Abstract
Tactile stimulation of the oropharynx (TSO) elicits the gag reflex and increases heart rate (HR) and mean arterial pressure (MAP) in anesthetized patients. However, the interaction between upper-airway defense reflexes and the sympathetic nervous system has not been investigated in conscious humans. In Experiment 1, beat-by-beat measurements of HR, MAP, muscle sympathetic nerve activity (MSNA), and renal vascular resistance (RVR) were measured during TSO and tactile stimulation of the hard palate (Sham) in the supine posture. In Experiment 2, TSO was performed before (pre) and after (post) inhalation of 4% lidocaine via nebulizer. Rate pressure product (RPP) was determined. Compared with Sham, TSO elicited the gag reflex and increased RPP [absolute change (Δ)36 ± 6 vs. 17 ± 5%], MSNA (Δ122 ± 39 vs. 19 ± 19%), and RVR (Δ55 ± 11 vs. 4 ± 4%). This effect occurred within one to two cardiac cycles of TSO. The ΔMAP (12 ± 3 vs. 6 ± 1 mmHg) and the ΔHR (10 ± 3 vs. 3 ± 3 beats/min) were also greater following TSO compared with Sham. Lidocaine inhalation blocked the gag reflex and attenuated increases in MAP (Δpre: 16 ± 2; Δpost: 5 ± 2 mmHg) and HR (Δpre: 12 ± 3; Δpost: 2 ± 2 beats/min) in response to TSO. When mechanically stimulated, afferents in the oropharynx not only serve to protect the airway but also cause reflex increases in MSNA, RVR, MAP, and HR. An augmented sympathoexcitatory response during intubation and laryngoscopy may contribute to perioperative cardiovascular morbidity and mortality.
Keywords: sympathetic nervous system, afferent, pharynx, local anesthesia, blood flow
many airway defense reflexes (e.g., cough reflex, gag reflex, swallow reflex) originate in the mouth, pharynx, and/or larynx (3, 7, 25). These upper-airway reflexes are operable under physiological conditions and effectively route food and air to the proper anatomical locations. During an acute perturbation, such as choking or coughing, blood pressure (BP) homeostasis is likely to be affected. Tactile stimulation of the oropharynx (TSO) is also a potent stimulus that increases heart rate (HR), BP, and rate pressure product (RPP) in humans. Specifically, endotracheal intubation, laryngoscopy, and bronchoscopy are clinical procedures that increase myocardial oxygen demand (24, 45, 47, 48). Considering that many patients are at risk for cardiac ischemia and arrhythmia during these procedures (29, 30, 35, 41), attenuating HR, BP, and RPP in response to TSO has received much attention (1, 8, 16, 47, 53, 55). However, the basic physiology is unclear, because patients in these cited experiments were premedicated, sedated, and/or under general anesthesia when TSO was applied.
A number of physiological stressors (i.e., orthostasis, exercise, hypoxemia) elicit sympathoexcitation, which serves to redistribute blood flow and maintain perfusion to critical organs. Studies in anesthetized patients have shown that muscle sympathetic nerve activity (MSNA) and BP increase rapidly and robustly during intubation and laryngoscopy (9, 11, 43), providing evidence that TSO elicits vasoconstriction within skeletal muscle. Another vascular bed—the kidney—receives ∼20% of cardiac output at rest, and alpha-adrenergic renal vasoconstriction occurs in responses to physiological stress (5, 27, 28, 40). In dogs, renal sympathetic nerve activity and BP both increase during intubation (42), but the renal vascular responses to TSO in humans are unknown. This may be clinically relevant, considering that perioperative renal failure (seemingly due to reductions in renal blood flow) is not uncommon.
The purpose of this study was to characterize the integrated neurovascular responses to TSO in conscious, unmedicated humans (Experiments 1) and to block this response using local anesthesia of the upper airway (Experiments 2 and 3). In these experiments, we tested two separate but related hypotheses. First, TSO will cause greater changes in BP, HR, MSNA, and renal vascular resistance (RVR) compared with tactile stimulation of the hard palate (Sham). Second, inhalation of 4% topical lidocaine prior to (pre) TSO will block the gag reflex and attenuate increases in HR and BP. Herein, we demonstrate that stimulation of mechanically sensitive afferents in the oropharynx elicits an increase in MSNA, along with acute hypertension, tachycardia, and renal vasoconstriction; these effects can be abolished with local anesthesia of the upper airway.
METHODS
All study protocols were approved in advance by the Institutional Review Board of the Penn State Milton S. Hershey Medical Center and conformed to the Declaration of Helsinki. A total of 23 healthy, unmedicated, young (range 22–33 years) subjects volunteered to participate and provided written, informed consent. Due to the unpleasant nature of gag-reflex testing, we attempted to recruit different groups of subjects for each study. In total, two subjects participated in both Experiments 1 and 2, and two different subjects participated in both Experiments 2 and 3. The sample size for Experiment 1 was determined based on power analyses of MSNA and RVR in response to TSO compared with Sham (i.e., power > 80% for each measure).
Experiment 1: Effect of TSO on MSNA and RVR
Thirteen individuals (eight men, five women; 25 ± 1 years, 1.76 ± 0.03 m, 74.3 ± 4.0 kg, 24.0 ± 0.7 kg/m2) participated in Experiment 1. All experiments were conducted in the supine posture. Subjects were first instrumented with a finger BP cuff (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands) to monitor beat-by-beat BP, a three-lead ECG (Cardiocap/5; GE Healthcare, Waukesha, WI) to measure HR, and a custom-designed pneumotrace to detect respiratory movement. Baseline recordings of MSNA (n = 6) or RVR (n = 8) were obtained for 5 min. For testing, the subject was instructed to “close the eyes, open the mouth, and stick out the tongue.” A registered nurse (same investigator for all trials) then applied stimulation to either the oropharynx (TSO; eliciting the gag reflex) or the hard palate of the mouth (Sham; no gag reflex) in a counterbalanced fashion. TSO was conducted using a wooden tongue blade or cotton swab, per standard clinical practice (7, 22), and was of very short duration (i.e., <2 s). Clinical intubation and laryngoscopy are typically 10–30 s in duration (21, 30, 47) and elicit the same airway defense reflex as TSO. The subject was not aware which stimulus was about to occur. A recovery period followed for 3–5 min, and once parameters returned to baseline, the opposite stimulus occurred. The goal was to administer one trial of TSO and one trial of Sham per subject, but additional trials were sometimes conducted if coughing (n = 2 trials) or limb movement (n = 3) occurred, since both of these cause measurement error. Although not the primary purpose of this study, during some MSNA trials, TSO was applied twice to explore whether hemodynamic and neural responses were attenuated in the second trial (Fig. 1). At the end of the experiment, subjects performed a maximal voluntary end-expiratory apnea to ensure that the quality of the MSNA recording was consistent from beginning to end.
Fig. 1.
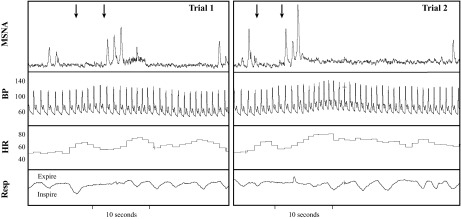
Representative beat-by-beat recordings of muscle sympathetic nerve activity (MSNA), blood pressure (BP), heart rate (HR), and respiratory movement (Resp) in the same subject. The 1st arrow denotes opening the mouth, and the 2nd arrow denotes tactile stimulation of the oropharynx (TSO; eliciting the gag reflex). Within 1–2 cardiac cycles after TSO, there was a rapid increase in MSNA, BP, and HR. Trials 1 and 2 were separated by 5 min.
Experiment 2: Effect of Lidocaine Inhalation on HR and BP during TSO
Ten individuals (six men, four women; 26 ± 1 years, 1.78 ± 0.03 m, 79.0 ± 5.2 kg, 24.8 ± 1.0 kg/m2) participated in Experiment 2. After baseline measurements of HR and BP, TSO was conducted in the supine posture. Next, the participants assumed the seated, upright posture and received 6 ml of 4% topical lidocaine HCl by nebulizer (AirLife Brand Misty Max 10). Lidocaine, a class Ib sodium channel blocker, has been administered locally and systemically to block sensory afferents that initiate sympathetic reflexes (10, 13, 36). Consistent with previous lidocaine-inhalation studies (4, 22, 54), compressed medical air was supplied at 4–8 l/min, and subjects breathed normally until the solution was gone (∼15 min). To keep the airway mucosa as dry as possible, suction was allowed ad libitum (Allegiance K86 Medi-Vac Yankauer Suction). Subjects then resumed the supine posture, and the TSO procedures were repeated within 2–3 min. Subjects were not allowed to leave the laboratory until the gag reflex returned (typically 20–40 min). TSO procedures were standardized, and beat-by-beat hemodynamics were always measured such that comparisons could be made within a given subject on the same day (i.e., test-retest reliability of hemodynamic responses to gag-reflex testing).
Experiment 3: Effect of Lidocaine Inhalation on HR and BP during Cold Pressor Test
On a separate day, four subjects underwent additional studies, which sought to determine whether the aforementioned lidocaine-inhalation protocol had a systemic effect (10, 26). After instrumentation and baseline measurements in the supine posture, subjects underwent the cold pressor test (CPT; hand into 1°C water) for 90 s. This procedure activates the sympathetic nervous system (23) and increase RPP, an index of myocardial oxygen demand (33). Immediately after the CPT, subjects were asked to rate their hand thermal sensation (where 0 = neutral/no sensation of cold, and 11 = unbearable cold) (15) and hand pain (where 0 = no pain, and 10 = unbearable pain) (18). Lidocaine inhalation was then conducted identically to the procedures listed above, and the CPT was repeated.
Measurements.
During Experiment 1, multifiber recordings of MSNA were obtained, with a tungsten microelectrode (FHC, Bowdoin, ME) inserted in the peroneal nerve of a leg. A reference electrode was placed subcutaneously, 2–3 cm from the recording electrode, which was adjusted until a site was found in which muscle sympathetic bursts were clearly identified using previously established criteria (52). Briefly, MSNA was distinguished from other nerve signals when there was increased burst activity in response to maximal voluntary end-expiratory apnea (to activate arterial chemoreflex) (17) and/or passive muscle stretch but not with skin stroking of the innervated area, rapid inspiration, or arousal stimuli (52). The nerve signal was amplified, band-pass filtered with a bandwidth of 500–5,000 Hz, and integrated with a time constant of 0.1 s (Model 662C-3; The University of Iowa Bioengineering, Iowa City, IA). The nerve signal was also routed to a loudspeaker and a computer for monitoring throughout the study.
During Experiment 1, transabdominal Doppler ultrasound (HDI 5000; ATL Ultrasound, Bothell, WA) was used to measure renal blood flow velocity (RBV), as described previously (28, 40). The artery was scanned with a curved array C5-2 transducer.
Prior to all experiments, resting measures of systolic BP (SBP) and diastolic BP (DBP) were obtained via automated sphygmomanometry of the brachial artery (SureSigns VS3; Philips Healthcare, Andover, MA) in triplicate. Beat-by-beat BP, HR, MSNA, and respiratory movement were sampled at 200 Hz by a data acquisition system (PowerLab; ADInstruments, Colorado Springs, CO).
Data collection and statistical analysis.
Beat-by-beat physiological parameters were analyzed offline using LabChart 7 (ADInstruments). The variables of interest included HR, SBP, DBP, mean arterial pressure (MAP), MSNA burst rate, and MSNA total activity. RPP was calculated as HR × SBP and was considered to be the primary outcome measure for two reasons. First, it was collected in all experiments, allowing consistent comparison (i.e., MSNA and RVR were only collected in Experiment 1). Second, it is a primary determinant of myocardial oxygen consumption that is relevant during upper-airway clinical procedures (29, 41). RBV was measured offline using Prosolv 3.0, and RVR was calculated as MAP/RBV; an increase in RVR is considered to be renal vasoconstriction (5, 27, 28, 40). Changes in MSNA total activity (area under the curve with baseline set to zero) and RVR were expressed as a percent change (%Δ) from baseline. All other data were expressed as an absolute change (Δ) from baseline.
Previous research has demonstrated that peak HR and BP responses occur within 5 s after the oropharynx is stimulated (21, 50). Therefore, we chose the peak cardiac cycle within this time frame as the peak hemodynamic response in the current study. Opening the mouth and sticking out the tongue (duration 3–5 s) were common to all experiments, so we also analyzed the five cardiac cycles preceding TSO (mouth open) and report an average. In a similar way, we analyzed the five cardiac cycles after the peak. When the gag reflex was not initiated (during Sham stimulation of Experiment 1 and after lidocaine in Experiment 2), the same latency after stimulation was reported as the peak response. For MSNA recordings, a bin containing 10 cardiac cycles (five before and five after TSO or Sham) was used as the peak. This approach was chosen, because a MSNA burst can only occur once/cardiac cycle.
Statistics were conducted using IBM SPSS 19.0, and graphics were produced with Microsoft Excel and Adobe CS5. For Experiment 1, two-way ANOVAs (time and stimulus) were conducted, followed by uncorrected paired t-tests on the peak ΔRPP, ΔMAP, ΔHR, %ΔMSNA total activity, %ΔRVR, and ΔRBV. For Experiment 2, a two (before lidocaine, after lidocaine)-by-five (time) repeated-measures ANOVA was used, followed by uncorrected paired t-tests when an interaction effect was found. With the use of data from Experiment 2, exploratory bivariate correlations were performed between ΔRPP, with TSO at the beginning of the study, and ΔRPP, with TSO at the end of the study (i.e., to clarify test-retest reliability of ΔRPP when undergoing gag-reflex testing within the same day). For Experiment 3, paired t-tests were used to compare SBP, DBP, MAP, HR, RPP, hand thermal sensation, and hand pain responses with CPT before and after lidocaine inhalation. Data are presented as means ± SE, and P < 0.05 was considered statistically significant.
RESULTS
Experiment 1: Effect of TSO on MSNA and RVR
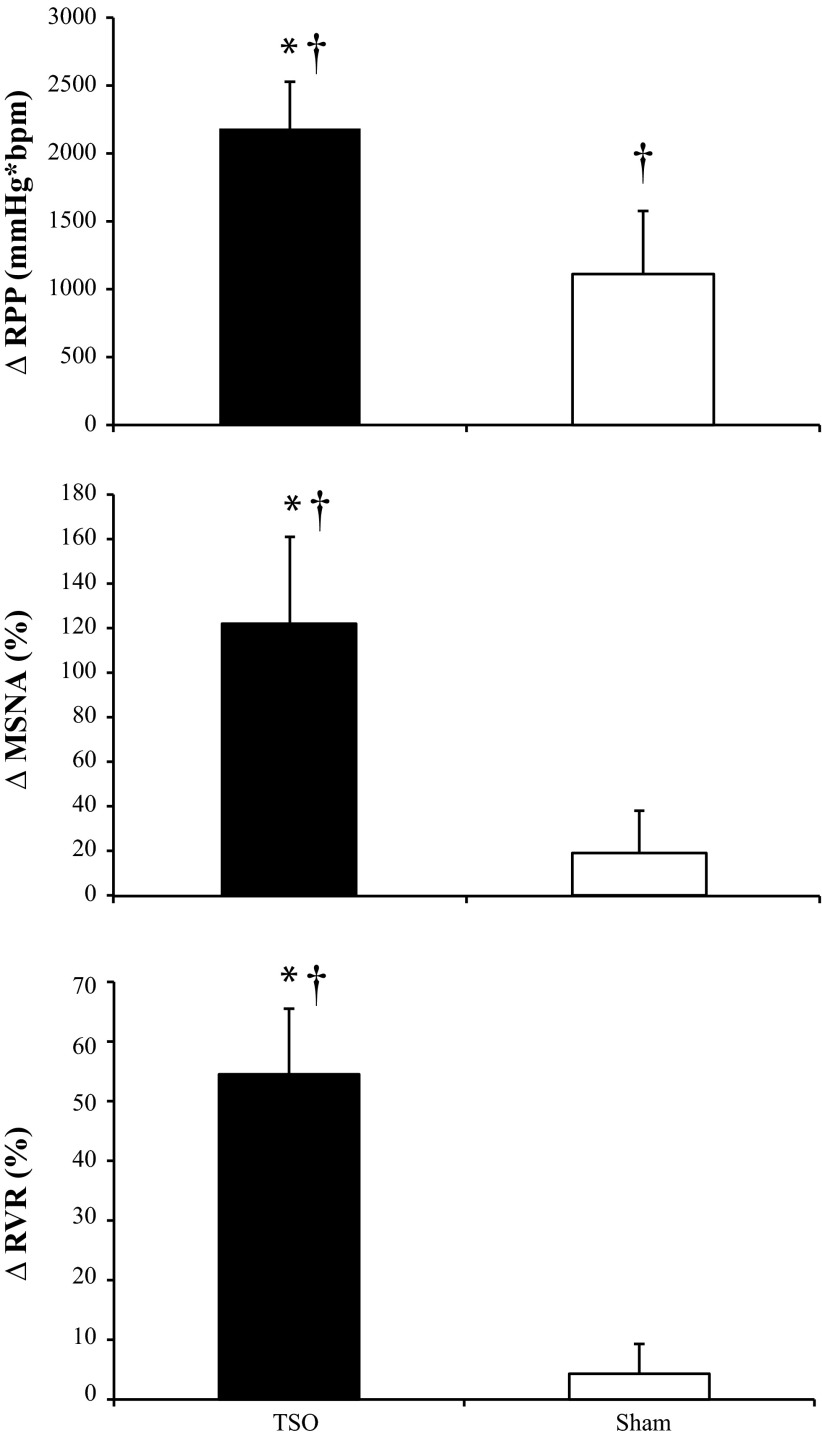
Resting baseline values for MAP (77 ± 1 mmHg), HR (59 ± 3 beats/min), MSNA burst rate (11 ± 2 burst/min), and RBV (69 ± 8 cm/s) were within normal levels for young, healthy subjects. In all subjects, TSO elicited the gag reflex, which was audible and confirmed by the subject and the investigators. Qualitative data (Fig. 1) indicate that TSO increased MSNA total activity as well as HR and MAP. Indeed, the ΔMAP (12 ± 3 vs. 6 ± 1 mmHg), ΔHR (10 ± 3 vs. 3 ± 3 beats/min), and ΔRBV (−15 ± 4 vs. 1 ± 1 cm/s) were greater following TSO compared with Sham (i.e., without eliciting the gag reflex). As shown in Fig. 2, the ΔRPP (P = 0.042), ΔMSNA (P = 0.040), and ΔRVR (P = 0.010) were significantly greater with TSO compared with Sham. Relative to baseline, Sham stimulation caused significant increases in RPP (P = 0.048) and MAP (P = 0.005) but not HR (P = 0.366), RBV (P = 0.691), or RVR (P = 0.304). Additionally, beat-by-beat analysis of MSNA revealed that in the first cardiac cycle following TSO, six of six subjects had a MSNA burst compared with zero of six subjects following Sham. In the second cardiac cycle following TSO, five of six subjects had a MSNA burst compared with two of six subjects following Sham.
Fig. 2.
Changes in rate-pressure product (ΔRPP; top, n = 13), ΔMSNA (middle, n = 6), and renal vascular resistance index (ΔRVR; bottom, n = 8) in response to TSO (black bars) and tactile stimulation of the hard palate (Sham; white bars) during Experiment 1. Data are means ± SE. *P < 0.05 between TSO and Sham; †P < 0.05 vs. respective baseline.
Experiment 2: Effect of Lidocaine Inhalation on HR and BP during TSO
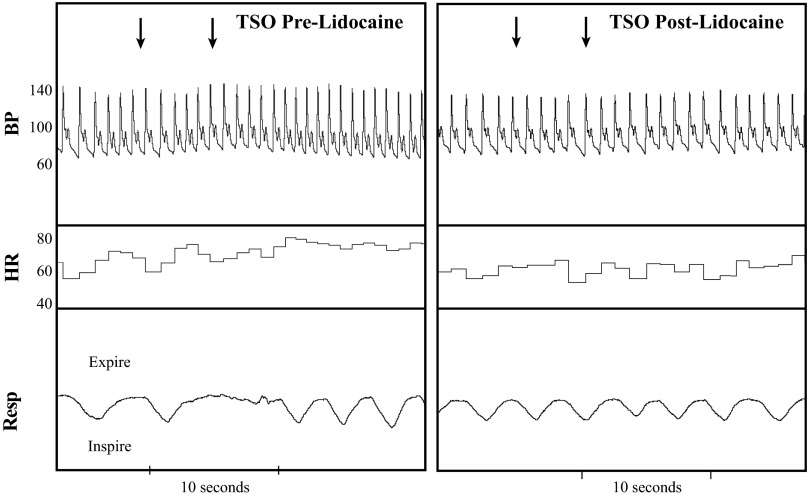
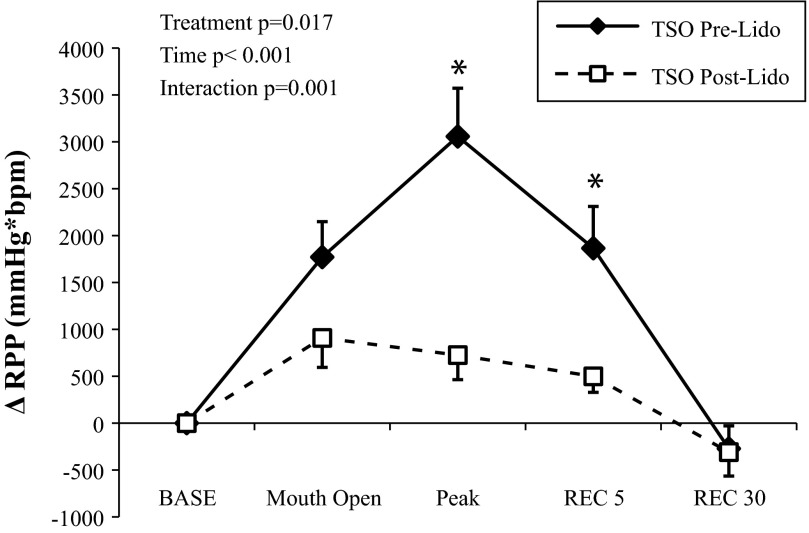
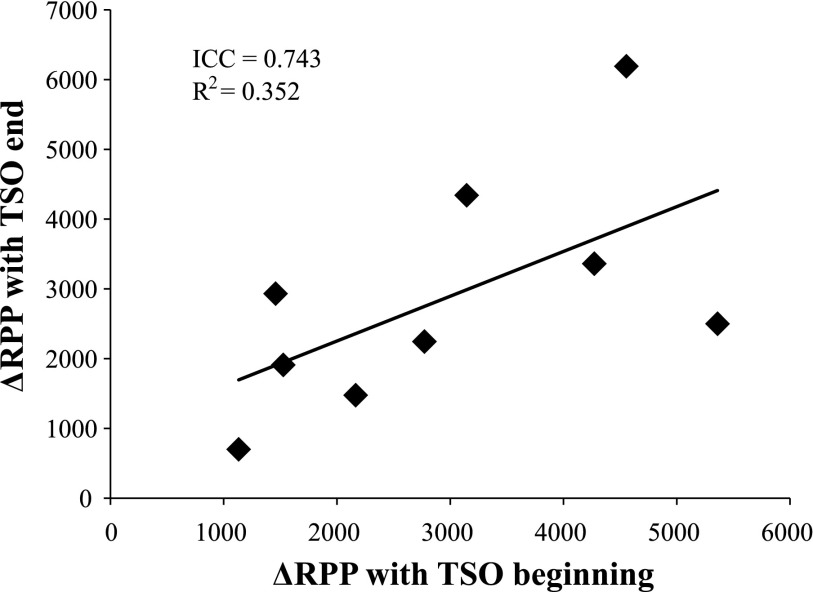
Inhalation of lidocaine did not affect supine resting MAP [pre: 77 ± 2; after (post): 78 ± 2 mmHg) or HR (pre: 58 ± 3; post: 56 ± 3 beats/min). During and after lidocaine inhalation, subjects reported a lack of sensation of the tongue and throat, and some individuals complained of hoarseness. Lidocaine blocked the gag reflex in all subjects (Fig. 3). The RPP response to TSO was reduced drastically following lidocaine (Fig. 4). Opening the mouth and sticking out the tongue elicited a modest increase in RPP, but this was not different between treatments (P = 0.122). Lidocaine reduced the peak RPP response to TSO (P = 0.004), as well as the RPP response within the first five cardiac cycles of recovery (P = 0.014). As expected, the peak ΔSBP (pre: 25 ± 4 vs. post: 7 ± 2 mmHg), peak ΔHR (pre: 12 ± 3 vs. post: 2 ± 2 beats/min), and peak ΔMAP (pre: 16 ± 2 vs. post: 5 ± 2 mmHg) response to TSO were also attenuated following lidocaine inhalation (all P < 0.01). As shown in Fig. 5, there was a strong, positive relationship between TSO-induced RPP responses within the same day (Cronbach's α = 0.743, P = 0.036).
Fig. 3.
Representative beat-by-beat recordings of BP, HR, and Resp in the same subject in response to TSO before (Pre; left) and after (Post; right) lidocaine inhalation. The 1st arrow denotes opening the mouth, and the 2nd arrow denotes TSO. Following lidocaine, TSO did not elicit the gag reflex.
Fig. 4.
ΔRPP in response to TSO before (solid lines) and after (dashed lines) 4% topical lidocaine inhalation (Experiment 2). Prior to lidocaine, TSO elicited the gag reflex; after lidocaine, the gag reflex was abolished. Data are means ± SE. *P < 0.05 between treatments. BASE, baseline; REC 5/30, recovery with 5/30 cardiac cycles.
Fig. 5.
Test-retest reliability of the ΔRPP in response to TSO within the same day. During Experiment 2, the gag reflex was elicited at the beginning of the experiment (before lidocaine; x-axis) and at the end of the experiment (after lidocaine had worn off; y-axis). ICC, intraclass correlation coefficient; R2, correlation coefficient.
Experiment 3: Effect of Lidocaine Inhalation on HR and BP during CPT
To determine whether inhaled lidocaine would attenuate hemodynamic and perceptual responses to the CPT, paired t-tests were conducted. The ΔRPP was not different before (2,347 ± 873 mmHg × beats/min) and after (2,559 ± 629 mmHg × beats/min) lidocaine (P = 0.591). Similarly, the ΔMAP (pre: 30 ± 6; post: 26 ± 8 mmHg) and the ΔHR (pre: 4 ± 6; post: 6 ± 4 beats/min) were also not statistically different. Furthermore, hand thermal sensation (pre: 9 ± 1; post: 8 ± 1) and hand pain (pre: 7 ± 1; post: 7 ± 1) were comparable before and after lidocaine, indicating that lidocaine inhalation did not cause systemic sympathoinhibition.
DISCUSSION
Previous research has demonstrated that laryngoscopy and upper-airway intubation cause rapid increases in HR, BP, MSNA, and plasma catecholamines (1, 8, 16, 21, 38, 53). These changes are likely to be reflex mediated, because they can be blunted or abolished by using different pharmacotherapies (i.e., ganglionic blockade, beta blockade) (46, 51). However, these cited studies were performed in anesthetized patients, and it is known that general anesthesia can affect reflex pathways (44). For this reason, we sought to document how conscious, unmedicated humans would respond to TSO. The primary, novel findings are: 1) TSO elicited reflex increases in MSNA, MAP, HR, and RVR compared with Sham; 2) inhalation of 4% topical lidocaine prior to TSO blocked the gag reflex and attenuated increases in HR and BP; and 3) inhalation of 4% topical lidocaine did not affect the hemodynamic or perceptual responses to the CPT. To our knowledge, this is the first report of TSO eliciting sympathoexcitation in conscious, unmedicated humans.
The afferent arm of the gag reflex is comprised of the glossopharyngeal nerve (cranial nerve IX) and the laryngeal branch of the vagus nerve (cranial nerve X). These nerves relay sensory information from the pharynx, tonsils, epiglottis, and base of the tongue to the medulla (31, 57). The efferent arm of the gag reflex includes the vagus nerve (cranial nerve X) and results in contraction of the posterior oral and pharyngeal musculature, thus preventing foreign bodies from entering the trachea (25). On the other hand, the hard palate is innervated by the maxillary branch of the trigeminal nerve, also called the nasopalatine nerve. The application of pressure to this nerve does not typically elicit the gag reflex. Therefore, Sham was considered to be the control in Experiment 1.
As noted in Fig. 1, TSO elicited rapid increases in HR and MAP, as detected by the Finometer device. Previous experiments using an arterial catheter have shown that upper-airway stimulation increases SBP by 30–60 mmHg and DBP by 10–30 mmHg, while causing a modest tachycardia (ΔHR 15–30 beats/min) (1, 8, 16, 21, 53). Our data are comparable, considering that TSO was of shorter duration than laryngoscopy and intubation (but elicited the same airway defense reflex). The nucleus tractus solitarius (NTS) receives inputs from cranial nerves IX and X, as well as the carotid chemoreceptors and baroreceptors that are well recognized to participate in circulatory homeostasis (34). We postulate that in response to TSO, the NTS activates both pharyngeal muscle contraction and sympathetic outflow to skeletal muscle and the kidney in a parallel manner.
Ebert et al. (9) were the first to demonstrate that MSNA increases during laryngoscopy and intubation in thiopental-anesthetized humans. This finding was corroborated in subsequent experiments that employed a pharyngeal suction stimulus (43). MSNA is a direct measure of sympathetic outflow to blood vessels within skeletal muscle and participates in reflex adjustments in BP. In the current study using conscious, unmedicated humans, we demonstrate that MSNA increased, and RVR also increased within one to two cardiac cycles of TSO (Fig. 2). Based on the temporal response, we hypothesize that TSO increased renal sympathetic nerve activity, thereby evoking renal vasoconstriction. A study in dogs documented that renal sympathetic nerve activity increased during both intubation and extubation (42). In our experiment, the magnitude of increase in RVR following TSO within one to two cardiac cycles was comparable with that seen after ∼60 s of isometric handgrip (40% maximal contraction) (27). During Sham stimulation, there was no increase in MSNA and no renal vasoconstriction, but we did document a small increase in RPP and MAP, attributable to participant anxiety and/or opening the mouth and protruding the tongue.
Lidocaine inhalation has been used in clinical anesthesia to lessen the circulatory responses resulting from intubation and laryngoscopy (4, 22, 54). Fundamentally, lidocaine blocks sodium channels and prevents afferent nerves from generating an action potential. Because of this, lidocaine has also been used in research studies to understand how peripheral afferents (e.g., within muscle or blood vessel) affect BP homeostasis (6, 10, 36). With the use of lidocaine inhalation to block sensory afferents in the oropharynx (presumably cranial nerves IX and X), we have demonstrated that TSO elicits a sympathoexcitatory reflex (in addition to the well-characterized gag reflex) in conscious, healthy humans. It is important to note that our lidocaine-inhalation protocol did not affect the physiological or perceptual responses to the CPT (Experiment 3). Regional administration of lidocaine into a limb is known to block the pressor and pain response to the CPT (14). Taken together, these data provide strong experimental evidence that inhalation of lidocaine is exerting a local, not systemic, anesthetic effect. Lidocaine inhalation is simple, well tolerated, and does not affect resting hemodynamics; these factors make it ideal to use in future research studies.
Experiment 2 required that each subject experience the gag reflex twice within the same day (i.e., before lidocaine was given and after it had worn off). Although not the intended purpose of our study, this experimental design allowed for test-retest reliability to be determined. As displayed in Fig. 5, the ΔRPP in response to TSO was similar within the same day, such that higher RPP responses to the first TSO were related to higher responses to the second TSO. This supports the previously established concept that individual differences in cardiovascular reactivity to upper-airway stimulation exist (19, 35).
On a physiological level, the current data indicate that short-duration tactile stimulation of the upper airway elevates sympathetic outflow to the kidney and skeletal muscle. These data in conscious human subjects extend upon prior publications, suggesting that the oropharynx is a sensory organ capable of initiating sympathetic reflexes (51, 56). On a clinical level, thousands of intubations are performed each day throughout the world, and many of these patients have underlying cardiovascular disease (29, 35). The sympathetic nervous system likely contributes to cardiac and renal complications observed in these patients. Specifically, longer intubation durations are linked with increased risk of myocardial infarction (2), and electrocardiographic abnormalities are most common during intubation (20, 37, 53). Clinical observations have also shown that postoperative renal failure (seemingly due to reductions in blood flow) is linked to increased mortality (49). Furthermore, ventricular tachycardia and cerebrovascular accident have been documented in one kidney-transplant patient following endotracheal intubation (12). During upper-airway procedures, it is desirable to prevent a large increase in RPP (i.e., with afferent blockade) rather than to give vasoactive medication or additional inhalation anesthetics (i.e., with efferent blockade, which would not normally be used) to combat an increased RPP. Whether oropharyngeal afferents also contribute to adverse outcomes resulting from other airway stimuli (e.g., cold-air inhalation, cigarette smoking, prolonged ventilator use) is yet to be determined.
The current experiments used a physiological approach to understand how TSO impacts cardiovascular homeostasis in young, healthy humans. As such, extrapolation to healthy, older adults or patient populations must be done with caution. It should be noted that TSO was of short duration in the current study, leading to similar yet smaller responses compared with previous studies (1, 8, 16, 21, 53). For ethical reasons, we chose not to intubate our conscious, healthy subjects and instead, focused on the physiological mechanisms underlying TSO. It is also possible that stimulation of the larynx or lower respiratory tract may elicit a different response, and this response may be modulated by the type of stimulus (e.g., pressure, temperature, irritation) (32, 33, 39). Additional studies are warranted to unravel how TSO impacts human physiology in both healthy and diseased states.
Clinical Implications
In the current study, TSO elicited acute increases in MSNA, MAP, HR, and RVR in conscious humans, and this effect could be blocked with local anesthesia of the upper airway. These data provide evidence that airway defense mechanisms (e.g., gag reflex) engage the sympathetic nervous system and elevate HR and BP. We speculate that a sensitized upper airway, due to allergies, cigarette smoking, or gingivitis, places a patient at an elevated cardiovascular risk during intubation and laryngoscopy via a sympathetic neural mechanism. To our knowledge, this concept has not been established previously, but the current physiological data relating upper-airway afferents to systemic vasoconstriction support this idea.
GRANTS
Support for this work was provided by National Heart, Lung, and Blood Institute Grants P01-HL096570 and R01-HL070222, National Center for Research Resources Grant UL1-RR033184, and National Center for Advancing Translational Sciences Grant UL1-TR000127 (to L. I. Sinoway), as well as National Center for Research Resources Grant C06-RR016499 and a Wilderness Medical Society Research in Training Grant (to M. D. Muller).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
Author contributions: M.D.M., P.M.M., and L.I.S. conception and design of research; M.D.M., J.L.M., J.C., and M.J.H. performed experiments; M.D.M. and M.J.H. analyzed data; M.D.M., J.L.M., J.C., M.J.H., P.M.M., and L.I.S. interpreted results of experiments; M.D.M. prepared figures; M.D.M. drafted manuscript; M.D.M., J.L.M., J.C., M.J.H., P.M.M., and L.I.S. edited and revised manuscript; M.D.M., J.L.M., J.C., M.J.H., P.M.M., and L.I.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors appreciate the nursing support provided by Cheryl Blaha and Todd Nicklas, as well as the technical support of Josh Oman, Charity Sauder, Dr. Rachel Drew, and Dr. Michael Herr. Gratitude is also extended to Anne Muller for preparing the graphics for this study. Finally, the authors appreciate the administrative guidance of Kris Gray and Jen Stoner.
REFERENCES
- 1.Abou-Madi M, Keszler H, Yacoub O. A method for prevention of cardiovascular reactions to laryngoscopy and intubation. Can Anaesth Soc J 22: 316–329, 1975 [DOI] [PubMed] [Google Scholar]
- 2.Bando K, Sun K, Binford RS, Sharp TG. Determinants of longer duration of endotracheal intubation after adult cardiac operations. Ann Thorac Surg 63: 1026–1033, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Bassi GS, Humphris GM, Longman LP. The etiology and management of gagging: a review of the literature. J Prosthet Dent 91: 459–467, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Chinn WM, Zavala DC, Ambre J. Plasma levels of lidocaine following nebulized aerosol administration. Chest 71: 346–348, 1977 [DOI] [PubMed] [Google Scholar]
- 5.Conboy EE, Fogelman AE, Sauder CL, Ray CA. Endurance training reduces renal vasoconstriction to orthostatic stress. Am J Physiol Renal Physiol 298: F279–F284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui J, McQuillan P, Blaha CA, Kunselman AR, Sinoway LI. Limb venous distension evokes sympathetic activation via stimulation of the limb afferents in humans. Am J Physiol Heart Circ Physiol 303: H457–H463, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies AE, Kidd D, Stone SP, MacMahon J. Pharyngeal sensation and gag reflex in healthy subjects. Lancet 345: 487–488, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Denlinger JK, Ellison N, Ominsky AJ. Effects of intratracheal lidocaine on circulatory responses to tracheal intubation. Anesthesiology 41: 409–412, 1974 [DOI] [PubMed] [Google Scholar]
- 9.Ebert TJ, Kanitz DD, Kampine JP. Inhibition of sympathetic neural outflow during thiopental anesthesia in humans. Anesth Analg 71: 319–326, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Ebert TJ, Mohanty PK, Kampine JP. Lidocaine attenuates efferent sympathetic responses to stress in humans. J Cardiothorac Vasc Anesth 5: 437–443, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Ebert TJ, Muzi M, Berens R, Goff D, Kampine JP. Sympathetic responses to induction of anesthesia in humans with propofol or etomidate. Anesthesiology 76: 725–733, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Fox EJ, Sklar GS, Hill CH, Villanueva R, King BD. Complications related to the pressor response to endotracheal intubation. Anesthesiology 47: 524–525, 1977 [DOI] [PubMed] [Google Scholar]
- 13.Friedman DB, Brennum J, Sztuk F, Hansen OB, Clifford PS, Bach FW, Arendt-Nielsen L, Mitchell JH, Secher NH. The effect of epidural anaesthesia with 1% lidocaine on the pressor response to dynamic exercise in man. J Physiol 470: 681–691, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman DB, Jensen FB, Mitchell JH, Secher NH. Heart rate and arterial blood pressure at the onset of static exercise in man with complete neural blockade. J Physiol 423: 543–550, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glickman-Weiss EL, Hearon CM, Nelson AG, Robertson RJ. A thermal perception scale for use during resting exposure to cold air. Percept Mot Skills 79: 547–560, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Hamaya Y, Dohi S. Differences in cardiovascular response to airway stimulation at different sites and blockade of the responses by lidocaine. Anesthesiology 93: 95–103, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Hardy JC, Gray K, Whisler S, Leuenberger U. Sympathetic and blood pressure responses to voluntary apnea are augmented by hypoxemia. J Appl Physiol 77: 2360–2365, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Havenith G, van de Linde EJ, Heus R. Pain, thermal sensation and cooling rates of hands while touching cold materials. Eur J Appl Physiol Occup Physiol 65: 43–51, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Ismail S, Azam SI, Khan FA. Effect of age on haemodynamic response to tracheal intubation. A comparison of young, middle-aged and elderly patients. Anaesth Intensive Care 30: 608–614, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Katz RL, Bigger JT., Jr Cardiac arrhythmias during anesthesia and operation. Anesthesiology 33: 193–213, 1970 [DOI] [PubMed] [Google Scholar]
- 21.King BD, Harris LC, Jr, Greifenstein FE, Elder JD, Jr, Dripps RD. Reflex circulatory responses to direct laryngoscopy and tracheal intubation performed during general anesthesia. Anesthesiology 12: 556–566, 1951 [DOI] [PubMed] [Google Scholar]
- 22.Kirkpatrick MB, Sanders RV, Bass JB., Jr Physiologic effects and serum lidocaine concentrations after inhalation of lidocaine from a compressed gas-powered jet nebulizer. Am Rev Respir Dis 136: 447–449, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Kregel KC, Seals DR, Callister R. Sympathetic nervous system activity during skin cooling in humans: relationship to stimulus intensity and pain sensation. J Physiol 454: 359–371, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laurito CE, Baughman VL, Becker GL, Polek WV, Riegler FX, VadeBoncouer TR. Effects of aerosolized and/or intravenous lidocaine on hemodynamic responses to laryngoscopy and intubation in outpatients. Anesth Analg 67: 389–392, 1988 [PubMed] [Google Scholar]
- 25.Miller AJ. Oral and pharyngeal reflexes in the mammalian nervous system: their diverse range in complexity and the pivotal role of the tongue. Crit Rev Oral Biol Med 13: 409–425, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Miller BD, Thames MD, Mark AL. Inhibition of cardiac sympathetic nerve activity during intravenous administration of lidocaine. J Clin Invest 71: 1247–1253, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Momen A, Bower D, Leuenberger UA, Boehmer J, Lerner S, Alfrey EJ, Handly B, Sinoway LI. Renal vascular response to static handgrip exercise: sympathetic vs. autoregulatory control. Am J Physiol Heart Circ Physiol 289: H1770–H1776, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Momen A, Leuenberger UA, Ray CA, Cha S, Sinoway LI. Renal vascular responses to static handgrip: role of the muscle mechanoreflex. Am J Physiol Heart Circ Physiol 285: H1247–H1253, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Mort TC. Emergency tracheal intubation: complications associated with repeated laryngoscopic attempts. Anesth Analg 99: 607–613, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Mort TC. The incidence and risk factors for cardiac arrest during emergency tracheal intubation: a justification for incorporating the ASA Guidelines in the remote location. J Clin Anesth 16: 508–516, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Mu L, Sanders I. Human tongue neuroanatomy: nerve supply and motor endplates. Clin Anat 23: 777–791, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller MD, Gao Z, Drew RC, Herr MD, Leuenberger UA, Sinoway LI. Effect of cold air inhalation and isometric exercise on coronary blood flow and myocardial function in humans. J Appl Physiol 111: 1694–1702, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller MD, Gao Z, Mast JL, Blaha CA, Drew RC, Leuenberger UA, Sinoway LI. Aging attenuates the coronary blood flow response to cold air breathing and isometric handgrip in healthy humans. Am J Physiol Heart Circ Physiol 302: H1737–H1746, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potts JT. Neural circuits controlling cardiorespiratory responses: baroreceptor and somatic afferents in the nucleus tractus solitarius. Clin Exp Pharmacol Physiol 29: 103–111, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Prys-Roberts C, Greene LT, Meloche R, Foex P. Studies of anaesthesia in relation to hypertension. II. Haemodynamic consequences of induction and endotracheal intubation. Br J Anaesth 43: 531–547, 1971 [DOI] [PubMed] [Google Scholar]
- 36.Ray CA, Secher NH, Mark AL. Modulation of sympathetic nerve activity during posthandgrip muscle ischemia in humans. Am J Physiol Heart Circ Physiol 266: H79–H83, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Roy WL, Edelist G, Gilbert B. Myocardial ischemia during non-cardiac surgical procedures in patients with coronary-artery disease. Anesthesiology 51: 393–397, 1979 [DOI] [PubMed] [Google Scholar]
- 38.Russell WJ, Morris RG, Frewin DB, Drew SE. Changes in plasma catecholamine concentrations during endotracheal intubation. Br J Anaesth 53: 837–839, 1981 [DOI] [PubMed] [Google Scholar]
- 39.Sant'Ambrogio G, Mathew OP, Sant'Ambrogio FB, Fisher JT. Laryngeal cold receptors. Respir Physiol 59: 35–44, 1985 [DOI] [PubMed] [Google Scholar]
- 40.Sauder CL, Conboy EE, Chin-Sang SA, Ray CA. Otolithic activation on visceral circulation in humans: effect of aging. Am J Physiol Renal Physiol 295: F1166–F1169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwab TM, Greaves TH. Cardiac arrest as a possible sequela of critical airway management and intubation. Am J Emerg Med 16: 609–612, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Seagard JL, Hopp FA, Bosnjak ZJ, Osborn JL, Kampine JP. Sympathetic efferent nerve activity in conscious and isoflurane-anesthetized dogs. Anesthesiology 61: 266–270, 1984 [DOI] [PubMed] [Google Scholar]
- 43.Sellgren J, Ponten J, Wallin BG. Characteristics of muscle nerve sympathetic activity during general anaesthesia in humans. Acta Anaesthesiol Scand 36: 336–345, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Shimokawa A, Kunitake T, Takasaki M, Kannan H. Differential effects of anesthetics on sympathetic nerve activity and arterial baroreceptor reflex in chronically instrumented rats. J Auton Nerv Syst 72: 46–54, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Shribman AJ, Smith G, Achola KJ. Cardiovascular and catecholamine responses to laryngoscopy with and without tracheal intubation. Br J Anaesth 59: 295–299, 1987 [DOI] [PubMed] [Google Scholar]
- 46.Siedlecki J. Disturbances in the function of cardiovascular system in patients following endotracheal intubation and attempts of their prevention by pharmacological bloackade of sympathetic system. Anaesth Resusc Intensive Ther 3: 107–123, 1975 [PubMed] [Google Scholar]
- 47.Sitzman BT, Rich GF, Rockwell JJ, Leisure GS, Durieux ME, DiFazio CA. Local anesthetic administration for awake direct laryngoscopy. Are glossopharyngeal nerve blocks superior? Anesthesiology 86: 34–40, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Sklar BZ, Lurie S, Ezri T, Krichelli D, Savir I, Soroker D. Lidocaine inhalation attenuates the circulatory response to laryngoscopy and endotracheal intubation. J Clin Anesth 4: 382–385, 1992 [DOI] [PubMed] [Google Scholar]
- 49.Slankamenac K, Breitenstein S, Held U, Beck-Schimmer B, Puhan MA, Clavien PA. Development and validation of a prediction score for postoperative acute renal failure following liver resection. Ann Surg 250: 720–728, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Stoelting RK. Blood pressure and heart rate changes during short-duration laryngoscopy for tracheal intubation: influence of viscous or intravenous lidocaine. Anesth Analg 57: 197–199, 1978 [DOI] [PubMed] [Google Scholar]
- 51.Tomori Z, Widdicombe JG. Muscular, bronchomotor and cardiovascular reflexes elicited by mechanical stimulation of the respiratory tract. J Physiol 200: 25–49, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vallbo AB, Hagbarth KE, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979 [DOI] [PubMed] [Google Scholar]
- 53.Venus B, Polassani V, Pham CG. Effects of aerosolized lidocaine on circulatory responses to laryngoscopy and tracheal intubation. Crit Care Med 12: 391–394, 1984 [DOI] [PubMed] [Google Scholar]
- 54.Vuckovic DD, Rooney SM, Goldiner PL, O'Sullivan D. Aerosol anesthesia of the airway using a small disposable nebulizer. Anesth Analg 59: 803–804, 1980 [PubMed] [Google Scholar]
- 55.Wahidi MM, Jain P, Jantz M, Lee P, Mackensen GB, Barbour SY, Lamb C, Silvestri GA. American College of Chest Physicians consensus statement on the use of topical anesthesia, analgesia, and sedation during flexible bronchoscopy in adult patients. Chest 140: 1342–1350, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Widdicombe J. Airway receptors. Respir Physiol 125: 3–15, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Wright SM. An examination of factors associated with retching in dental patients. J Dent 7: 194–207, 1979 [DOI] [PubMed] [Google Scholar]