Abstract
Heavy exercise increases ventilation-perfusion mismatch and decreases pulmonary gas exchange efficiency. Previous work using magnetic resonance imaging (MRI) arterial spin labeling in athletes has shown that, after 45 min of heavy exercise, the spatial heterogeneity of pulmonary blood flow was increased in recovery. We hypothesized that the heterogeneity of regional specific ventilation (SV, the local tidal volume over functional residual capacity ratio) would also be increased following sustained exercise, consistent with the previously documented changes in blood flow heterogeneity. Trained subjects (n = 6, maximal O2 consumption = 61 ± 7 ml·kg−1·min−1) cycled 45 min at their individually determined ventilatory threshold. Oxygen-enhanced MRI was used to quantify SV in a sagittal slice of the right lung in supine posture pre- (preexercise) and 15- and 60-min postexercise. Arterial spin labeling was used to measure pulmonary blood flow in the same slice bracketing the SV measures. Heterogeneity of SV and blood flow were quantified by relative dispersion (RD = SD/mean). The alveolar-arterial oxygen difference was increased during exercise, 23.3 ± 5.3 Torr, compared with rest, 6.3 ± 3.7 Torr, indicating a gas exchange impairment during exercise. No significant change in RD of SV was seen after exercise: preexercise 0.78 ± 0.15, 15 min postexercise 0.81 ± 0.13, 60 min postexercise 0.78 ± 0.08 (P = 0.5). The RD of blood flow increased significantly postexercise: preexercise 1.00 ± 0.12, 15 min postexercise 1.15 ± 0.10, 45 min postexercise 1.10 ± 0.10, 60 min postexercise 1.19 ± 0.11, 90 min postexercise 1.11 ± 0.12 (P < 0.005). The lack of a significant change in RD of SV postexercise, despite an increase in the RD of blood flow, suggests that airways may be less susceptible to the effects of exercise than blood vessels.
Keywords: magnetic resonance imaging, pulmonary gas exchange, ventilation-perfusion mismatch, lung imaging
the stress of exercise has numerous effects on the pulmonary system, many of which are increased by longer exercise duration and higher intensity. For example, in most individuals, exercise increases ventilation-perfusion (V̇a/Q̇) mismatch and decreases the efficiency of gas exchange (14, 20, 38), which manifests as an increase in the arterial-alveolar oxygen difference (A-aDo2) (10, 14, 23). In subjects exercising at an intensity below an O2 consumption (V̇o2) of 4.0 l/min, V̇a/Q̇ mismatch comprises most of the A-aDo2 (41). Additionally, V̇a/Q̇ mismatch has been shown to increase with increasing duration of moderate or heavy exercise (20), and changes in the patterns of V̇a/Q̇ matching persist after exercise lasting into the recovery period (38). We have previously shown using a magnetic resonance imaging (MRI) technique known as arterial spin labeling (ASL) that, in the recovery period after 45 min of heavy exercise at the ventilatory threshold [∼75% maximal V̇o2 (V̇o2 max)], the spatial heterogeneity of pulmonary perfusion is increased compared with preexercise baseline, and this effect persists for at least 1 h postexercise (7).
Although controversial, there is evidence that the mechanism responsible for exercise-induced V̇a/Q̇ mismatch is interstitial pulmonary edema (26, 27, 37, 38). This is suggested to be because translocation of fluid into the interstitial space acts to mechanically compress small blood vessels and airways, thus altering the local resistances, affecting V̇a/Q̇ matching (38). Since the branching structure of the lung is such that the airways and blood vessels branch together, the spatial distribution of ventilation may show a similar increase in heterogeneity after exercise to that of blood flow. However, changes in the spatial distribution of ventilation after exercise are unknown. We hypothesized that the heterogeneity of regional specific ventilation (SV) following sustained exercise would be increased compared with preexercise resting conditions, in a similar way as the previously reported effect on perfusion (7).
To test this, we used a recently developed functional MRI technique known as SV imaging (SVI) that allows the assessment of the spatial distribution of SV (35), the local tidal volume-to-functional residual capacity ratio. Using SVI and ASL (2, 6), we measured the spatial distribution both of SV and of pulmonary blood flow in healthy athletic subjects before and after sustained heavy exercise consisting of 45 min of cycling at ventilatory threshold (7). This is an exercise intensity and duration that have been previously shown to induce V̇a/Q̇ mismatch in most athletic individuals (20) and thus might be expected to cause postexercise disruptions in the distribution of ventilation and perfusion.
METHODS
Subjects
This study was approved by the Institutional Review Boards at the University of California, San Diego, and San Diego State University. Six healthy, athletic subjects, 4 men, 2 women (age = 23.5 ± 2.5 yr, mean ± SD, weight = 74.0 ± 15.8 kg, V̇o2 max = 61.4 ± 6.9 ml·kg−1·min−1) were recruited. Subjects gave written, informed consent and were screened for magnetic resonance (MR) contraindications with MRI safety questionnaires. Subjects also completed physical activity readiness questionnaires, pulmonary function testing, medical history, and a physical examination before their involvement in the study to rule out any previously undiagnosed disease or conditions. Subject characteristics are depicted in Table 1.
Table 1.
Subject characteristics
| Means ± SD | |
|---|---|
| n | 6 (4 men, 2 women) |
| Age, yr | 23.5 ± 2.5 |
| Weight, kg | 74.0 ± 15.8 |
| V̇o2max, l/min | 4.53 ± 1.00 |
| V̇o2max, ml·kg−1·min−1 | 61.4 ± 6.9 |
| Maximum heart rate, beats/min | 196 ± 3 |
| Maximum power, W | 423 ± 65 |
| V̇o2max at VT, % | 75 ± 6 |
| Heart rate at VT, beats/min | 163 ± 16 |
| V̇o2 at VT, l/min | 3.5 ± 0.8 |
| Workload at VT, W | 255 ± 62 |
V̇o2, O2 consumption; V̇o2max, maximal V̇o2; VT, ventilatory threshold.
Study Design
At a preliminary testing session, subjects underwent a progressive incremental test to V̇o2 max on a cycle ergometer to determine their ventilatory threshold. The ventilatory threshold is used by athletes to define the maximal sustainable intensity for prolonged aerobic exercise and thus was used to provide a workload for the exercise task. At least 48 h later, subjects returned for the imaging/exercise session. Spirometry was performed before baseline imaging. After the baseline imaging session (performed in supine posture), an arterial catheter was inserted into the radial artery for blood sampling, and then subjects performed 45 min of exercise at a V̇o2 corresponding to their previously determined ventilatory threshold. Following the exercise task, subjects completed spirometry again before the imaging protocol was repeated twice, at 15 and 60 min postexercise. A time line of data acquisition is given in Fig. 1.
Fig. 1.

Procedure time line. During MRI, DEN is density, Asl is arterial spin labeling measured blood flow, and SVI is specific ventilation imaging measured specific ventilation. During pre- and postexercise measures, subjects lay supine in the scanner. During cycling exercise, subjects were in the upright posture. Arrows indicate timing of arterial blood-gas measurements.
Preliminary Testing
The subjects performed a standard test of V̇o2 max (ParvoMedics TruMax 2400 system, Sandy, UT) using a progressive incremental test to volitional fatigue on a cycle ergometer (Excaliber, Quinton Instruments, Groningen, The Netherlands). The starting workload was 50–150 W, depending on body size and sex, and the workload was increased by 25 W (women) or 30 W (men) every minute until exhaustion. Workload (Watts), heart rate (beats/min), and V̇o2 (calculated in liters per minute and milliliters per kilogram per minute) were recorded during testing. Ventilatory thresholds of the subjects were determined using the V-slope method (4), also noting the target corresponding workload and heart rate to be used for the 45-min exercise task. Table 1 contains V̇o2 max and heart rate data obtained from this preliminary test and the workload used to determine the 45-min exercise task.
Data Collection
No less than 48 h after preliminary testing, subjects returned to the laboratory for further testing, consisting of spirometry, MRI measurements of lung density, pulmonary blood flow, and SV before and after 45 min of heavy exercise at ∼75% of V̇o2 max.
Spirometry
Spirometry was performed in the standing position before and immediately after exercise using an EasyOne spirometer (NDD Medical Technologies, Zurich, Switzerland). Forced expiratory volume in 1 s, forced vital capacity, ratio of forced expiratory volume in 1 s to forced vital capacity, and, forced expiratory volume 25 to 75th percent are reported in Table 2.
Table 2.
Spirometry
| Preexercise |
5 min Postexercise |
||||
|---|---|---|---|---|---|
| Means ± SD | %Predicted | Means ± SD | %Predicted | P Value | |
| FVC, liters | 5.56 ± 1.00 | 108 ± 9 | 5.41 ± 0.87 | 103 ± 14 | 0.17 |
| FEV1, liters | 4.47 ± 0.79 | 103 ± 9 | 4.56 ± 0.81 | 104 ± 13 | 0.46 |
| FEV1/FVC, % | 81 ± 6 | 96 ± 6 | 84 ± 6 | 101 ± 8 | 0.39 |
| FEF25–75, liters | 4.33 ± 1.24 | 87 ± 16 | 4.98 ± 1.30 | 106 ± 22 | 0.08 |
Values are means ± SD, with percentage of predicted values also reported. Pulmonary function tests were performed by spirometry pre- and 5 min postexercise. FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; FEF25–75, forced expiratory volume in the 25 to 75 percent. No statistically significant changes were seen in any measures of pulmonary function after exercise.
MRI Data Collection
The subject was placed supine in the MRI scanner wearing a face mask (6500 series V2 mask, Hans Rudolph), equipped with a non-rebreathing valve (Hans Rudolph, 2700). The inspiratory port of the valve was connected to a remote controlled valve, allowing a change from breathing room air to breathing 100% oxygen from a large gas-tight bag, for SVI. The outlet of the valve was connected to a 6-m-long, low-resistance expiratory line, leading out of the scanner room, where metabolic data were measured using the ParvoMedics Metabolic Measurement System, (ParvoMedics, Sandy, UT). Reference phantoms were placed on the anterior chest wall so as to be within the field of view (FOV) of the scans, permitting absolute quantification of blood flow and lung density (17). The subject lay on the posterior element of an eight-channel cardiac coil, and the anterior elements were placed directly on the chest wall. Images for a single 15-mm sagittal slice were acquired during brief (∼1 s) breath holds at functional residual capacity with the subject voluntarily respiratory gating. The right lung was chosen to eliminate motion artifacts from the aorta and heart in the left hemithorax. The image slice was positioned in the midclavicular line to capture the maximum anterior-posterior diameter of the lung, and the image slice position was referenced to the spinal cord so that it could be duplicated when the subject was repositioned in the scanner after exercise. All MR data were collected using a GE Signa 1.5T HD EXCITE clinical scanner (General Electric, Milwaukee, WI), with Operating System 14x-M5. This system is a short-bore design with a bore diameter of 60 cm. The RF subsystem (“EXCITE”) provides 16 quadrature (8 independent) high-bandwidth (1-MHz sampled) receiver channels.
Regional SV Quantification Using MRI
SV is the ratio of the volume of fresh gas (ΔV) entering a region of lung to the end-expiratory volume (V0) of that region (i.e., SV = ΔV/V0) (28, 33). Thus SV is a dimensionless quantity that measures the tidal expansion of that region relative to its end-expiratory volume (35). SVI is a proton MR technique capable of quantifying regional SV in a lung slice (35). In short, this technique is based on the following: the presence of oxygen dissolved in tissues shortens the longitudinal relaxation time (T1) of tissues, which alters the local MR signal intensity (11). A regional higher O2 tissue concentration results in a shorter T1, resulting in an increase in signal intensity for T1-weighted images (11). The rate of change of the alveolar O2 concentration following a sudden change in fraction of inspired oxygen is determined almost entirely by the local SV (35): lung regions with a higher SV reach a new equilibrium faster than those with lower SV. Thus the regional SV is quantified on a voxel-by-voxel basis by determining the MR signal intensity change on T1-weighted images. These images are acquired at end expiration (FRC) on a self-gated series of successive MR images, following fraction of inspired oxygen changes (0.21 to 1.00), while simultaneously measuring tidal volume (40). Validation of SVI against multiple breath nitrogen washout demonstrates that SVI describes the mean and width of the SV distribution with a high degree of validity (36).
The complete description of the technique used can be found in Ref. 40. Briefly, the MR images were acquired by an inversion recovery single-shot fast spin echo half Fourier acquisition, with a FOV of 40 cm × 40 cm, in a 15-mm-thick tissue slice. Images were acquired at a 256 × 128 resolution and reconstructed to 256 × 256; thus each voxel was ∼1.6 mm × 1.6 mm × 15 mm. The inversion pulse was cardiac-triggered to maximize the amount of stationary blood and was acquired following an inversion time delay of T1 = 1,100 ms, (close to the T1 of blood), ensuring maximal signal difference between the O2-enhanced breaths and the room-air breaths (9). A functional MRI block design approach was implemented with an image acquired every 5 s: 20 images while breathing room air, followed by 20 breaths of 100% O2. This cycle was repeated five times with an additional 20 breaths on oxygen added to the last cycle to give added resolution of slowly equilibrating lung units (low SV). A total of 220 images were acquired, over 18 min (35). SVI data were acquired at three time points: during preexercise (baseline) and at 15 and 45 min post exercise.
MR Blood Flow Imaging
Pulmonary blood flow was measured using a two-dimensional FAIRER (flow-sensitive alternating inversion recovery with an extra radio-frequency pulse) ASL sequence, with a half-Fourier acquisition single shot turbo spin echo readout (2). This technique has been extensively described in previous reports (1, 7, 29) and has been validated against a flow phantom (24). Cardiac triggered tag and control images were acquired in short breath holds (1–2 s), acquired 5-s apart, at end-expiration following normal tidal breaths. An imaging delay time of 700–960 ms was used (equal to 80% of the R-R interval, set individually for each subject), and repetition time = 5 s. The slice thickness was 15 mm, and FOV was 40 cm × 40 cm. To ensure data quality, tag and control pairs were acquired in triplicate during each ASL measurement. Each ASL blood flow image was constructed from the subtraction of two consecutive ASL tag, and control images and the results from the triplicate subtracted images were averaged. The ASL imaging was performed twice at each time point, before and after each SVI acquisition, thus bracketing these measures. The resulting data [mean, standard deviation, relative dispersion (RD), and fractal dimension] from the two preexercise measures were averaged, thus giving five time points for pulmonary blood flow: preexercise, 15 min postexercise, 45 min postexercise, 60 min postexercise, and 90 min postexercise.
Since large conduit vessels can be a significant source of signal that is not capillary perfusion to the slice, voxels with a signal intensity greater than 35% of the maximum were eliminated as described (8). Blood flow data were expressed as milliliters per minute per cubic centimeter and also normalized for regional lung density (measured below), and expressed as milliliters per minute per gram.
MR Lung Density
In addition to the ASL acquisition at each time point, a proton density image was also acquired in the same slice using a multigradient echo sequence to quantify regional lung water (18, 40). This technique has been validated, is highly reliable, and shows excellent agreement with gravimetric measures of total lung water, but cannot distinguish between water in intravascular and extravascular compartments (18). The decay rate of the signal is back-extrapolated to an echo time of zero on a voxel-by-voxel basis by fitting the signal acquired at two echoes to a single exponential and converted to a measure of lung water density using the measured signal from the reference phantom. Sequence parameters are repetition time = 10 ms, flip angle = 10°, slice thickness = 15 mm, FOV = 40 cm × 40 cm, receiver bandwidth = 125 kHz, and a full acquisition matrix of 64 × 64.
Coil Inhomogeneity
A multichannel torso coil was used to maximize signal-to-noise ratio for the measurements of SV and pulmonary blood flow. Images acquired with the torso coil are reconstructed from eight coils that may have different sensitivity profiles and thus suffer from signal inhomogeneity, based on proximity of lung regions to the coil elements. While not a problem for SV, since it measures the rate of change and not the absolute change in signal intensity, the blood-flow images must be corrected for this inhomogeneity. Paired lung density images from the torso coil were acquired preexercise, 15 min postexercise, and 60 min postexercise and used with the density images acquired from the body coil described above to correct for torso coil inhomogeneity in all acquired blood flow images on an individual subject basis, as described in earlier studies (7, 21).
Arterial Sampling
After the baseline, resting MRI data were acquired, and a radial artery catheter (20 gauge) was inserted percutaneously with local anesthetic using sterile technique. A sterile thermocouple (IT-18, PhysiTemp, Clifton, NJ) was inserted into the hub of the arterial catheter to monitor blood temperature. Arterial blood samples were taken in duplicate; at rest; during the exercise task at 10, 20, 30, and 45 min of exercise; and at 15, 45, and 90 min postexercise. A total of 16 samples were drawn into 3-ml preheparinized syringes for arterial blood-gas assessment and stored on ice until analyzed for arterial Po2 (PaO2), arterial Pco2 (PaCO2), and pH using an IL GEM 3000 blood gas analyzer (Instrumentation Laboratories, Lexington, MA). All blood-gas values were corrected to the subject's blood temperature measured during sample collection. The results from the duplicate samples at each time point were averaged.
Exercise Task
Following the insertion of the arterial catheter, subjects performed 45 min of cycling exercise at a V̇o2 consistent with their previously determined ventilatory threshold. The workload averaged ∼255 ± 62 W across subjects over the duration of the test. Throughout the exercise task, heart rate and metabolic parameters (V̇o2, V̇o2, carbon dioxide production, respiratory exchange ratio, and minute ventilation) were monitored to confirm that the subjects reached the target level of effort.
Data Analysis
Mean SV and lung blood flow were calculated for the images acquired, as previously described (2, 35). SV for nondependent, middle, and dependent thirds in the sagittal plane was also calculated to detect a possible effect across gravitational regions. Additionally, because changes specific to the lung base might be expected to arise from cycling in a largely upright posture, SV in apical, middle, and basal lung thirds was also calculated.
To characterize the heterogeneity of the distribution of SV and pulmonary blood flow, two indexes were used: 1) the spatial relative dispersion (RD = SD/mean); relative dispersion is a global measure of spatial heterogeneity without considering the specific anatomical location of heterogeneity, with larger relative dispersion values designating greater heterogeneity (29); and 2) fractal dimension, which allows for the analysis of pulmonary blood flow and SV heterogeneity distribution independent of voxel size and is indicative of branching structure (12). Fractal dimension was computed by calculating the relative dispersion of the image in progressively larger blocks centered on each voxel for the SV and blood flow images. Values for fractal dimension of a distribution range between 1.0 and 1.5; a fractal dimension of 1.0 suggests a homogeneous distribution, and 1.5 a spatially random distribution (12, 21).
Statistical Analysis
Statistical analysis was performed using repeated-measures ANOVA (Statview 5.0, SAS Institute, Cary, NC). For SV and related measures of spatial heterogeneity (RD and fractal dimension), there were two repeated measures, time of measurement and lung region with three time points (levels) for time of measurement (preexercise, 15 min postexercise, and 60 min postexercise), and three regions for lung region (in the gravitational direction: nondependent, middle, dependent lung; in the cephalocaudal direction: apical, middle, and basal). For pulmonary blood flow, there was one repeated measure, time of measurement, with five levels (preexercise, 15 min postexercise, 45 min postexercise, 60 min postexercise, and 90 min postexercise). For arterial blood-gas data, PaO2, PaCO2, and A-aDo2, there was also one repeated measure for time of measurement with eight levels: preexercise; 10, 20, 30, and 45 min during exercise; and 15, 45, and 90 min postexercise. When overall significance was present, post hoc testing was done with Fischer's protected least squares difference. All data are presented as means ± SD, with the null hypothesis rejected if P < 0.05, two tailed.
RESULTS
All subjects tolerated the study well. Descriptive characteristics of subjects, including exercise capacity, are reported in Table 1. There were no significant changes in the measured spirometry variables comparing pre- and 5 min postexercise (Table 2).
Arterial Blood Gases
Arterial blood-gas data are given in Table 3. There was a significant decrease in temperature-corrected PaO2, from 106.5 ± 3.2 Torr preexercise to 88.2 ± 8.0 Torr averaged over the duration of the exercise task (P < 0.01), but, by 15 min postexercise (103.2 ± 6.1 Torr), PaO2 was not different from preexercise values. A-aDo2 increased significantly from 6.3 ± 3.7 Torr preexercise to 23.3 ± 5.3 Torr averaged during exercise (P < 0.001) and returned to 4.2 ± 4.4 Torr by 15 min postexercise (Table 3). No statistically significant differences were seen in PaCO2 throughout the upright cycling exercise task compared with preexercise or recovery.
Table 3.
Metabolic data and arterial blood gases before exercise, during exercise, and during recovery
| Rest | Exercise | 15 min Postexercise | 45 min Postexercise | 90 min Postexercise | |
|---|---|---|---|---|---|
| V̇o2, l/min stpd | 0.39 ± 0.12 | 3.48 ± 0.84 | 0.32 ± 0.07 | 0.36 ± 0.10 | 0.29 ± 0.06 |
| V̇e, l/min btps | 13.7 ± 3.4 | 97.4 ± 24.6 | 10.5 ± 2.2 | 10.1 ± 3.5 | 8.9 ± 2.6 |
| HR, beats/min | 71.5 ± 11.4 | 177.8 ± 9.4 | 77.7 ± 11.9 | 70.0 ± 2.0 | 64.7 ± 1.5 |
| A-aDo2, Torr | 6.3 ± 3.7 | 23.3 ± 5.3* | 4.2 ± 4.4 | 8.0 ± 4.4 | 10.0 ± 4.4 |
| PaO2, Torr | 112.3 ± 2.8 | 112.0 ± 5.4 | 107.5 ± 7.1 | 107.5 ± 7.9 | 110.7 ± 5.0 |
| PaO2, Torr | 106.5 ± 3.2 | 88.2 ± 8.0* | 103.2 ± 6.1 | 99.7 ± 7.2 | 99.5 ± 7.9 |
| PaCO2, Torr | 35.8 ± 2.6 | 36.4 ± 3.2 | 38.0 ± 2.8 | 39.0 ± 4.2 | 37.2 ± 2.3 |
| pH | 7.45 ± 0.01 | 7.35 ± 0.04* | 7.39 ± 0.02* | 7.43 ± 0.01 | 7.44 ± 0.03 |
| Temperature, °C | 36.1 ± 0.5 | 37.9 ± 0.4* | 37.0 ± 0.4* | 36.6 ± 0.7† | 36.5 ± 0.4 |
| Lactate, mmol/l | 1.1 ± 0.3 | 4.2 ± 2.3 | 2.0 ± 0.6 | 1.3 ± 0.6 | 0.9 ± 0.4 |
| HCO3− | 24.7 ± 1.3 | 20.3 ± 2.6 | 22.8 ± 1.6 | 24.9 ± 1.8 | 25.2 ± 1.2 |
| Base excess | 0.6 ± 1.2 | −4.5 ± 3.0 | −1.9 ± 1.6 | 0.4 ± 1.7 | 1.0 ± 1.4 |
Values are means ± SD. Exercise values were calculated from average of four time points during exercise: 10, 20, 30, and 45 min. V̇e, minute ventilation; HR, heart rate; A-aDo2, alveolar-arterial difference for O2; PaO2, alveolar partial pressure of O2, PaO2, arterial partial pressure of O2, PaCO2, arterial partial pressure of CO2. Significantly different from rest:
P < 0.05,
P < 0.01.
SV
Mean values.
Example SV images are given in Fig. 2. Mean SV data are given in Table 4. There were no significant changes in mean SV (preexercise 0.34 ± 0.07, 15 min postexercise 0.33 ± 0.07, 60 min postexercise 0.31 ± 0.05, P = 0.11), indicating that mean SV had returned to baseline values at the time of postexercise imaging.
Fig. 2.
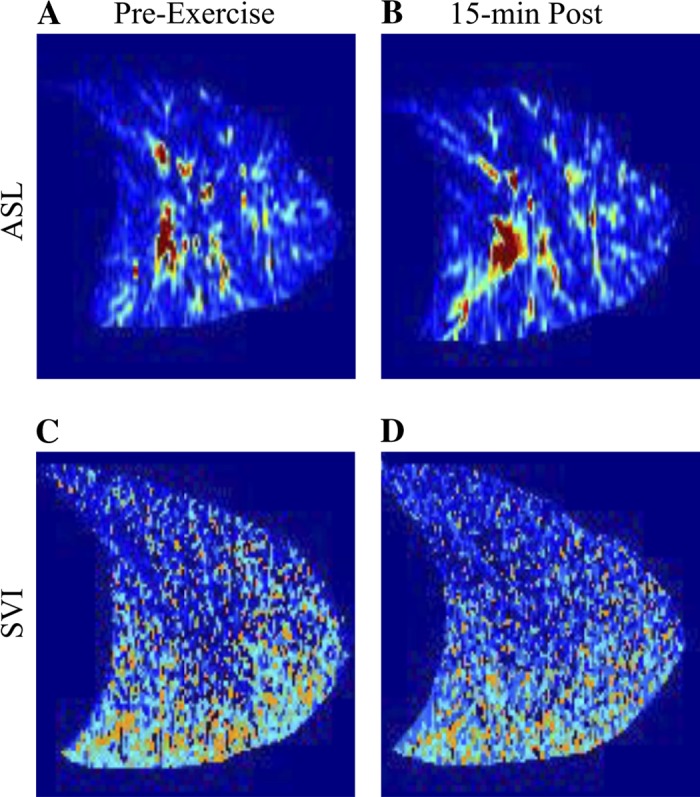
Representative supine images of pulmonary blood flow preexercise (A) and 15 min postexercise (B) and specific ventilation preexercise (C) and 15 min postexercise (D) in subject 6. In all images, the apex of the lung is to the right, and base of the lung is to the left. Signal from the large conduit blood vessels is removed from the blood flow images in postprocessing.
Table 4.
Specific ventilation, blood flow, and density
| Rest | 15 min Postexercise | 45 min Postexercise | 60 min Postexercise | 90 min Postexercise | P Value | |
|---|---|---|---|---|---|---|
| Mean data | ||||||
| Specific ventilation | 0.34 ± 0.07 | 0.33 ± 0.07 | 0.31 ± 0.05 | 0.11 | ||
| Blood flow ml·min−1·cm−3 | 2.55 ± 0.92 | 3.35 ± 0.80 | 3.38 ± 0.96 | 3.15 ± 0.73 | 3.24 ± 0.99 | 0.08 |
| Density, g H2O/cm3 lung | 0.23 ± 0.07 | 0.24 ± 0.04 | 0.22 ± 0.03 | 0.26 | ||
| Blood flow, ml·min−1·g−1 | 12.40 ± 4.67 | 14.37 ± 1.92 | 15.75 ± 3.32 | 16.19 ± 3.24 | 16.61 ± 3.74 | 0.20 |
| Heterogeneity: relative dispersion | ||||||
| Specific ventilation | 0.78 ± 0.15 | 0.81 ± 0.13 | 0.78 ± 0.08 | 0.50 | ||
| Blood flow, ml·min−1·cm−3 | 1.00 ± 0.12 | 1.15 ± 0.10* | 1.10 ± 0.10* | 1.19 ± 0.11* | 1.11 ± 0.12* | <0.005 |
| Density, g H2O/cm3 lung | 0.30 ± 0.06 | 0.28 ± 0.05 | 0.29 ± 0.06 | 0.65 | ||
| Blood flow, ml·min−1·g−1 | 0.87 ± 0.14 | 1.00 ± 0.12* | 0.96 ± 0.07* | 1.01 ± 0.10* | 0.98 ± 0.12* | <0.05 |
| Heterogeneity: fractal dimension | ||||||
| Specific ventilation | 1.17 ± 0.06 | 1.18 ± 0.05 | 1.15 ± 0.04 | 0.44 | ||
| Blood flow, ml·min−1·cm−3 | 1.17 ± 0.01 | 1.18 ± 0.03 | 1.18 ± 0.03 | 1.18 ± 0.03 | 1.16 ± 0.03 | 0.21 |
| Blood flow, ml·min−1·g−1 | 1.21 ± 0.03 | 1.22 ± 0.03 | 1.22 ± 0.04 | 1.23 ± 0.04 | 1.21 ± 0.05 | 0.95 |
Values are means ± SD. Relative dispersion = SD/mean (unitless). Specific ventilation is a unitless ratio. Fractal dimension is unitless.
Statistically different from rest, P < 0.05.
Lung regions.
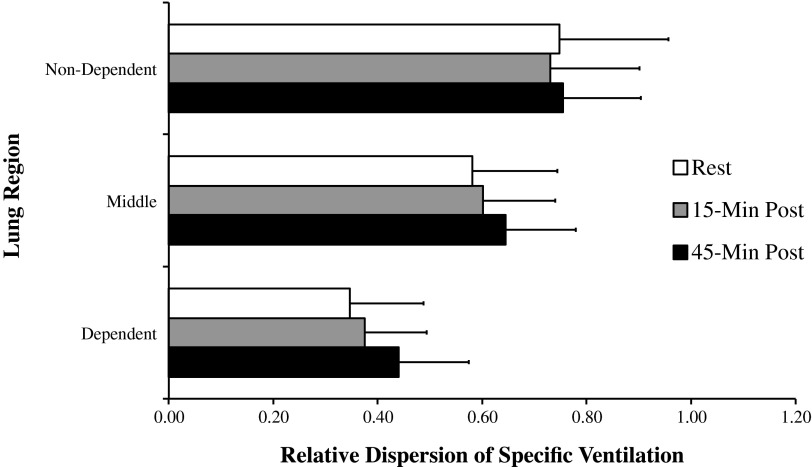
As expected, SV was statistically different (P < 0.001) across lung regions, nondependent, middle, and dependent, consistent with the previously known gravitational gradient in ventilation (35), but there was no significant interaction between lung region and time postexercise (P = 0.69), indicating that the gravitational gradient in SV was not significantly altered after exercise.
Measures of heterogeneity.
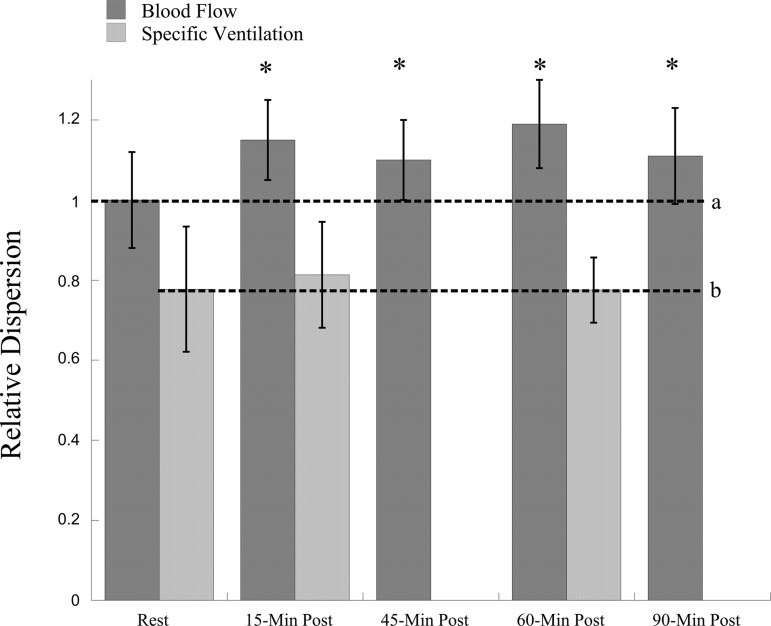
A summary of mean values for relative dispersion of SV are given in Fig. 3 and Table 4 and for fractal dimension in Table 4. The heterogeneity of SV as measured by relative dispersion (Fig. 3) and fractal dimension was not significantly altered postexercise (relative dispersion: preexercise 0.78 ± 0.15, 15 min postexercise 0.81 ± 0.13, and 60 min postexercise 0.78 ± 0.08, P = 0.50; fractal dimension preexercise: 1.17 ± 0.06, 15 min postexercise: 1.18 ± 0.05, 60 min postexercise: 1.15 ± 0.04, P = 0.44). Individual data are shown in Fig. 4. Three subjects showed a small increase in the relative dispersion of SV: one a decrease, and two no change. Figure 5 shows data for the three time points of measurement of relative dispersion of SV across gravitational regions. There was no significant effect time of measurement on relative dispersion of SV (P = 0.69) in this regional analysis, indicating that these lung regions showed a similar pattern of response.
Fig. 3.
Relative dispersion of pulmonary blood flow (ml·min·cm−3) and specific ventilation. *Relative dispersion of mean pulmonary blood flow postexercise is statistically different than rest, P = 0.0048. Post hoc analysis showed that pulmonary blood flow was different at 15 min (P < 0.001), 45 min (P = 0.001), 60 min (P = 0.001), and 90 min postexercise (P = 0.003), compared with rest. Relative dispersion of mean specific ventilation did not change after exercise (P = 0.50). a, Dotted line indicates baseline relative dispersion of pulmonary blood flow. b, Dotted line indicates baseline relative dispersion of specific ventilation.
Fig. 4.
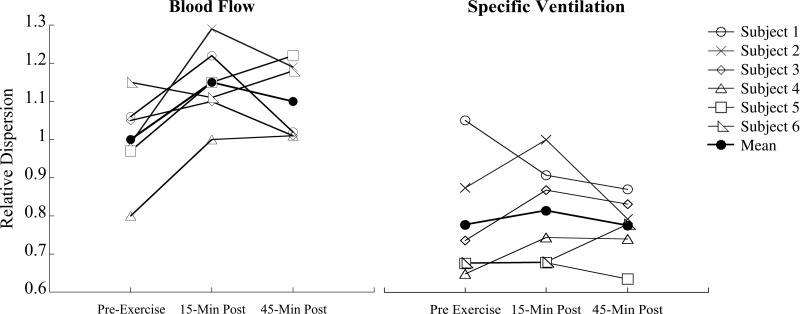
Individual responses of relative dispersion of blood flow (ml·min·cm−3) and specific ventilation preexercise and 15 and 45 min postexercise.
Fig. 5.
Relative dispersion of specific ventilation per one-third of lung. Relative dispersion of specific ventilation was significantly different between lung regions at all time points (P < 0.001), but no interaction effect was seen with lung region by time postexercise (P = 0.46).
Given that the exercise task was done upright, RD was evaluated in the apical, mid, and basal one-thirds of the lung, corresponding to nondependent, middle, and dependent gravitational regions in upright posture. There was also no significant effect of time of measurement on RD of SV (P = 0.42) when this regional analysis was considered (data not shown).
Pulmonary Blood Flow
Mean values.
Mean pulmonary blood flow data are shown in Table 4. Mean pulmonary blood flow both in milliliters per minute per cubic centimeter and milliliters per minute per gram was not significantly different at any time point after 45 min of heavy exercise (P = 0.08 and P = 0.2, respectively), suggesting that cardiac output had returned to baseline levels by the time scanning took place.
Measures of heterogeneity.
Mean values for RD of pulmonary blood flow are given in Fig. 3 and Table 4 and for fractal dimension in Table 4. Individual responses for RD of pulmonary blood flow (ml·min−1·cm−3) are shown in Fig. 4. The RD of mean pulmonary blood flow (both in ml·min−1·cm−3 and ml·min−1·g−1) was significantly increased postexercise compared with rest (P < 0.005 and P < 0.05, respectively). Additionally, RD was still elevated 90 min postexercise. However, fractal dimension of pulmonary blood flow did not change significantly (P = 0.21 and P = 0.95 for ml·min−1·cm−3 and ml·min−1·g−1, respectively) postexercise.
Lung Density and Calculated Total Lung Water
Proton density data are given in Table 4. There were no statistically significant differences in mean lung density in the imaging slice from preexercise, 0.23 ± 0.07 g H2O/cm3 lung, 15 min postexercise, 0.24 ± 0.04 g H2O/cm3 lung, and 45 min postexercise, 0.22 ± 0.03 g H2O/cm3 lung (P = 0.26). As expected, lung density was significantly different between dependent, middle, and nondependent lung regions at all time points (P < 0.001), but was not significantly different at any time postexercise in the different regions (P = 0.88). Calculated total lung water in the imaged lung slice was also not significantly different between rest, 48.2 ± 10.7 g, 15 min postexercise, 41.5 ± 5.5 g, and 45 min postexercise, 43.2 ± 7.0 g (P = 0.25).
DISCUSSION
Consistent with our laboratory's previous study (7), the relative dispersion of pulmonary blood flow was significantly increased after exercise and remained elevated through recovery. However, in the present study, after sustained heavy exercise, we found no statistically significant changes in the heterogeneity of the spatial distribution of SV, as measured by the relative dispersion. The lack of significant changes in SV heterogeneity, combined with changes in pulmonary blood flow heterogeneity, suggest that the effect of heavy exercise on the airways is less marked than the effect on the blood vessels.
Exercise and the Lung
It is well known that exercise causes a decrease in the efficiency of gas exchange, resulting in an increase in the A-aDo2 (10, 14, 19, 38). V̇a/Q̇ mismatch has been shown to comprise most of the A-aDo2 during exercise in sedentary subjects and up to 70% of the A-aDo2 during maximal exercise in highly trained endurance athletes (41). V̇a/Q̇ mismatch has been shown to worsen with increasing duration and intensity of exercise and, during prolonged submaximal exercise, accounts for virtually all the A-aDo2 (20). The mechanism of the increase in V̇a/Q̇ mismatch with exercise is not completely elucidated. However, the accumulation of interstitial edema resulting from an increase in pulmonary vascular pressure leading to fluid transudation exceeding lymphatic transport capacity (22, 38) has been suggested as the most likely explanation. Such fluid would be expected to alter local resistances in small blood vessels and perhaps airways, affecting local flow and V̇a/Q̇ matching. This has been discussed at length (27, 39), and there is considerable experimental evidence to support this idea. For example, exercise in normobaric hypoxia increases V̇a/Q̇ mismatch significantly, and this effect is mitigated by breathing 100% oxygen (15). Since hypoxia increases pulmonary arterial and mean capillary pressures, and this effect is alleviated by 100% oxygen breathing, this is consistent with vascular pressures as being important in the development of V̇a/Q̇ mismatch. Subjects who develop V̇a/Q̇ mismatch during exercise have greater V̇a/Q̇ mismatch in recovery compared with those who do not (38). This difference extends up to 20 min postexercise, long after cardiac output and ventilation normalize to resting levels (7, 20, 38).
This idea is further supported by histological studies in animal models showing that, in the lungs of pigs postexercise, there was a significantly greater number of pulmonary arteries with perivascular cuffing, compared with nonexercise control animals (38). Such cuffs would be expected to cause mechanical compression and, by altering local resistances and flow, disrupt V̇a/Q̇ matching (38). Consistent with this idea, our laboratory has previously shown in a similar population of subjects, that the same exercise task increases the spatial heterogeneity of pulmonary blood flow after exercise, and the magnitude of the changes were correlated with V̇a/Q̇ mismatch measured using MIGET (7). Taken together, this evidence suggests that pulmonary edema is likely the primary mechanism of V̇a/Q̇ mismatch in exercise (25, 31, 37, 38), but is not proof of this mechanism. Since airways and blood vessels travel together in the lung parenchyma, collectively this information suggests that both the spatial distribution of ventilation, as well as that of pulmonary blood flow, has the potential to be adversely affected by heavy exercise and thus might be expected to show changes in the recovery period.
Different Responses of Blood Flow and Ventilation
In the present study, we found an increase in the spatial heterogeneity of pulmonary blood flow postexercise in all but one subject (see Fig. 4), which was statistically significant, consistent with our previous work (7). However, contrary to our hypothesis, in the recovery period after 45 min of heavy exercise, the heterogeneity of SV was not significantly increased. Individually, the relative dispersion for SV after exercise increased in three subjects, did not change for two subjects, and decreased for one subject (Fig. 4). The reasons for this individual variability are unknown. It is notable that the changes in the heterogeneity of SV were not well correlated with the changes in heterogeneity of perfusion (R2 = 0.28). However, this finding raises the possibility that some subjects may be vulnerable to the development of increased heterogeneity in SV after exercise, while others are not. In addition, these findings may not be characteristic of a broader population, particularly of nonathletes.
Despite any individual variability, the results of the present study suggest that blood vessels are affected differently during and after exercise than airways. There are several possibilities that might explain this finding. For example, bronchodilation is well known to occur as a result of exercise (5, 13, 42), would be expected to increase the caliber of conducting airways, and may offset any potential effect of exercise acting to alter the distribution of SV. However, arguing against this, in our study, there were no statistically significant changes observed in spirometry after exercise (Table 2).
Alternately, if the cause of our findings of increased heterogeneity of pulmonary blood flow after exercise is the accumulation of interstitial pulmonary edema during exercise, blood vessels may be more vulnerable than airways, to either the development of edema in the adjacent interstitial space, or the effects once edema has accumulated. In the previously mentioned animal study, although the number of perivascular cuffs was increased in exercised animals, interestingly, there was no significant increase in the number of airways with peribronchial cuffing compared with resting controls (37). In resting dogs, when infusion of saline is used to induce pulmonary edema, there is a pattern of central migration of the edema fluid around blood vessels, but not airways (32). Finally, there is evidence that perivascular fluid cuffs cause an increase in alveolar tissue resistance in rats, without an increase in airway resistance (30). Our finding of increased blood flow heterogeneity in the absence of significant increases in SV heterogeneity postexercise is consistent with the finding of perivascular, but not peribronchial, cuffing in these animal studies, but is of course not proof of this mechanism. Altogether, this suggests that the airways and blood vessels, and thus the distributions of ventilation and perfusion, may respond differently following heavy exercise.
Compared with SVI, which takes 18 min, pulmonary blood flow using ASL is acquired from two images obtained in ∼10 s. Consequently, because of the different acquisition times for blood flow and SV data, temporal averaging is different between our blood flow and ventilation measurements. Thus one possible explanation for the differences we observed after heavy exercise between the two measures may be an artifactual result of differences in temporal averaging. To gain an estimate of the magnitude of this problem, we used data from a recently published paper to evaluate the effect of a longer period of temporal averaging on the measured heterogeneity of blood flow measurements (3). This prior study acquired multiple ASL tag and control pairs for ∼15 min (180 breaths, 90 subtracted tag and control pairs), and thus was of a comparable duration to the SVI acquisition. Individual subject data were aggregated into a single temporally averaged blood flow image, representing 15 min of temporal averaging. The relative dispersion of the temporally averaged image was compared with the average relative dispersion for the 90 individual images in each of the time series. The relative dispersion of the temporally averaged image was lower, but the decrease was small (relative dispersion = 0.80 for the individual blood flow maps, 0.74 for the 90 image average, <8% difference). Although in this prior study data were collected at rest, and thus transient changes were not expected, since the relative dispersion of blood flow in the present study increased by 15% after exercise compared with ∼4% change in SV, the differences in temporal averaging between the two types of data are unlikely to explain the results of our study. This is especially true since each type of image (SV or blood flow) was compared with the same type of image before and after exercise.
Since preparation of the subject for MRI scanning after the exercise task took ∼10 min, blood flow and density measurements took ∼5 min, and 18 min were required to complete a SV acquisition, another possibility is that any changes in airways incurred during exercise may have resolved by the time SV data were acquired. Indeed, it is notable that, at the time of our first measurement, the A-aDo2 was not significantly different from the preexercise baseline value. Thus the timing of our measurement may have missed significant changes in the distribution of SV that were present during exercise and the early postexercise period, but resolved by the time of imaging. Although V̇a/Q̇ mismatch (as measured by the multiple inert-gas method) has been shown to persist for at least 20 min after exercise (38), and our laboratory has previously shown that pulmonary blood flow heterogeneity is elevated for at least 1 h after a similar exercise task (7), we are unable to rule this out as a possibility, and this is a limitation of the present study. However, if this is the case, since the changes in blood flow heterogeneity in the present study persisted for at least 1 h postexercise, this also implies that recovery from exercise in airways and blood vessels differs.
Exercise and Gas Exchange
In the present study, there was a marked increased in A-aDo2 (23.3 ± 5.3 Torr) during 45 min of heavy exercise at ventilatory threshold, consistent with previous studies (10, 19, 22). When gas exchange during sustained heavy exercise was evaluated with the multiple inert-gas technique (20), V̇a/Q̇ mismatch explained all of the A-aDo2. Since the present study used a similar exercise task and athlete population as that prior study, the most likely cause for the increased A-aDo2 in our subjects is also V̇a/Q̇ mismatch and not the other possible contributors to the A-aDo2, such as diffusion limitation or shunt. Disruption in V̇a/Q̇ matching can result from changes in the distribution of ventilation, or of the distribution of perfusion, or a combination of both.
In the present study, A-aDo2 returned to normal after exercise, indicating no significant gas exchange impairment in recovery. This might seem paradoxical since the changes in blood flow heterogeneity are present at least 1 h into recovery. However, if as suggested, previous heavy exercise creates cuffing affecting blood vessels (7), but not airways, this would be expected to create areas of high V̇a/Q̇ ratio. These high V̇a/Q̇ regions will have a normal or even elevated alveolar Po2, and thus will have little effect on the A-aDo2. Any redistribution of blood away from cuffed regions would tend to create regions of low V̇a/Q̇ ratio, affecting gas exchange during exercise while overall flow was high, but with little effect when cardiac output fell in recovery. Although this idea is speculative, we evaluated the previously published multiple inert-gas data of Hopkins et al. (20), which used a similar exercise task and duration as the present study, to determine whether there was development of regions of high V̇a/Q̇ ratio (with a V̇a/Q̇ ratio between 10 and 100) during prolonged heavy exercise. In that prior study, both ventilation and blood flow to high V̇a/Q̇ regions increased from rest after heavy exercise (ventilation: 0.7 ± 0.6% rest, 6.7 ± 2.4% 1 h, P < 0.005; blood flow 0.1 ± 0.1% rest, 2.4 ± 2.0% exercise, P < 0.05), supporting this idea.
Lung Water
It might be expected that, if interstitial edema was the cause of the changes seen in the distribution of pulmonary blood flow postexercise, there would be a net increase in total lung water in our subjects. We did not see a significant increase in total lung water (which includes contributions from both intravascular and extravascular water) in the present study, which is potentially inconsistent with interstitial edema as a cause of our findings. However, fluid shifts from the intravascular space into the interstitium could result in interstitial edema with no net change in the total fluid content of the lung. Pulmonary blood volume has been reported to decrease following exercise in athletes (16), consistent with this idea. Some studies have shown an increase in extravascular lung water (+0.021 g/ml) of extravascular water (normalized for ml of lung) seen with exercise during a similar exercise task (31). In our lung slice, this would correspond to an increase of ∼4 g of extravascular water, and it may be that this change is too subtle to detect using our measurement, especially if there were any corresponding reduction in the intravascular space. Independent measures of intravascular and extravascular lung water would quantify any net shift; however, it is not currently possible to distinguish these two compartments with our technique.
Assumptions and Limitations
There are some potential sources of error and technical limitations to our study that warrant discussion. Presently, SVI measurements cannot be made during exercise because of motion artifact, resulting in image blurring and registration issues. Additionally, supine exercise is difficult to perform at high workloads (34), and breath holding during heavy exercise between the long series of images taken to measure SV presents an additional challenge. Thus, in the present study, we can only infer that the changes observed postexercise reflect any changes that occurred during exercise.
The algorithm used to calculate SV assumes that every subject reproduces identical tidal breaths to FRC for all 220 repetitions. Subject movement, deviations in tidal volume, and/or failure to return to FRC before each acquisition may result in small mis-registration of consecutive images. For this reason, the true resolution of the SVI measurement is lower than the nominal image resolution, as the response of neighboring voxels is averaged as a consequence.
SVI acquisition is limited to a single 15-mm slice of the lung, representing ∼10% of the lung. In our study, we imaged the right lung to avoid image artifact from cardiac motion, and the changes in this slice may not necessarily represent changes in SV across the entire lung. However, in these young, healthy subjects, there is no reason to suspect large differences in physiological response between lung regions, provided care is taken to sample the lung appropriately, such that both dependent and nondependent lung regions are included in the images slice.
The limitations and errors associated with the ASL-FAIRER method to measure pulmonary blood flow have been described in a recent publication (8). As discussed in that paper, the signal from larger conduit vessels does not reflect capillary perfusion, as the blood contained in these vessels is destined for a distal capillary bed. To address this issue, we masked out voxels from the image with signal intensity greater than 35% of the maximum signal in the blood flow images. For our relatively lateral slice location, this threshold results in the removal of large conducting vessels, while minimizing the amount of perfusion (defined as delivery of blood to a capillary bed) that is removed from the analysis (8). Thus the signal that remains largely represents blood flow (∼90%) (8).
Another issue that needs to be addressed is whether or not this study had adequate statistical power to support the conclusion reached, particularly in light of the small number of subjects studied. The repeated-measures design of this study, combined with good reliability of measurements (test-retest correlations for SVI were 0.76) in part offsets the potential lack of power risked by small numbers of subjects. There was a highly significant change in blood flow heterogeneity, which increased over 15% from the preexercise baseline, demonstrating that statistical power was adequate for this variable. However, we did not see a significant change in the relative dispersion of SV, which was, on average, 3.8% greater at the first postexercise time point compared with preexercise. For this change to be significant, if in fact it was maintained in a larger population of subjects, post hoc power calculations indicate that 88 subjects would be required for adequate power to show a change of this statistically. Thus this change is small and not likely to be a factor of major biological importance.
Conclusions
We examined the effect of recovery from heavy sustained exercise on the spatial distribution of SV in human subjects. We found no statistically significant changes in mean SV or heterogeneity of SV after exercise. However, we did find significant increases in pulmonary blood flow heterogeneity during recovery from heavy exercise. Combined with the lack of changes in distribution of SV, and associated measures of heterogeneity, our results suggest that the effect of heavy exercise on the airways is less marked that the effect on the blood vessels.
GRANTS
Funding for this study was provided by National Heart, Lung, and Blood Institute Grant R01 HL-081171, PI, to S. R. Hopkins, and HL-080203, PI, to G. K. Prisk. R. C. Sá's work was supported by the National Space Biomedical Research Institute though National Aeronautics and Space Administration Grant NCC 9-58.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.T., R.C.S., R.J.T., G.K.P., and S.R.H. conception and design of research; V.T., R.C.S., T.J.A., S.H., R.J.T., W.T.C., P.D.W., C.K.D., G.K.P., and S.R.H. performed experiments; V.T., R.C.S., T.J.A., S.H., R.J.T., W.T.C., G.K.P., and S.R.H. analyzed data; V.T., R.C.S., T.J.A., R.J.T., P.D.W., G.K.P., and S.R.H. interpreted results of experiments; V.T. and S.R.H. prepared figures; V.T., R.J.T., and S.R.H. drafted manuscript; V.T., R.C.S., T.J.A., R.J.T., P.D.W., G.K.P., and S.R.H. edited and revised manuscript; V.T., R.C.S., T.J.A., S.H., R.J.T., W.T.C., P.D.W., C.K.D., G.K.P., and S.R.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the contributions of Jeff Struthers and Harrieth Wagner for technical expertise, the hard work of the subjects in this study, and Dr. Michael J. Buono and Dr. Fred W. Kolkhorst for mentorship and fruitful discussions in the analysis of data.
REFERENCES
- 1. Arai TJ, Henderson AC, Dubowitz DJ, Levin DL, Friedman PJ, Buxton RB, Prisk GK, Hopkins SR. Hypoxic pulmonary vasoconstriction does not contribute to pulmonary blood flow heterogeneity in normoxia in normal supine humans. J Appl Physiol 106: 1057–1064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arai TJ, Prisk GK, Holverda S, Sá RC, Theilmann RJ, Henderson AC, Cronin MV, Buxton RB, Hopkins SR. Magnetic resonance imaging quantification of pulmonary perfusion using calibrated arterial spin labeling. J Vis Exp 51: 2712, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asadi AK, Cronin MV, Sá RC, Theilmann RJ, Holverda S, Hopkins SR, Buxton RB, Prisk GK. Spatial-temporal dynamics of pulmonary blood flow in the healthy human lung in response to altered FiO2. J Appl Physiol 114: 107–118, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60: 2020–2027, 1986 [DOI] [PubMed] [Google Scholar]
- 5. Beck KC. Control of airway function during and after exercise in asthmatics. Med Sci Sports Exerc 31: S4–S11, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Bolar DS, Levin DL, Hopkins SR, Frank L, Liu T, Wong E, Buxton RB. Quantification of regional pulmonary blood flow using ASL-FAIRER. Magn Reson Med 55: 1308–1317, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Burnham KJ, Arai TJ, Dubowitz DJ, Henderson AC, Holverda S, Buxton RB, Prisk GK, Hopkins SR. Pulmonary perfusion heterogeneity is increased by sustained, heavy exercise in humans. J Appl Physiol 107: 1559–1568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burrowes KS, Buxton RB, Prisk GK. Assessing potential errors of MRI-based measurements of pulmonary blood flow using a detailed network flow model. J Appl Physiol 113: 130–141, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Q, Jakob PM, Griswold MA, Levin DL, Hatabu H, Edelman RR. Oxygen enhanced MR ventilation imaging of the lung. MAGMA 7: 153–161, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Dempsey JA, Hanson PG, Henderson KS. Exercise-induced arterial hypoxaemia in healthy human subjects at sea level. J Physiol 355: 161–175, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edelman RR, Hatabu H, Tadamura E, Li W, Prasad PV. Noninvasive assessment of regional ventilation in the human lung using oxygen-enhanced magnetic resonance imaging. Nat Med 2: 1236–1239, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Glenny RW, Robertson HT. Fractal modeling of pulmonary blood flow heterogeneity. J Appl Physiol 70: 1024–1030, 1991 [DOI] [PubMed] [Google Scholar]
- 13. Haas F, Pasierski S, Levine N, Bishop M, Axen K, Pineda H, Haas A. Effect of aerobic training on forced expiratory airflow in exercising asthmatic humans. J Appl Physiol 63: 1230–1235, 1987 [DOI] [PubMed] [Google Scholar]
- 14. Hammond MD, Gale GE, Kapitan KS, Ries A, Wagner PD. Pulmonary gas exchange in humans during exercise at sea level. J Appl Physiol 60: 1590–1598, 1986 [DOI] [PubMed] [Google Scholar]
- 15. Hammond MD, Gale GE, Kapitan KS, Ries A, Wagner PD. Pulmonary gas exchange in humans during normobaric hypoxic exercise. J Appl Physiol 61: 1749–1757, 1986 [DOI] [PubMed] [Google Scholar]
- 16. Hanel B, Teunissen I, Rabol A, Warberg J, Secher NH. Restricted postexercise pulmonary diffusion capacity and central blood volume depletion. J Appl Physiol 83: 11–17, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Henderson AC, Prisk GK, Levin DL, Hopkins SR, Buxton RB. Characterizing pulmonary blood flow distribution measured using arterial spin labeling. NMR Biomed 22: 1025–1035, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holverda S, Theilmann RJ, Sá RC, Arai TJ, Hall ET, Dubowitz DJ, Prisk GK, Hopkins SR. Measuring lung water: ex vivo validation of multi-image gradient echo MRI. J Magn Reson Imaging 34: 220–224, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hopkins SR, Barker RC, Brutsaert TD, Gavin TP, Entin P, Olfert IM, Veisel S, Wagner PD. Pulmonary gas exchange during exercise in women: effects of exercise type and work increment. J Appl Physiol 89: 721–730, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Hopkins SR, Gavin TP, Siafakas NM, Haseler LJ, Olfert IM, Wagner H, Wagner PD. Effect of prolonged, heavy exercise on pulmonary gas exchange in athletes. J Appl Physiol 85: 1523–1532, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Hopkins SR, Henderson AC, Levin DL, Yamada K, Arai TJ, Buxton RB, Prisk GK. Vertical gradients in regional lung density and perfusion in the supine human lung: the Slinky effect. J Appl Physiol 103: 240–248, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hopkins SR, McKenzie DC, Schoene RB, Glenny RW, Robertson HT. Pulmonary gas exchange during exercise in athletes. I. Ventilation-perfusion mismatch and diffusion limitation. J Appl Physiol 77: 912–917, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Hopkins SR, McKenzie DC. Hypoxic ventilatory response and arterial desaturation during heavy work. J Appl Physiol 67: 1119–1124, 1989 [DOI] [PubMed] [Google Scholar]
- 24. Hopkins SR, Prisk GK. Lung perfusion measured using magnetic resonance imaging: new tools for physiological insights into the pulmonary circulation. J Magn Reson Imaging 32: 1287–1301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hopkins SR, Stary CM, Falor E, Wagner H, Wagner PD, McKirnan MD. Pulmonary gas exchange during exercise in pigs. J Appl Physiol 86: 93–100, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Hopkins SR. Exercise induced arterial hypoxemia: the role of ventilation-perfusion inequality and pulmonary diffusion limitation. Adv Exp Med Biol 588: 17–30, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Hopkins SR. Point: Pulmonary edema does occur in human athletes performing heavy sea-level exercise. J Appl Physiol 109: 1270–1272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaneko K, Milic-Emili J, Dolovich MB, Dawson A, Bates DV. Regional distribution of ventilation and perfusion as a function of body position. J Appl Physiol 21: 767–777, 1966 [DOI] [PubMed] [Google Scholar]
- 29. Levin DL, Buxton RB, Spiess JP, Arai TJ, Balouch J, Hopkins SR. Effects of age on pulmonary perfusion heterogeneity measured by magnetic resonance imaging. J Appl Physiol 102: 2064–2070, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Lowe K, Alvarez DF, King JA, Stevens T. Perivascular fluid cuffs decrease lung compliance by increasing tissue resistance. Crit Care Med 38: 1458–1466, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McKenzie DC, O'Hare TJ, Mayo J. The effect of sustained heavy exercise on the development of pulmonary edema in trained male cyclists. Respir Physiol Neurobiol 145: 209–218, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Michel RP, Zocchi L, Rossi A, Cardinal GA, Ploy-Song-Sang Y, Poulsen RS, Milic-Emili J, Staub NC. Does interstitial lung edema compress airways and arteries? A morphometric study. J Appl Physiol 62: 108–115, 1987 [DOI] [PubMed] [Google Scholar]
- 33. Milic-Emili J, Henderson JA, Dolovich MB, Trop D, Kaneko K. Regional distribution of inspired gas in the lung. J Appl Physiol 21: 749–759, 1966 [DOI] [PubMed] [Google Scholar]
- 34. Ray CA, Cureton KJ. Interactive effects of body posture and exercise training on maximal oxygen uptake. J Appl Physiol 71: 596–600, 1991 [DOI] [PubMed] [Google Scholar]
- 35. Sá RC, Cronin MV, Henderson AC, Holverda S, Theilmann RJ, Arai TJ, Dubowitz DJ, Hopkins SR, Buxton RB, Prisk GK. Vertical distribution of specific ventilation in normal supine humans measured by oxygen-enhanced proton MRI. J Appl Physiol 109: 1950–1959, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sá RC, Hopkins SR, Prisk GK, Darquenne C. Validation of the distribution of specific ventilation obtained by proton MR imaging (Abstract). Am J Respir Crit Care Med 183: A2035, 2012 [Google Scholar]
- 37. Schaffartzik W, Arcos JP, Tsukimoto K, Mathieu-Costello O, Wagner PD. Pulmonary interstitial edema in the pig after heavy exercise. J Appl Physiol 75: 2535–2540, 1993 [DOI] [PubMed] [Google Scholar]
- 38. Schaffartzik W, Poole DC, Derion T, Tsukimoto K, Hogan MC, Arcos JP, Bebout DE, Wagner PD. V̇a/Q̇ distribution during heavy exercise and recovery in humans: implications for pulmonary edema. J Appl Physiol 72: 1657–1667, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Sheel AW, McKenzie DC. Counterpoint: Pulmonary edema does not occur in human athletes performing heavy sea-level exercise. J Appl Physiol 109: 1272–1273, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Theilmann RJ, Arai TJ, Samiee A, Dubowitz DJ, Hopkins SR, Buxton RB, Prisk GK. Quantitative MRI measurement of lung density must account for the change in T(2) (*) with lung inflation. J Magn Reson Imaging 30: 527–534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW, Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol 61: 260–270, 1986 [DOI] [PubMed] [Google Scholar]
- 42. Warren J, Jennings S. Normal human airway response to exercise. J Appl Physiol 56: 1686, 1984 [DOI] [PubMed] [Google Scholar]