Abstract
Tendon is a highly specialized, hierarchical tissue designed to transfer forces from muscle to bone; complex viscoelastic and anisotropic behaviors have been extensively characterized for specific subsets of tendons. Reported mechanical data consistently show a pseudoelastic, stress-vs.-strain behavior with a linear slope after an initial toe region. Many studies report a linear, elastic modulus, or Young's modulus (hereafter called elastic modulus) and ultimate stress for their tendon specimens. Individually, these studies are unable to provide a broader, interstudy understanding of tendon mechanical behavior. Herein we present a metaanalysis of pooled mechanical data from a representative sample of tendons from different species. These data include healthy tendons and those altered by injury and healing, genetic modification, allograft preparation, mechanical environment, and age. Fifty studies were selected and analyzed. Despite a wide range of mechanical properties between and within species, elastic modulus and ultimate stress are highly correlated (R2 = 0.785), suggesting that tendon failure is highly strain-dependent. Furthermore, this relationship was observed to be predictable over controlled ranges of elastic moduli, as would be typical of any individual species. With the knowledge gained through this metaanalysis, noninvasive tools could measure elastic modulus in vivo and reasonably predict ultimate stress (or structural compromise) for diseased or injured tendon.
Keywords: tendon, biomechanics, modulus, stress, strain
tendon is a highly specialized, hierarchical tissue with a predominantly mechanical function in the body (i.e., translating muscle contraction into joint movement). A key functional metric for pathologic or damaged tendon is therefore ultimate strength. This parameter bounds the mechanical capabilities of normal tissue and defines risk of failure in damaged or diseased tissue. Along with ultimate strength, tendon elastic modulus is also commonly reported in many in vitro studies while examining various tendons from different species and in different states of damage or repair.
Applying data from multiple sources under different testing conditions to an in vivo subject is problematic. Whereas some studies have suggested a possible relationship between ultimate stress and elastic modulus in tendon (71), this issue has not been rigorously explored on an interstudy basis. One group reported tendon having relatively constant elastic modulus over three orders of magnitude of body mass (58). However, the range of elastic moduli and ultimate stresses reported in the literature warrant further investigation into whether tendons have constant elastic properties or whether they exhibit a more complex behavior. Additionally, the dependence of tendon failure on strain, suggested by Sharma and Maffulli (65), requires further investigation; if tendon failure is a function of strain and if mechanical behavior is sufficiently linear, elastic modulus will be directly related to ultimate stress.
Animal musculoskeletal systems have evolved to accommodate a wide range of forces. Tendon dimensions have adapted under the influence of daily loading that scales with animal size (57, 58) and factors such as mechanical advantage (6, 7), safety (44), and energy storage (8). However, tendon dimensions alone do not explain the wide variety of mechanical properties reported in the previously published literature. It follows that different tendons have not only adapted in dimensions to allow them to sustain required loads, but also in areas such as composition, microstructure, and cross-linking to exhibit different mechanical properties.
The unique mechanical behavior of healthy tendon can change dramatically due to either natural processes such as aging or traumatic events such as injury or disease. Recently, investigators have been able to quantify the resulting morphological attributes of tendon and correlate them with mechanical changes. Some of these factors include collagen fibril volume fraction (36, 45), collagen fibril diameter (21, 24), tissue hydration (17, 39, 69), and collagen cross-linking (1, 4, 18, 40). Although interstudy inconsistencies mean that none of these factors necessarily predict tendon mechanical properties, they do provide an extensive database of tendon mechanical properties from reliable sources. No investigations have pooled these data broadly to characterize interstudy relationships. This study provides this detailed characterization of tendon failure mechanics, the ultimate goal of which is to relate clinically measurable mechanical properties of tendon to failure properties. Elastic modulus and ultimate stress are compared on the basis of published results for tendon from different species and conditions of health and injury.
METHODS
Identification and selection of studies.
Prior to conducting a literature search, general study inclusion/exclusion criteria were established. Studies were selected only if there was sufficient information to calculate all relevant structural and mechanical properties (gauge length, cross-sectional area, stiffness, ultimate stress, elastic modulus, and ultimate load). For example, if ultimate stress was not given explicitly, the study could be used if cross-sectional area and ultimate load were reported. The strain rate used for the pull-to-failure test in the study was also required for inclusion. It was the goal of this metaanalysis to collect a highly heterogeneous study pool that included many different animals and tendon groups, to determine whether any observed interrelationships between elastic modulus and ultimate stress could be broadly applied.
A search of published literature using the Google Scholar database (studies from 1980 to the present) was performed. The key words tendon, mechanical properties, ultimate stress, and elastic modulus were used in separate searches and in conjunction with injury or healing to identify all relevant articles. An additional search of articles published since 2000 was performed to avoid overlooking any recent relevant research. Articles were examined and selected on the basis of inclusion/exclusion criteria. A final group of studies was selected on the basis of inclusion/exclusion criteria from the articles cited by this initial group of studies.
Data extraction.
The extracted data consisted of objective data only, so all extraction was performed by a single reviewer. Extracted data included animal, tendon, sample size, gauge length (mm), cross-sectional area (mm2), strain rate (%/sec), elastic modulus (MPa), stiffness (N/mm), ultimate stress (MPa), and failure load (N). In some cases, structural data (load, stiffness) were collected to calculate the mechanical properties of the tendon (stress, modulus) by the following relationships:
| 1 |
| 2 |
where σ represents stress, F represents force, A represents original area, E represents elastic modulus, Lgage represents gage length, and k represents stiffness.
In some studies, the cross-sectional area or gage length of a specific tendon was not explicitly given. In these cases, studies that used the same tendon and mechanical testing setup were consulted. Cross-sectional areas and gage lengths were taken from these related studies and used in the above calculations. Available data were typically given in engineering units (i.e., small deformation metrics for stress and strain), so those metrics are also used in this analysis.
Modulus used to predict failure of tendons.
The failure properties of tendons in individual studies were predicted using one of two methods, both of which assume a constant ratio of ultimate stress to elastic modulus. Although this ratio does not necessarily equal ultimate strain because of the pseudoelastic behavior of tendon and mechanical nonlinearity, it is a conservative estimate of ultimate strain and will be treated as such for the duration of the analysis.
In the first method, the failure stress of a tendon that had been treated (i.e., healing, cross-linked, etc.) was predicted from its modulus value using a ratio based on a control (untreated) data point with known ultimate stress (σC) and elastic modulus (EC). Hence, the regression prediction follows the equation:
| 3 |
where σP is the predicted ultimate stress of the tendon and EM is the measured modulus of elasticity for the specific tendon. This method uses data specific to a study (the control group information from the study along with the modulus of the specific tendon).
The second prediction method predicts the ultimate stress of a tendon using a constant ratio (i.e., ultimate strain) on the basis of the relationship between ultimate stress and elastic modulus in the pooled data (Fig. 1A):
| 4 |
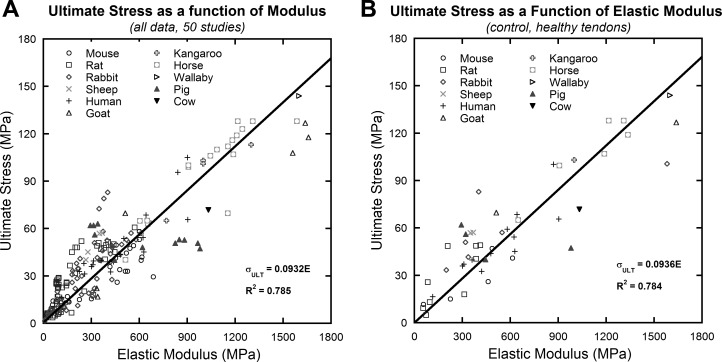
Fig. 1.
Compiled data collected from literature search of 50 studies. Each data point represents the average mechanical behavior of a single group in each study. A linear relationship between elastic modulus and ultimate stress was observed in the compiled data (A) and in the healthy control tendon data (B). Linear curve fits were forced through the origin.
This method uses general information from pooled data along with the modulus of the specific tendon.
To determine the accuracy of each prediction method, both were applied to each data set.
Statistical analysis.
A number of different analyses were used test the accuracy of each prediction method. First, the ratio of predicted to actual stress was calculated. Next, the predicted ultimate stress was plotted against the actual values and fitted with a linear regression, yielding an R2 value. Finally, the root-mean-square error (RMSE) calculation was used to test the variance of the predicted compared with the actual values.
Ultimate strain values at different elastic modulus values were compared using ANOVA with Tukey's post hoc analysis using Kaleidagraph (v4.03, Synergy Software, Reading, PA). Significance was set at P = 0.05, trends at P = 0.10.
RESULTS
Literature search results.
The Google Scholar search of published literature yielded 4,660 articles that included the key words used in the search (tendon, mechanical properties, ultimate stress, elastic modulus) along with either the word injury or healing. Fifty of these studies fit the inclusion/exclusion criteria and were included in the metaanalysis (2, 3, 5, 9–13, 15, 16, 19, 20, 22, 23, 25–32, 34, 35, 37–39, 42, 43, 45, 46, 48, 53, 54, 59–64, 66–68, 71–77). Within the numerous animal and tendon groups in these studies, tendons were subjected to a number of modifications including but not limited to the effects of injury and healing, genetic alterations, allograft preparation, in vivo mechanical environment, and age (Table 1).
Table 1.
Studies associated by group
| Group | Studies |
|---|---|
| Injury | Young et al. (1998), Stone et al. (1999), Dahlgren et al. (2002), Awad et al. (2003), Forslund and Aspenberg (2003), Chen et al. (2004), Ferry et al. (2007), Fu et al. (2008), Ansorge et al. (2009), Dyment et al. (2012) |
| Genetics | Robinson et al. (2004), Lin et al. (2005), Mikic et al. (2008), Rigozzi et al. (2010) |
| Allografts | Gibbons et al. (1991), Roe et al. (1992), Salehpour et al. (1995), Seto et al. (2009) |
| Mechanical | Cherdchutham et al. (2001), Lavagnino et al. (2005), Eliasson et al. (2007), Legerlotz et al. (2007), Trudel et al. (2007), Rumian et al. (2009) |
| Age | Danielsen and Andreassen (1988), Johnson et al. (1994), Cherdchutham et al. (2001) |
| Other | Galeski et al. (1977), Woo et al. (1980), Butler et al. (1986), Bosch et al. (1992), Yamamoto et al. (1992), Blevins et al. (1994), Itoi et al. (1995), Wang and Ker (1995), Crevier et al. (1996), McGough et al. (1996), Smith et al. (1996), Crevier-Denoix et al. (1997), Haut and Haut (1997), Donahue et al. (2001), Dowling et al. (2002), Batson et al. (2003), Carpenter et al. (2005), Hashemi et al. (2005), Diehl et al. (2006), Birch et al. (2008), Su et al. (2008), Hansen et al. (2010), Thorpe et al. (2010) |
Relationship between modulus and ultimate stress.
Ultimate stress and elastic modulus were found to correlate strongly in the data set, including all data, R2 = 0.785 (Fig. 1A); and also in healthy control tendons, R2 = 0.784 (Fig. 1B). A comparison of animal groups showed that the ratio of ultimate stress to elastic modulus (ultimate strain, εult) varied across animal groups (P < 0.001). When divided into groups on the basis of size, ultimate strain remained significantly different between smaller animals (P < 0.001). Rat tendons (εult = 17.96 ± 7.95%) have a significantly higher ultimate strain compared with mouse (εult = 10.47 ± 4.89%) and rabbit (εult = 12.82 ± 4.86%) tendons (P < 0.001 for both). Results are reported as means ± standard deviation. No significant differences in ultimate strain were found for tendons from the larger animals (P = 0.482).
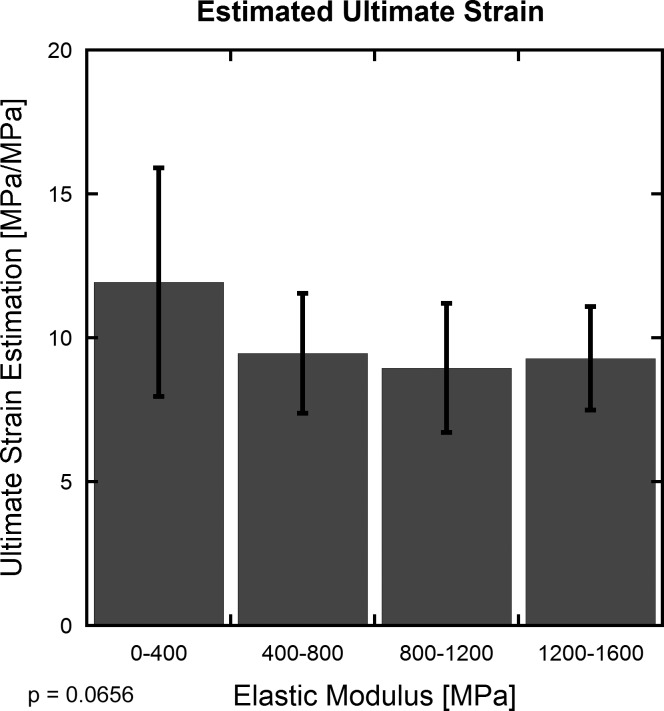
When the pooled data are divided into five groups on the basis of elastic modulus, groups with higher elastic modulus exhibited lower ultimate strain (Fig. 2), and a decreasing trend is observed over the entire data set (P = 0.066). No significance or trend was found between any modulus groups (Fig. 2; P ≥ 0.11).
Fig. 2.
The ratio of ultimate stress to elastic modulus (estimate of ultimate strain) shows a decreasing trend with increasing elastic modulus (standard deviation shown). ANOVA analysis revealed this trend when the whole range of elastic moduli were considered (P = 0.0656), but post hoc analysis found no significance or trends between any pair of groups (P ≥ 0.11). The decrease in estimated ultimate strain is most pronounced in the lowest modulus values, which coincides with the smallest animals and also with groups that underwent more drastic treatments (i.e., genetic knockouts, allografts, etc.). Smaller specimen size may amplify potential edge effects, and more extreme treatments may further result in altered tendon behaviors.
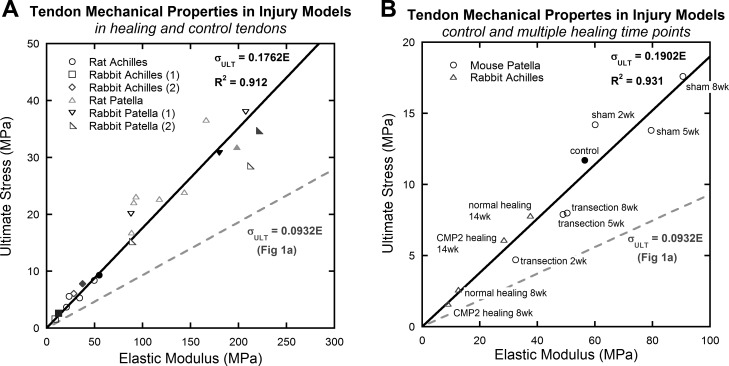
The mechanical behavior of normal and healing (from transection or collagenase-injection injury models) tendons is shown in Fig. 3. Elastic modulus and ultimate stress in these normal and injured populations show a strong linear correlation (R2 = 0.912; Fig. 3A), although they have a higher ratio of ultimate stress to elastic modulus than in the original linear curve fit (Fig. 1A). The higher ratio of ultimate stress to elastic modulus (high ultimate strain) in these data follows the trend shown in Fig. 2, because tendons from rabbits, rats, and mice have lower elastic modulus and exhibit higher ultimate strain (noted by the higher ratio of ultimate stress to elastic modulus). Further comparisons showed that for an average elastic modulus of ∼100 MPa, the estimated ultimate strains were similar between injury studies (εult = 18%) and the overall data set (εult = 16%). A detailed examination of the treatment groups of two example studies (28, 31) appears in Fig. 3B.
Fig. 3.
A: mechanical properties of normal and healing (after transection- or collagenase-induced injury) tendon (15, 28, 31, 32, 67). Each data point represents the average mechanical behavior of a single group (i.e., control, “sham” surgery, or healing group) in each study. Tendons induced with an injury and subject to different healing protocols followed the same linear relationship as was observed before. B: details of two studies (28, 31). Control data points are solid, treatment groups are open. Linear curve fits were forced through the origin. Solid lines fit all data in the individual graph, and the trend from Fig. 1A is superimposed on each graph as a dashed line.
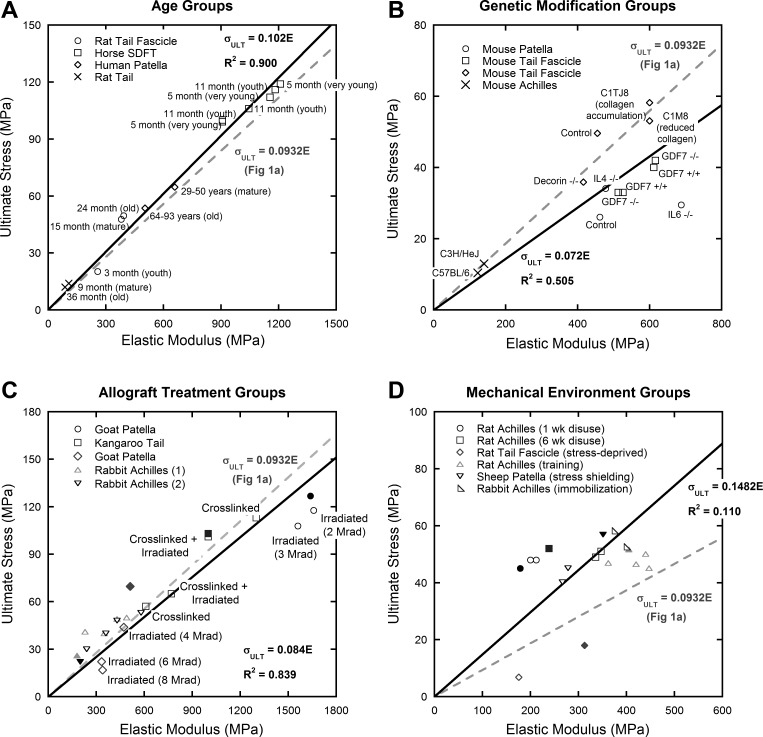
Figure 4 presents data from multiple studies that tested the mechanical properties of tendons that differed in age (sampling from young, mature, and old individuals on the basis of life span of each particular animal), genetic modification (altered expression of interleukins, GDF-7, collagen, and decorin), allograft treatment (cross-linking or irradiation methods prior to implantation), and in vivo mechanical environments (i.e., disuse, vibration, strength training). The ratio of ultimate stress to elastic modulus in studies of animals over multiple age levels had the most linear trend (R2 = 0.900; Fig. 4A), followed closely by data from allograft preparation studies (R2 = 0.839; Fig. 4C). This is intuitive, because the underlying tendon structure should be similar across groups. The effects of genetic alterations (R2 = 0.505; Fig. 4B) and the in vivo mechanical environment (R2 = 0.110; Fig. 4D) on tendon mechanics were not highly linear and showed much more variation, which may be due to changes to the tendon structure itself.
Fig. 4.
Mechanical properties of tendons grouped by age (A), genetics (B), allograft treatment (C), and in vivo mechanical environments (D). Each data point represents the average mechanical behavior of a single group in each study. Control data points are solid, treatment groups are open. Linear curve fits were forced through the origin. Solid lines fit all data in the individual graph, and the trend from Fig. 1A was superimposed on each graph as a dashed line. A: age (16, 23, 43): tendon specimens from a range of animal life spans. Rat specimens included youth (3 mo), adult (15 mo), and elderly (24 mo); horse specimens included very young (5 mo) and youth (11 mo); human specimens included adult (29–50 years) and elderly (64–93 years). B: genetics (48, 54, 59, 60): altered expression of interleukins, GDF-7, collagen, and decorin results in altered mechanical behavior, but decreases (or increases) in modulus remain strongly correlated to decreases (or increases) in ultimate stress. C3H/HeJ (C3H) and C57BL/6J (B6) animals are two inbred strains commonly used in skeletal structure function studies; C3H animals demonstrate larger collagen fibrils than B6 animals. “+/+” Indicates an animal that is homozygous positive for a gene, “−/−” indicates a homozygous knockout for that gene. C1TJ8 animals had a mutation at the collagen cleavage site that resulted in accumulation of collagen in the soft tissues, C1M8 animals had a mutation that inhibited collagen formation, which resulted in a 50% reduction in type I collagen. C: allograft treatments (35, 61, 63, 64): common allograft treatments including irradiation (gamma, electron beam) and cross-linking [gluteraldehyde, 1-ethyl-3-[3-dimethyl aminopropyl] carbodiimide (EDC)]. The density of data points prohibits labeling of each point, but combinations of irradiation and cross-linking were used. D: mechanical environment (29, 45, 46, 62, 72): environmental conditions of tendons were varied using stress shielding/immobilization/unloading or physical training methods. Mechanical effects of age and allograft preparation were highly linear. Effects of genetic alterations and differences in mechanical environment were less well defined.
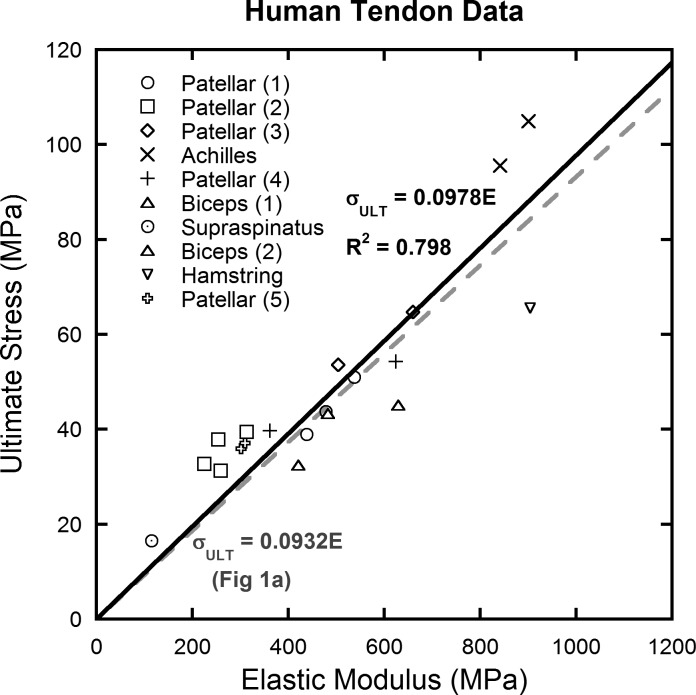
Because clinical translation to human tissue is our highest interest, human data were extracted from the larger data set (Fig. 5) and examined separately (10, 12, 13, 26, 37–39, 42, 43, 53, 75). The data follow the linear trend well (R2 = 0.798) and suggest that human tendons fail at about 9.78% strain.
Fig. 5.
Mechanical data extracted from studies on human tendon (10, 12, 13, 26, 37–39, 42, 43, 53, 75). Whereas different tendons have different mechanical properties, they have the same ratio of ultimate stress to elastic modulus (ultimate strain).
Modulus used to predict failure of tendons.
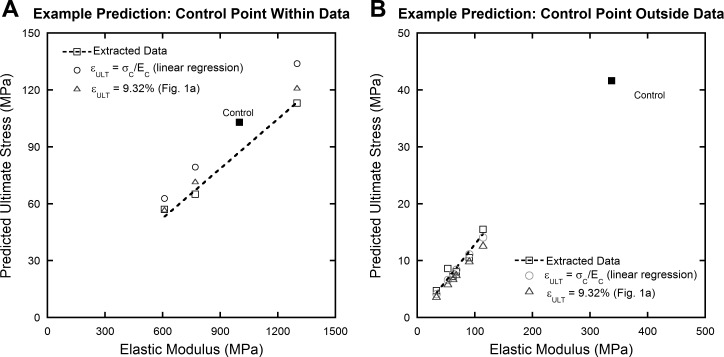
To better understand the prediction of ultimate stress from elastic modulus (and assumed constant ultimate strain), two representative studies were used (Fig. 6). Both of the previously described prediction methods work reasonably well over a range of scales; both high-strength kangaroo tail tendon allografts (61) and smaller, healing rabbit Achilles tendons (77) were accurately predicted. Prediction using a linear regression was also successful whether the control data point, on which the linear regression is based, is within the data set (Fig. 6A) or far outside of it (Fig. 6B).
Fig. 6.
Example predictions using data from studies investigating allograft preparations (A) (61) and Achilles tendon repair (B) (77). Two data sets demonstrate that the prediction methods work well over a range of scales and that the location of the control data point is inconsequential. Control data points are solid, treatment groups are open.
The ratio of predicted to actual values (predicted/actual), R2 values, and RMSE for different animal and treatment groups are presented in Tables 2 and 3, respectively. Predictions that assumed a constant ultimate strain (εult = 9.32%; Fig. 1A) performed well in estimating failure properties of altered tendons (R2 = 0.832; RMSE = 13.3). Predictions based on a control tendon ultimate strain (linear regression through zero) were even more consistent (R2 = 0.959; RMSE = 9.1). The linear regression overpredicted ultimate stress by ∼5% (predicted/actual ratio = 1.048), whereas the prediction assuming εult = 9.32% underestimated ultimate stress by about 7% (predicted/actual ratio = 0.930). If a healthy control point is not known, the ultimate strain of 9.32% acquired from the pooled data appears to accurately approximate ultimate stress from elastic modulus.
Table 2.
Accuracy of prediction methods for different animal groups
| Group | Predicted/Actual Stress | R2 | RMSE | |
|---|---|---|---|---|
| ε = σc/Ec (linear regression) | Horse | 1.085 | 0.938 | 12.6 |
| Human | 0.996 | 0.989 | 2.2 | |
| Mouse | 1.050 | 0.928 | 5.3 | |
| Rabbit | 1.126 | 0.907 | 9.6 | |
| Rat | 1.022 | 0.844 | 8.0 | |
| Other | 1.125 | 0.881 | 13.4 | |
| All | 1.048 | 0.959 | 9.1 | |
| ε = 9.32% (Fig. 1A) | Horse | 0.998 | 0.759 | 13.2 |
| Human | 0.925 | 0.802 | 10.1 | |
| Mouse | 1.060 | 0.789 | 10.6 | |
| Rabbit | 0.653 | 0.763 | 14.3 | |
| Rat | 0.679 | 0.707 | 12.7 | |
| Other | 1.071 | 0.880 | 15.6 | |
| All | 0.930 | 0.832 | 13.3 |
RMSE, root-mean-square error.
Table 3.
Accuracy of prediction methods for different treatment groups
| Group | Predicted/Actual Stress | R2 | RMSE | |
|---|---|---|---|---|
| ε = σc/Ec (linear regression) | Age | 1.063 | 0.942 | 12.9 |
| Allografts | 1.142 | 0.877 | 15.1 | |
| Genetics | 1.061 | 0.737 | 6.7 | |
| Injury | 0.994 | 0.876 | 5.0 | |
| Mechanics | 1.135 | 0.914 | 13.3 | |
| Other | 0.988 | 0.991 | 2.3 | |
| All | 1.048 | 0.959 | 9.1 | |
| ε = 9.32% (Fig. 1A) | Age | 0.890 | 0.983 | 8.4 |
| Allografts | 0.992 | 0.905 | 15.5 | |
| Genetics | 1.205 | 0.041 | 13.4 | |
| Injury | 0.745 | 0.735 | 10.2 | |
| Mechanics | 0.804 | 0.797 | 19.4 | |
| Other | 0.871 | 0.895 | 10.7 | |
| All | 0.930 | 0.832 | 13.3 |
RMSE, root-mean-square error.
When comparing animal groups (Table 2), the linear regression was most reliable for human studies (R2 = 0.989; RMSE = 2.2), with prediction values underpredicted by less than 1%. This method also provided good predictions for mouse tendons (R2 = 0.928; RMSE = 5.3) with only a 5% overprediction. The linear regression provided more accurate predictions for all animal groups compared with predictions assuming εult = 9.32%, according to RMSE (9.1 vs. 13.3).
When considering treatment groups (Table 3), the failure properties of injured tendons were well predicted by the linear regression (R2 = 0.876; RMSE = 5.0). Tendons with genetic modifications were less accurately predicted (R2 = 0.737; RMSE = 6.7), with a prediction/actual ultimate stress ratio that overestimated values by ∼6%. Interestingly, the studies grouped into the Other category (Table 3) were most accurately predicted by the linear regression (R2 = 0.991; RMSE = 2.3). With the exception of age, the linear regression more accurately predicted failure properties for all treatment groups compared with predictions assuming εult = 9.32%.
DISCUSSION
In the pooled data, a fundamental relationship between ultimate stress and elastic modulus is reported, which correlated strongly (R2 = 0.785; Fig. 1A). The same trend held true for the healthy control tendons as a subset (Fig. 1B). This relationship has been suggested to be true in the past (71), but to our knowledge, has never before been shown across multiple studies. Collagen fibrils, the main component contributing to tendon mechanical properties, begin macroscopic failure at 8–10% strain (65); it would therefore appear intuitive that tendons across species and tendon groups would experience failure in a similar strain range and thus demonstrate a correlation between ultimate stress and elastic modulus. This metaanalysis showed that this relationship between ultimate stress and elastic modulus also holds true for a number of different states of pathology, damage, or healing (other than healthy tendon). Within confidence limits, this relationship can be applied to pathomechanics. For example, an injured tendon whose modulus decreases by a certain percentage will also show a proportional decrease in ultimate stress.
This analysis also used the ratio of ultimate stress to elastic modulus as a conservative estimate for ultimate strain. The strain estimate is linearly elastic and based on the linear portion of the stress-strain curve in which the elastic modulus is calculated. This is not inclusive of the strain-stiffening toe region or the strain-softening region before failure at ultimate stress. Tangent modulus values in the nonlinear toe region are much lower than in the linear portion of the stress-strain curve (and therefore undergo more strain for the same stress level), thus contributing to larger actual ultimate stress values being measured than are estimated with this linear model. Whereas ultimate strain tends to decrease with increasing elastic modulus, this trend is not significant when considering specific studies or individual species. Smaller ranges of elastic moduli within these groups make estimations of ultimate strain much more accurate (Fig. 2). Although none of the subsets of data precisely follow the overall trend in Fig. 1A, the variations in ratio of ultimate stress to elastic modulus (ultimate strain) follow the trend found in Fig. 2. For the entire data set, tendons with higher elastic moduli exhibit lower ultimate strain. This can be observed clearly in specific subsets of tendons as well. Injury data, for example (Fig. 3), correlate strongly and show an ultimate strain higher than the pooled data with much smaller values of elastic moduli in those studies. It is also valuable to note that with increasing elastic modulus estimation of ultimate strain becomes much more accurate (Fig. 2), thus making this analysis more applicable to human pathology. The larger variance below ∼400 MPa could be due to inaccuracy in measurements of cross-sectional area, leading to errors in stress calculation, or of initial length, leading to errors in strain calculation.
One goal of this study was to obtain mechanical data from a highly heterogeneous population of tendons. With this heterogeneity comes an inherent lack of continuity in methods between studies. As such, a number of factors could not be controlled or corrected for easily. First, strain rate has been observed to have significant effects on tendon mechanics (39, 60, 70, 75). When the mechanical properties of tendon were normalized assuming a linear relationship with the logarithm of strain rate, the correlation between elastic modulus and ultimate stress just in the strain-rate–focused studies increased only moderately (from R2 = 0.562 to R2 = 0.666). When the normalization method was applied to the studies with the highest strain rates, ultimate stress did change by an average of 16.9 ± 7.25%, but elastic modulus remained relatively unchanged, differing by an average of less than 3.76 ± 1.60%.
The other factor that was difficult to correct was the differences in measurement techniques. Cross-sectional area measurements varied from study to study, including methods using calibrated digital images (3, 28, 45, 48, 46, 60), cast molds (5, 62, 71), caliper measurements (27, 29, 31, 32, 35, 38, 54, 68, 77), gravimetric measurements (16, 61, 73), ultrasound imaging (19, 20, 22, 75), MRI imaging (72), laser micrometer (2, 43, 64, 67), and specialized area calipers (30, 63, 76). Strain measurements also differed between studies. In general, tendon failure was observed to fall in the range of 10–15% strain, but it did vary from study to study, especially when strain was reported with grip-to-grip vs. on-tissue marker measurements. Specimen length has been observed to have significant effects on measurement of failure strain (47); however, these errors are likely indicative of artifacts due to gripping or other end-effects that are inherent to any mechanical testing system, the correction of which extends beyond the scope of this metaanalysis. If the extracted data were limited by specimen length, the heterogeneity of the pooled data would have been severely limited.
Whereas data on tendon injury and healing are important to consider for their clinical relevance, this metaanalysis included a number of other types of studies that tested differences in age, genetics, allograft preparation, and in vivo mechanical environments (Fig. 4). Of all the groups, those changes in mechanical properties due to age effects seemed to follow a linear trend the best followed closely by allograft preparation effects (R2 = 0.900 and 0.839, respectively). Human data (Fig. 5) also show consistent linear behavior for a limited number of available studies (R2 = 0.798). In contrast, the groups with genetic alterations (R2 = 0.505) and changes in in vivo mechanical environment (R2 = 0.110) were not highly linear and showed more variation.
The linear regression through zero prediction method performed more consistently than predictions based on εult = 9.32% from the pooled data (Fig. 1A). However, in predicting the mechanical effects of aging, an assumed constant ultimate strain of 9.32% provided a better prediction than linear regressions from control points (perhaps because the control points are subjective whether they are young, adult, or elderly). It is important to note that the linear regression overpredicted the ultimate stress of tendons by ∼5% (predicted/actual ratio = 1.048), whereas the prediction assuming εult = 9.32% underestimated the ultimate stress by close to the same amount (predicted/actual ratio = 0.930). Although the linear regression is more consistent in predicting failure, it does not provide a safety factor. When applying this linear regression method, this should be taken into account.
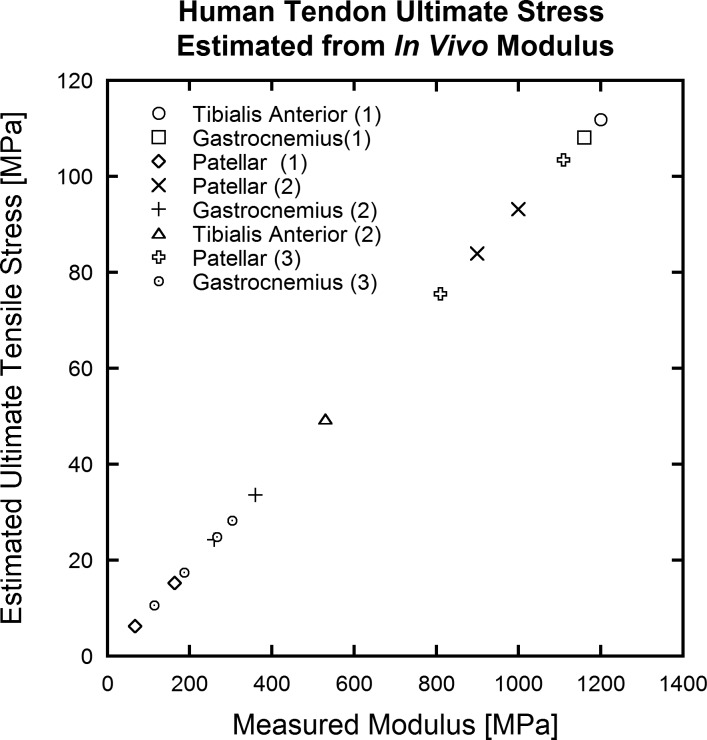
As strain-tracking and force estimation methods continue to improve, the use of ultrasound to mechanically characterize tendon has become a promising technique (14, 33, 41, 49–52, 55, 56) that can be applied in a clinical setting. This metaanalysis suggests that a predictable link exists between elastic modulus and tendon strength. Using these data, ultrasound analysis may then be able to predict failure properties of tendons from subfailure ultrasound measurements of elastic modulus (collected in the linear portion of the stress-strain curve) as in Fig. 7. Such analysis would add functional/mechanical insight to many types of tendon modification, including injury, genetics, altered mechanical environment, and age effects. Similar analysis could also help to evaluate the treatments for tendon injuries.
Fig. 7.
Estimations of ultimate stress on the basis of elastic modulus values collected in vivo. Ultimate stress values fall within the range observed among human data shown in Fig. 5.
Conclusions.
In this metaanalysis we have shown that a linear relationship exists between elastic modulus and ultimate stress that is consistent among tendons of various species, age, and injury status. It is also possible to predict the ultimate mechanical properties of a specific tendon group from a control point and knowledge of the elastic modulus of the tendon, which can be measured noninvasively using ultrasound. Human tendons and those subjected to injury and healing (in animal models) proved to be some of the most predictable types of tendons that closely follow the relationship between elastic modulus and ultimate stress.
GRANTS
Support for this study was provided by National Science Foundation Grant CMS-0553016 and by National Institutes of Health Grants R21-EB008548 and R01-AR059916. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.S.L., S.E.D.-K., R.S.L., and R.V. conception and design of research; A.S.L. performed experiments; A.S.L. analyzed data; A.S.L., S.E.D.-K., R.S.L., and R.V. interpreted results of experiments; A.S.L. and S.E.D.-K. prepared figures; A.S.L. and S.E.D.-K. drafted manuscript; A.S.L., S.E.D.-K., R.S.L., and R.V. edited and revised manuscript; A.S.L., S.E.D.-K., R.S.L., and R.V. approved final version of manuscript.
REFERENCES
- 1.Andreassen TT, Seyer-Hansen K, Bailey AJ. Thermal stability, mechanical properties and reducible cross-links of rat tail tendon in experimental diabetes. Biochim Biophys Acta 677: 313–317, 1981. [DOI] [PubMed] [Google Scholar]
- 2.Ansorge HL, Beredjiklian PK, Soslowsky LJ. CD44 deficiency improves healing tendon mechanics and increases matrix and cytokine expression in a mouse patellar tendon injury model. J Orthop Res 27: 1386–1391, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awad HA, Boivin GP, Dressler MR, Smith FN, Young RG, Butler DL. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res 21: 420–431, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bailey AJ. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev 122: 735–755, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Batson EL, Paramour RJ, Smith TJ, Birch HL, Patterson-Kane JC, Goodship AE. Are the material properties and matrix composition of equine flexor and extensor tendons determined by their functions? Equine Vet J 35: 314–318, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Biewener A. Biomechanics of mammalian terrestrial locomotion. Science 250: 1097–1103, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Biewener A. Muscle-tendon stresses and elastic energy storage during locomotion in the horse. Comp Biochem Physiol B Biochem Mol Biol 120: 73–87, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Biewener AA. Biomechanical consequences of scaling. J Exp Biol 208: 1665–1676, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Birch HL, Wilson AM, Goodship AE. Physical activity: does long-term, high-intensity exercise in horses result in tendon degeneration? J Appl Physiol 105: 1927–1933, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Blevins FT, Hecker AT, Bigler GT, Boland AL, Hayes WC. The effects of donor age and strain rate on the biomechanical properties of bone-patellar tendon-bone allografts. Am J Sports Med 22: 328–333, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Bosch U, Decker B, Kasperczyk W, Nerlich A, Oestern HJ, Tscherne H. The relationship of mechanical properties to morphology in patellar tendon autografts after posterior cruciate ligament replacement in sheep. J Biomech 25: 821–830, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Butler DL, Kay MD, Stouffer DC. Comparison of material properties in fascicle-bone units from human patellar tendon and knee ligaments. J Biomech 19: 425–432, 1986 [DOI] [PubMed] [Google Scholar]
- 13.Carpenter JE, Wening JD, Mell AG, Langenderfer JE, Kuhn JE, Hughes RE. Changes in the long head of the biceps tendon in rotator cuff tear shoulders. Clin Biomech (Bristol, Avon) 20: 162–165, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol 105: 1907–1915, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YJ, Wang CJ, Yang KD, Kuo YR, Huang HC, Huang YT, Sun YC, Wang FS. Extracorporeal shock waves promote healing of collagenase-induced Achilles tendinitis and increase TGF-beta1 and IGF-I expression. J Orthop Res 22: 854–861, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Cherdchutham W, Meershoek LS, Van Weeren PR, Barneveld A. Effects of exercise on biomechanical properties of the superficial digital flexor tendon in foals. Am J Vet Res 62: 1859–1864, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Chimich D, Shrive N, Frank C, Marchuk L, Bray R. Water content alters viscoelastic behaviour of the normal adolescent rabbit medial collateral ligament. J Biomech 25: 831–837, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Couppé C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol 107: 880–886, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Crevier N, Pourcelot P, Denoix JM, Geiger D, Bortolussi C, Ribot X, Sanaa M. Segmental variations of in vitro mechanical properties in equine superficial digital flexor tendons. Am J Vet Res 57: 1111–1117, 1996 [PubMed] [Google Scholar]
- 20.Crevier-Denoix N, Collobert C, Pourcelot P, Denoix MJ, Sanaa M, Geiger D, Bernard N, Ribot X, Bortolussi C, Bousseau B. Mechanical properties of pathological equine superficial digital flexor tendons. Equine Vet J Suppl 29: 23–26, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Curwin SL, Roy RR, Vailas AC. Regional and age variations in growing tendon. J Morphol 221: 309–320, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Dahlgren LA, Van der Meulen MC, Bertram JE, Starrak GS, Nixon AJ. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. J Orthop Res 20: 910–919, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Danielsen CC, Andreassen TT. Mechanical properties of rat tail tendon in relation to proximal-distal sampling position and age. J Biomech 21: 207–212, 1988 [DOI] [PubMed] [Google Scholar]
- 24.Derwin KA, Soslowsky LJ. A quantitative investigation of structure-function relationships in a tendon fascicle model. J Biomech Eng 121: 598–604, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Diehl P, Steinhauser E, Gollwitzer H, Heister C, Schauwecker J, Milz S, Mittelmeier W, Schmitt M. Biomechanical and immunohistochemical analysis of high hydrostatic pressure-treated Achilles tendons. J Orthop Sci 11: 380–385, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Donahue TL, Gregersen C, Hull ML, Howell SM. Comparison of viscoelastic, structural, and material properties of double-looped anterior cruciate ligament grafts made from bovine digital extensor and human hamstring tendons. J Biomech Eng 123: 162–169, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Dowling BA, Dart AJ, Hodgson DR, Rose RJ, Walsh WR. Recombinant equine growth hormone does not affect the in vitro biomechanical properties of equine superficial digital flexor tendon. Vet Surg 31: 325–330, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Dyment NA, Kazemi N, Aschbacher-Smith LE, Barthelery NJ, Kenter K, Gooch C, Shearn JT, Wylie C, Butler DL. The relationships among spatiotemporal collagen gene expression, histology, and biomechanics following full-length injury in the murine patellar tendon. J Orthop Res 30: 28–36, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eliasson P, Fahlgren A, Pasternak B, Aspenberg P. Unloaded rat Achilles tendons continue to grow, but lose viscoelasticity. J Appl Physiol 103: 459–463, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Ferry ST, Dahners LE, Afshari HM, Weinhold PS. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med 35: 1326–1333, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Forslund C, Aspenberg P. Improved healing of transected rabbit Achilles tendon after a single injection of cartilage-derived morphogenetic protein-2. Am J Sports Med 31: 555–559, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Fu SC, Shum WT, Hung LK, Wong MW, Qin L, Chan KM. Low-intensity pulsed ultrasound on tendon healing. Am J Sports Med 36: 1742–1749, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Fukashiro S, Rob M, Ichinose Y, Kawakami Y, Fukunaga T. Ultrasonography gives directly but noninvasively elastic characteristic of human tendon in vivo. Eur J Appl Physiol Occup Physiol 71: 555–557, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Galeski A, Kastelic J, Baer E, Kohn RR. Mechanical and structural changes in rat tail tendon induced by alloxan diabetes and aging. J Biomech 10: 775–782, 1977 [DOI] [PubMed] [Google Scholar]
- 35.Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone-patellar tendon-bone allografts. J Orthop Res 9: 209–218, 1991 [DOI] [PubMed] [Google Scholar]
- 36.Goh KL, Holmes DF, Lu HY, Richardson S, Kadler KE, Purslow PP, Wess TJ. Ageing changes in the tensile properties of tendons: influence of collagen fibril volume fraction. J Biomech Eng 130: 021011–021018, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Hansen P, Haraldsson BT, Aagaard P, Kovanen V, Avery NC, Qvortrup K, Larsen JO, Krogsgaard M, Kjaer M, Magnusson SP. Lower strength of the human posterior patellar tendon seems unrelated to mature collagen cross-linking and fibril morphology. J Appl Physiol 108: 47–52, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Hashemi J, Chandrashekar N, Slauterbeck J. The mechanical properties of the human patellar tendon are correlated to its mass density and are independent of sex. Clin Biomech (Bristol, Avon) 20: 645–652, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Haut TL, Haut RC. The state of tissue hydration determines the strain-rate-sensitive stiffness of human patellar tendon. J Biomech 30: 79–81, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Haut RC, Lancaster RL, DeCamp CE. Mechanical properties of the canine patellar tendon: some correlations with age and the content of collagen. J Biomech 25: 163–173, 1992 [DOI] [PubMed] [Google Scholar]
- 41.Ito M, Kawakami Y, Ichinose Y, Fukashiro S, Fukunaga T. Nonisometric behavior of fascicles during isometric contractions of a human muscle. J Appl Physiol 85: 1230–1235, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Itoi E, Berglund LJ, Grabowski JJ, Schultz FM, Growney ES, Morrey BF, An KN. Tensile properties of the supraspinatus tendon. J Orthop Res 13: 578–584, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, Woo SL. Tensile and viscoelastic properties of human patellar tendon. J Orthop Res 12: 796–803, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Ker RF, Alexander RM, Bennett MB. Why are mammalian tendons so thick? J Zool 216: 309–324, 1988 [Google Scholar]
- 45.Lavagnino M, Arnoczky SP, Frank K, Tian T. Collagen fibril diameter distribution does not reflect changes in the mechanical properties of in vitro stress-deprived tendons. J Biomech 38: 69–75, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Legerlotz K, Schjerling P, Langberg H, Brüggemann GP, Niehoff A. The effect of running, strength, and vibration strength training on the mechanical, morphological, and biochemical properties of the Achilles tendon in rats. J Appl Physiol 102: 564–572, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Legerlotz K, Riley GP, Screen HR. Specimen dimensions influence the measurement of material properties in tendon fascicles. J Biomech 43: 2274–2280, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin TW, Cardenas L, Soslowsky LJ. Tendon properties in interleukin-4 and interleukin-6 knockout mice. J Biomech 38: 99–105, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Maganaris CN. Tensile properties of in vivo human tendinous tissue. J Biomech 35: 1019–1027, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol 521: 307–313, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maganaris CN, Paul JP. Load-elongation characteristics of in vivo human tendon and aponeurosis. J Exp Biol 203: 751–756, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Maganaris CN, Reeves ND, Rittweger J, Sargeant AJ, Jones DA, Gerrits K, De Haan A. Adaptive response of human tendon to paralysis. Muscle Nerve 33: 85–92, 2006 [DOI] [PubMed] [Google Scholar]
- 53.McGough RL, Debski RE, Taskiran E, Fu FH, Woo SL. Mechanical properties of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc 3: 226–229, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Mikic B, Entwistle R, Rossmeier K, Bierwert L. Effect of GDF-7 deficiency on tail tendon phenotype in mice. J Orthop Res 26: 834–839, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Onambélé GN, Burgess K, Pearson SJ. Gender-specific in vivo measurement of the structural and mechanical properties of the human patellar tendon. J Orthop Res 25: 1635–1642, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Onambélé GL, Narici MV, Maganaris CN. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol 100: 2048–2056, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Pollock CM, Shadwick RE. Allometry of muscle, tendon, and elastic energy storage capacity in mammals. Am J Physiol Regul Integr Comp Physiol 266: R1022–R1031, 1994 [DOI] [PubMed] [Google Scholar]
- 58.Pollock CM, Shadwick RE. Relationship between body mass and biomechanical properties of limb tendons in adult mammals. Am J Physiol Regul Integr Comp Physiol 266: R1016–R1021, 1994 [DOI] [PubMed] [Google Scholar]
- 59.Rigozzi S, Müller R, Snedeker JG. Collagen fibril morphology and mechanical properties of the Achilles tendon in two inbred mouse strains. J Anat 216: 724–731, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson PS, Lin TW, Reynolds PR, Derwin KA, Iozzo RV, Soslowsky LJ. Strain-rate sensitive mechanical properties of tendon fascicles from mice with genetically engineered alterations in collagen and decorin. J Biomech Eng 126: 252–257, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Roe SC, Milthorpe BK, True K, Rogers GJ, Schindhelm K. The effect of gamma irradiation on a xenograft tendon bioprosthesis. Clin Mater 9: 149–154, 1992 [DOI] [PubMed] [Google Scholar]
- 62.Rumian AP, Draper ER, Wallace AL, Goodship AE. The influence of the mechanical environment on remodelling of the patellar tendon. J Bone Joint Surg Br 91: 557–564, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Salehpour A, Butler DL, Proch FS, Schwartz HE, Feder SM, Doxey CM, Ratcliffe A. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone-patellar tendon-bone allografts. J Orthop Res 13: 898–906, 1995 [DOI] [PubMed] [Google Scholar]
- 64.Seto A, Gatt CJ, Dunn MG. Improved tendon radioprotection by combined cross-linking and free radical scavenging. Clin Orthop Relat Res 467: 2994–3001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact 6: 181–190, 2006 [PubMed] [Google Scholar]
- 66.Smith CW, Young IS, Kearney JN. Mechanical properties of tendons: changes with sterilization and preservation. J Biomech Eng 118: 56–61, 1996 [DOI] [PubMed] [Google Scholar]
- 67.Stone D, Green C, Rao U, Aizawa H, Yamaji T, Niyibizi C, Carlin G, Woo SL. Cytokine-induced tendinitis: a preliminary study in rabbits. J Orthop Res 17: 168–177, 1999 [DOI] [PubMed] [Google Scholar]
- 68.Su WR, Chen HH, Luo ZP. Effect of cyclic stretching on the tensile properties of patellar tendon and medial collateral ligament in rat. Clin Biomech (Bristol, Avon) 23: 911–917, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Svensson RB, Hassenkam T, Grant CA, Magnusson SP. Tensile properties of human collagen fibrils and fascicles are insensitive to environmental salts. Biophys J 99: 4020–4027, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Svensson RB, Hassenkam T, Hansen P, Magnusson SP. Viscoelastic behavior of discrete human collagen fibrils. J Mech Behav Biomed Mater 3: 112–115, 2010 [DOI] [PubMed] [Google Scholar]
- 71.Thorpe CT, Stark RJ, Goodship AE, Birch HL. Mechanical properties of the equine superficial digital flexor tendon relate to specific collagen cross-link levels. Equine Vet J 42 (Suppl 38): 538–543, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Trudel G, Koike Y, Ramachandran N, Doherty G, Dinh L, Lecompte M, Uhthoff HK. Mechanical alterations of rabbit Achilles' tendon after immobilization correlate with bone mineral density but not with magnetic resonance or ultrasound Imaging. Arch Phys Med Rehabil 88: 1720–1726, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Wang XT, Ker RF. Creep rupture of wallaby tail tendons. J Exp Biol 198: 831–845, 1995 [DOI] [PubMed] [Google Scholar]
- 74.Woo SY, Ritter MA, Amiel D, Sanders TM, Gomez MA, Kuei SC, Garfin SR, Akeson WH. The biomechanical and biochemical properties of swine tendons-long term effects of exercise on the digital extensors. Connect Tissue Res 7: 177–183, 1980 [DOI] [PubMed] [Google Scholar]
- 75.Wren TA, Yerby SA, Beaupré GS, Carter DR. Mechanical properties of the human achilles tendon. Clin Biomech (Bristol, Avon) 16: 245–251, 2001 [DOI] [PubMed] [Google Scholar]
- 76.Yamamoto N, Hayashi K, Kuriyama H, Ohno K, Yasuda K, Kaneda K. Mechanical properties of the rabbit patellar tendon. J Biomech Eng 114: 332–337, 1992 [DOI] [PubMed] [Google Scholar]
- 77.Young RG, Butler DL, Weber W, Caplan AI, Gordon SL, Fink DJ. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res 16: 406–413, 1998 [DOI] [PubMed] [Google Scholar]