Abstract
Impaired glucose tolerance (IGT) is characterized by decreased oxidative capacity and reduced carbohydrate utilization during exercise. However, it is unclear if the presence of impaired fasting glucose (IFG) affects fuel utilization during exercise in adults with IGT. We tested the hypothesis that the presence of IFG in adults with IGT decreases reliance on carbohydrate during exercise. Middle-aged, obese, sedentary individuals (n = 6, IGT and n = 6, IFG+IGT) were compared during exercise at 60% peak O2 consumption for 45 min on a cycle ergometer. Glucose rates of appearance and disposal and muscle glycogen were assessed by stable isotope dilution methods, and fat utilization was estimated via indirect calorimetry. A 75-g oral glucose tolerance test was used to determine fasting and 2-h glucose concentrations. A glucose intolerance severity z-score was calculated from the oral glucose tolerance test. Glucose flux (i.e., rates of appearance and disposal) was not different between groups. However, individuals with IFG+IGT had lower muscle glycogen use (P < 0.05) and elevated fat oxidation (P < 0.01) during exercise compared with those with isolated IGT. Plasma nonesterified fatty acids and glucose were significantly higher during exercise in subjects with IFG+IGT vs. IGT alone (P < 0.05). Fat utilization during exercise correlated with fasting glucose (r = 0.57, P = 0.05), glucose intolerance severity z-score (r = 0.66, P = 0.01), and nonesterified fatty acids (trend; r = 0.55, P = 0.08). The presence of IFG shifts fuel selection toward increased fat oxidation and decreased muscle glycogen utilization during exercise in adults with IGT. Whether these differences in substrate use contribute to, or are the result of, movement along the continuum from prediabetes to type 2 diabetes awaits further work.
Keywords: prediabetes, diabetes, metabolic flexibility, hyperglycemia
type 2 diabetes risk is higher in adults with impaired glucose tolerance [IGT; oral glucose tolerance test (OGTT) 2-h glucose value 7.8–11.1 mM] compared with people with impaired fasting glucose (IFG; 5.6–6.9 mM) (2, 10, 15). This increased propensity to develop diabetes in adults with IGT appears to be even greater when combined with IFG (2, 39). Insulin resistance is an important etiological factor in the progression of glucose intolerance to type 2 diabetes (3, 34). However, the inability to switch between lipid and carbohydrate fuel sources during insulin stimulation may precede insulin resistance, as demonstrated in adults with isolated IFG who were insulin sensitive relative to age and body weight-matched IGT counterparts (9). This observation suggests that the presence of IFG may independently affect fuel selection in adults with IGT. It remains unknown, however, if the combination of IFG+IGT represents a distinct phenotype with altered glucose and lipid metabolism compared with age- and weight-matched adults with isolated IGT.
The primary energy source during moderate- to high-intensity exercise is carbohydrate from the breakdown of muscle glycogen. However, adults with insulin resistance, IGT, and type 2 diabetes have reduced muscle glycogen utilization and, therefore, rely more on fat oxidation during exercise (4–6, 21). The exact cause of altered glycogen utilization in adults with IGT is unclear, but most studies reporting reduced carbohydrate oxidation between groups are confounded by differences in mild to severe fasting hyperglycemia (5, 13, 31). When IFG is combined with IGT, chronically elevated glucose concentrations may influence the capacity of skeletal muscle to utilize carbohydrate, which, in turn, can influence glucose homeostasis (1, 8, 16, 24, 33). Recently, Malin and Kirwan showed that the presence of IFG attenuated the normalization of postprandial glucose tolerance in adults with IGT after 12 wk of exercise training compared with age- and weight-matched subjects with IGT only (26). It remains unknown, however, if carbohydrate or muscle glycogen utilization differs during exercise in adults with IFG+IGT vs. IGT. Therefore, we tested the hypothesis that fasting hyperglycemia is an independent predictor of less glycogen and more fat utilization during exercise in adults with IFG+IGT matched on age, sex, body fat, aerobic fitness, and insulin resistance compared with individuals with isolated IGT.
METHODS
Subjects.
These men and women were a part of a larger study designed to assess the impact of exercise with or without metformin on insulin sensitivity (27). A subset of these adults with IGT at baseline underwent an acute exercise bout to characterize substrate metabolism. Sixteen subjects were ranked according to fasting glucose concentrations, and only the upper and lower tertiles (n = 6 in each group) were included to test if the presence of IFG alters fuel selection during exercise in adults with IGT. All subjects in the upper tertile met criteria for IFG (>5.6 mM or 100 mg/dl). Subjects were excluded if they smoked, had metabolic disease (e.g., cardiovascular disease or type 2 diabetes), and took dietary supplements/medications known to affect substrate metabolism or exercise capacity (e.g., chromium, niacin, ephedrine, oral contraceptives). Before testing, all subjects were verbally briefed and signed informed consent documents that were approved by the University of Massachusetts Amherst Institutional Review Board.
Preliminary testing.
A standard 75-g OGTT was conducted after an overnight fast. Blood glucose concentrations were collected to determine individuals with IGT (2-h glucose concentrations: 7.8–11.1 mM or 140–199 mg/dl) and the absence or presence of IFG (5.6–6.9 mM or 100–126 mg/dl). Glucose intolerance severity was expressed using z-score calculations and defined by the American Diabetes Association criteria for impaired fasting and 2-h glucose levels. The standard deviation of our study cohort was used as the denominator for each outcome, and the equation was as follows: [fasting glucose: 100(mg/dl)/11.14] + [2-h glucose: 140(mg/dl)/25.45]. Peak oxygen consumption (V̇o2 peak) on a cycle ergometer (SensorMedics 800, Yorba Linda, CA) and body composition (via DEXA; Lunar Prodigy, Madison, WI) were measured as previously described (27). Insulin sensitivity was measured after an overnight fast during a 2-h euglycemic-hyperinsulinemic clamp (insulin infusion 80 mU·m−2·min−1) enriched with glucose isotope ([6,6-2H]glucose). This insulin infusion was selected to determine peripheral insulin sensitivity, as endogenous glucose production is almost completely suppressed (27). Pedometers (Omron HJ112, Lake Forest, IL) were provided to all subjects and worn around the waistband for 7 days. These data were averaged to characterize habitual ambulation.
Submaximal exercise protocol.
Subjects were instructed to avoid strenuous activity 24–36 h before testing. After a 10- to 12-h overnight fast, indwelling catheters were placed in a superficial vein of each forearm for continuous infusion of glucose stable isotope solution ([6,6-2H]glucose) and venous blood sampling. Baseline blood samples were collected, and a priming bolus of 200-mg [6,6-2H]glucose was given, followed by a 90 min infusion of [6,6-2H]glucose at a rate of 3.0 mg/min, delivered by peristaltic infusion pump (Harvard Apparatus Pump 22, Holliston, MA). Resting blood samples were collected at baseline and 75 and 90 min. Subjects were then moved to the cycle ergometer for collection of baseline respiratory gases via indirect calorimetry (ParvoMedics Truemax 2400, Consentius Technologies, Sandy, UT). Isotopic infusion rate was doubled to 6.0 mg/min, and subjects warmed up for 5 min on the cycle ergometer at 25 W. Cycle ergometer testing was performed at 60% V̇o2 peak for 45 min, and substrate oxidation was determined by indirect calorimetry. Breath and blood samples were collected at 15, 25, 35, and 45 min. Breath samples were collected for 7 min before each time point. The last 2 min of each sampling period were used to calculate the respiratory exchange ratio (RER), rate of carbohydrate and fat oxidation, and percentage of energy derived from carbohydrate or fat, as described previously (25). Blood concentrations of glucose, lactate, nonesterified fatty acids (NEFA), and insulin, as well as the rating of perceived exertion and heart rate were also collected during this time. Subjects recorded dietary intake 4 days before exercise testing and were instructed to consume at least 200 g of carbohydrate.
Blood analysis.
Plasma glucose and lactate were determined enzymatically (GL5 Analyzer, Analox Instruments, Lunenberg, MA). Plasma insulin was measured by radioimmunoassay (Millipore, St. Charles, MO). NEFA were measured by enzymatic colorimetry (Wako Chemicals, Richmond, VA).
Calculations.
Standard equations were used to assess rates of glucose appearance (Ra) and disposal (Rd) (40).
where F is the isotope infusion rate, IE1 and IE2 are enrichments of plasma glucose with isotope label at time t1 and t2, respectively, C1 and C2 are plasma glucose concentrations, and V is the estimated volume of distribution for glucose (180 ml/kg). Substrate oxidation rates were derived from standard equations (30). Estimated muscle glycogen utilization was calculated as follows: rate of total carbohydrate oxidation − blood glucose Rd. This calculation is based on the assumption that all blood glucose cleared from the blood is oxidized, which is likely untrue (i.e., %glucose Rd oxidized is ∼70–90%). Thus our calculation of estimated muscle glycogen utilization underestimates glycogen use (5). Glucose isotopic enrichment was measured using a Shodex Asahipak NH2P-50 4E Analytical column, (4.6 × 250 mm, Thompson Instrument) installed on a Agilent 1100 series high-performance liquid chromatography equipped with a mass spectrometer detector, as discussed before (35).
Statistical analysis.
Data were analyzed using the R statistical package (version 2.4.0, The R foundation, Vienna, Austria, 2006). Unpaired t-tests were used to determine statistical differences between subject characteristics, exercise heart rate, oxygen consumption, expressed relative to body weight, and percent peak. Ra and Rd as well as the relative percentage of energy from blood glucose, muscle glycogen, and fat were averaged across minutes 25, 35, and 45 and compared by unpaired t-tests. A two-factor (group by time) repeated-measures analysis of variance was used to determine group mean differences for all other outcomes. Pairwise comparisons were conducted in the event of a significant interaction. Spearman's rank correlation was used to assess relationships between glucose intolerance severity, fat use, and glycogen use during exercise. Significant differences were accepted as P ≤ 0.05.
RESULTS
Subject characteristics.
Groups were of similar age, sex, body fat, habitual ambulation, and aerobic fitness (Table 1). By study design, IFG+IGT had significantly higher fasting plasma glucose concentrations compared with IGT (P < 0.01), but 2-h glucose concentrations were not different. Glucose intolerance severity was higher in IFG+IGT than IGT alone (2.4 ± 0.4 vs. 1.0 ± 0.4; P < 0.05). Whole body insulin sensitivity was not different between IFG+IGT and IGT groups (6.72 ± 1.72 vs. 7.15 ± 1.63 mg·kg FFM−1·min−1·pmol−1·min−1; P = 0.86). There was no difference between IFG+IGT and IGT in total energy intake (2,307.6 ± 144.2 vs. 2,315.2 ± 204.6 kcal; P = 0.98) or carbohydrate consumption (247.9 ± 24.6 vs. 232.9 ± 24.3 g; P = 0.67) before testing.
Table 1.
Subject characteristics
| IFG+IGT | IGT | |
|---|---|---|
| Sex (F/M) | 3/3 | 3/3 |
| Age, yr | 47.5 ± 7.7 | 48.0 ± 2.6 |
| Body weight, kg | 92.5 ± 12.9 | 92.6 ± 21.2 |
| Body mass index, kg/m2 | 33.6 ± 4.8 | 33.9 ± 5.9 |
| Total body fat, % | 43.2 ± 6.3 | 39.9 ± 7.4 |
| Central fat, % | 45.8 ± 6.2 | 43.6 ± 6.4 |
| Waist circumference, cm | 106.4 ± 12.6 | 109.2 ± 17.3 |
| Fat-free mass, kg | 50.8 ± 9.8 | 55.9 ± 13.0 |
| Fasting glucose, mM | 5.9 ± 0.5* | 4.9 ± 0.2 |
| 2-h Glucose, mM | 9.4 ± 1.0 | 10.0 ± 1.6 |
| Glucose intolerance severity (z-score) | 2.4 ± 0.4* | 1.0 ± 0.4 |
| V̇o2peak, l/min | 2.4 ± 0.9 | 2.6 ± 0.5 |
| V̇o2peak, ml•kg−1•min−1 | 26.1 ± 8.4 | 27.1 ± 5.9 |
| Heart rate peak, beats/min | 175.5 ± 21.7 | 162.7 ± 9.9 |
| Steps per day | 5,916.7 ± 522.4 | 5,622.6 ± 871.8 |
Values are means ± SD; n = 6 subjects for both groups.
IFG+IGT, impaired fasting glucose + impaired glucose tolerant; IGT, impaired glucose tolerant only; F, female; M, male; V̇o2peak, peak O2 consumption. Glucose intolerance severity (z-score) was calculated from fasting and 2-h blood glucose concentrations during a 75-g oral glucose tolerance test (see methods).
Significant compared with IGT, P < 0.05.
Exercise characteristics.
Oxygen consumption, relative to body weight (16.3 ± 1.0 vs. 16.3 ± 1.8 mg·kg−1·min−1; P = 0.9) or percentage of V̇o2 peak (61.4 ± 0.4 vs. 61.4 ± 0.4%; P = 0.8), was similar between IFG+IGT and IGT, respectively. Heart rate during exercise was not statistically different between IFG+IGT and IGT (138.4 ± 8.6 vs. 128.6 ± 4.2 beats/min; group effect; P = 0.33). However, IFG+IGT perceived the exercise to be more difficult (via RPE) than IGT across the duration of exercise (IFG+IGT = 12.2 ± 0.9, 12.5 ± 0.5, 14.0 ± 0.4, and 14.7 ± 0.6 vs. IGT = 14.3 ± 0.5, 14.2 ± 0.6, 14.4 ± 0.6, 14.8 ± 0.7 arbitrary units at minutes 15, 25, 35, and 45, respectively; group × test interaction; P < 0.05).
Substrate utilization.
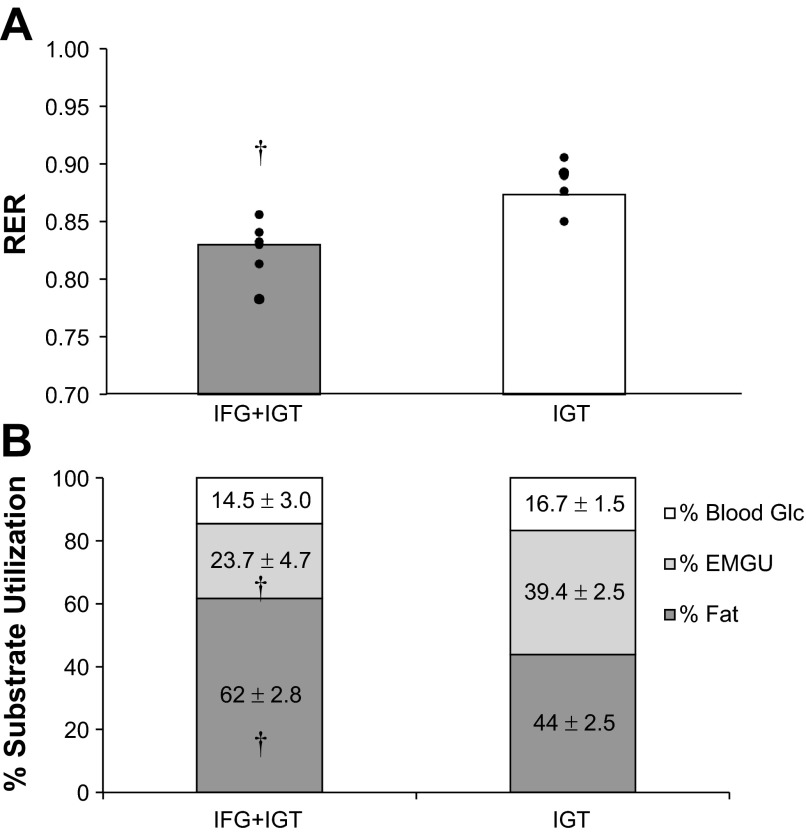
Fasting RER was not different between groups (IFG+IGT = 0.76 ± 0.03 vs. IGT = 0.74 ± 0.04; P = 0.76). However, individuals with IFG+IGT had lower RER values during exercise compared with IGT (group effect: P < 0.05; Fig. 1A). Thus fat was the primary fuel source in adults with IFG+IGT (∼62%) compared with isolated IGT (∼44%) during exercise (P < 0.01; Fig. 1B). Blood glucose utilization was similar between IFG+IGT (∼15%) and IGT (∼17%), but the contribution of estimated skeletal muscle glycogen use was less in the IFG+IGT (∼24%) than IGT (∼39%) group (P < 0.05; Fig. 1B).
Fig. 1.
Respiratory exchange ratio (RER; A) and the relative percentage of energy expended (B) during submaximal exercise. Values are means with individual data shown. IGT, impaired glucose tolerant only; IFG+IGT, impaired fasting glucose + impaired glucose tolerant; Glc, glucose; EMGU, estimated muscle glycogen utilization. †Significant compared with IGT, P < 0.05.
Exercise glucose flux.
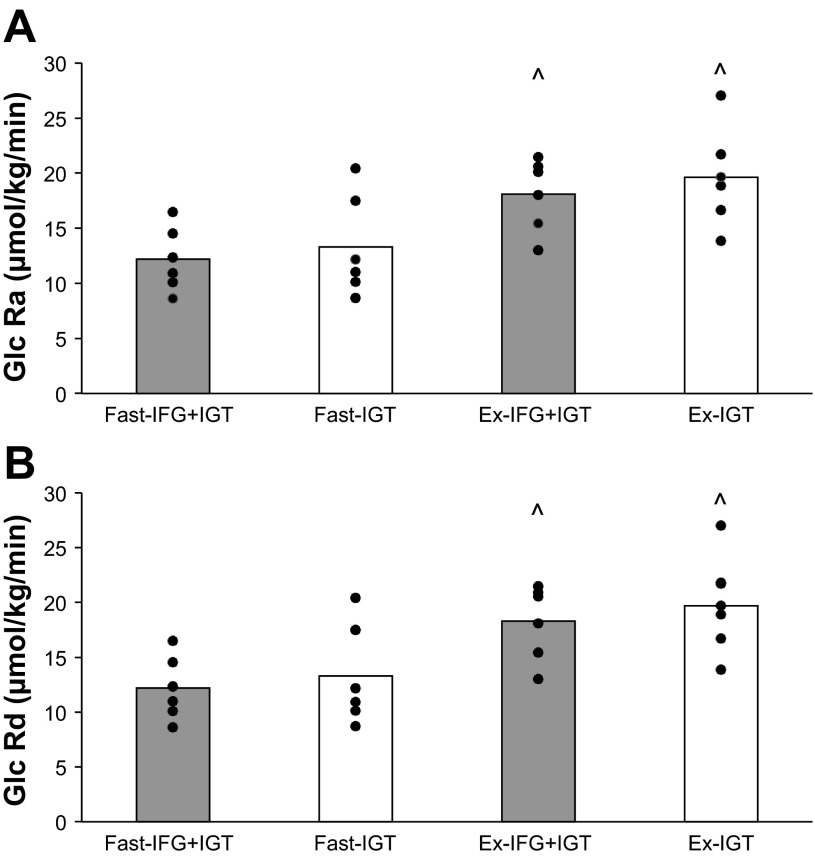
Plasma isotopic enrichment (IFG+IGT = 0.019 ± 0.002 vs. IGT = 0.020 ± 0.002; P = 0.35) was not different between groups. Glucose Ra and Rd increased during exercise in each group, compared with rest (P < 0.01); however, glucose Ra (group effect: P < 0.52) and Rd (group effect: P < 0.55) were similar between IFG+IGT and IGT during rest and exercise, respectively (Fig. 2, A and B, respectively).
Fig. 2.
Glucose rates of appearance (Ra; A) and disposal (Rd; B) during submaximal exercise. Ex, exercise; Fast, fasting state. Values are means with individual data shown. ^ Significant compared with Fast, P < 0.05.
Substrates and hormone during exercise.
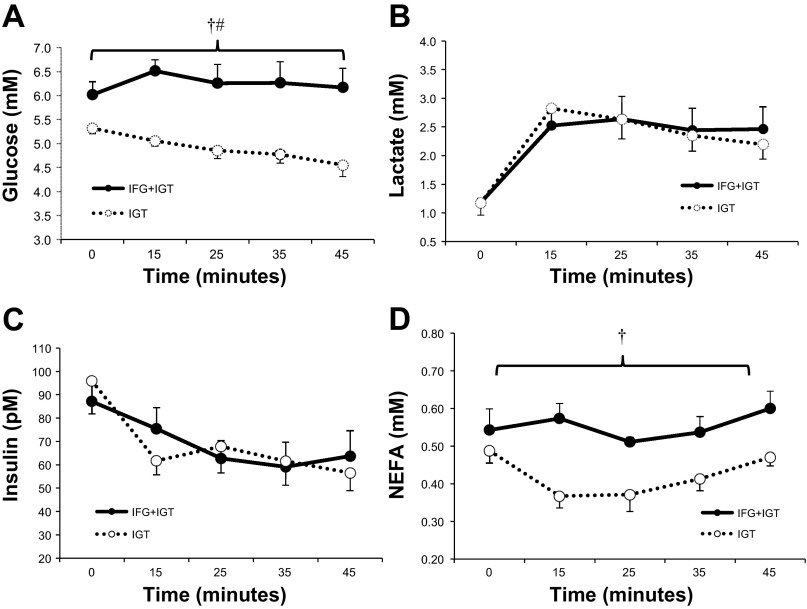
Individuals with IFG+IGT had higher plasma glucose and NEFA concentrations during exercise compared with IGT (group effect; P < 0.05; Fig. 3). Plasma lactate and insulin were not statistically different between groups during exercise.
Fig. 3.
Metabolites and hormones during submaximal exercise. A: glucose; B: lactate; C: insulin; D: nonesterified fatty acid (NEFA). Values are mean ± SE. †Significant compared with IGT, P < 0.05. #Time effect, P < 0.05.
Correlational analysis.
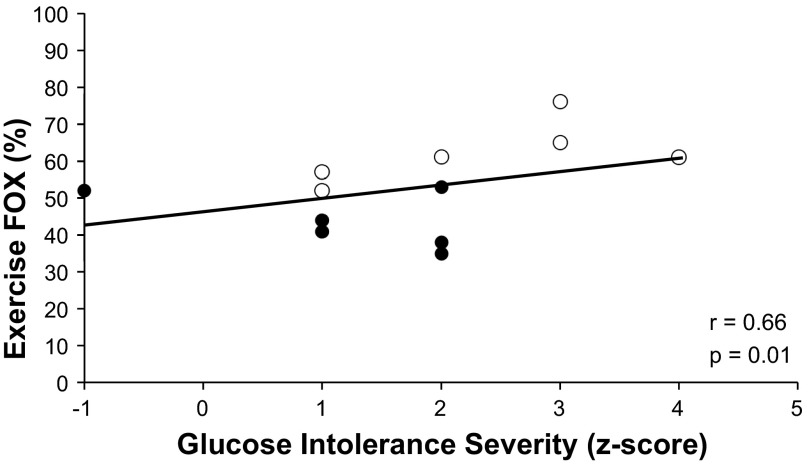
Fasting hyperglycemia correlated with elevated fat use during exercise (r = 0.57, P = 0.05) and exercise NEFA concentrations (r = 0.80, P = 0.004). Increased fat utilization during exercise correlated with reduced glycogen reliance (r = −0.83, P = 0.001), exercise NEFAs (trend: r = 0.55, P = 0.08), and increased glucose intolerance severity (r = 0.66, P = 0.01; Fig. 4).
Fig. 4.
Glucose intolerance severity correlates with fat oxidation (FOX) during exercise. ○, IFG+IGT; ●, IGT. Glucose intolerance severity (z-score) was calculated from fasting and 2-h blood glucose concentrations during a 75-g oral glucose tolerance test.
DISCUSSION
The major finding in this study is that individuals with IFG+IGT use less glycogen and more fat during exercise than adults with IGT (Fig. 1, A and B). Although our work may at first seem paradoxical since lipid oxidation is often reported to decrease as individuals progress toward type 2 diabetes [see reviews (17, 19)], our findings are consistent with several well-controlled studies reporting that fat utilization is similar (6, 20, 21) or elevated during exercise in adults at risk for diabetes compared with healthier counterparts (13, 18, 37). Insulin resistance is considered an important underlying factor contributing to the inability to switch from predominantly fat use in the fasted state to mainly carbohydrate oxidation during insulin stimulation (i.e., metabolic inflexibility) (11, 23). Previously, our group demonstrated that insulin-resistant women matched for body fat, aerobic fitness, and habitual physical activity had impaired metabolic flexibility (i.e., increased reliance on lipids) during low- to moderate-intensity exercise compared with insulin-sensitive women (5). In the present study, however, adults with IFG+IGT and isolated IGT had similar peripheral insulin resistance as measured by the euglycemic clamp with glucose kinetics (Table 1). Our findings are novel since groups were also matched on age, sex, body fat, habitual ambulation, and aerobic fitness, thereby allowing us to test the independent effects of glucose intolerance severity on fuel selection during exercise in adults at high risk of developing type 2 diabetes. Thus our findings expand on previous work (5) and indicate that factors other than peripheral insulin resistance explain the differences in fuel selection during exercise in adults with IFG+IGT vs. IGT.
The mechanism responsible for reduced carbohydrate utilization in individuals with IFG+IGT, compared with adults with IGT, is unclear, but it may be related to the interaction of glucose intolerance severity and impaired skeletal muscle oxidative capacity (1, 8, 16, 24, 33). In vitro work by Aas et al. (1) suggests that chronic hyperglycemia reduces metabolic flexibility in human myotubes by reducing mitochondrial electron transport system (i.e., complex I) activity. In accordance with these latter findings, in vivo reductions in fasting glucose levels were significantly correlated with increased mitochondrial respiration in type 2 diabetic adults treated with insulin, suggesting that lower glucose intolerance severity is linked to improved mitochondrial oxidative capacity (16, 33). It is not possible to know if altered fuel utilization causes insulin resistance or vice a versa from our data, but, given that hyperglycemia impairs metabolic flexibility (1, 16, 33), it seems reasonable that glucose intolerance would contribute to differences in fuel utilization. In the present study, we did not observe a direct correlation between fasting glucose concentrations or glucose intolerance severity and estimated muscle glycogen use. Therefore, based on the association between fat utilization and glucose intolerance severity (Fig. 4), our findings suggest that adults with IFG+IGT may have lipid-specific effects on cellular regulators of carbohydrate oxidation (7, 29). Lipid oversupply to skeletal muscle has been reported to increase incomplete fat oxidation and promote impaired glucose oxidation (29). This is supported by work of Constantin-Teodosiu et al. (7), whereby high fat feeding elevated NEFA levels and decreased the activation of pyruvate dehydrogenase, which, in turn, downregulated carbohydrate oxidation. Since elevated plasma NEFA concentrations have been linked with mitochondrial dysfunction and metabolic inflexibility (22, 38), and we observed elevated NEFAs during exercise, our findings highlight the need for future work to use lipid tracers and examine skeletal muscle biopsies in adults with IFG+IGT to elucidate mechanisms regulating substrate metabolism compared with individuals with IGT.
We recognize that the elevated fat utilization observed in individuals with IFG+IGT may not be a direct result of elevated fat oxidation, but rather a compensatory rise due to decreased carbohydrate oxidation. There were no statistical differences in glucose flux during exercise between groups, suggesting that the mild hyperglycemia seen during exercise in adults with IFG+IGT did not promote mass action effects that stimulated glucose uptake. Subsequently, blood glucose utilization was not a limiting factor for fuel utilization (6), and these data indicate that the reduced reliance on carbohydrate (i.e., lower RER, Fig. 1, A and B) in adults with IFG+IGT was the result of decreased estimated muscle glycogen utilization. Muscle glycogen use during exercise is related to resting glycogen concentrations (36). If our participants with IFG+IGT began exercise with lower glycogen concentrations, a decrease in glycogen use would be expected. Muscle biopsies would be needed to rule out the role of muscle glycogen content on carbohydrate oxidation during exercise, but both groups in this study consumed ∼230 g of carbohydrate on the days before testing. Thus differences in initial skeletal muscle glycogen content at the onset of exercise are unlikely to explain the decreased carbohydrate utilization seen between IFG+IGT and IGT groups in this study.
It is worth noting that we did not detect differences in fasting RER. Our finding is similar to previous work reporting that differences in fuel utilization only manifest under insulin-stimulated conditions in adults with isolated IFG or IGT vs. normal glucose tolerance (9). We recognize that inclusion of IFG-only subjects in the present study would aid in the understanding of fuel selection differences across the prediabetes phenotypes, although IFG individuals tend to have high peripheral insulin sensitivity compared with IGT individuals, and this would have confounded the results herein (9, 32). The implication of elevated fat oxidation during exercise in adults with IFG+IGT on postexercise insulin-stimulated glucose uptake is also unknown. We cannot distinguish between the lipid sources (i.e., NEFA and/or intramyocellular lipid content) that contributed to the elevated whole body fat oxidation seen in individuals with IFG+IGT in this study (12, 28, 31), but we speculate that exercise increases the use of these lipids in adults with IFG+IGT to “rescue” the cell from lipid abnormalities related to insulin resistance (14). Whether exercise training improves peripheral insulin sensitivity in adults with IFG+IGT to the same degree as adults with IGT, and this change in peripheral insulin sensitivity is related to changes in substrate utilization, remains to be seen.
In conclusion, the presence of IFG shifts energy metabolism away from carbohydrate and toward fat utilization during exercise in adults with IGT and occurs independent of insulin resistance, aerobic fitness, and body fat. Given that the presence of IFG attenuates the decline in postprandial glucose levels in adults with IGT (26), our data suggest that further work is required to determine the role of fuel utilization on insulin-stimulated glucose uptake across the prediabetes phenotypes after exercise. Whether these differences in substrate use contribute to, or are the result of, movement along the continuum from prediabetes to overt type 2 diabetes awaits further work in this area. Taken together, our findings suggest that, although IFG+IGT and IGT alone are often considered together under the umbrella of “prediabetes,” the presence of IFG is a potentially confounding variable, and it would be appropriate to treat individuals with IFG+IGT vs. IGT-only as distinct subtypes of prediabetes.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 5 R56 DK-081038 (B. Braun) and the American College of Sports Medicine Student Foundation Grant (S. K. Malin).
DISCLOSURES
B. Braun receives consultant fees from Pfizer, Inc.
AUTHOR CONTRIBUTIONS
Author contributions: S.K.M. and B.B. conception and design of research; S.K.M., R.V., C.O., and B.B. performed experiments; S.K.M., R.V., C.O., and B.B. analyzed data; S.K.M., R.V., C.O., and B.B. interpreted results of experiments; S.K.M. prepared figures; S.K.M. drafted manuscript; S.K.M., R.V., C.O., and B.B. edited and revised manuscript; S.K.M., R.V., C.O., and B.B. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Kirsten Granados, Robert Gerber, and Joy Nightingale for technical assistance. We thank Stuart Chipkin for overseeing medical supervision, and Sung-Eun Choi for diet analysis. We also extend our appreciation to the dedicated participants for time and effort.
REFERENCES
- 1. Aas V, Hessvik N, Wettergreen M, Hvammen A, Hallen S, Thoresen GH, Rustan A. Chronic hyperglycemia reduces substrate oxidation and impairs metabolic switching of human myotubes. Biochim Biophys Acta 1812: 94–105, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Blake DR, Meigs JB, Muller DC, Najjar SS, Andres R, Nathan DM. Impaired glucose tolerance, but not impaired fasting glucose, is associated with increased levels of coronary heart disease risk factors: results from the Baltimore Longitudinal Study on Aging. Diabetes 53: 2095–2100, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Rizza R. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 55: 3536–3549, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Borghouts LB, Wagenmakers AJ, Goyens PL, Keizer HA. Substrate utilization in non-obese Type II diabetic patients at rest and during exercise. Clin Sci (Lond) 103: 559–569, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Braun B, Sharoff CG, Chipkin SR, Beaudoin F. Effects of insulin resistance on substrate utilization during exercise in overweight women. J Appl Physiol 97: 991–997, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Colberg SR, Hagberg JM, McCole SD, Zmuda JM, Thompson PD, Kelley DE. Utilization of glycogen but not plasma glucose is reduced in individuals with NIDDM during mild-intensity exercise. J Appl Physiol 81: 2027–2033, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Constantin-Teodosiu D, Constantin D, Stephens F, Laithwaite D, Greenhaff PL. The role of FOXO and PPAR transcription factors in diet-mediated inhibition of PDC activation and carbohydrate oxidation during exercise in humans and the role of pharmacological activation of PDC in overriding these changes. Diabetes 61: 1017–1024, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cusi KJ, Pratipanawatr T, Koval J, Printz R, Ardehali H, Granner DK, Defronzo RA, Mandarino LJ. Exercise increases hexokinase II mRNA, but not activity in obesity and type 2 diabetes. Metabolism 50: 602–606, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Faerch K, Vaag A. Metabolic inflexibility is a common feature of impaired fasting glycaemia and impaired glucose tolerance. Acta Diabetol 48: 349–353, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Festa A, D'Agostino R, Howard G, Mykknen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 102: 42–47, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Galgani J, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab 295: E1009–E1017, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86: 5755–5761, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Goodpaster BH, Wolfe RR, Kelley DE. Effects of obesity on substrate utilization during exercise. Obes Res 10: 575–584, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Goodpaster B, Coen P. Do acute exercise and diet reveal the molecular basis for metabolic flexibility in skeletal muscle? Diabetes 61: 983–983, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haffner SM. Insulin resistance, inflammation, and the prediabetic state. Am J Cardiol 92: 18J–26J, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hernandez-Alvarez MI, Thabit H, Burns N, Shah S, Brema I, Hatunic M, Finucane F, Liesa M, Chiellini C, Naon D, Zorzano A, Nolan JJ. Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1α/mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care 33: 645–651, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holloway GP, Bonen A, Spriet LL. Regulation of skeletal muscle mitochondrial fatty acid metabolism in lean and obese individuals. Am J Clin Nutr 89: 455S–462S, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Horowitz JF, Klein S. Oxidation of nonplasma fatty acids during exercise is increased in women with abdominal obesity. J Appl Physiol 89: 2276–2282, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Houmard JA. Intramuscular lipid oxidation and obesity. Am J Physiol Regul Integr Comp Physiol 294: R1111–R1116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanaley JA, Cryer PE, Jensen MD. Fatty acid kinetic responses to exercise. Effects of obesity, body fat distribution, and energy-restricted diet. J Clin Invest 92: 255–261, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang J, Kelley DE, Robertson RJ, Goss FL, Suminski RR, Utter AC, Dasilva SG. Substrate utilization and glucose turnover during exercise of varying intensities in individuals with NIDDM. Med Sci Sports Exerc 31: 82–89, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest 92: 91–98, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49: 677–683, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Kulkarni SS, Salehzadeh F, Fritz T, Zierath JR, Krook A, Osler ME. Mitochondrial regulators of fatty acid metabolism reflect metabolic dysfunction in type 2 diabetes mellitus. Metabolism 61: 175–185, 2012 [DOI] [PubMed] [Google Scholar]
- 25. Malin SK, Stephens BR, Sharoff CG, Hagobian TA, Chipkin SR, Braun B. Metformin's effect on exercise and postexercise substrate oxidation. Int J Sport Nutr Exerc Metab 20: 63–71, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Malin SK, Kirwan JP. Fasting hyperglycaemia blunts the reversal of impaired glucose tolerance after exercise training in obese older adults. Diabetes Obes Metab 14: 835–841, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malin SK, Gerber R, Chipkin SR, Braun B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care 35: 131–136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mensink M, Blaak EE, van Baak MA, Wagenmakers AJ, Saris WH. Plasma free fatty acid uptake and oxidation are already diminished in subjects at high risk for developing type 2 diabetes. Diabetes 50: 2548–2554, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 15: 595–605, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci 16: 23–29, 1991 [PubMed] [Google Scholar]
- 31. Perreault L, Bergman BC, Hunerdosse DM, Playdon MC, Eckel RH. Inflexibility in intramuscular triglyceride fractional synthesis distinguishes prediabetes from obesity in humans. Obesity (Silver Spring) 18: 1524–1531, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perreault L, Bergman BC, Playdon MM, Dalla Man C, Cobelli C, Eckel RH. Impaired fasting glucose with or without impaired glucose tolerance: progressive or parallel states of prediabetes? Am J Physiol Endocrinol Metab 295: E428–E435, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raboll R, Hojberg PM, Almdal T, Boushel R, Haugaard SB, Madsbad S, Dela F. Effect of hyperglycemia on mitochondrial respiration in type 2 diabetes. J Clin Endocrinol Metab 94: 1372–1378, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Rizza R. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes 59: 2697–2707, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharoff CG, Hagobian TA, Malin SK, Chipkin SR, Yu H, Hirshman MF, Goodyear LJ, Braun B. Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin resistant individuals. Am J Physiol Endocrinol Metab 298: E815–E823, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sherman WM, Costill DL, Fink WJ, Miller JM. Effect of exercise-diet manipulation on muscle glycogen and its subsequent utilization during performance. Int J Sports Med 2: 114–118, 1981 [DOI] [PubMed] [Google Scholar]
- 37. Thyfault JP, Kraus RM, Hickner RC, Howell AW, Wolfe RR, Dohm GL. Impaired plasma fatty acid oxidation in extremely obese women. Am J Physiol Endocrinol Metab 287: E1076–E1081, 2004 [DOI] [PubMed] [Google Scholar]
- 38. van de Weijer T, Sparks L, Phielix E, Meex R, van Herpen N, Hesselink MKC, Schrauwen P, Schrauwen Hinderling V. Relationships between mitochondrial function and metabolic flexibility in type 2 diabetes mellitus. PLos One 8: e51648–e51648, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weyer C, Bogardus C, Pratley RE. Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 48: 2197–2203, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine. New York: Wiley-Liss, 1992, p. 119–144 [Google Scholar]