Abstract
Stent deployments with geographical miss (GM) are associated with increased risk of target-vessel revascularization and periprocedural myocardial infarction. The aim of the current study was to investigate the underlying biomechanical mechanisms for adverse events with GM. The hypothesis is that stent deployment with GM [longitudinal GM, or LGM (i.e., stent not centered on the lesion); or radial GM, RGM (i.e., stent oversizing)] results in unfavorable fluid wall shear stress (WSS), WSS gradient (WSSG), oscillatory shear index (OSI), and intramural circumferential wall stress (CWS). Three-dimensional computational models of stents and plaque were created using a computer-assisted design package. The models were then solved with validated finite element and computational fluid dynamic packages. The dynamic process of large deformation stent deployment was modeled to expand the stent to the desired vessel size. Stent deployed with GM resulted in a 45% increase in vessel CWS compared with stents that were centered and fully covered the lesion. A 20% oversized stent resulted in 72% higher CWS than a correct sized stent. The linkages between the struts had much higher stress than the main struts (i.e., 180 MPa vs. 80 MPa). Additionally, LGM and RGM reduced endothelial WSS and increased WSSG and OSI. The simulations suggest that both LGM and RGM adversely reduce WSS but increase WSSG, OSI, and CWS. These findings highlight the potential mechanical mechanism of the higher adverse events and underscore the importance of stent positioning and sizing for improved clinical outcome.
Keywords: geographic miss, restenosis, STLLR trial, adverse events, target-vessel revascularization
although the term geographic miss (GM) was first introduced to describe failure to fully cover the diseased arterial segment with intravascular radiation therapy, the GM phenomenon may also account for some stent adverse effects (13). The STLLR trial on the effect of stent deployment techniques on clinical outcomes was a multicenter prospective study that found that suboptimal drug-eluting stent (DES) deployment with geographical miss was associated with increased risk of target-vessel revascularization (TVR) defined as any repeated intervention or bypass surgery of the target vessel and myocardial infarction (MI) (13). The trial included 1,557 patients in 41 U.S. hospitals and found that 67% of the patients had GM [longitudinal GM, LGM (i.e., stent not centered on the lesion); and/or radial GM, RGM (i.e., stent oversizing)]. The TVR rates in patients without GM were 45% lower than in patients with GM and the myocardial infarction rates were 60% lower for patients without GM. The STLLR report suggested that the lack of intimal hyperplasia in patients treated with DES likely contributed to a false perception that suboptimal percutaneous coronary intervention technique would no longer affect clinical outcomes (30). Another large clinical trial (2,157 patients) found negative residual stenosis (RGM) was associated with a higher extent of in-stent restenosis (30), consistent with the finding of another trial that negative residual stenosis was associated with impaired myocardial perfusion and increased mortality (16).
These clinical data suggest that either LGM or RGM was a common occurrence during stent deployment ( of the time) and led to a significantly higher risk for restenosis, target-lesion revascularization (TLR), MI, and stent thrombosis. The mechanism for the higher clinical event rates, however, remains unclear. The objective of the current study was to investigate the potential underlying biomechanical mechanism for the higher rate of adverse events. Our hypothesis is that stent deployment with GM (LGM or RGM) results in unfavorable fluid WSS, WSSG, OSI, and solid intramural circumferential wall stress (CWS), which may lead to higher clinical adverse effects. GM may also cause higher stresses in the stent struts, potentially lead to stent fractures.
METHODS
Three-dimensional (3-D) computational models of stents and plaque were created in Pro/Engineer (Needham, MA) which is a computer-assisted design package. The models were then interfaced, meshed, and solved in validated finite element and computational fluid dynamic package ANSYS (Canonsburg, PA). The stent geometry simulated was a design with the general pattern similar to that of commercial stents (Boston Scientific Taxus and Johnson & Johnson Cypher). Because of the circumferential symmetry of the stent and plaque, quarter symmetry models were developed to reduce computational costs. The stent diameter and length modeled were 3 mm and 5 mm, respectively. About 1.8 million mesh elements were used in the simulations. A mesh convergence test showed that doubling the number of elements resulted in only <2% difference in wall stress. Hence, the mesh density was deemed sufficient for the simulations.
The governing equations for blood are the Navier-Stokes (conservation of momentum) and Continuity equations (conservation of mass) (6); namely:
| (1) |
| (2) |
where V is blood velocity, t is time, P is blood pressure, ρ is blood mass density, η is blood dynamic viscosity, ∇→ is the gradient operator, and D is the blood rate of deformation tensor.
The governing equations for the stent and plaque were the Momentum and Equilibrium equations (7) (i.e., Newton's laws of Mechanics):
| (3) |
| (4) |
where ai is acceleration, fi is force per unit mass, sΩ(t) is the vessel domain at time t, nj is normal vector, ti is surface traction vector, and σij is stress. The plaque was modeled as calcified tissue with material properties on the basis of reports by Ebenstein et al. (14) where nanoindentation was used to measure plaque mechanical properties. The average modulus for calcified plaque was about 180 MPa, and the stent was considered as stainless steel with the Poisson ratio of 0.3 (14).
For blood, a non-Newtonian Carreau model was used to account for the shear thinning behavior of blood (6, 8). The model has been known to accurately describe the behavior of red blood cell suspensions and the viscosity matched closely with experimental measurements (10). The density of the blood was taken as 1,060 kg/m3. The blood was modeled as incompressible with flow on the basis of human left coronary artery velocity measurements applied at the inlet of vessel (3). For the outlet of the vessels, a traction-free boundary condition was imposed (33). At the wall interface, no slip was assumed between blood and the endothelium, and the vessel wall was considered to be impermeable. The mathematical definitions of WSS, WSSG, and OSI were detailed in previous publications (4, 8). The differences among WSS, WSSG, and OSI were compared in centered and GM stents and in correct and oversized stents. Oversized stents (10% and 20%) were modeled because these are likely the more common interventional scenarios.
The dynamic process of stent deployment was modeled by a linear displacement-driven time function imposed on the struts to expand the stent to the desired size. The stent struts interact and deform the vessel wall by the Augmented Lagrangian contact algorithm. Penetration between the stent and vessel elements was not allowed by the contact algorithm. If penetration was detected, the overlapping elements were returned to their positions at the previous time step. A large deformation analysis was performed and solved using a Newton-Raphson's method. To model interactions between specific model portions, contact surfaces were introduced. The artery was constrained in the proximal and distal sections to prevent rotations and the stent was constrained to allow radial displacements.
RESULTS
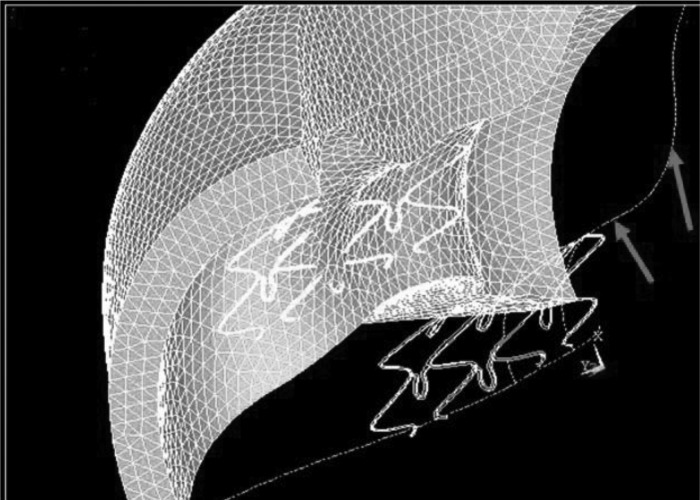
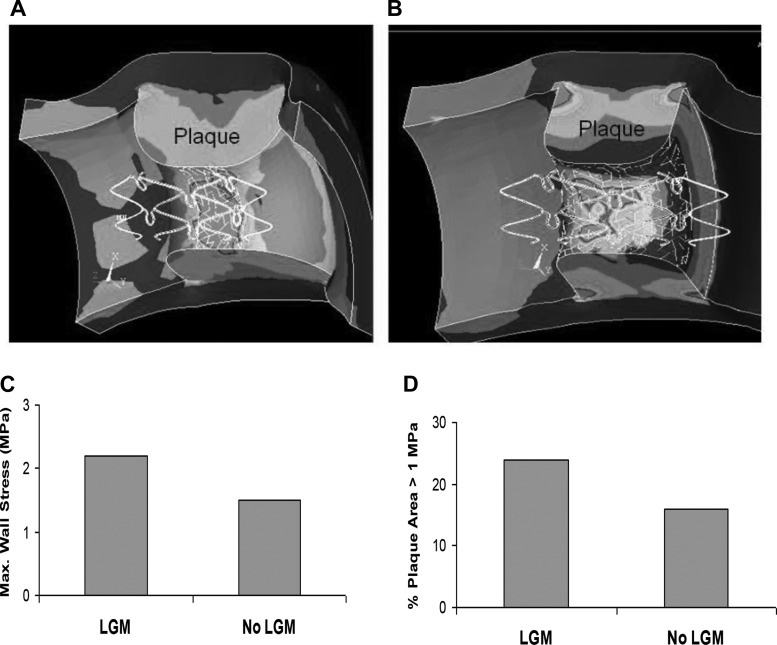
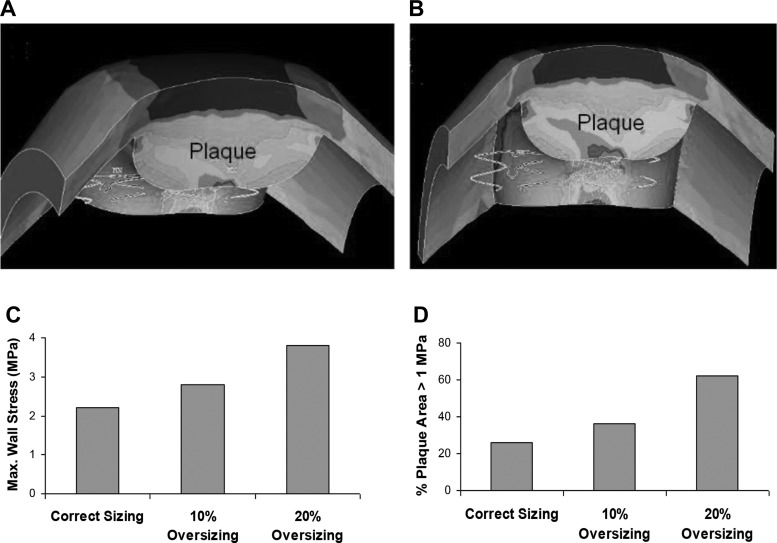
A large deformation simulation of the dynamic stenting plaque process is shown in Fig. 1. The dynamic process of stent deployment was simulated to obtain accurate stress distribution resulting from the process of struts radially deforming the plaque. The stress distributions are compared between a correctly centered stent and LGM (Fig. 2, A and B), with the plaque labeled. A stent deployed with LGM resulted in a 45% increase in wall stress compared with a stent that was centered and fully covered the lesion (2.2 MPa vs. 1.5 MPa), as shown in Fig. 2C. As expected, the CWS was highest at the contact region between the plaque and stent. The percentages of plaque area with stress >1 MPa were 24% vs. 16% for LGM and centered stents, respectively (Fig. 2D). The effects of RGM (stent oversizing) are shown in Fig. 3 for correct sized, and 10% and 20% oversizing in the presence of LGM. CWS was highest at the contact region between the plaque and stent. The 20% oversizing resulted in about 41% and 72% increases in CWS compared with 10% oversizing and correct sizing (3.8 vs. 2.7 and 2.2 MPa, respectively), as shown in Fig. 3C. The differences between 10% and 20% were incremental because even with 10% oversizing, the plaque was already pushed much beyond the unstented configuration. The percentages of plaque area with stress >1 MPa were 24%, 37%, and 62% for correct sized, and 10% and 20% oversized stents, respectively (Fig. 3D).
Fig. 1.
A large deformation simulation of stenting plaque. The dashed lines show the original undeformed configuration (the plaque protruding into the lumen, causing a stenosis; arrows); the solid lines and finite element mesh are of the final deformed state with the stenosis eliminated.
Fig. 2.
Circumferential stress distributions with geographic miss (GM) (A) and with centered stent (B). The plaque is labeled. C: longitudinal GM (LGM) resulted in a 45% increase in wall stress (2.10 MPa vs. 1.45 MPa). Stresses were highest at the contact region between the plaque and stent. D: percentage of plaque area with stress >1 MPa (24% vs. 16%).
Fig. 3.
Effects of radial geographical miss or stent oversizing: 10% oversizing (A) and 20% oversizing (B). The plaque is labeled. C: 20% oversizing resulted in about 40% and 73% increases in wall stress compared with 10% oversizing and correct sizing (3.8 vs. 2.7 and 2.2 MPa for 20% increase, 10% increase, and correct sizing, respectively); oversizing was for stenting with LGM. D: % of plaque area with stress >1 MPa (24%, 37%, and 62% for correct sized, and 10% and 20% oversized stents, respectively).
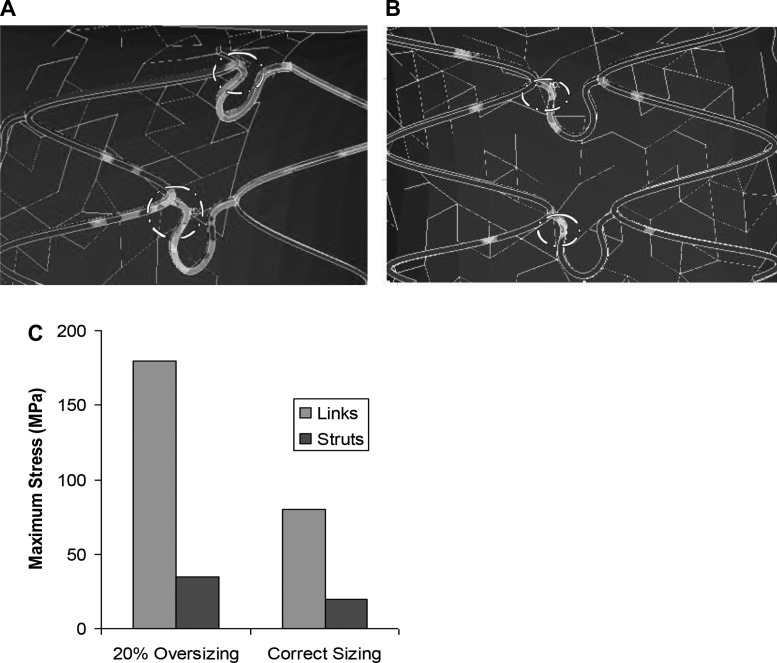
The stress distributions on the stent struts for 20% oversizing and correct sizing in the presence of LGM are shown in Figure 4, A and B, respectively. The stresses concentrate at the struts in contact with plaque (dash circled in Fig. 4, A and B). The linkages between the struts have the highest stress of about 180 MPa in RGM, likely due to a much smaller area for stress distribution on the links compared with struts (Fig. 4A). Therefore the links are more vulnerable to fracture, which can cause protrusion and perforation of the plaque. The stress concentrations are highlighted in dashed circles. The highest stress without RGM was only about 80 MPa (Fig. 4C).
Fig. 4.
Stress distributions on stent struts. Stent sizing was for stenting with LGM. The stresses concentrate at the struts in contact with plaque. Linkages between struts have much higher stress than the main struts. Stress concentrations are highlighted in dashed circles.
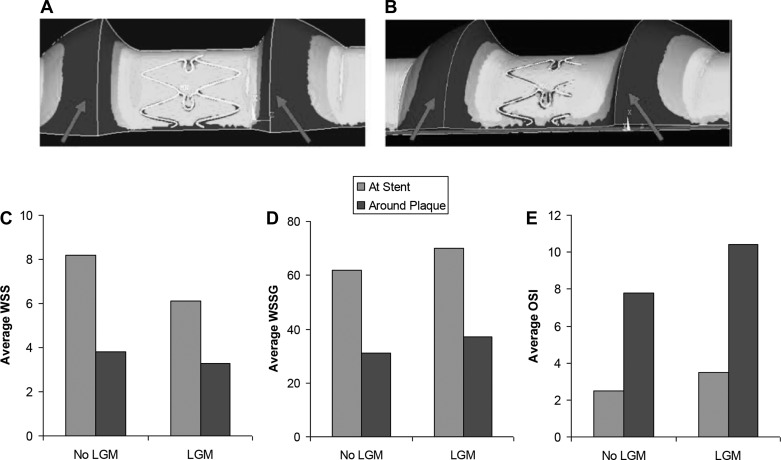
WSS at the stented region and around the plaque (labeled by arrows) were lower for LGM compared with non-LGM (8.2 vs. 6.1 dyn/cm2, and 3.8 vs. 3.3 dyn/cm2, respectively, Fig. 5, A–C). This is likely due to the partially submerged struts, which disturb the flow and result in lower WSS. The WSSG, on the other hand, increased at stented region and around the plaque for LGM compared with correct size stent (72 vs. 61 dyn/cm3, and 35 vs. 32 dyn/cm3, respectively, Fig. 5D). OSI also increased at the stented region and around the plaque for LGM (3.5% vs. 2.6% and 10.4% vs. 7.8%, respectively; Fig. 5E).
Fig. 5.
A–C: wall shear stress (WSS) at the stented region and around the plaque (arrows) were lower in LGM (A) than non-LGM (B) stents. WSS is measured in dyn/cm2. WSS gradient (WSSG) (D) and oscillatory shear index (OSI) (E) at the stented region and around the plaque were both higher for LGM compared with non-LGM. WSSG is measured in dyn/cm3; OSI is measured in %.
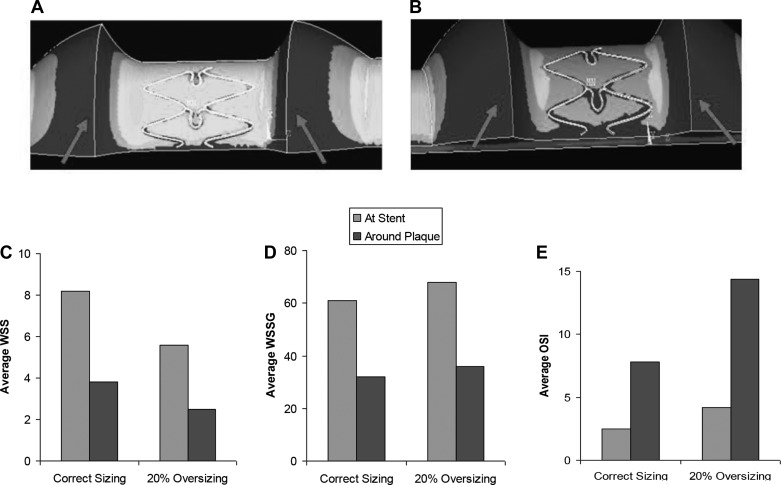
WSS at the stented region and around the plaque (labeled by arrows) were lower for RGM (20% oversizing) compared with correct size stent (5.6 vs. 8.2 dyn/cm2, and 2.4 vs. 3.8 dyn/cm2, respectively; Fig. 6, A–C). This is due to the increase in lumen diameter that results in lower blood WSS. WSSG measures at the stented region and around the plaque were higher for RGM compared with correct size stent (68 vs. 61 dyn/cm3, and 36 vs. 32 dyn/cm3, respectively; Fig. 6D). OSI also increased at the stented region and around the plaque for RGM (4.2% vs. 2.6%, and 14.3% vs. 7.8%, respectively; Fig. 6E).
Fig. 6.
A–C: WSS at the stented region and around the plaque (arrows) was lower in radial geographical miss (RGM) (A) than non-RGM (B) stents. WSS is measured in dyn/cm2. WSSG (D) and OSI (E) at the stented region and around the plaque were higher in RGM stents compared with non-RGM stents. WSSG is measured in dyn/cm3; OSI is measured in %.
DISCUSSION
This study found that both longitudinal and radial GM increase CWS, WSSG, and OSI, but decrease WSS. These mechanical parameters may contribute to the underlying mechanisms for the significantly increased clinical adverse events associated with GM. For example, the STLLR trial found a strong association between GM and MI rates and there was a threefold increase in GM (2.4% vs. 0.8%). For stent thrombosis, there was a 50% increase in GM (1.2% vs. 0.8%) 1 yr poststenting. For RGM, clinical trials showed that stent oversizing was associated with increased TVR, MI, and impaired myocardial perfusion (16, 30). A greater propensity for disruptions of plaque associated with oversizing may also lead to increased distal microembolization (16).
The current study provides quantifications of the effects of fluid and solid stresses that can be correlated with clinical data as detailed below. Longitudinal GM resulted in ∼45% increase in CWS (Fig. 2). The reason for the stress increase is due to the asymmetrical and uneven stent deployment. The stresses are highest at the contact area between the plaque and stent. This region may be most vulnerable to plaque remodeling and enlargement. Because we previously simulated the effects of stent oversizing (RGM) on CWS, the current study focused on LGM, and LGM in the presence of RGM (Fig. 3) (8). The present study focused on various LGM cases because LGM is the predominant form of GM.
LGM also led to lower WSS and higher WSSG and OSI (Fig. 5). The STLLR trial found that the TLR and MI rates in patients without LGM were about 59% and 67% lower, respectively, than patients with GM. The increase in CWS and WSSG and decrease in WSS are potentially key culprits for the increased adverse clinical events in GM. In addition, nonuniform WSS has been found to contribute to cell migration and differentiation (22). The stent struts lower WSS and create unsmooth distribution of WSS, thereby elevating WSSG, which has been found to expand intracellular gap junctions and disrupt intracellular junctions, making the vessel wall more vulnerable to the infiltration of inflammatory cells and lipids (36). GM, especially RGM, resulted in higher OSI, a measure of the extent of oscillatory behavior of flow, which has been found to promote pathological processes in arteries. The oscillatory shear and associated reversed flow were found to reduce nitric oxide, which may lead to endothelial dysfunction and platelet adhesion (20, 23).
The current study also found that negative residual stenosis (RGM) resulted in lower WSS and higher WSSG, OSI, and intramural CWS (Figs. 3 and 6). In a clinical trial of 748 patients, Gibson et al. initially hypothesized that negative residual stenosis (RGM) may be associated with improved outcome (16). The trial found that stent oversizing, however, was associated with impaired myocardial perfusion (16). The incidence of abnormal flow in patients post-percutaneous coronary intervention was 37% higher for the oversizing group. In comparison, our simulation study found that the fluid shear was 35% less with oversizing. The findings by Gibson et al. were consistent with another clinical trial (2,157 patients treated with stents) that showed stent oversizing was associated with more extensive intimal hyperplasia and in-stent restenosis (30). It was found that the maximum area restenosis of 20% vs. 10% stent oversizing was 36% higher. In comparison, our simulation study found that the intramural stress for 20% vs. 10% stent oversizing was 33% higher. On the basis of these clinical data, the simulations are remarkably consistent with clinical finding.
RGM creates higher intramural stresses in the vessel wall where solid wall stresses are known stimuli for inflammatory responses and smooth muscle cell proliferation, and contribute to the atherogenesis process (5, 8, 15, 18). Additionally, the higher intramural stress can potentially cause plaque cracking and fracture, leading to thrombosis due to platelet deposition at the fissure.
Implications for stent fractures.
There has been increasing awareness of stent fractures, especially in the DES era (9, 26). Because angiography has limited resolution to detect cracks and fractures, there has been under-recognition of stent fractures (1, 25). The current simulations found that RGM resulted in much higher stresses on the struts, especially the links that had the highest stress of about 180 MPa in RGM (circled in Fig. 4), not far below the 210 MPa fatigue fracture stress of the stent. Fatigue fracture does not imply that the stent would fracture immediately, but rather may fracture after a period of time from a few days to months. Therefore the links are much more vulnerable to fatigue fracture in RGM. For example, Hoshi et al. reported that mechanical stress was the cause for the coronary stent fracture observed in their study (17). As shown in histology studies, the fractures often occurred at the links rather than at the main struts (25). This was true for various degrees of fractures from partial limited fractures to complete separation and split of the struts. The autopsy study also found 29% of stents implanted had fractures, which is much higher than reported clinically (25). Additionally, Park et al. found that stent fractures were associated with higher TLR and MI rates (26).
More cases of Cypher stent fractures have been reported. The Cypher design features long intersinusoidal linker segments that may be more susceptible to fractures. The higher detected incidences have been attributed to the Sirolimus eluting stent being more radiopaque (easier to detect on angiogram) (1, 32, 37). Because an angiogram is a 2-D projection, it is easy to miss fracture on the other side of the stent. The recent autopsy study found that the Cypher stent indeed had a higher incidence of stent fracture (64%), although other stents are not immune. Thirty-six percent of fractured stents were found to be the Taxus stent (25). The overlapping stents were also found to be more susceptible to fractures because higher stresses can result when the struts of the two stents contact each other. It has also been reported that DES stents have higher incidences of stent fractures (9, 37). The present simulations in conjunction with fatigue testing can lead to improved designs and reduced failure. Because the intent was to examine the maximum stresses in the struts to determine whether it is near the fatigue fracture stress, RGM in combination with LGM (as in Fig. 3) was simulated. Although the maximum stress was still below the fatigue fracture stress, it was not far below (180 vs. 210 MPa).
Potential GM effects on DES.
It is important to note that the stents used in the STLLR trial were DES (13), and hence the mechanical pertubations associated with GM apply to DES. Indeed, the authors of the STLLR report suggested that the lack of intimal hyperplasia in patients treated with DES likely contributed to a false sense of security that percutaneous coronary intervention technique would no longer affect clinical outcomes (13). It has been found that stent fatigue fracture is a more significant problem for DES (2, 28). In bare metal stents, the stent struts may become embedded in a thick layer of intimal hyperplasia, which can prevent the struts from fractures. For DES when intimal hyperplasia is largely inhibited over a longer period of time, the struts are not embedded in intimal hyperplasia and thus are more vulnerable to fatigue fracture.
The absence of intimal hyperplasia to cover the struts of DES also affects the fluid shear stress where the low WSS caused by the struts may persist for months and possibly years, which can predispose the stented region to stent thrombosis. It was found that DES caused incomplete re-endothelization, which predisposes to late thrombosis (12, 19, 24, 35). Additionally, DES are believed to be antihealing and inhibit both smooth muscle cells and endothelium (29, 35). The latter can make the vessel wall more vulnerable to the infiltration of low density lipoprotein and inflammatory cells (19). In conjunction, low and oscillatory shear increases the residence time for low density lipoprotein and inflammatory cells (20, 23). In addition, high WSSG opens cell gap junctions for the infiltration of these cells (36). The combination of low shear, high WSSG and OSI, and compromised endothelium may potentially worsen the progression of plaque in the long term. Therefore it is important to have optimal stent sizing and positioning to minimize these negative effects. Interestingly, a recent clinical study found that intravascular ultrasound–guided DES implantation was associated with significantly fewer deaths and MIs at 1 yr (3.3% vs. 6.5%) and 2 yrs (5.0% vs. 8.8%) (11).
In the STLLR trial and a substudy, the RGM lumped together both oversizing and undersizing, whereas the current study focused on oversizing because it is the most common mis-sizing cases in clinical practice (13, 34). Two independent clinical trials performed by Gibson et al. (16) and Sick et al. (30) both found that stent oversizing was associated with increased TVR. For LGM, the STLLR trial also included cases that had balloon protrusions beyond either the proximal or distal edge of the stent. These cases had balloon injury areas uncovered by the stents. It was found that balloon injuries also contribute to the increase in TLR rates (13).
Limitations.
First, stent tapering was not considered in the current model, although that is unlikely to be clinically significant. Second, prolapse of tissue between struts was not modeled, which may influence intrastrut WSS. This effect is not expected to be as significant as the effect of stent struts. Third, although the present mechanical findings are relevant to both bare metal and drug-eluting stents, the kinetics and transport of drug elution were not simulated and are not expected to affect the mechanical conclusions. Fourth, an idealized plaque geometry was assumed in the current study. There are numerous variations of plaque geometry and components (lipid pools, calcifications, etc.). The purpose of the current study was to provide an overall trend for patients with GM rather than to study a particular patient-specific case. Asymmetric lipid pool in eccentric plaques was found by Loree et al. to induce local stress concentrations (22). This is especially relevant for studies of vulnerable plaque and potential plaque rupture, which are beyond the scope of the current study. Our simulations of symmetric plaque represents an average stress lower than that observed in eccentric plaque. Hence, the conclusions of the current study will not be affected by more complex plaques, albeit the absolute numerical results are likely to be higher. Fifth, the cyclic motion of cardiac muscle was not considered. The contractions and bending of the arteries and stents may affect the local mechanics. Finally, the current simulation study is hypothesis-generating, and future experimental and clinical studies are needed to further verify the stipulated hypotheses. We previously published a validation study in swine animal experiments demonstrating that stent mis-sizing (RGM) promotes intimal hyperplasia, which correlated significantly with lower fluid WSS and higher intramural wall stress (8).
Significance.
These findings shed light on the potential underlying mechanism of the higher clinical adverse events associated with mis-sizing and mis-positioning and offer an opportunity for improved stent deployment via more accurate stent positioning and sizing. The occurrence of GM is operator-dependent and can be improved by the development of procedural guidelines and the use of latest vessel sizing technologies. The current model predictions of solid and fluid mechanics correlated well with findings of large clinical trials using DES (13, 16, 30). The lack of remodeling and hyperplasia in DES is likely to make the adverse solid and fluid effects after stent implantation much more persistent. Additionally, the lack of intimal hyperplasia makes the stents more vulnerable to fracture and thrombosis, a more significant problem in the DES era. (1, 17, 25, 28). A potential benefit of these simulations is in design optimization of stents. For example, the finding that the links between struts are the most vulnerable to fracture suggests that a design feature requires further optimization. The geometric models in the current study were created using a 3-D computer-assisted design software based on parametric model construction. Design optimizations are possible using parametric software such as Pro-Engineer and an optimization package. Future studies in this direction are warranted.
GRANTS
This research was supported in part by National Heart, Lung, and Blood Institute Grants HL087235 and HL084529.
DISCLOSURES
Dr. Deepak L. Bhatt discloses the following relationships: Advisory Board: Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Chest Pain Centers; Chair: American Heart Association Get With The Guidelines Science Subcommittee; Honoraria: American College of Cardiology (Editor, Clinical Trials, Cardiosource), Duke Clinical Research Institute (clinical trial steering committees), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), WebMD (CME steering committees); Other: Senior Associate Editor, Journal of Invasive Cardiology; Data Monitoring Committees: Duke Clinical Research Institute (CHARGE-AMI); Mayo Clinic (TAILOR-PCI); Population Health Research Institute (COMPLETE, TOTAL); Research Grants: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, The Medicines Company; Unfunded Research: FlowCo, PLx Pharma, Takeda.
AUTHOR CONTRIBUTIONS
Author contributions: H.Y.C., B.-K.K., and D.B. conception and design of research; H.Y.C., B.-K.K., and D.B. performed experiments; H.Y.C., B.-K.K., and D.B. analyzed data; H.Y.C., B.-K.K., D.B., and G.S.K. interpreted results of experiments; H.Y.C. prepared figures; H.Y.C. drafted manuscript; H.Y.C., D.B., and G.S.K. edited and revised manuscript; H.Y.C. and G.S.K. approved final version of manuscript.
REFERENCES
- 1. Carroll JD. Coronary stent fracture: the hidden truth of a problem more common than stent thrombosis. Catheter Cardiovasc Interv 73: 88–89, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Carter AJ. Drug-eluting stent fracture promise and performance. J Am Coll Cardiol 54: 1932–1934, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Chatzizisis YS, Giannoglou GD, Parcharidis GE, Louridas GE. Is left coronary system more susceptible to atherosclerosis than right? A pathophysiological insight. Int J Cardiol 116: 7–13, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Chen HY, Hermiller J, Sinha AK, Sturek M, Zhu L, Kassab GS. Effects of intravascular stent sizing on endothelial and vessel wall stress: potential mechanisms of in-stent restenosis. J Appl Physiol 106: 1686–1691, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen HY, Kassab GS. Computational modeling of coronary stents. In: Computational Biomechanics, edited by Mofrad MR, Guilak FS. New York: Springer, 2010 [Google Scholar]
- 6. Chen HY, Moussa ID, Davidson C, Kassab GS. Impact of main branch stenting on endothelial shear stress: role of side branch diameter, angle and lesion. J R Soc Interface 9: 1187–1193, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen HY, Navia JA, Shafique S, Kassab GS. Fluid-structure interaction (FSI) in aortic cross-clamping: potential injury mechanisms. J Biomech 43: 221–227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen HY, Sinha AK, Choy JS, Zheng H, Sturek M, Bigelow B, Bhatt DL, Kassab GS. Mis-sizing of stent promotes intimal hyperplasia: impact of endothelial shear and intramural stress. Am J Physiol Heart Circ Physiol 301: H2254–H2263, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chhatriwalla AK, Cam A, Unzek S, Bhatt DL, Raymond RE, Lincoff AM, Whitlow PL, Ellis SG, Tuzcu EM, Kapadia SR. Drug-eluting stent fracture and acute coronary syndrome. Cardiovasc Revasc Med 10: 166–171, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Chien S, Usami S, Taylor M, Lundberg JL, Gergersem MI. Effects of hematocrit and plasma proteins on human blood rheology at low shear rates. J Appl Physiol 21: 81–87, 1966 [DOI] [PubMed] [Google Scholar]
- 11. Claessen BE, Mehran R, Mintz GS, Weisz G, Leon MB, Dogan O, de Ribamar Costa J, Jr, Stone GW, Apostolidou I, Morales A, Chantziara V, Syros G, Sanidas E, Xu K, Tijssen JG, Henriques JP, Piek JJ, Moses JW, Maehara A, Dangas GD. Impact of intravascular ultrasound imaging on early and late clinical outcomes following percutaneous coronary intervention with drug-eluting stents. JACC Cardiovasc Interv 4: 974–981, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Cook S, Ladich E, Nakazawa G, Eshtehardi P, Neidhart M, Vogel R, Togni M, Wenaweser P, Billinger M, Seiler C, Gay S, Meier B, Pichler WJ, Jüni P, Virmani R, Windecker S. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation 120: 391–399, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Costa MA, Angiolillo DJ, Tannenbaum M, Driesman M, Chu A, Patterson J, Kuehl W, Battaglia J, Dabbons S, Shamoon F, Flieshman B, Niederman A, Bass TA, STLLR Investigators Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial). Am J Cardiol 101: 1704–1711, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Ebenstein DM, Coughlin D, Chapman J, Li C, Pruitt LA. Nanomechanical properties of calcification, fibrous tissue, and hematoma from atherosclerotic plaques. J Biomed Mater Res A 91: 1028–1037, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Fung YC. Biomechanics: Flow Stress, Growth. New York: Springer, 1998 [Google Scholar]
- 16. Gibson CM, Kirtane AJ, Boundy K, Ly H, Karmpaliotis D, Murphy SA, Giugliano RP, Cannon CP, Antman EM, Braunwald E, TIMI Study Group Association of a negative residual stenosis following rescue/adjunctive percutaneous coronary intervention with impaired myocardial perfusion and adverse outcomes among ST-segment elevation myocardial infarction patients. J Am Coll Cardiol 45: 357–362, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Hoshi T, Sato A, Nishina H, Kakefuda Y, Noguchi Y, Aonuma K. Fatal ostial right coronary artery coronary stent fracture and perforation induced by mechanical stress between the sternum and dilated aortic root. Circulation 123: 1679–1682, 2011 [DOI] [PubMed] [Google Scholar]
- 18. Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation 103: 1051–1056, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 48: 193–202, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Kassab GS, Navia JA, Lu X. Proper orientation of the graft artery is important to ensure physiological flow direction. Ann Biomed Eng 34: 953–957, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Lin MC, Almus-Jacobs F, Parry GC, Mackman N, Shyy JY, Chien S. Shear stress induction of the tissue factor gene. J Clin Invest 99: 737–744, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loree HM, Kamm RD, Stringfellow RG, Lee RT. Effects of fibrous cap thickness on peak circumferential stress in model atherosclerotic vessels. Circ Res 71: 850–858, 1992 [DOI] [PubMed] [Google Scholar]
- 23. Lu X, Kassab GS. Nitric oxide is significantly reduced in ex vivo porcine arteries during reverse flow because of increased superoxide production. J Physiol 561: 575–582, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moses JW, Dangas G, Mehran R, Mintz GS. Drug-eluting stents in the real world: how intravascular ultrasound can improve clinical outcome. Am J Cardiol 102, Suppl 9: 24J–28J, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Nakazawa G, Finn AV, Vorpahl M, Ladich E, Kutys R, Balazs I, Kolodgie FD, Virmani R. Incidence and predictors of drug-eluting stent fracture in human coronary artery a pathologic analysis. J Am Coll Cardiol 54: 1924–1931, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Park KW, Park JJ, Chae IH, Seo JB, Yang HM, Lee HY, Kang HJ, Cho YS, Yeon TJ, Chung WY, Koo BK, Choi DJ, Oh BH, Park YB, Kim HS. Clinical characteristics of coronary drug-eluting stent fracture: insights from a two-center DES registry. J Korean Med Sci 26: 53–58, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perrin CL, Tardy PM, Sorbie KS, Crawshaw JC. Experimental and modeling study of Newtonian and non-Newtonian fluid flow in pore network micromodels. J Colloid Interface Sci 295: 542–550, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Pitney M, Pitney K, Jepson N, Friedman D, Nguyen-Dang T, Matthews J, Giles R, Taylor D. Major stent deformation/pseudofracture of 7 Crown Endeavor/Micro Driver stent platform: incidence and causative factors. EuroIntervention 7: 256–262, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Seth DA. “Anti-healing” versus “pro-healing”. Catheter Cardiovasc Interv 71: 605–606, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Sick P, Hüttl T, Niebauer J, Thiele H, Lauer B, Hambrecht R, Hentschel B, Schuler G. Influence of residual stenosis after percutaneous coronary intervention with stent implantation on development of restenosis and stent thrombosis. Am J Cardiol 91: 148–153, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Stähli BE, Camici GG, Steffel J, Akhmedov A, Shojaati K, Graber M, Lüscher TF, Tanner FC. Paclitaxel enhances thrombin-induced endothelial tissue factor expression via c-Jun terminal NH2 kinase activation. Circ Res 99: 149–155, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Stribling WK, Prinz AW, Topaz O, Khan R, Gentili A, Jovin IS. Bypass graft stent fracture leading to saphenous vein graft pseudoaneurysm. Circulation 122: e476–e477, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Stroud JS, Berger SA, Saloner D. Numerical analysis of flow through a severely stenotic carotid artery bifurcation. J Biomech Eng 124: 9–20, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Tahara S, Bezerra HG, Kyono H, Carrigan T, Mehanna E, Wang W, Costa MA. Impact of acute gain on clinical outcomes of patients treated with sirolimus-eluting stent. A sub-analysis study from the STLLR trial. Circ J 75: 2113–2119, 2011 [DOI] [PubMed] [Google Scholar]
- 35. Virmani R, Farb A, Guagliumi G, Kolodgie FD. Drug-eluting stents: caution and concerns for long-term outcome. Coron Artery Dis 15: 313–318, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Wells DR, Archie JP, Jr, Kleinstreuer C. Effect of carotid artery geometry on the magnitude and distribution of wall shear stress gradients. J Vasc Surg 23: 667–678, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Wu MC, Cheng CC, Huang TY. Fracture of zotarolimus-eluting stent after implantation. Tex Heart Inst J 36: 618–620, 2009 [PMC free article] [PubMed] [Google Scholar]