Abstract
Sympathetically mediated renal vasoconstriction may contribute to the pathogenesis of hypertension in older adults, but empirical data in support of this concept are lacking. In 10 young (26 ± 1 yr) and 11 older (67 ± 2 yr) subjects, we quantified acute hemodynamic responses to three sympathoexcitatory stimuli: local cooling of the forehead, cold pressor test (CPT), and voluntary apnea. We hypothesized that all stimuli would increase mean arterial blood pressure (MAP) and renal vascular resistance index (RVRI) and that aging would augment these effects. Beat-by-beat MAP, heart rate (HR), and renal blood flow velocity (from Doppler) were measured in the supine posture, and changes from baseline were compared between groups. In response to 1°C forehead cooling, aging was associated with an augmented MAP (20 ± 3 vs. 6 ± 2 mmHg) and RVRI (35 ± 6 vs. 16 ± 9%) but not HR. In older adults, there was a positive correlation between the cold-induced pressor response and forehead pain (R = 0.726), but this effect was not observed in young subjects. The CPT raised RVRI in both young (56 ± 13%) and older (45 ± 8%) subjects, but this was not different between groups. Relative to baseline, end-expiratory apnea increased RVRI to a similar extent in both young (46 ± 14%) and older (41 ± 9%) subjects. During sympathetic activation, renal vasoconstriction occurred in both groups. Forehead cooling caused an augmented pressor response in older adults that was related to pain perception.
Keywords: cold face test, renal blood flow, vasoconstriction, peripheral chemoreflex, noxious cooling
the kidney is a vital organ for salt and water homeostasis and receives ∼20% of cardiac output in resting humans (60). Furthermore, the renal vasculature is innervated by sympathetic nerves that allow for alpha adrenergic-mediated vasoconstriction during times of stress (i.e., exercise and orthostasis) (6, 43). As such, acute adjustments in renal blood flow importantly contribute to whole-body fluid balance and blood pressure control. It follows that chronic impairments in this process (e.g., enhanced water reabsorption and/or enhanced vasoconstriction) predispose an individual to hypertension. Considering that aging itself is a risk factor for hypertension (44, 67), and glomerular filtration rate declines with age (12, 14), baseline impairments in renal function combined with sympathetic activation may negatively affect the elderly. Therefore, a better understanding of the renal circulation in older adults may have clinical relevance. However, there is currently little experimental data (42, 61) regarding the effect of healthy aging on renal vasoconstriction during sympathoexcitatory stress.
Cold temperatures are seasonally experienced in most regions of the world. The compensatory physiological responses to cold involve the sympathetic nervous system and serve to prevent a reduction in core body temperature (4). Exposure to cold temperatures typically elicits an increase in mean arterial blood pressure (MAP) (22, 32, 71). Coincident with this pressor response, local (3) and systemic (74) cold exposure has been shown to elicit renal vasoconstriction in young people. These previous studies (3, 74), using Doppler ultrasound, indicate that the visceral circulation can acutely and rapidly adapt to local or systemic cold stress. It is clear that healthy, older adults have an augmented MAP response to whole-body cooling (i.e., systemic skin temperature reduction that is not painful and does not induce shivering) (16, 25, 73), but the acute hemodynamic and renal vascular responses to local cooling in older adults are unknown.
The purpose of the current study was 1) to determine MAP, heart rate (HR), and renal vascular resistance index (RVRI) in response to local cooling of the forehead, local cooling of the hand [i.e., cold pressor test (CPT)], and end-expiratory apnea (i.e., stimuli known to increase vasoconstrictor nerve traffic) and 2) to determine if healthy aging modifies these physiological responses. We hypothesized that 1) all stimuli would increase MAP and RVRI, and 2) aging would augment the MAP and RVRI response to local cooling and apnea. To address these hypotheses, we conducted acute physiological experiments using transabdominal Doppler ultrasound in healthy young and healthy older humans (experiment 1). Based on the data from experiment 1, additional forehead-cooling trials were performed to determine whether forehead pain and/or cold perception correlated to the observed MAP responses (experiment 2).
METHODS
Subjects and design.
This study used a repeated-measures crossover design, and age served as a between-subjects factor. All study protocols were approved in advance by the Institutional Review Board of the Penn State Milton S. Hershey Medical Center and conformed to the Declaration of Helsinki. Each participant provided written, informed consent. Data were collected in a thermoneutral laboratory (22–25°C) from May to August 2012 to minimize any seasonal effects on cardiovascular control (72) or thermoregulation (69). Ten young [six men and four women; 26 ± 1 yr, 174 ± 3 cm height, 77.2 ± 5.2 kg body wt, 25.3 ± 1.0 body mass index (BMI)] and 11 older volunteers (six men and five women; 67 ± 2 yr, 173 ± 2 cm height, 72.5 ± 2.5 kg body wt, 24.4 ± 0.8 BMI) participated in experiment 1. For experiment 2, an additional 16 young subjects (eight men and eight women; 25 ± 1 yr, 172 ± 3 cm height, 76.5 ± 2.7 kg body wt, 24.6 ± 0.7 BMI) and 13 older subjects (six men and seven women; 66 ± 2 yr, 173 ± 2 cm height, 72.7 ± 2.7 kg body wt, 24.5 ± 0.8 BMI) were recruited. All subjects were normotensive, nonasthmatic, nonobese nonsmokers, not taking any prescription or vasoactive medication, and were in good health, as determined by history and physical examination. After the consent process but before enrollment, the older adults underwent a stress electrocardiogram (modfied Bruce protocol). The test was interpreted by a cardiologist to rule out coronary artery disease. All subjects reported being physically active, but none were competitive athletes. Subjects refrained from caffeine, alcohol, and exercise for 24 h before the study and arrived to the laboratory in a semifasted state (i.e., 4–6 h after their last meal).
Procedures.
Subjects were supine and clothed in a high-density, tube-lined suit (Med-Eng Systems, Ottawa, Ontario, Canada) that covered the entire body except for the feet, hands, and head. Neutral water (34–35°C) was perfused through the suit to maintain mean skin temperature at a constant level and ensure that the obtained responses were reflex mediated. Two-way zippers in the suit allowed for abdominal access during testing. Following familiarization trials, baseline measurements were obtained after 15 min of quiet rest. Subjects then underwent the following five stimuli in random order, separated by at least 15 min of quiet rest (to ensure parameters returned to baseline): 1) forehead cooling at 1°C; 2) forehead cooling at 15°C; 3) neutral forehead time control (35°C); 4) CPT (hand in 1°C ice water); and 5) maximal voluntary end-expiratory apnea.
Local forehead cooling was conducted for 60 s, as described previously (21, 30, 31). Briefly, a plastic bag filled with ∼250 ml ice and water (1°C) was placed on the forehead. Care was taken to avoid contact with the eyes (i.e., to avoid the oculocardiac reflex), and subjects were monitored to ensure normal breathing. This forehead-cooling procedure is thought to stimulate trigeminal afferents that are involved in the diving reflex (i.e., bradycardia and peripheral vasoconstriction), but bradycardia is not a universal finding in healthy people (11, 22, 65). Because forehead cooling at 1°C was expected to be painful, we also performed forehead cooling at 15°C in all subjects, which is a temperature that stimulates cold-sensitive afferents but does not elicit pain (23, 32, 35). Exposure of the forehead to neutral water (35°C) was also conducted to account for time and pressure effects. Water temperature was measured via thermistors (TC-2000; Sable Systems International, Las Vegas, NV). During experiment 2, only MAP, HR, forehead pain, and forehead cold sensation were measured during 60 s of forehead cooling at 1°C (i.e., RVRI was not obtained).
Local cooling of the hand (the CPT) was chosen, because it is a sympathoexcitatory stimulus that is perceived as both cold and painful (32, 70). Prior experiments from our laboratory (49, 51) and others (10, 55) have indicated that young and older subjects have similar MAP and HR responses to the CPT (i.e, peak responses obtained immediately before removing the hand from the water minus baseline level). For the CPT, the left hand up to the styloid process was placed into 1°C water and remained submerged for 2 min. Data from the last 20 s of immersion were used as the peak response.
The end-expiratory apnea was chosen because it is a nonthermal stimulus that increases sympathetic outflow (18, 46). For the voluntary end-expiratory apnea, individuals held their breath as long as possible, and the peak three cardiac cycles were used in analysis (i.e., always immediately before resuming inspiration) (36, 37).
Measurements.
Resting measures of systolic blood pressure (SBP) and diastolic blood pressure (DBP) were obtained via automated sphygmomanometry (Philips SureSigns VS3; Philips Healthcare, Andover, MA) in triplicate. Beat-by-beat blood pressure was obtained via photoplethysmography (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands), HR was measured via three-lead ECG (Cardiocap/5; GE Healthcare, Little Chalfont, UK), and spontaneous respiration was tracked via pneumography. These parameters were sampled at 200 Hz by a data acquisition system (PowerLab; ADInstruments, Colorado Springs, CO) and were synchronized with the Doppler images using verbal and written time stamps. Transabdominal Doppler ultrasound (HDI 5000; Advanced Technology Laboratories Ultrasound, Bothell, WA) was used to measure renal blood flow velocity (RBV), as described previously (43, 61). Briefly, the depth and angle of insonation (<60°) were held constant within each subject, and images were acquired and saved at end expiration (end of spontaneous respiratory cycle). The same investigator (M. D. Muller) performed all RBV measurements and was not blinded during data analysis. RVRI was calculated offline as MAP/RBV; an increase in RVRI is considered to indicate renal vasoconstriction (6, 41, 43, 61). Thermal sensation (0 = neutral/no sensation of cold, and 11 = unbearable cold) and pain perception (0 = no pain, and 10 = unbearable pain) were obtained immediately after each stimulus (17, 20).
Statistical analysis.
All statistical analyses were conducted using IBM SPSS 19.0, and graphics were produced using Microsoft Excel and Adobe Illustrator CS5. Normality was confirmed by the Kolmogorov-Smirnov test (i.e., P > 0.05 for all physiological measurements). Baseline anthropometric and hemodynamic parameters were determined with independent sample t-tests. Forehead-cooling data were averaged into six bins of 10-s duration (i.e., 0–10, 10–20, 20–30, 30–40, 40–50, and 50–60 s), and peak changes (Δ) from baseline were calculated. These bins were chosen based on prior publications (29, 31). For forehead cooling, the effects of group and time were determined using repeated-measures ANOVA. When the assumption of sphericity was violated, the Greenhouse-Geisser adjustment was used. When a group-by-time interaction was found, post hoc testing was conducted via appropriate t-tests. For CPT and apnea trials, independent t-tests were used to compare Δ between groups. Forehead-cooling data from experiments 1 and 2 were analyzed using bivariate correlations to compare ΔMAP with both forehead pain and thermal sensation. In contrast to experiment 1, where temperature was considered to be the stimulus, and physiological variables were the response, this correlation analysis assumed that perceptual variables were the stimulus, and ΔMAP was the response. A ratio of ΔMAP to forehead pain was also derived and compared between groups. For this ratio, larger numbers indicate a larger pressor response for a given level of forehead pain. Data are presented as means ± SE throughout, and P < 0.05 was considered statistically significant.
RESULTS
Baseline measurements.
The older adults in experiment 1 had higher SBP (115 ± 3 vs. 105 ± 2 mmHg, P = 0.031) and DBP (71 ± 2 vs. 61 ± 1 mmHg, P < 0.001) compared with their younger counterparts. Resting RBV was not different between young (58 ± 3 cm/s) and older (53 ± 4 cm/s) subjects (P = 0.313), but resting RVRI was higher in older subjects (1.79 ± 0.13 vs. 1.37 ± 0.07 mmHg·cm−1·s−1, P = 0.045). Older adults in experiment 2 had higher SBP (116 ± 2 vs. 104 ± 2 mmHg, P < 0.001), DBP (72 ± 2 vs. 60 ± 1 mmHg, P < 0.001), and MAP (86 ± 3 vs. 76 ± 2 mmHg, P < 0.001) compared with their younger counterparts.
Forehead cooling.
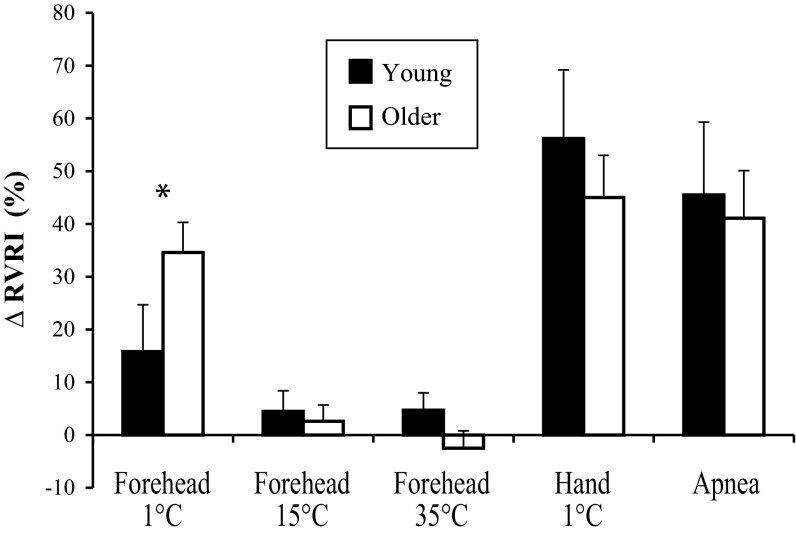
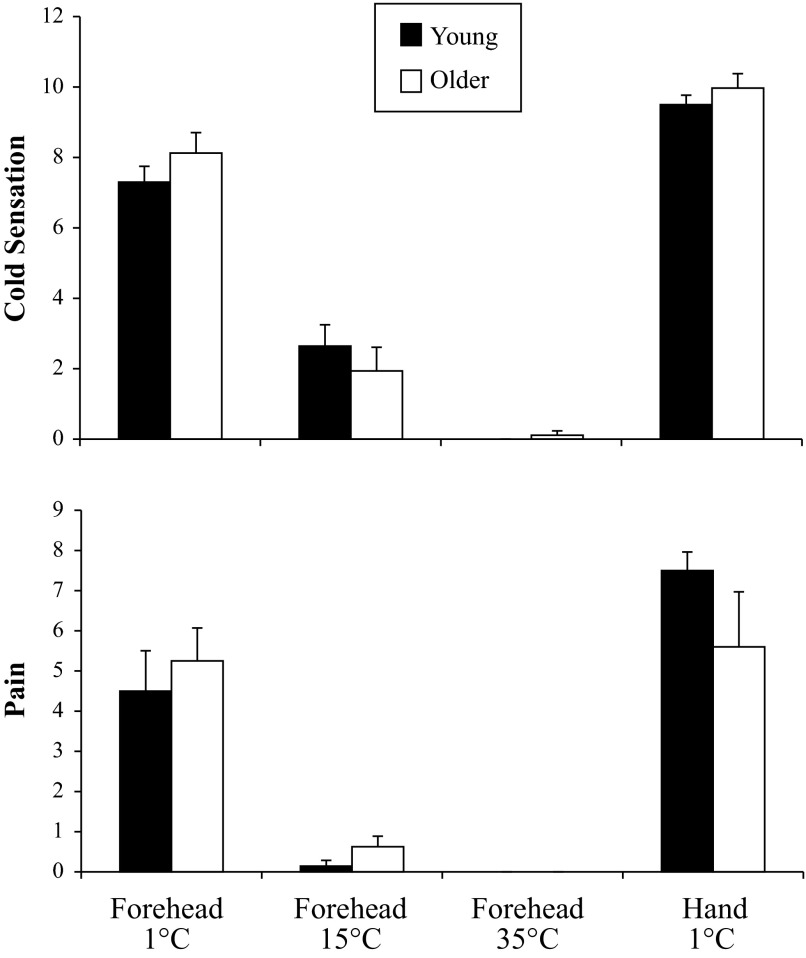
In response to forehead cooling at 1°C, ΔMAP showed a group-by-time interaction (P < 0.001; Fig. 1), such that older adults had an augmented pressor response. In a similar way, ΔSBP (19 ± 5 vs. 11 ± 3 mmHg) was augmented significantly in the older subjects; however, ΔDBP (6 ± 1 vs. 9 ± 2 mmHg) was similar between groups. HR did not change across time in either group (Fig. 1). The ΔRVRI was also greater in older subjects (P = 0.039; Fig. 2). On average, both groups perceived the 1°C stimulus as “very cold” and “painful” (Fig. 3). Neither forehead cooling at 15°C nor 35°C time-control studies had an effect on ΔMAP, ΔHR, or ΔRVRI. Exposure to forehead cooling at 15°C was perceived as “very cool” and “not painful,” and time control with 35°C was rated as “neutral” and not painful (Fig. 3).
Fig. 1.
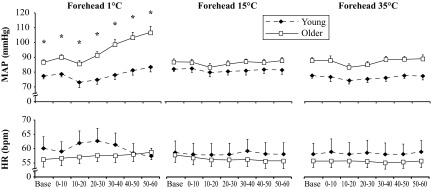
Mean arterial blood pressure (MAP) and heart rate (HR) were measured in young (black diamonds, n = 10) and older (white squares, n = 11) subjects during 60 s of forehead cooling (1°C and 15°C water in a plastic bag), as well as a neutral (35°C water) time control. Means ± SE; *group difference at a given time point, P < 0.05.
Fig. 2.
Peak changes in renal vascular resistance index (ΔRVRI) in young (black bars, n = 10) and older (white bars, n = 11) subjects. *Group difference, P < 0.05.
Fig. 3.
Local cold sensation (top) and local pain perception (bottom) in young (black bars, n = 10) and older (white bars, n = 11) subjects. For cold sensation, 0 = neutral, 5 = cold, 10 = very, very cold. For pain, 0 = no pain, 6 = painful, and 10 = unbearably painful. There were no group differences.
Experiment 2 was conducted to evaluate the relationship between physiological (ΔMAP and ΔHR) and perceptual (pain and thermal sensation) responses. Similar to experiment 1, older subjects had an augmented ΔMAP to 1°C forehead cooling (16 ± 4 vs. 8 ± 2 mmHg, P = 0.033), whereas ΔHR (−4 ± 2 vs. −1 ± 1 beats/min, P = 0.089) forehead pain [3.8 ± 0.6 vs. 4.1 ± 0.8 arbitrary units (au), P = 0.809] and forehead cold sensation (7.0 ± 0.5 vs. 7.8 ± 0.5 au, P = 0.201) were not different between groups. When combining all available data from experiments 1 and 2 (n = 26 young, and n = 24 older), there were no group differences in cold sensation (young: 7.1 ± 0.4; older: 7.8 ± 0.4 units, P = 0.120) or pain perception (young: 4.2 ± 0.6; older: 4.7 ± 0.6 units, P = 0.499). As shown in Fig. 4, there was a significant relationship between ΔMAP and forehead pain in the older subjects (R = 0.726, P < 0.001) but not the young subjects (R = 0.242, P = 0.224). When comparing ΔMAP and forehead cold sensation, neither young subjects (R = 0.188, P = 0.347) nor older subjects (R = 0.370, P = 0.075) demonstrated a significant relationship. The ratio of ΔMAP to forehead pain was augmented significantly in the older subjects (3.5 ± 0.6 vs. 1.8 ± 0.4 au, P = 0.023). These calculated values are to be expected since ΔMAP was also augmented in the older adults (16 ± 2 vs. 8 ± 1 mmHg, P = 0.003). Thus despite both groups perceiving similar levels of forehead pain in response to 60 s of forehead cooling at 1°C, the older adults had a pressor response that was approximately two times larger compared with the young subjects.
Fig. 4.
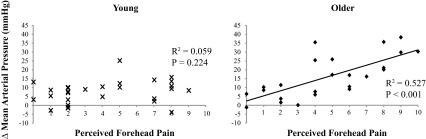
Relationship between forehead pain and ΔMAP in response to forehead cooling at 1°C. Data from experiments 1 and 2 are presented together.
Cold pressor test.
Immersion of the hand into 1°C water for 2 min increased MAP in both groups (young: 80 ± 1 to 111 ± 3 mmHg; older: 87 ± 2 to 106 ± 3 mmHg) and caused a modest tachycardia (young: 64 ± 4 to 70 ± 3 beats/min; older: 57 ± 2 to 60 ± 3 beats/min). The CPT elicited renal vasoconstriction of similar magnitude in both groups (Fig. 2). On average, both groups perceived the CPT as “very, very cold” and “painful to very painful” (Fig. 3).
Voluntary apnea.
End-expiratory apnea duration was not different between young (27 ± 4 s) and older (33 ± 2 s) subjects. The ΔMAP was comparable between young (from 78 ± 2 to 89 ± 4 mmHg) and older (from 87 ± 2 to 103 ± 4 mmHg) subjects. The ΔHR was also similar between young (from 63 ± 5 to 70 ± 2 beats/min) and older (from 57 ± 2 to 65 ± 3 beats/min) subjects. As shown in Fig. 2, apnea elicited a similar magnitude of renal vasoconstriction in both groups.
DISCUSSION
The purpose of this study was to determine the effect of healthy aging on reflex cardiovascular and renal adjustments to three unique stimuli: local cooling of forehead, local cooling of hand (i.e., CPT), and voluntary end-expiratory apnea. We hypothesized that these three sympathoexcitatory stimuli would increase MAP and elicit renal vasoconstriction and that older subjects would have augmented responses. The current experiments provide four novel findings. First, aging is associated with an augmented MAP response to forehead cooling at 1°C that is strongly correlated to the individual's pain perception. Second, older adults have an augmented increase in RVRI in response to forehead cooling at 1°C. Third, the CPT elicited renal vasoconstriction of similar magnitude in both groups. Fourth, maximal end-expiratory apnea elicited renal vasoconstriction of similar magnitude in both groups. On a physiological level, these data indicate that painful cooling of the forehead is a unique thermal stimulus that elicits age-related differences in blood pressure and renal blood flow. On a clinical level, exposure of the forehead to a cold and/or painful stimulus may contribute to the higher blood pressures observed in older people (75) and hemodialysis patients (1, 5, 58) during the winter compared with summer.
Forehead cooling.
Forehead cooling activates the sympathetic nervous system (13, 22), and prior publications have suggested that this short-duration stimulus can be used to detect autonomic dysfunction (28, 30). Because of our interest in both cold stress physiology and autonomic control of the circulation, we reasoned that forehead cooling would be a unique and valuable stressor to identify potential age differences in cardiovascular function. Studying how thermal stress and aging impact the human body is an area of active investigation because of the clear adverse effects of extreme temperatures on cardiovascular morbidity and mortality (39, 64).
Consistent with previous studies showing augmented pressor responses to whole-body skin cooling (16, 25, 73), the current data indicate that older adults also have an augmented MAP response to forehead cooling at 1°C. This pressor response in older adults was coincident with an augmented renal vasoconstriction (Fig. 2), which is often considered to be evidence of increased renal sympathetic nerve activity (6, 43, 61), but one must consider that augmented renal vasoconstriction with aging could be the cause or consequence of an augmented MAP in these subjects. This caveat is particularly important because the older subjects had higher RVRI at baseline. As forehead cooling at 1°C was a painful cold stimulus, subjects also underwent the CPT to determine whether MAP and RVRI responses to this stimulus were different between age groups. As shown in Fig. 2, MAP and RVRI responses to the CPT were comparable between groups. By using a nonpainful, nonthermal stimulus to activate the sympathetic nervous system (i.e., maximal apnea that activates the chemoreflex), we reinforce further that some unique aspect of forehead cooling underlies the group differences in MAP and RVRI as opposed to a general effect of aging. Other age-related changes, such as increased arterial stiffness, impaired baroreflex sensitivity, reduced left-ventricular compliance, reduced adrenergic sensitivity, and elevated resting muscle sympathetic nerve activity (MSNA), are unlikely to explain our findings, because these factors would have been evident in CPT and apnea trials as well (8, 44, 54, 57, 63, 76).
The correlation data from experiments 1 and 2 reveal that forehead pain perception contributes to, but does not entirely explain, the observed MAP response in older adults. This finding was evident, despite mean pain ratings being similar between groups. Experiments in monkeys demonstrated that cold pain was mediated by mechanothermal nociceptors (35), but there is currently no data showing that these types of afferents are altered with aging. It is possible that the trigeminal nerve receptive field differs between young and older subjects (26), such that an identical bag of 1°C ice water elicits a different input to the central nervous system in older people, but this concept seems unlikely, because ratings of forehead pain were similar between groups. It is well established that noxious pressure or electrical stimulation to the supraorbital ridge activates the sympathetic nervous system in humans (56). Specifically, Nordin and Fagius (56) demonstrated a 300–400% increase in MSNA and a 21-mmHg increase in MAP in young men. Whether reflex responses to painful stimuli are modified by the aging process is unclear, but the current data suggest that the forehead is a unique anatomical location to begin future investigation.
Facial cooling of the trigeminal region resembles the “human diving reflex” in that it often involves bradycardia (increased cardiac vagal activity) and vasoconstriction of selected vascular beds (increased sympathetic outflow) in an attempt to redistribute and conserve oxygen (13, 15). This reflex is well established in diving mammals (9) and ducks (2). Importantly, the diving reflex elicits profound bradycardia and reductions in renal blood flow in Weddell seals and sea lions (7, 66, 77). In the current study, we were not able to replicate the bradycardia seen in previous human experiments using identical forehead-cooling methodology (21, 29–31, 59). Careful examination of these cited studies reveals that mean baseline HR was ∼72 beats/min compared with 60 beats/min in our study. Higher resting HR may allow a greater decrease in HR in response to facial cooling at 1°C (i.e., there would be more cardiac vagal tone to activate and/or more cardiac sympathetic tone to inhibit). In the study by Stemper et al. (65), with baseline HR more similar to our subjects, there was a modest bradycardia from a baseline of 64 to 61 beats/min in response to facial cooling at 1°C in young subjects. Another study by Edwards et al. (11) actually showed a modest tachycardia (from 57 to 60 beats/min) in responses to forehead cooling in young subjects. Hence, there may be a “floor effect,” such that subjects with low, resting HR are not able to activate the efferent vagus to lower HR further in response to forehead cooling. Alternatively, the pain response (sympathetic activation) may simply over-ride any vagal activation that does occur.
Cold pressor test.
The CPT is a widely used laboratory stimulus that increases sympathetic outflow to skeletal muscle (32, 70) and increases myocardial oxygen demand (45, 48). From a practical standpoint, people exposed to cold ambient conditions also experience hand pain (52) and reduced manual dexterity (53), thereby increasing their risk for accident (19). Thus physiological and perceptual responses to hand cooling have clinical and occupational applications. Boddi et al. (3) demonstrated that the CPT elicits renal vasoconstriction in young people; we further the literature by showing that a similar level of renal vasoconstriction occurs in older people. The proposed mechanism is via increases in renal sympathetic nerve activity in both groups. Kregel et al. (32) demonstrated that the increased MSNA during sustained, severe skin cooling (hand in ice water at 1°C) was associated with pain perception. Our thermal and pain sensation data agree with Kregel et al. (32) and Ng et al. (55) in that CPT is a “very painful” and very cold stimulus that produces a consistent pressor response in both young and older subjects.
Wilson et al. (74) documented significant vasoconstriction in the splanchnic and renal arteries during skin-surface cooling in healthy, young adults. With regard to aging, there is growing evidence that renal vasoconstriction in response to isometric handgrip (i.e., sympathoexcitatory stimulus) is enhanced in older adults (34, 42). To our knowledge, we are the first to document renal responses to the CPT in older humans. Although not the intended purpose of the study, we also found that local cooling of the forehead does not elicit the same hemodynamic and renal responses as cooling of the hand (Fig. 2). These findings are consistent with a previous study by Heindl et al. (22), who showed differences in MSNA during local cooling of the hand and forehead. The lack of similarity between these two different cold stimuli may be due to differences in surface area, type, and distribution of cold receptors and/or threshold for pain receptors.
Voluntary apnea.
End-expiratory apnea was used in this study as a nonthermal, sympathoexcitatory stimulus. In humans, voluntary apnea elicits hypercapnia- and hypoxia- induced increases in MSNA (18, 36), resulting in acute hypertension. The MSNA response is mediated primarily by reductions in arterial oxygen saturation (i.e., hypoxia), which stimulates chemoreceptors located in the carotid bodies (36). Hypercapnia plays a minor role in the MSNA response; Morgan et al. (46) showed an abolished neurocirculatory response to apnea with supplemental oxygen. To our knowledge, we are the first to show renal vasoconstriction in response to voluntary apnea in young people. Additionally, we demonstrate a similar level of renal vasoconstriction in older adults. The increase in RVRI is most likely due to hypoxia-induced sympathetic activation during apnea. Our data are consistent with animal studies showing renal vasoconstriction during diving (7, 66, 77), and these findings fit the theoretical purpose of the diving reflex (i.e., conserving oxygen by diverting it to more critical organs) (15).
Experimental considerations.
The inability to measure renal artery diameter reliably is a limitation of the current study. With Doppler ultrasound, the spatial resolution decreases as the frequency of the transducer decreases (33). A low-frequency transducer (2.5 MHz) was used to obtain optimal velocity signals at the expense of spatial resolution; hence, we could not measure renal artery diameter and instead used ΔRVRI as an index of reflex-mediated vasoconstriction. Whereas MSNA can be measured directly by microneurography in humans, renal sympathetic nerve activity cannot be acquired. As noted in prior publications (6, 34), the renal artery is a conduit artery, and reductions in flow velocity are thought to be due to increases in downstream resistance. This concept is supported by an invasive study, demonstrating that pharmacological vasoconstriction (intra-arterial injection of adenosine to reduce flow velocity) does not alter renal artery diameter (40). We also acknowledge that the kidney is under autoregulatory control; because of this, acute ΔMAP may differentially influence RVRI between young and older subjects, independent of the autonomic nervous system. Lastly, a cause and effect mechanism for the observed age-related differences is still yet to be determined.
Clinical significance.
The current study may shed light into altered renal physiology with aging. The combination of increased baseline sympathetic nerve activity (54, 63) and declining glomerular filtration rate (14) in older adults may lead to susceptibility to renal complications when faced with an acute sympathoexcitatory challenge. Indeed, during sympathetic stress, renal sympathetic nerve activity increases, and urinary sodium excretion decreases in healthy rats, and this effect is exacerbated in hypertensive rats via a sympathetic mechanism (38). Whether a similar mechanism occurs in humans was not tested directly in the current study, but recent studies indicate that renal nerve ablation reduces MSNA and MAP, improves baroreflex sensitivity, and reduces left-ventricular mass in hypertensive patients (62) and also reduces MAP in patients with chronic kidney disease (24). Many of these patients are elderly and have additional cardiovascular risk factors beyond what was studied in our experiment. Taken together, the relationship among sympathetic activation, the renal circulation, and high blood pressure is an area of continued medical and physiological importance. In addition to adverse effects of sympathetic activation on the kidney, stressors associated with sympathetic activation (e.g., exercise, cold exposure, mental stress) are risk factors for adverse cardiac outcomes (27). Recent publications have outlined the potential mechanism, whereby a surge in blood pressure (e.g., due to local cold exposure) leads to thrombosis and potentially, a fatal cardiac event (47, 68). The fact that 60 s of forehead cooling elicits such a large pressor response and renal vasoconstriction in older adults requires further investigation.
Conclusions.
These physiological studies revealed four novel findings. First, aging is associated with an augmented MAP response to forehead cooling at 1°C that is strongly correlated to the individual's pain perception. Second, older adults have an augmented increase in RVRI in response to forehead cooling at 1°C. Third, the CPT, a known sympathoexcitatory stimulus, elicits renal vasoconstriction of similar magnitude in healthy, young and older subjects. Fourth, voluntary apnea elicited renal vasoconstriction that was similar in both groups. These findings, as well as prior publications (6, 41–43, 50, 61, 74), indicate that renal vasoconstriction occurs in response to sympathoexcitatory stimuli. The current data further our understanding of how the aging process affects renal vasoconstriction in responses to acute physiological stress and also suggest that forehead afferents may be altered with aging.
GRANTS
Support for this work was provided by National Heart, Lung, and Blood Institute Grant P01-HL096570 (to L. I. Sinoway), National Center for Research Resources Grant UL1-RR033184 (to L. I. Sinoway), and National Center for Advancing Translational Sciences Grant UL1-TR000127 (to L. I. Sinoway), as well as a Wilderness Medical Society Research in Training Grant (to M. D. Muller).
DISCLOSURES
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Author contributions: H.P. and M.D.M. conception and design of research; H.P., J.L.M., and M.D.M. performed experiments; H.P. and M.D.M. analyzed data; H.P., J.L.M., L.I.S., and M.D.M. interpreted results of experiments; M.D.M. prepared figures; H.P. and M.D.M. drafted manuscript; H.P., J.L.M., L.I.S., and M.D.M. edited and revised manuscript; H.P., J.L.M., L.I.S., and M.D.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful for the nursing support provided by Cheryl Blaha and Todd Nicklas and the technical assistance of Charity Sauder, Josh Oman, and Matt Heffernan. Gratitude is also extended to Anne Muller for preparing the graphics for this study. Finally, the authors acknowledge the administrative guidance of Kris Gray and Jen Stoner.
REFERENCES
- 1. Argiles A, Mourad G, Mion C. Seasonal changes in blood pressure in patients with end-stage renal disease treated with hemodialysis. N Engl J Med 339: 1364–1370, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Blix AS. The importance of asphyxia for the development of diving bradycardia in ducks. Acta Physiol Scand 95: 41–45, 1975 [DOI] [PubMed] [Google Scholar]
- 3. Boddi M, Sacchi S, Lammel RM, Mohseni R, Serneri GG. Age-related and vasomotor stimuli-induced changes in renal vascular resistance detected by Doppler ultrasound. Am J Hypertens 9: 461–466, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc 78: 603–612, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Cheung AK, Yan G, Greene T, Daugirdas JT, Dwyer JT, Levin NW, Ornt DB, Schulman G, Eknoyan G. Seasonal variations in clinical and laboratory variables among chronic hemodialysis patients. J Am Soc Nephrol 13: 2345–2352, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Conboy EE, Fogelman AE, Sauder CL, Ray CA. Endurance training reduces renal vasoconstriction to orthostatic stress. Am J Physiol Renal Physiol 298: F279–F284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis RW, Castellini MA, Kooyman GL, Maue R. Renal glomerular filtration rate and hepatic blood flow during voluntary diving in Weddell seals. Am J Physiol Regul Integr Comp Physiol 245: R743–R748, 1983 [DOI] [PubMed] [Google Scholar]
- 8. Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation 106: 1349–1354, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Drummond PC, Jones DR. The initiation and maintenance of bradycardia in a diving mammal, the muskrat, Ondatra zibethica. J Physiol 290: 253–271, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ. Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol Heart Circ Physiol 263: H798–H803, 1992 [DOI] [PubMed] [Google Scholar]
- 11. Edwards DG, Roy MS, Prasad RY. Wave reflection augments central systolic and pulse pressures during facial cooling. Am J Physiol Heart Circ Physiol 294: H2535–H2539, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Esposito C, Plati A, Mazzullo T, Fasoli G, De Mauri A, Grosjean F, Mangione F, Castoldi F, Serpieri N, Cornacchia F, Dal Canton A. Renal function and functional reserve in healthy elderly individuals. J Nephrol 20: 617–625, 2007 [PubMed] [Google Scholar]
- 13. Fagius J, Sundlof G. The diving response in man: effects on sympathetic activity in muscle and skin nerve fascicles. J Physiol 377: 429–443, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fehrman-Ekholm I, Skeppholm L. Renal function in the elderly (>70 years old) measured by means of iohexol clearance, serum creatinine, serum urea and estimated clearance. Scand J Urol Nephrol 38: 73–77, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Foster GE, Sheel AW. The human diving response, its function, and its control. Scand J Med Sci Sports 15: 3–12, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Gao Z, Wilson TE, Drew RC, Ettinger J, Monahan KD. Altered coronary vascular control during cold stress in healthy older adults. Am J Physiol Heart Circ Physiol 302: H312–H318, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glickman-Weiss EL, Hearon CM, Nelson AG, Robertson RJ. A thermal perception scale for use during resting exposure to cold air. Percept Mot Skills 79: 547–560, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Greaney JL, Ray CA, Prettyman AV, Edwards DG, Farquhar WB. Influence of increased plasma osmolality on sympathetic outflow during apnea. Am J Physiol Regul Integr Comp Physiol 299: R1091–R1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Havenith G, Heus R, Daanen HA. The hand in the cold, performance and risk. Arctic Med Res 54, Suppl 2: 37–47, 1995 [PubMed] [Google Scholar]
- 20. Havenith G, van de Linde EJ, Heus R. Pain, thermal sensation and cooling rates of hands while touching cold materials. Eur J Appl Physiol Occup Physiol 65: 43–51, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Heath ME, Downey JA. The cold face test (diving reflex) in clinical autonomic assessment: methodological considerations and repeatability of responses. Clin Sci (Lond) 78: 139–147, 1990 [DOI] [PubMed] [Google Scholar]
- 22. Heindl S, Struck J, Wellhoner P, Sayk F, Dodt C. Effect of facial cooling and cold air inhalation on sympathetic nerve activity in men. Respir Physiol Neurobiol 142: 69–80, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Hensel H, Boman KK. Afferent impulses in cutaneous sensory nerves in human subjects. J Neurophysiol 23: 564–578, 1960 [DOI] [PubMed] [Google Scholar]
- 24. Hering D, Mahfoud F, Walton AS, Krum H, Lambert GW, Lambert EA, Sobotka PA, Bohm M, Cremers B, Esler MD, Schlaich MP. Renal denervation in moderate to severe CKD. J Am Soc Nephrol 23: 1250–1257, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol 107: 1076–1082, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kakizawa Y, Seguchi T, Kodama K, Ogiwara T, Sasaki T, Goto T, Hongo K. Anatomical study of the trigeminal and facial cranial nerves with the aid of 3.0-tesla magnetic resonance imaging. J Neurosurg 108: 483–490, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Kario K, McEwen BS, Pickering TG. Disasters and the heart: a review of the effects of earthquake-induced stress on cardiovascular disease. Hypertens Res 26: 355–367, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Khurana R, Watabiki S, Garcia J, Nelson E. Cold face test in vagal dysfunction. Trans Am Neurol Assoc 102: 142–146, 1977 [PubMed] [Google Scholar]
- 29. Khurana RK. Cold face test: adrenergic phase. Clin Auton Res 17: 211–216, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Khurana RK, Watabiki S, Hebel JR, Toro R, Nelson E. Cold face test in the assessment of trigeminal-brainstem-vagal function in humans. Ann Neurol 7: 144–149, 1980 [DOI] [PubMed] [Google Scholar]
- 31. Khurana RK, Wu R. The cold face test: a non-baroreflex mediated test of cardiac vagal function. Clin Auton Res 16: 202–207, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Kregel KC, Seals DR, Callister R. Sympathetic nervous system activity during skin cooling in humans: relationship to stimulus intensity and pain sensation. J Physiol 454: 359–371, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kremkau FW. Diagnostic Ultrasound Principles and Instruments (5th ed.). Philadelphia: WB Saunders, 1998, chapt. 3 and 4 [Google Scholar]
- 34. Kuipers NT, Sauder CL, Kearney ML, Ray CA. Interactive effect of aging and local muscle heating on renal vasoconstriction during isometric handgrip. Am J Physiol Renal Physiol 297: F327–F332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. LaMotte RH, Thalhammer JG. Response properties of high-threshold cutaneous cold receptors in the primate. Brain Res 244: 279–287, 1982 [DOI] [PubMed] [Google Scholar]
- 36. Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol 79: 581–588, 1995 [DOI] [PubMed] [Google Scholar]
- 37. Leuenberger UA, Hardy JC, Herr MD, Gray KS, Sinoway LI. Hypoxia augments apnea-induced peripheral vasoconstriction in humans. J Appl Physiol 90: 1516–1522, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Lundin S, Thoren P. Renal function and sympathetic activity during mental stress in normotensive and spontaneously hypertensive rats. Acta Physiol Scand 115: 115–124, 1982 [DOI] [PubMed] [Google Scholar]
- 39. Mannino JA, Washburn RA. Environmental temperature and mortality from acute myocardial infarction. Int J Biometeorol 33: 32–35, 1989 [DOI] [PubMed] [Google Scholar]
- 40. Marraccini P, Fedele S, Marzilli M, Orsini E, Dukic G, Serasini L, L'Abbate A. Adenosine-induced renal vasoconstriction in man. Cardiovasc Res 32: 949–953, 1996 [PubMed] [Google Scholar]
- 41. Momen A, Bower D, Leuenberger UA, Boehmer J, Lerner S, Alfrey EJ, Handly B, Sinoway LI. Renal vascular response to static handgrip exercise: sympathetic vs. autoregulatory control. Am J Physiol Heart Circ Physiol 289: H1770–H1776, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Momen A, Leuenberger UA, Handly B, Sinoway LI. Effect of aging on renal blood flow velocity during static exercise. Am J Physiol Heart Circ Physiol 287: H735–H740, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Momen A, Leuenberger UA, Ray CA, Cha S, Handly B, Sinoway LI. Renal vascular responses to static handgrip: role of muscle mechanoreflex. Am J Physiol Heart Circ Physiol 285: H1247–H1253, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol 293: R3–R12, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Monahan KD, Feehan RP, Sinoway LI, Gao Z. Contribution of sympathetic activation to coronary vasodilatation during the cold pressor test in healthy men: effect of ageing. J Physiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morgan BJ, Denahan T, Ebert TJ. Neurocirculatory consequences of negative intrathoracic pressure vs. asphyxia during voluntary apnea. J Appl Physiol 74: 2969–2975, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Muller JE, Abela GS, Nesto RW, Tofler GH. Triggers, acute risk factors and vulnerable plaques: the lexicon of a new frontier. J Am Coll Cardiol 23: 809–813, 1994 [DOI] [PubMed] [Google Scholar]
- 48. Muller MD, Gao Z, Drew RC, Herr MD, Leuenberger UA, Sinoway LI. Effect of cold air inhalation and isometric exercise on coronary blood flow and myocardial function in humans. J Appl Physiol 111: 1694–1702, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Muller MD, Gao Z, Mast JL, Blaha CA, Drew RC, Leuenberger UA, Sinoway LI. Aging attenuates the coronary blood flow response to cold air breathing and isometric handgrip in healthy humans. Am J Physiol Heart Circ Physiol 302: H1737–H1746, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Muller MD, Mast JL, Cui J, Heffernan MJ, McQuillan PM, Sinoway LI. Tactile stimulation of the oropharynx elicits sympathoexcitation in conscious humans. J Appl Physiol; 10.1152/japplphysiol.00197.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Muller MD, Mast JL, Patel H, Sinoway LI. Cardiac mechanics are impaired during fatiguing exercise and cold pressor test in healthy older adults. J Appl Physiol 114: 186–194, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Muller MD, Muller SM, Ryan EJ, Bellar DM, Kim CH, Glickman EL. Pain and thermal sensation in the cold: the effect of interval versus continuous exercise. Eur J Appl Physiol 111: 979–987, 2011 [DOI] [PubMed] [Google Scholar]
- 53. Muller MD, Ryan EJ, Bellar DM, Kim CH, Blankfield RP, Muller SM, Glickman EL. The influence of interval versus continuous exercise on thermoregulation, torso hemodynamics, and finger dexterity in the cold. Eur J Appl Physiol 109: 857–867, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21: 498–503, 1993 [DOI] [PubMed] [Google Scholar]
- 55. Ng AV, Callister R, Johnson DG, Seals DR. Sympathetic neural reactivity to stress does not increase with age in healthy humans. Am J Physiol Heart Circ Physiol 267: H344–H353, 1994 [DOI] [PubMed] [Google Scholar]
- 56. Nordin M, Fagius J. Effect of noxious stimulation on sympathetic vasoconstrictor outflow to human muscles. J Physiol 489: 885–894, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. Am J Cardiol 99: 1629–1636, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Prasad GV, Nash MM, Zaltzman JS. Seasonal variation in outpatient blood pressure in stable renal transplant recipients. Transplantation 72: 1792–1794, 2001 [DOI] [PubMed] [Google Scholar]
- 59. Reyners AK, Tio RA, Vlutters FG, van der Woude GF, Reitsma WD, Smit AJ. Re-evaluation of the cold face test in humans. Eur J Appl Physiol 82: 487–492, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Rowell LB. (editor). Control of Individual Vascular Beds: Splanchnic and Renal Circulations. New York: Oxford, 1986, chapt. 4, p. 78–85 [Google Scholar]
- 61. Sauder CL, Conboy EE, Chin-Sang SA, Ray CA. Otolithic activation on visceral circulation in humans: effect of aging. Am J Physiol Renal Physiol 295: F1166–F1169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med 361: 932–934, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol 528: 407–417, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, Wilhelm JL. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med 335: 84–90, 1996 [DOI] [PubMed] [Google Scholar]
- 65. Stemper B, Hilz MJ, Rauhut U, Neundorfer B. Evaluation of cold face test bradycardia by means of spectral analysis. Clin Auton Res 12: 78–83, 2002 [DOI] [PubMed] [Google Scholar]
- 66. Stone HL, Gray K, Stabe R, Chandler JM., Jr Renal blood flow in a diving trained sea lion. Nature 242: 530–531, 1973 [DOI] [PubMed] [Google Scholar]
- 67. Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995 [DOI] [PubMed] [Google Scholar]
- 68. Tofler GH, Muller JE. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation 114: 1863–1872, 2006 [DOI] [PubMed] [Google Scholar]
- 69. van Ooijen AM, van Marken Lichtenbelt WD, van Steenhoven AA, Westerterp KR. Seasonal changes in metabolic and temperature responses to cold air in humans. Physiol Behav 82: 545–553, 2004 [DOI] [PubMed] [Google Scholar]
- 70. Victor RG, Leimbach WN, Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 9: 429–436, 1987 [DOI] [PubMed] [Google Scholar]
- 71. Wagner JA, Horvath SM. Cardiovascular reactions to cold exposures differ with age and gender. J Appl Physiol 58: 187–192, 1985 [DOI] [PubMed] [Google Scholar]
- 72. Widlansky ME, Vita JA, Keyes MJ, Larson MG, Hamburg NM, Levy D, Mitchell GF, Osypiuk EW, Vasan RS, Benjamin EJ. Relation of season and temperature to endothelium-dependent flow-mediated vasodilation in subjects without clinical evidence of cardiovascular disease (from the Framingham Heart Study). Am J Cardiol 100: 518–523, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wilson TE, Gao Z, Hess KL, Monahan KD. Effect of aging on cardiac function during cold stress in humans. Am J Physiol Regul Integr Comp Physiol 298: R1627–R1633, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wilson TE, Sauder CL, Kearney ML, Kuipers NT, Leuenberger UA, Monahan KD, Ray CA. Skin-surface cooling elicits peripheral and visceral vasoconstriction in humans. J Appl Physiol 103: 1257–1262, 2007 [DOI] [PubMed] [Google Scholar]
- 75. Woodhouse PR, Khaw KT, Plummer M. Seasonal variation of blood pressure and its relationship to ambient temperature in an elderly population. J Hypertens 11: 1267–1274, 1993 [PubMed] [Google Scholar]
- 76. Xiao RP, Lakatta EG. Deterioration of beta-adrenergic modulation of cardiovascular function with aging. Ann N Y Acad Sci 673: 293–310, 1992 [DOI] [PubMed] [Google Scholar]
- 77. Zapol WM, Liggins GC, Schneider RC, Qvist J, Snider MT, Creasy RK, Hochachka PW. Regional blood flow during simulated diving in the conscious Weddell seal. J Appl Physiol 47: 968–973, 1979 [DOI] [PubMed] [Google Scholar]