatory rhinitis. Rhinology 48: 7–10, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci 27: 9874–9884, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kenia P, Houghton T, Beardsmore C. Does inhaling menthol affect nasal patency or cough? Pediatr Pulmonol 43: 532–537, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Knowlton WM, Daniels RL, Palkar R, McCoy DD, McKemy DD. Pharmacological blockade of TRPM8 ion channels alters cold and cold pain responses in mice. PLoS One 6: e25894, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol 493: 596–606, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Kwong K, Kollarik M, Nassenstein C, Ru F, Undem BJ. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol 295: L858–L865, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laude EA, Morice AH, Grattan TJ. The antitussive effects of menthol, camphor and cineole in conscious guinea-pigs. Pulm Pharmacol 7: 179–184, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Lippmann M, Schlesinger RB. Toxicological bases for the setting of health-related air pollution standards. Annu Rev Public Health 21: 309–333, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Marlowe KF. Urticaria and asthma exacerbation after ingestion of menthol-containing lozenges. Am J Health Syst Pharm 60: 1657–1659, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Matthews JM, Qin N, Colburn RW, Dax SL, Hawkins M, McNally JJ, Reany L, Youngman MA, Baker J, Hutchinson T, Liu Y, Lubin ML, Neeper M, Brandt MR, Stone DJ, Flores CM. The design and synthesis of novel, phosphonate-containing transient receptor potential melastatin 8 (TRPM8) antagonists. Bioorg Med Chem Lett 22: 2922–2926, 2012 [DOI] [PubMed] [Google Scholar]
- 26. Mazzone SB, Mori N, Canning BJ. Synergistic interactions between airway afferent nerve subtypes regulating the cough reflex in guinea-pigs. J Physiol 569, Pt 2: 559–573, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mazzone SB, Reynolds SM, Mori N, Kollarik M, Farmer DG, Myers AC, Canning BJ. Selective expression of a sodium pump isozyme by cough receptors and evidence for its essential role in regulating cough. J Neurosci 29: 13662–13671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCoy DD, Knowlton WM, McKemy DD. Scraping through the ice: uncovering the role of TRPM8 in cold transduction. Am J Physiol Regul Integr Comp Physiol 300: R1278–R1287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Millqvist E, Ternesten-Hasséus E, Bende M. Inhalation of menthol reduces capsaicin cough sensitivity and influences inspiratory flows in chronic cough. Respir Med 107: 433–438, 2013 [DOI] [PubMed] [Google Scholar]
- 30. Morice AH, Marshall AE, Higgins KS, Grattan TJ. Effect of inhaled menthol on citric acid induced cough in normal subjects. Thorax 49: 1024–1026, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muroi Y, Ru F, Chou YL, Carr MJ, Undem BJ, Canning BJ. Selective inhibition of vagal afferent nerve pathways regulating cough using NaV1.7 shRNA silencing in guinea pig nodose ganglia. Am J Physiol Regul Integr Comp Physiol. First published April 10, 2013; 10.1152/ajpregu.00028.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Namer B, Seifert F, Handwerker HO, Maihöfner C. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. Neuroreport 16: 955–959, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol 586: 1595–1604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nassenstein C, Taylor-Clark TE, Myers AC, Ru F, Nandigama R, Bettner W, Undem BJ. Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. J Physiol 588, Pt 23: 4769–4783, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nishino T, Tagaito Y, Sakurai Y. Nasal inhalation of l-menthol reduces respiratory discomfort associated with loaded breathing. Am J Respir Crit Care Med 156: 309–313, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Orani GP, Anderson JW, Sant'Ambrogio G, Sant'Ambrogio FB. Upper airway cooling and l-menthol reduce ventilation in the guinea pig. J Appl Physiol 70: 2080–2086, 1991 [DOI] [PubMed] [Google Scholar]
- 37. Paul IM, Beiler JS, King TS, Clapp ER, Vallati J, Berlin CM., Jr Vapor rub, petrolatum, and no treatment for children with nocturnal cough and cold symptoms. Pediatrics 126: 1092–1099, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Plevkova J, Brozmanova M, Pecova R, Tatar M. Effects of intranasal capsaicin challenge on cough reflex in healthy human volunteers. J Physiol Pharmacol 55, Suppl 3: 101–106, 2004 [PubMed] [Google Scholar]
- 39. Plevkova J, Kollarik M, Brozmanova M, Revallo M, Varechova S, Tatar M. Modulation of experimentally-induced cough by stimulation of nasal mucosa in cats and guinea pigs. Respir Physiol Neurobiol 142: 225–235, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Plevkova J, Poliacek I, Antosiewicz J, Adamkov M, Jakus J, Svirlochova K, Tatar M. Intranasal TRPV1 agonist capsaicin challenge and its effect on c-fos expression in the guinea pig brainstem. Respir Physiol Neurobiol 173: 11–15, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Redolfi S, Raux M, Donzel-Raynaud C, Morelot-Panzini C, Zelter M, Derenne JP, Similowski T, Straus C. Effects of upper airway anaesthesia on respiratory-related evoked potentials in humans. Eur Respir J 26: 1097–1103, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Sekizawa S, Tsubone H, Kuwahara M, Sugano S. Nasal receptors responding to cold and l-menthol airflow in the guinea pig. Respir Physiol 103: 211–219, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Tatar M, Webber SE, Widdicombe JG. Lung C-fibre receptor activation and defensive reflexes in anaesthetized cats. J Physiol 402: 411–420, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tatar M, Sant'Ambrogio G, Sant'Ambrogio FB. Laryngeal and tracheobronchial cough in anesthetized dogs. J Appl Physiol 76: 2672–2679, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Taylor-Clark TE, Kollarik M, MacGlashan DW, Jr, Undem BJ. Nasal sensory nerve populations responding to histamine and capsaicin. J Allergy Clin Immunol 116: 1282–1288, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Trevisani M, Milan A, Gatti R, Zanasi A, Harrison S, Fontana G, Morice AH, Geppetti P. Antitussive activity of iodo-resiniferatoxin in guinea pigs. Thorax 59: 769–772, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Voets T, Owsianik G, Nilius B. TRPM8. Handb Exp Pharmacol 179: 329–344, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Willis DN, Liu B, Ha MA, Jordt SE, Morris JB. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J 25: 4434–4444, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Winickoff JP, McMillen RC, Vallone DM, Pearson JL, Tanski SE, Dempsey JH, Healton C, Klein JD, Abrams D. US attitudes about banning menthol in cigarettes: results from a nationally representative survey. Am J Public Health 101: 1234–1236, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wise PM, Breslin PA, Dalton P. Sweet taste and menthol increase cough reflex thresholds. Pulm Pharmacol Ther 25: 236–241, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wise PM, Preti G, Eades J, Wysocki CJ. The effect of menthol vapor on nasal sensitivity to chemical irritation. Nicotine Tob Res 13: 989–997, 2011 [DOI] [PubMed] [Google Scholar]
- 53. Xing H, Ling J, Chen M, Gu JG. Chemical and cold sensitivity of two distinct populations of TRPM8-expressing somatosensory neurons. J Neurophysiol 95: 1221–1230, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Xing H, Ling JX, Chen M, Johnson RD, Tominaga M, Wang CY, Gu J. TRPM8 mechanism of autonomic nerve response to cold in respiratory airway. Mol Pain 4: 22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zanotto KL, Merrill AW, Carstens MI, Carstens E. Neurons in superficial trigeminal subnucleus caudalis responsive to oral cooling, menthol, and other irritant stimuli. J Neurophysiol 97: 966–978, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Zhao H, Sprunger LK, Simasko SM. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am J Physiol Gastrointest Liver Physiol 298: G212–G221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou Y, Sun B, Li Q, Luo P, Dong L, Rong W. Sensitivity of bronchopulmonary receptors to cold and heat mediated by transient receptor potential cation channel subtypes in an ex vivo rat lung preparation. Respir Physiol Neurobiol 177: 327–332, 2011 [DOI] [PubMed] [Google Scholar]