Abstract
To determine how the obstructive sleep apnea (OSA) patient's pathophysiological traits predict the success of the treatment aimed at stabilization or increase in respiratory motor outputs, we studied 26 newly diagnosed OSA patients [apnea-hypopnea index (AHI) 42 ± 5 events/h with 92% of apneas obstructive] who were treated with O2 supplementation, an isocapnic rebreathing system in which CO2 was added only during hyperpnea to prevent transient hypocapnia, and a continuous rebreathing system. We also measured each patient's controller gain below eupnea [change in minute volume/change in end-tidal Pco2 (ΔV̇e/ΔPetCO2)], CO2 reserve (eupnea-apnea threshold PetCO2), and plant gain (ΔPetCO2/ΔV̇e), as well as passive upper airway closing pressure (Pcrit). With isocapnic rebreathing, 14/26 reduced their AHI to 31 ± 6% of control (P < 0.01) (responder); 12/26 did not show significant change (nonresponder). The responders vs. nonresponders had a greater controller gain (6.5 ± 1.7 vs. 2.1 ± 0.2 l·min−1·mmHg−1, P < 0.01) and a smaller CO2 reserve (1.9 ± 0.3 vs. 4.3 ± 0.4 mmHg, P < 0.01) with no differences in Pcrit (−0.1 ± 1.2 vs. 0.2 ± 0.9 cmH2O, P > 0.05). Hypercapnic rebreathing (+4.2 ± 1 mmHg PetCO2) reduced AHI to 15 ± 4% of control (P < 0.001) in 17/21 subjects with a wide range of CO2 reserve. Hyperoxia (SaO2 ∼95–98%) reduced AHI to 36 ± 11% of control in 7/19 OSA patients tested. We concluded that stabilizing central respiratory motor output via prevention of transient hypocapnia prevents most OSA in selected patients with a high chemosensitivity and a collapsible upper airway, whereas increasing respiratory motor output via moderate hypercapnia eliminates OSA in most patients with a wider range of chemosensitivity and CO2 reserve. Reducing chemosensitivity via hyperoxia had a limited and unpredictable effect on OSA.
Keywords: isocapnia, hypercapnia, hyperoxia
there are several lines of evidence linking the stability of central respiratory motor output with airway obstruction during sleep. First, there are significant (albeit modest) correlations of loop gain, an estimate of control system stability, with the severity of obstructive sleep apnea (OSA) (3, 68, 78), and continuous positive-airway pressure (CPAP) therapy reduces loop gain in many OSA patients as well as in animals (16, 57, 65). Second, central apneas or unstable breathing during sleep may result in airway obstruction at the nadir of respiratory drive in snoring subjects (1, 6, 26, 52, 66). Third, increasing or stabilizing respiratory drive or reducing chemosensitivity reduces airway resistance and/or relieves airway obstruction in some sleep apnea patients (5, 15, 28, 69). However, which OSA patients might benefit from reducing the propensity for unstable central respiratory motor output has not been adequately addressed.
Based on these background findings we hypothesized that stabilizing central respiratory motor output in OSA patents who are characterized by a combination of mild to moderate airway collapsibility (Pcrit = 0 ± 2 cmH2O) and increased controller gain (chemosensitivity) would relieve airway obstruction. To this end we determined the critical closing pressure of the upper airway (UAW), the CO2 responsiveness below eupnea (controller gain), and plant gain in newly diagnosed, untreated OSA patients. Then, in these same patients we tested the effect of three treatments on their OSA. These included preventing transient hypocapnia (via selective rebreathing during the hyperpneic phase), raising end-tidal Pco2 (PetCO2) (via continuous deadspace rebreathing) and preventing hypoxemia [via supplemental fraction of inspired O2 (FiO2)]. We found the isocapnic and especially the hypercapnic treatments to be effective in reducing OSA; these treatments were more likely to be successful in those patients with mild to moderately collapsible upper airways and high controller gains and narrowed CO2 reserves.
METHODS
Subjects.
Twenty-six newly diagnosed adult patients (18–72 yr) with mild to severe OSA (AHI ≥ 10 events/h of sleep of predominantly obstructive type) were studied before receiving any treatment. Subjects were excluded if they had acute or chronic heart dysfunction or failure, cerebrovascular disease, asthma, or chronic obstructive pulmonary disease. All subjects provided written informed consent prior to participation. The experimental protocol was approved by the University of Wisconsin Center for Health Sciences Human Subjects Committee.
All subjects underwent 2–4 overnight studies in our laboratory, and each time, they reported to the laboratory in the evening (8–9 pm), having refrained from any alcohol and caffeinated beverages during or after their evening meal. Subjects deprived themselves of ∼1 h of their normal sleep duration before each study night. Subjects slept with a facemask, through which they were connected to a breathing circuit that was modified for each protocol as described below.
Experimental Setup
Polysomnographic methods and respiratory monitoring.
Standard polysomnography technique was used to document the sleep/wake state and arousal (29). In addition, ventilation was measured with a pneumotachograph (model 3700, Hans Rudolph). Mask pressure was measured with a differential pressure transducer (DP 103, Validyne) from its side port. Calibrated respiratory inductance plethysmography (Inductotrace, Ambulatory Monitoring) was used to assess respiratory effort. Arterial oxygen saturation (SaO2) was measured by pulse oximetry (Ohmeda Oxicap no. 4700). End-tidal gases were measured by a gas analyzer (AMETEK, model CD-3A), which was calibrated using known gases.
Hypercapnic rebreathing.
As described in our previous publication (34), subjects slept with a single-port facemask attached to an adjustable plastic cylinder with a variable volume of 450–700 ml. The cylinder was open to room air through a hole of 2-cm diameter.
Isocapnic rebreathing.
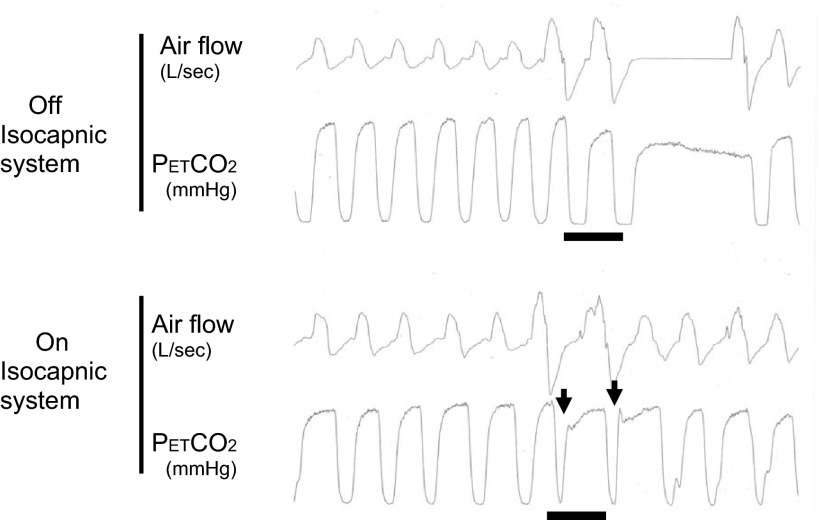
The subject breathed through a pneumotach connected to T tube with a two-way nonrebreathing Hans Rudolph valve that was open to room air on one port and to a gas reservoir (2.5 liters) on the other. The inspired port of the valve was connected to a pneumatic balloon valve with an inflatable balloon in each port. Normally the port to room air was open and closed to the reservoir. Once the inspired tidal volume exceeded the target volume (of 1.5× eupneic control) an electrical signal from a flow sensor (model 8410) was sent to activate the balloon valve through a solenoid, thereby closing the port open to room air and opening the port to the CO2-enriched reservoir. When the tidal volume (Vt) returned to <1.5× control the balloon valve switched back to the normal configuration with room air as the inspired gas. Therefore, as shown in Fig. 1 this system allowed extra CO2 to be added only during the hyperpneic phase and normocapnia was maintained. This isocapnic rebreathing system is able to deliver CO2 in a timely fashion to any random hyperpnea. Therefore, our system distinguishes itself from “dynamic CO2 therapy”, which used an automated algorithm as predicted from the patient's predetermined periodic breathing cycle, to deliver CO2 to the hyperpnea phase only (20, 47).
Fig. 1.
How does isosystem work? Maintaining isocapnia via isocapnic rebreathing system. During the hyperpneic phase when breathing room air, end-tidal Pco2 (PetCO2) was reduced below the apneic threshold when a sufficiently large ventilatory overshoot occurred in the control condition (top panel); PetCO2 was maintained above the apneic threshold and near normocapnia via selective rebreathing during the ventilatory overshoot (bottom panel). The horizontal lines indicate the fraction of end-tidal CO2 (FetCO2) traces of the two breaths with tidal volume (Vt) > two times control. In the bottom panel, the arrows indicate a sharp increase in FiCO2 after onset of inspiration in the two augmented tidal volumes.
Hyperoxic inhalation.
Patients breathed ambient air for the initial 1–1.5 h of sleep. We then supplied a gas mixture with 40–50% O2, balanced by N2, through a full face mask, at flow rates sufficient to maintain SaO2 95–98% during sleep throughout most of the rest of the night with a total of 190 ± 21 min of hyperoxia, returning to room air breathing later in the night.
Physiologic classification of patients.
The propensity for developing breathing instability during non-rapid eye movement (NREM) sleep was assessed by measuring the controller gain and plant gain and estimating the “CO2 reserve” below eupnea. Upper airway collapsibility was estimated by measuring upper airway critical closing pressure (Pcrit). Both methods have been described previously in detail (73).
Briefly, to determine controller and plant gains, a positive-pressure ventilator in the assist mode was attached to the subject through a sealed mask. CO2 reserve was measured in a lateral posture to avoid the need for application of high levels of CPAP to stabilize the airway and breathing. Following the baseline CPAP period (usually at 4–8 cmH2O), a transient hyperventilation was initiated in the pressure support mode to bring PetCO2 down in steps of 1–2 mmHg to the apneic threshold (73, 75). The average PetCO2 on the three breaths immediately preceding the apnea was taken as the apneic Pco2 threshold. The difference between eupneic PetCO2 during stable breathing and the apneic threshold PetCO2 was calculated as CO2 reserve. The controller gain was calculated as the slope of minute ventilation (ΔV̇e) to ΔPetCO2 from eupnea to apnea, and the plant gain was determined by the ΔV̇e required to decrease PetCO2 during pressure support hyperventilation.
To determine Pcrit, the subject was connected to a modified BiPAP device (Respironics, Murrysville, PA) that was able to deliver both negative and positive (−20 to 20 cmH2O) pressures though a tight-fitting full facemask. Pcrit was determined by reducing airway pressure to the point of zero flow rate in each subject. Pcrit was assessed in a supine posture with the head positioned on a contoured foam pillow to ensure a constant position and neck flexion.
Zolpidem (10 mg) was given prior to bedtime to facilitate sleep and to suppress the arousability from sleep. Zolpidem at this dosage has been shown to have no significant effect on ventilation or ventilatory stability, blood gases, occlusion pressure, ventilatory responses to CO2, or ventilatory stability (7, 12, 43, 46, 53).
Protocol for Treatment Studies
Visit 1 (split night).
Subjects received isocapnic rebreathing to prevent transient hypocapnia, hypercapnic rebreathing to increase respiratory drive, and room air breathing to provide control data. The three interventions were given in a random order, and the duration of each condition was allotted about one-third of each individual's total sleep time, as was inferred from the subject's clinical polysomnography study (292 ± 28 min total sleep/night). A 5-min washout period was allowed for the transition between any two conditions. In addition, four subjects had two split nights with one night of room air vs. isocapnia and the other night of room air vs. hypercapnia, because their total sleep time was too short to complete the three conditions in a single night. Studies were separated by 2–15 days. To verify the findings obtained during the split night, one subject underwent an overnight with isocapnic rebreathing; another subject underwent an overnight with hypercapnic rebreathing treatment.
Visit 2.
Visit 2 was used to quantify UAW collapsibility (Pcrit) and controller and plant gains and CO2 reserve below eupnea during sleep (73).
Visit 3.
After 60–90 min of room air breathing each of 19 subjects underwent a hyperoxia intervention for the reminder of their sleep period during which O2 was supplied through the breathing valve at a flow rate sufficient to maintain SaO2 between 95 and 98%.
Data analysis.
During the treatment night, AHI, apnea index (AI), and hypopnea index (HI) were compared in the same sleep stage in the same position under conditions of room air (control), and for each treatment using one-way repeated-measures ANOVA, along with Tukey test if necessary. The eupneic V̇e and PetCO2 were averaged under each condition during 3–5 min stable breathing, and the mean values were compared among control, isocapnia, and hypercapnia in the split night using one-way repeated-measures ANOVA; and compared between control and hyperoxia using paired t-test. Arousal index was also compared under the three conditions using the above-mentioned statistical methods. For the four subjects who had two split nights, the control values were the means of the two night room air studies.
To assess differences in Pcrit, controller, plant and loop gains as well as CO2 reserve between those patients who did and those who did not respond to each treatment, we used a cutoff of a >30% reduction in AHI below control as a meaningful treatment effect. We also identified those patients who experienced a reduction of AHI to <10 events/h with each of the treatments as an indication of a complete resolution of OSA. An unpaired t-test was applied to compare the above-mentioned characteristics between the two groups. In addition, we determined the correlation coefficient among all subjects between reductions in AHI and the CO2 reserve, controller gain and Pcrit.
All data are expressed as means ± SE.
RESULTS
Characteristics of Subjects
We studied 26 subjects (20 men and 6 women), average age 58 ± 2 yr, body mass index (BMI) = 33 ± 1 kg/m2. Based on the results from the diagnostic overnight polysomnogram the subjects had an average AHI of 42 ± 5 events/h of sleep, AI of 23 ± 20, and HI of 19 ± 14; 92 ± 3% of the apneas were scored as obstructive.
Sleep Times/Respiratory Arousals
Subjects slept for similar times (88–90 min total sleep time) and for similar durations in each sleep stage for control, isocapnic, and hypercapnic conditions (see Fig. 2). Sleep was predominantly in Stage I and II NREM with much shorter periods in Stage III and REM. Transient arousals (3–15 s) associated with a respiratory disturbance event averaged 23 ± 3 events/h under room air control, were unchanged with the isocapnic treatment (19 ± 4) and significantly reduced with the hypercapnic treatment (12 ± 4). Arousals not associated with respiratory disturbance were unchanged across all conditions, and averaged 6–7 events/h.
Fig. 2.
The total sleep time and sleep stage distribution of room air control, isocapnia, and hypercapnia treatment conditions. There was no difference in terms of total sleep time and sleep stage distribution among room air, isocapnic rebreathing, and hypercapnic rebreathing. S1 and S2, Stage I and Stage II non-rapid eye movement sleep; S3, Stage III sleep; REM, rapid eye movement sleep.
Polygraph Recordings of Isocapnic and Hypercapnic Treatment Effects
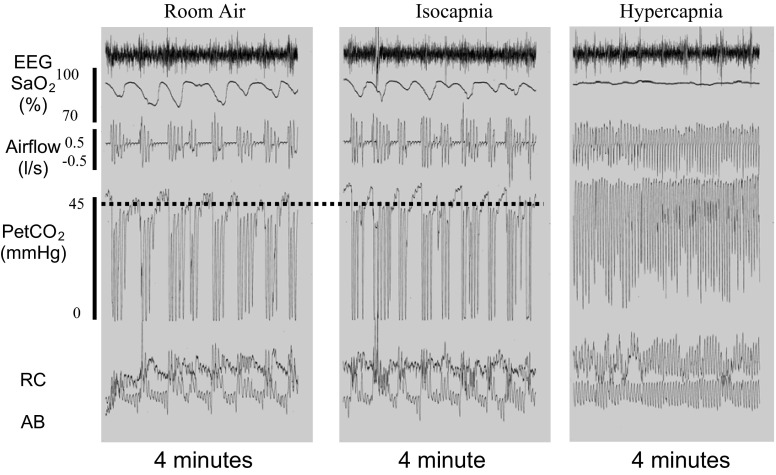
Figure 3 shows the response to selective isocapnic treatment in an OSA patient who is typical of those who responded positively. Under room air control, note the cyclical apneas with paradoxical motion of ribcage and abdomen during the apneas. With the selective isocapnic treatment, PetCO2 was maintained and almost all apneas were eliminated, although some underlying instability in flow rate and Vt is noticeable. Figure 4 shows the typical patient with severe OSA who failed to reduce AHI in response to the selective rebreathe isocapnic treatment, but did reduce their AHI to <10 events/h with continuous rebreathing and hypercapnia (in this case +5 mmHg PetCO2).
Fig. 3.
Polysomnograph (PSG) records from one representative subject of those who showed a marked reduction of apnea-hypopnea index (AHI) with isocapnic rebreathing. Repetitive obstructive apneas were indicated by the absence of flow despite respiratory effort. Most of these obstructive apneas were eliminated by isocapnic rebreathing without raising PetCO2. The 4-min periods shown for both control and isocapnic treatment conditions were representative of the breathing pattern, PetCO2, and AHI experienced during the total 90- to 95-min periods of control and treatment conditions studied in this patient. EEG, electroencephalogram; SaO2, arterial oxyhemoglobin saturation; Ab, abdominal movement; RC, rib cage movement.
Fig. 4.
Three PSG records from another representative subject of those who showed no change in AHI with isocapnic rebreathing but marked reduction of AHI with hypercapnic rebreathing. Periodic breathing with recurrent obstructive sleep apnea (OSA) during room air breathing (left panel) continued during isocapnic rebreathing (middle panel). These apneas were eliminated by hypercapnic rebreathing with an increase in PetCO2 of 5.2 mmHg (right panel). The 4-min periods shown for control, isocapnic, and hypercapnic treatments were representative of the breathing pattern, PetCO2, and AHI experienced during the total 90- to 95-min periods of control and treatment conditions studied in this patient. The dashed line represents the average PetCO2 achieved during a period of stable room air breathing in NREM sleep.
Effectiveness of Maintaining Isocapnia on AHI
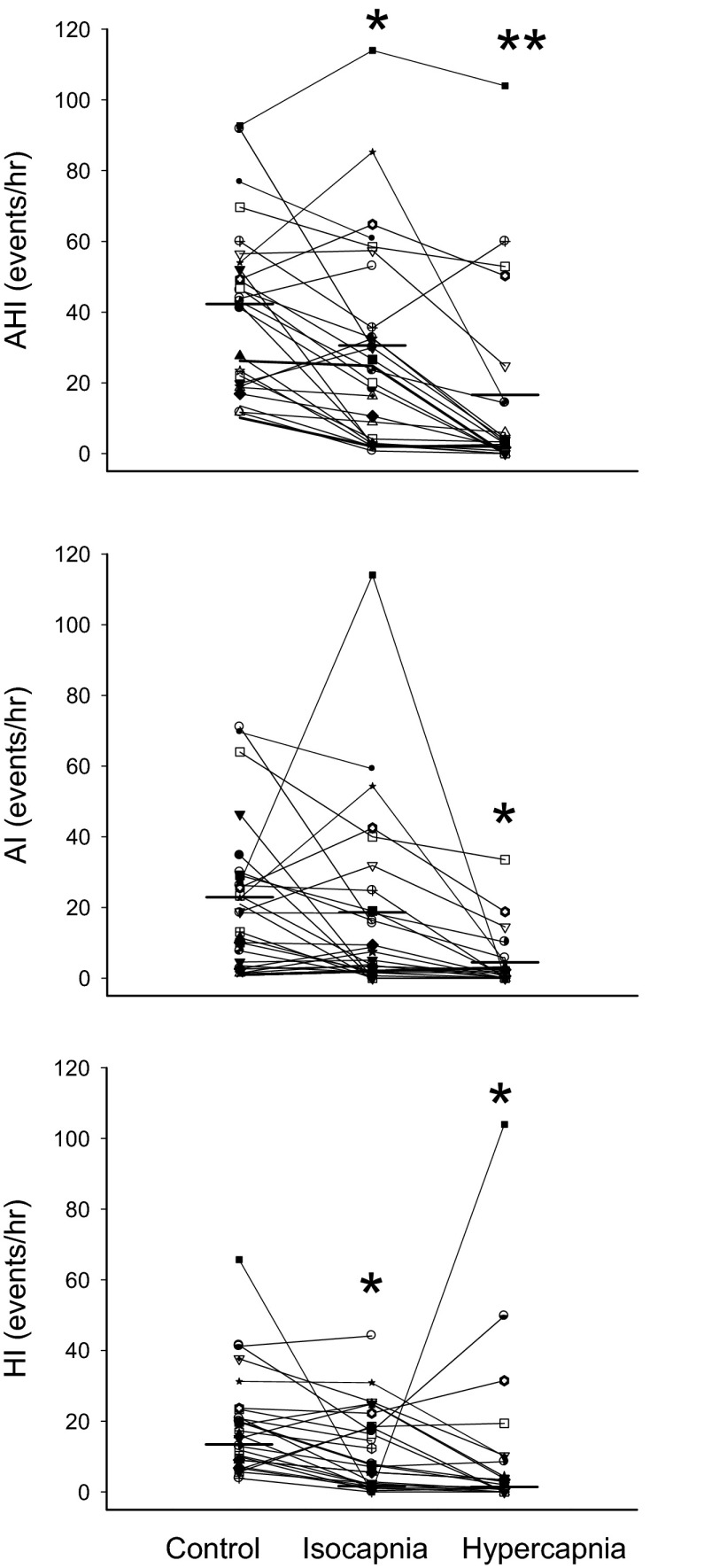
Selective rebreathing, isocapnic trials produced no change from room air control in the mean eupneic PetCO2 or V̇e (see Table 1). Individual subject changes in AHI in response to isocapnia and hypercapnia are shown for all subjects in Fig. 5 with group mean values for eupneic breathing shown in Table 1. In 14 of 26 patients the isocapnic treatment method reduced AHI by >30% below room air control (−69 ± 6%; range −31 to −95%). Seven of these 14 “responders” reduced their AHI to <10 events/h. Mean AHI fell from 38 ± 6 to 13 ± 3 events/h, AI from 24 ± 5 to 7 ± 2/h, and HI from 14 ± 2 to 6 ± 2/h. None of the remaining 12 patients reduced their AHI > 30% below control, as neither apnea nor hypopnea indexes were significantly reduced in these subjects.
Table 1.
Effects of three treatments on eupnic breathing
| Room Air Control for CO2 Treatments | Isocapnia | Hypercapnia | Room Air Control for O2 Treatment | Hyperoxia | |
|---|---|---|---|---|---|
| Sleep time, min | 90.7 ± 8.2 | 92.6 ± 10.0 | 86.0 ± 10.1 | 87 ± 6.1 | 190 ± 21† |
| Vt, liters | 0.47 ± 0.16 | 0.50 ± 0.18 | 0.67 ± 0.21* | 0.56 ± 0.04 | 0.56 ± 0.05 |
| Freq, no./min | 15.1 ± 2.0 | 15.5 ± 2.5 | 15.2 ± 2.0 | 14.8 ± 0.6 | 13.8 ± 0.5† |
| V̇e, l/min | 7.2 ± 2.5 | 7.8 ± 2.9 | 9.8 ± 3.1* | 8.1 ± 0.6 | 7.5 ± 0.6 |
| PetO2 | 105.5 ± 4.8 | 109.0 ± 7.8 | 100.3 ± 5.0 | 107.4 ± 1.6 | 189.4 ± 11.6† |
| PetCO2 | 40.9 ± 3.5 | 41.7 ± 3.8 | 45.1 ± 4.6* | 45.0 ± 1.2 | 44.1 ± 1.1 |
Values are means ± SE; n =26 for isocapnia, 21 for hypercapnia, and 19 for hyperoxia. Vt, tidal volume; Freq, frequency; V̇e, minute volume; PetO2, end-tidal Po2; PetCO2, end-tidal Pco2.
P < 0.05 compared with room air and isocapnia.
P < 0.05 compared with room air.
Fig. 5.
Effect of isocapnic and hypercapnic rebreathing on AHI in all subjects. With isocapnic treatment, 12 of 26 subjects showed a reduction in AHI by more than 30% of room air control and 7 of the 14 reduced AHI to <10 events/hr. With hypercapnic treatment 17 of 21 subjects had AHI reduced by more than 30% of control and 14 of the 17 reduced AHI to <10 events/h. AI, apnea index; HI, hypopnea index. *P < 0.05, **P < 0.01 compared with room air.
Effectiveness of Hypercapnia on AHI
The hypercapnia achieved via continuous deadspace rebreathing caused a 4.2 ± 1 mmHg increase in eupneic PetCO2 and a 13.6 ± 3% increase in V̇e (see Table 1). All but four of the 21 subjects who received the hypercapnia treatment showed a reduction in AHI in excess of 30% below control (see Fig. 5). In these 17 responsive patients AHI was reduced by 94 ± 3% below control (range −33 to −100%), and this reduction was attributable primarily to a reduction in AI. Fourteen of these 17 patients reduced their AHI to <10 events/h. In all 14 patients who had responded significantly to the isocapnic treatment (see above), hypercapnia reduced AHI substantially further from an average of 13 ± 3 with isocapnia to 2 ± 1 events/h with hypercapnia. Of the 12 nonresponsive subjects to isocapnia, 8 showed >30% reductions in AHI with hypercapnia due to further reductions in both AI (38 ± 19%) and HI (30 ± 3%); and 3 of these 8 patients reduced their AHI to <10 events/h.
Physiological Characteristics Distinguishing the Responsive from Nonresponsive Groups to Isocapnic and Hypercapnic Treatments
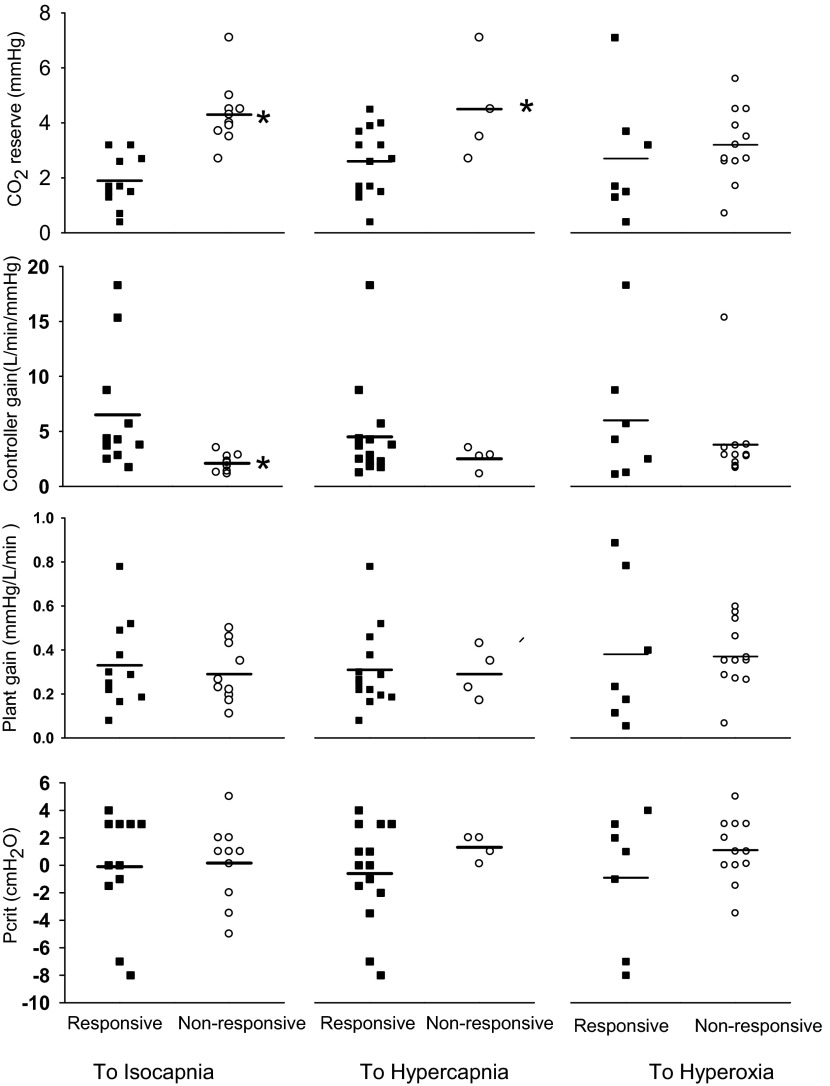
Isocapnia.
The left part of Table 2 and the left panel of Fig. 6 show the comparisons between the responsive and nonresponsive groups for the isocapnic treatment. Subjects in both groups were of comparable age and BMI. The overnight PSG baseline data showed a similar AHI (38 ± 6 vs. 48 ± 8 events/h, P = 0.26) and similar spectrum of severity (i.e., AHI ranged from 12 to 92 events/h in both groups). Classification studies revealed that there were no differences in Pcrit (response vs. nonresponse: −0.1 ± 1.2 vs. 0.2 ± 0.9 cmH2O, P = 0.85) or CPAP holding pressure (12.8 ± 0.8 vs. 11.9 ± 0.7 cmH2O, P = 0.36), indicating a similar UAW passive collapsibility. The responsive group to isocapnia had a greater controller gain below eupnea (6.5 ± 1.7 vs. 2.1 ± 0.2 l·min−1·mmHg−1, P < 0.01) and higher loop gain (2.2 ± 0.8 vs. 0.6 ± 0.2, P = 0.01) as well as a smaller CO2 reserve (1.9 ± 0.3 vs. 4.3 ± 0.4 mmHg, P < 0.01) compared with the nonresponsive group. However, the groups did not differ in their plant gain (0.33 ± 0.06 vs. 0.29 ± 0.04 mmHg·l−1·min−1, P = 0.6). Among all subjects studied, CO2 reserve was negatively correlated to the reduction in AHI (R = −0.56, P < 0.01); the relationship between reduction of AHI (% of baseline) and the controller gain was weaker (R = 0.40, P = 0.08) and there was no correlation between the improvement of AHI and either Pcrit (R = 0.006; P = 0.98) or plant gain (R = 0.07, P = 0.77).
Table 2.
Control values for subjects who were responsive and nonresponsive to isocapnic, hypercapnic, and hyperoxic treatments
| To Isocapnia |
To Hypercapnia |
To Hyperoxia |
||||
|---|---|---|---|---|---|---|
| Responsive | Nonresponsive | Responsive | Nonresponsive | Responsive | Nonresponsive | |
| N | 14 (3F + l1M) | 12 (3F + 9M) | 17 (5F + 12M) | 4 (0F + 4M) | 7 (3F + 4M) | 12 (0F + 12M) |
| Age, yr | 59 ± 2 | 58 ± 3 | 58 ± 2 | 56 ± 2 | 62 ± 2 | 55 ± 3 |
| BMI, kg/m2 | 33 ± 2 | 34 ± 2 | 33 ± 2 | 36 ± 1 | 31 ± 2 | 34 ± 2 |
| AHI, no./h | 38 ± 6 | 48 ± 8 | 34 ± 4 | 68 ± 9 | 36 ± 7 | 52 ± 8 |
| CA/total apnea, % | 10.9 ± 3.7 | 3.9 ± 2.8 | 9.5 ± 3.2 | 0.2 ± 0.2 | 4.6 ± 3.3 | 5.2 ± 3.0 |
| AHI range, min–max | 12–92 | 12–93 | 12–57 | 49–93 | 12–60 | 10–85 |
| Mean SaO2 | 93 ± 1 | 91 ± 1 | 93 ± 1 | 87 ± 3* | 92 ± 2 | 93 ± 1 |
| Controller gain, l• min−1•mmHg−1 | 6.5 ± 1.7 | 2.1 ± 0.2** | 4.5 ± 1.2 | 2.6 ± 0.5 | 6.0 ± 2.3 | 3.6 ± 1.1 |
| Plant gain, mmHg•l−1•min | 0.33 ± 0.06 | 0.29 ± 0.04 | 0.31 ± 0.05 | 0.29 ± 0.06 | 0.38 ± 0.13 | 0.37 ± 0.04 |
| CO2 reserve, mmHg | 1.9 ± 0.3 | 4.3 ± 0.4** | 2.6 ± 0.3 | 4.5 ± 1.0 | 2.7 ± 0.8 | 3.2 ± 0.4 |
| Pcrit, cmH2O | −0.1 ± 1.2 (3 to −8) | 0.2 ± 0.9 (5 to −5) | −0.6 ± 1.0 (4 to −8) | 1.3 ± 0.5 (2 to 0.1) | −0.9 ± 1.8 (5 to −3.5) | 1.1 ± 0.7 (4 to −8) |
Values are means ± SE. Responsive group, apnea-hypopnea index (AHI) reduced by 30% of baseline value or more in response to isocapnic rebreathing (31 ± 6% of control) or hypercapnia (15 ± 4% of control) or hyperoxia (36 ± 11% of control); Nonresponsive group, the reduction in AHI was <30% of baseline during isocapnic rebreathing (112 ± 10% of control) or hypercapnia (98 ± 8% of control) or hyperoxia (149 ± 21% of control). BMI, body mass index; CA, central apnea; CO2 reserve, the proximity between eupneic PetCO2 and apneic threshold PetCO2. Pcrit, critical closing pressure.
Fig. 6.
Classification characteristics of subjects who did/did not respond to each of the three treatments. For the isocapnic treatment, the responsive group demonstrated a smaller CO2 reserve (1.9 ± 0.3 vs. 4.3 ± 0.4 mmHg, *P < 0.01) and greater controller gain (6.5 ± 1.7 vs. 2.1 ± 0.2 l·min−1·mmHg−1, *P < 0.01) compared with the nonresponsive group. The same tendency was also seen in response to the hypercapnic and hyperoxic treatments, but there was substantially more overlap of individual subject values for controller gain and CO2 reserve for the responsive vs. the nonresponsive groups. Plant gain and critical closing pressure (Pcrit) values were similar for responders and nonresponders to all three treatments.
Hypercapnia.
The middle panels in Table 2 and Fig. 6 contrast characteristics determined under control conditions for responders and nonresponders to hypercapnia. Note, that as with the isocapnic treatment, the responsive group to hypercapnea tended to have slightly higher controller gains and narrower CO2 reserves although there was considerable overlap between the two groups. Hypercapnia was more effective than isocapnia in reducing AHI in some OSA patients with relatively low controller gains and wide CO2 reserves. As a result, there was no difference in either controller gain, loop gain, plant gain, CO2 reserve or Pcrit between the responsive and the nonresponsive groups to hypercapnia. Of further note are the uniformly positive Pcrits (and holding pressures of 11–15 cmH2O) as well as relatively wide CO2 reserves in all four nonresponders to iso- or hypercapnic treatments.
Effectiveness of Hyperoxia in Treating OSA
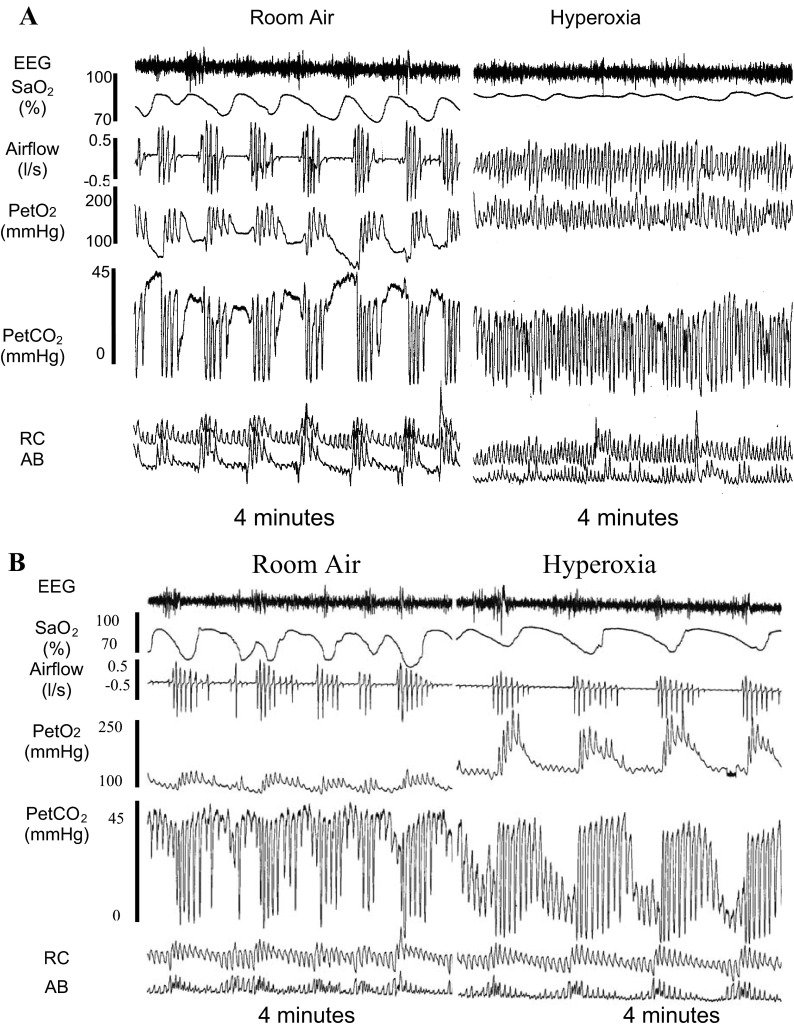
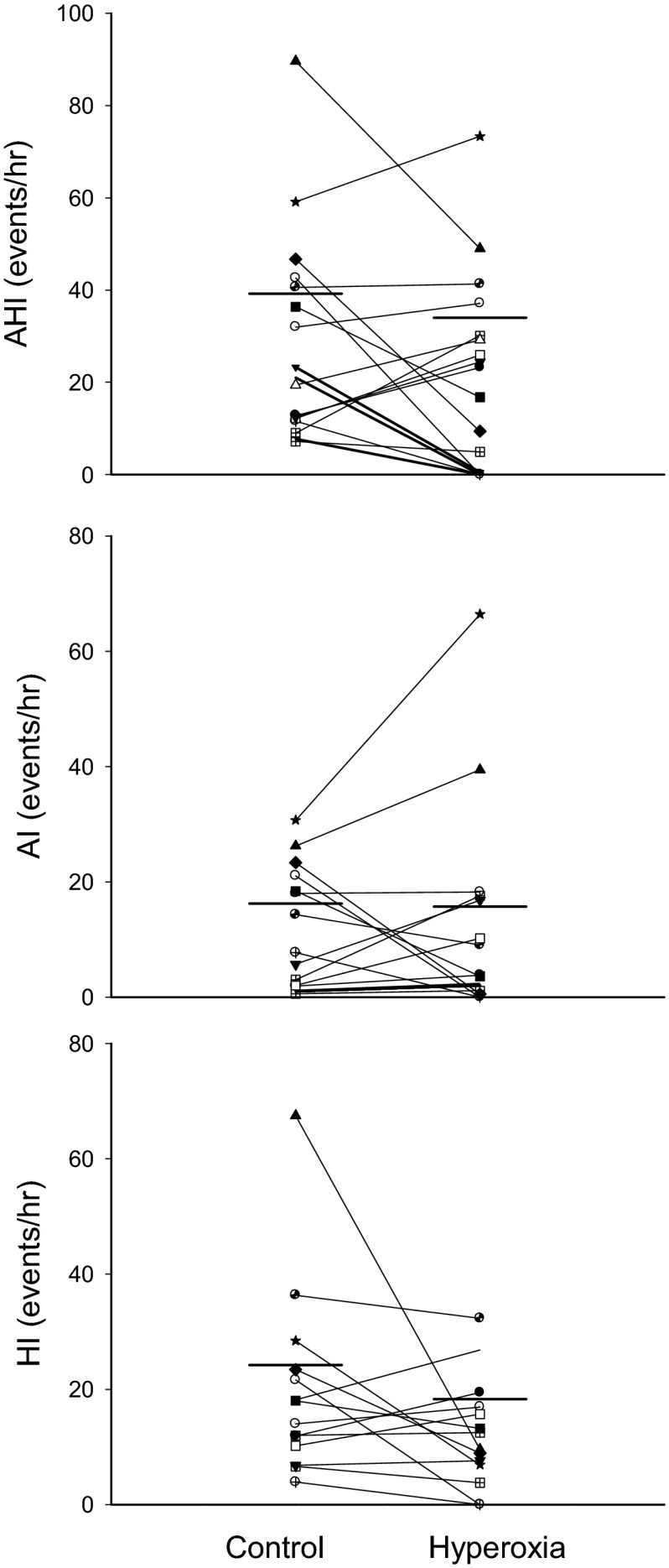
Hyperoxic inhalation consistently increased end-tidal Po2 (PetO2) and maintained SaO2 between 95 and 98%, but did not affect other respiratory parameters, except for a slight (by 1 breath/min) yet significant (P < 0.05) reduction in the breathing frequency and higher PetO2/SaO2 (see Table 1). Hyperoxia did not reduce the group mean AHI significantly (room air vs. hyperoxia: 39 ± 6 vs. 34 ± 6 events/h, P = 0.25). However, as shown in Figs. 7A and 8, 7/19 subjects reduced AHI by >30% of baseline via hyperoxic inhalation (−64 ± 11%; range −100% to −32%). In these 7 subjects, AHI was reduced from 36 ± 7/h during overnight room air control study or from 36 ± 11 during the same (split) night control room air breathing to 14 ± 6 events/h (P < 0.05). However, in the remaining 12 subjects, their AHI was not altered significantly (46 ± 6/h) compared with either the overnight baseline studies (52 ± 8 events/h) or the 1.45 h of room air breathing within the same split night (41 ± 8 events/h) as shown in Fig. 7B. Apnea length increased in both responders and nonresponders to hyperoxic treatment, as the average length increased from 24.1 ± 3.6 to 30.1 ± 4.3 s (P < 0.05).
Fig. 7.
PSG records from two representative subjects during room air vs hyperoxia. A: room air vs. hyperoxia in one subject/responder. B: room air vs. hyperoxia in one subject/nonresponder. Repetitive obstructive apneas were noted in both A and B. In A, apneas and hypopneas were eliminated by hyperoxia; while in B, the apneas were persistent despite the high FiO2 and SaO2. Note in B, apnea length was increased by hyperoxic inhalation.
Fig. 8.
Effect of hyperoxia on AHI, apneas, and hypopneas in all subjects. Seven of 19 subjects showed a reduction in AHI by more than 30% of baseline value in response to hyperoxia.
As shown in the right panels of Table 1 and Fig. 6, there were no clear group differences in plant gain or Pcrit distinguishing the responsive from the nonresponsive group to hyperoxia. However, we note that all but one of the 12 nonresponsive group members tended to show a relatively low controller gain or CO2 chemoreflex slope below eupnea.
Overnight vs. Split Night
The AHI under control, air-breathing conditions averaged 35 ± 26 events/h for the overnight polysomnography study (5.2 ± 0.8 h) and 42 ± 23 for the shorter split night (1.5 ± 0.1 h) (P = 0.10). The control AHI magnitude was significantly correlated among subjects between overnight and split night sessions (r = 0.66, P < 0.01).
In the one subject with overnight isocapnic treatment the AHI was reduced from 74 (control night) to 15 events/h, which compared favorably with the split night trial (115 min study time), with reductions in AHI from 92 to 30 events/h (AI: from 71 to 16 event/h). Similarly, for the single subject with overnight hypercapnic treatment, AHI fell from 52 to 18 events/h (AI 46 to 8 events/h) for the overnight studies, compared with reductions from 47 to 0 events/h for AHI (24 to 0 event/h for AI) for the split night study (99 min).
DISCUSSION
Our study was aimed at 1) evaluating the effects of stabilizing or increasing central respiratory motor output and of reducing chemoreflex gain on OSA in newly diagnosed, untreated patients; and 2) determining whether certain characteristics of passive airway collapsibility and control system gains would be predictive of treatment success. There were three major findings. First, stabilization of respiratory motor output through selective isocapnic rebreathing limited to the transient hyperpneic phase, reduced OSA substantially in those patients with a high controller gain and a narrowed CO2 reserve. Second, enhancement of respiratory motor output via continuous hypercapnia (achieved via continuous dead space rebreathing) was effective in reducing obstructive apneas in the vast majority of OSA patients (17/21) with a wide range of chemoreflex gains and upper airway collapsibility. Third, hyperoxia (SaO2 95–98%) showed a mixed outcome in terms of its effect on the frequency of obstructive apneas/hypopneas (7 of 19 patients reduced AHI by >30%) but had a consistent effect in prolonging apnea length. Finally, in a minority of OSA patients (4/26) maintaining isocapnia or creating hypercapnia or hyperoxia were all ineffective at reducing OSA. These findings have implications for evaluating the potential contributions of controller gain and both the stability and magnitude of respiratory motor output to the pathogenesis and treatment of OSA.
Limitations
There were advantages as well as limitations to our experimental design which, for almost all patients, compared control, room air breathing conditions to each of the three treatments within three separate nights of sleep. On the one hand, this approach avoided night to night variability in sleep-disordered breathing and its determinants and allowed us to assess the consistency of any treatment effects on the transition from room air controls to each of the treatments and also upon return to control conditions. Our design also compared treatment vs. controls under similar conditions of sleep posture and sleep state, which ruled out any contributions of these variables to the observed treatment effects on airway obstruction. On the other hand it is well documented that within-night variability of sleep-disordered breathing and/or upper airway resistance can also be significant in OSA patients and cannot always be explained by variations in posture or sleep stage. Indeed, some OSA patients have significant periods with no or few episodes of obstructive apneas or transient arousals (76, 79). So, by limiting our observations of each treatment effect to only 1.5–2 h of sleep, we have not achieved an ideal design for quantifying treatment effects on AHI. This would require an additional session of observations over an entire night (or more) of sleep for each treatment. These additional nights of study were not a realistic goal for this initial study in the great majority of our newly diagnosed, untreated OSA patients, who were awaiting assignment to CPAP treatment. For now we can only point out that the diagnostic polysomnography night revealed that our OSA patients, especially those with >40 AHI, tended to have cyclical obstructive apneic episodes fairly consistently throughout the great majority of the night. Importantly, their AHI indexes under control, room air breathing conditions were significantly correlated and comparable in magnitude over the course of the entire polysomnography night vs. during the shorter periods in the split night. We attribute the higher average AHI during the split night to the requirement for a supine sleeping position during this sleep session (see below). We also note the close agreement achieved for isocapnic or hypercapnic treatments on reducing AHI in two of our OSA patients when comparisons between control vs. treatment were made between overnight trials of 5 to 6 h each vs. the split night studies.
The mask we used for selective rebreathing to maintain isocapnia was equipped with a solenoid valve and pneumotachograph and its use required our patients to sleep primarily in the supine position. Because of the discomfort created by the apparatus and its potentially disruptive effect on sleep state, we were unable to accurately evaluate the effects of the iso- or hypercapnic treatments, per se, on sleep efficiency. Previous studies using CO2 administration in patients with congestive heart failure have shown that elimination of central apnea and periodic breathing may (42) or may not (62, 63) eliminate or significantly reduce the prevalence of transient arousals associated with disordered breathing events. Our findings showed no effect of iso- or hypercapnic treatments on nonrespiratory arousals and significant reductions in transient arousals associated with respiratory disturbances during the hypercapnic rebreathing with no change during the isocapnic treatment.
The primary variable used to assess the effectiveness of the three interventions was the change in AHI. If the AHI was reduced by ≥30% of the baseline level, we considered this to be a meaningful alleviation of the patient's OSA because it corresponds to changes found via the use of compliant CPAP treatment in patients with moderate OSA (54). The biological basis for this standard, however, needs to be further verified. We also point out that half of our “responsive” patients to isocapnia and all but two of our responders to hypercapnea reduced their AHI to <10 events/h. Further, even if we had used a cut-off of a 50% reduction in AHI to designate responders vs. nonresponders to isocapnia, the responders (10 subjects now) would still have been separated by a high controller gain (7.6 ± 2.1 vs. 2.4 ± 0.3, P < 0.01) and narrower CO2 reserve (1.8 ± 0.4 vs. 3.9 ± 0.4, P < 0.01).
Finally, we note that two of our OSA patients had highly negative Pcrits of −7 to −8 cmH2O, which we confirmed with repeated measures upon reducing airway pressure to achieve absolute zero flow conditions. One of these patients was our mildest OSA patient (with only 12 AHI) who had a high controller gain (5.7 l·min−1·mmHg−1) and small CO2 reserve (1.5 mmHg), but the other had an AHI of 47 and AI of 23 and with isocapnic treatment reduced his AHI and HI by more than 70%. We are puzzled by this finding and can only refer to the comprehensive study of Kirkness et al. (37) who observed that 5 of 150 OSA patients with AHI > 10 showed Pcrit values more negative than −5 cmH2O.
Determinants of Propensity for Central Respiratory Instability
Although specific mechanisms will vary across the various conditions of sleep-induced breathing instability, in general the major determinants of central respiratory motor output instability include enhanced chemoreceptor sensitivities (ΔV̇e/ΔPaCO2, controller gain), gas exchange efficiency (ΔPaCO2/ΔV̇e, plant gain) and/or mixing gain (i.e., circulatory delay from lungs to chemoreceptors) (10, 17, 36, 44). Indirect estimates for assessing loop gain, or the risk of ventilatory instability, have included the ratio of ventilatory decline to ventilatory response achieved via variations in airway pressure with CPAP (67) or a proportional assist ventilator (49), pseudorandom binary stimulation using inspired CO2 (19) and mathematical models of the patient's spontaneous, sinusoidal periodic patterns (44, 58). Our method for estimating controller and plant gains (between eupnea and apnea) used assist control, positive pressure ventilation to gradually reduce PetCO2 in a stepwise fashion in the sleeping patient until the apneic threshold and periodic breathing were achieved. The PetCO2 reduction resulting from the corresponding increase in V̇e to reach the apneic threshold provided an estimate of the control system plant gain. The slope of the ventilatory decline between eupnea and apnea was assumed to be linear and provided an estimate of controller gain below eupnea; and this slope also likely reflected the gain above eupnea as well, although this has not been directly tested (51, 75). In turn, these measurements allow calculation of what we have termed the “CO2 reserve” or the proximity of the eupneic PaCO2 to apneic threshold PaCO2, which reflects the magnitude of the controller and plant gains.
We emphasize that it is not the apneic threshold or even the CO2 reserve, by themselves, that cause instability; rather it is the controller and plant gains which are responsible for system instability as well as for dictating alterations in the apneic threshold and the CO2 reserve (13, 17, 44, 51, 67). Thus these gains and the CO2 reserve are interdependent, and we think it is important to consider all three of these parameters when assessing the propensity for instability. For example, according to classic linear control theory (35, 36) a small ventilatory disturbance in the face of high controller and/or plant gains can initiate ventilatory oscillations, but this theory no longer applies once apnea (and a limit cycle) occurs. Apneas then introduce potential perpetuators of transient arousals, ventilatory overshoots and continued ventilatory oscillations because of the marked synergistic effects on respiratory motor output and sensory input to the central nervous system produced by changes in chemoreceptor stimuli. These apneas, per se, are then important mediators of ventilatory instability, and we believe it is instructive to quantify their determinants in terms of Pco2 threshold and the CO2 reserve below spontaneous eupnea.
How Can the Concepts of Plant Gain and CO2 Reserve Be Applied To Explain How Raising FiCO2 Diminishes or Removes Instability in Central Respiratory Motor Output?
As inspired Pco2 (PiCO2) increases and the PiCO2 to alveolar Pco2 (PacO2) gradient is reduced, each liter of alveolar ventilation excretes less net CO2, amounting to a rightward shift and reduced slope of the isometabolic curve relating PacO2 to alveolar ventilation (V̇a) (35, 36, 44). So, theoretically at least, the stabilizing effect of increased PiCO2 appears to result from two related mechanisms. First, plant gain is reduced, requiring a much greater increase in alveolar ventilation for a given reduction in PacO2. This influence of reduced plant gain is equivalent to the stabilization and apnea-reducing effects of a pharmacologically induced hyperventilation and hypocapnia (31, 51). Second, with elevations in FiCO2, the operating levels of PaCO2 and ventilation rise further above the apneic threshold, thereby widening the CO2 reserve. In our experiments, we would expect both iso- and hypercapnia protocols to provide a stabilizing effect on central respiratory motor output. That is, 1) with isocapnia, an augmented PiCO2 reduced plant gain; and 2) with hypercapnia, an even greater stabilizing effect on central respiratory motor output will occur as the PiCO2 to PaCO2 gradient would be further reduced and the raised PaCO2 is moved further away from the apneic threshold.
Central Instability and CO2 Effects on OSA
We observed that the selective rebreathe isocapnic treatment conditions, which prevented the cyclical reductions in PetCO2 commensurate with transient ventilatory overshoots, were effective in significantly reducing AHI below air-breathing control in some of the OSA patients, whereas continuous rebreathing and hypercapnia were effective in eliminating OSA in almost all patients. What might explain the effectiveness and relative effectiveness of these two types of manipulations of CO2?
First, we think the available evidence in sleeping animals and humans supports a significant role for transient reductions in PacO2 as critical mediators of central apneas and periodicities during NREM sleep. Supportive evidence includes the apneas and periodicities achieved via transient hypocapnic hyperpneas elicited via either assisted mechanical ventilation (24, 49, 51, 61, 75) or following airway occlusion (11, 30). These post hyperpnea central apneas are prevented via controlling PetCO2 at or very near eucapnic levels (8, 21, 51) or by carotid chemoreceptor denervation (9, 50). Pressure support-assisted hyperpneas with raised Vt and with PetCO2 controlled at normocapnic levels will, by themselves, also elicit significant neuromechanical inhibition of diaphragm EMG (and Pdi) amplitude but without significant TE prolongation or apnea (55, 72). Second, it has also been demonstrated that hypocapnic-induced central periodicities or apneas will precipitate upper airway narrowing and/or obstruction at the nadir of respiratory drive in subjects with airways susceptible to collapse (6, 27, 40, 59, 66). Third, in snorers with elevated upper airway resistance during air breathing, hypercapnia induced via increased FiCO2 reduced upper airway resistance during sleep (4, 5, 48). This effect of hypercapnia on reducing airway resistance is consistent with its reported stimulating effect on the recruitment of hypoglossal motor nerve activity and on airway dilator muscle EMG. Some human and animal data support a linear CO2 driven recruitment of the diaphragm as opposed to a highly nonlinear, threshold-like response of upper airway muscle EMG to increased chemoreceptor stimuli (23, 25, 41, 45).
We believe our present findings in OSA patients are explained by these fundamental concepts which combine chemoreflex/controller gain effects on central instability, together with substantial variability in chemosensitivity among patients in their CO2-dependent recruitment of upper airway as well as respiratory pump muscles. Accordingly, we would attribute our isocapnic treatment effects of reducing OSA to maintaining a stable central respiratory motor output, presumably to both the chest wall and upper airway musculature. Most of the OSA patients who responded positively to this isocapnic “stabilizing” treatment had collapsible airways (passive Pcrit = −0.1 ± 1.2 cmH2O and CPAP holding pressures > 10 cmH2O) in combination with a relatively high chemoreflex controller gain to reduced PacO2 below eupnea, resulting in an abnormally narrowed CO2 reserve. Those patients who failed to reduce their OSA significantly with maintained isocapnia had levels of passive Pcrit equivalent to those of the responsive group, but they had controller gains that were substantially lower and CO2 reserves that were more than double those of the responsive group.
We interpret these group differences to mean 1) that high controller gain leading to central control instability was a more important underlying mechanism contributing to OSA in the responsive group vs. the nonresponsive group; and 2) that a raised PaCO2 not only stabilized any underlying central periodicity but importantly, added a powerful recruitment of both chest wall and upper airway dilators which raised V̇e significantly and completely or near completely eliminated airway obstruction in almost all patients.
Classification of Pathophysiological Traits and Treatment Outcomes
We believe our findings illustrate the importance of classifying patients to select treatments aimed at control system stability and/or upper airway muscle recruitment which might be effective in diminishing obstructive events. However, there are other important characteristics that are known to influence the propensity for cyclical obstructive apneas that we have not considered. These mechanisms include individual differences in the ability to effectively recruit upper airway dilators during an apnea prior to arousal, effectiveness in converting neural drive into dilator muscle shortening and airway reopening and arousal threshold sensitivity to chemoreceptor stimuli (15, 67, 77, 78). Our evaluation of controller and plant gains should be somewhat predictive of the propensity for ventilatory overshoot and hypocapnic-induced ventilatory depression and apnea following an obstructive apnea. However, our assessments did not consider any changes in these control system gains that might have occurred because of individual sensitivities to subtle sleep state changes during CO2 administration. Furthermore, we assessed Pcrit only under “passive” conditions which would not account for differences in “active” muscle recruitment and stiffening of the upper airway in response to iso- or hypercapnic treatments. Thus we speculate that the presence of one or more of these important destabilizing characteristics as described above may explain why our isocapnic treatment was not effective in a few patients with normal controller and plant gains and CO2 reserves. Abnormalities in some of these additional traits may also explain why four of our OSA patients failed to reduce AHI even with the hypercapnic treatment. Based on what appeared to be severely recessed mandibles i.e., “small jaw,” in these four patients we suggest that their failure to respond positively likely reflected a marked craniofacial impingement on pharyngeal patency.
CO2 vs. Pharmacological Treatments?
Hypercapnia was highly effective at eliminating OSA and normalizing AHI in almost all of our patients regardless of their control system gains or their airway collapsibility. Indeed hypercapnia reduced AHI to < 10 even in the 4 patients with Pcrit in excess of +3 cmH2O, implying that airway dilator muscles were effectively recruited and the “active” Pcrit substantially reduced below its “passive” value (33). However, hypercapnia also has potential side effects such as chemoreceptor driven increases in sympathetic nerve activity or circulating catecholamines or possibly sleep state disruption (2, 62) and in practical terms would be difficult to deliver in a controlled fashion and especially to monitor outside of the laboratory. Accordingly, it needs to be asked if there might be a pharmacological means of increasing central respiratory motor output and reducing control system gains which might be equally effective as induced hypercapnia? The carbonic anhydrase inhibitor, acetazolamide, induces metabolic acidosis and stimulates ventilation. It has been shown to be highly effective in treating most central apneas in congestive heart failure patients (31, 70) and has also been used in small numbers of OSA patients with mixed effects (56, 60, 64, 71). Most recently, Edwards et al. (15) showed that administration of acetazolamide administered in relatively high doses to 13 OSA patients (who were already undergoing CPAP treatment) over a 1-wk period resulted in a 50% reduction in median AHI, with about half of the patients showing greater than 30% reductions. In this comprehensive study the authors attributed treatment effects solely to a substantial reduction in plant gain as eupneic PetCO2 fell an average of 8 mmHg, with no significant alteration with treatment in pharyngeal collapsibility or arousal thresholds. The effectiveness of acetazolamide in reducing OSA appears to be comparable to what we have found with maintaining isocapnia and, as discussed above, we would attribute the effects of both of these treatments primarily to reduced plant gain effects on stabilizing motor output to both the upper airway and the chest wall. In turn, the consistency or magnitude of reduction in AHI with either of these treatments was substantially less than what we have found with hypercapnia and we would attribute this difference to a much more powerful effect of hypercapnia vs. either acetazolamide or isocapnia in recruiting upper airway dilator musculature and reducing airway resistance in addition to its effect on plant gain and central instability (see section above). We are aware of a prior case study which also reported complete relief of OSA with a few minutes of CO2 administration (39); however, we are not aware of pharmacological treatments to date (14, 38) which have been as consistent in markedly reducing OSA as that currently found with moderate hypercapnia.
Effects of Maintaining SaO2 > 95% Via Supplemental O2
Acute hyperoxia, even in healthy nonapneic subjects, will reduce chemoreflex controller gain for CO2 and widen the CO2 reserve (51, 74). Furthermore, carotid body denervation in canines will prevent apnea and periodic breathing induced via transient ventilatory overshoot and hypocapnia (9, 50). Accordingly, preventing intermittent hypoxemia via supplemental O2 administration has been shown to reduce many, but not all, central apneas and periodicities in most congestive heart failure patients with Cheyne-Stokes respiration (18, 22, 32, 42). However, with OSA, hyperoxia had relatively minor effects on AHI and was effective in a smaller percentage of patients and in some cases even increased the occurrence of obstructive apneas (and increased apnea length) (18, 28, 69). Wellman et al. (69) reported that supplemental O2 sufficient to maintain SaO2 ∼98% improved obstructive events significantly in half of his sample of 12 OSA patients and only in those patients with a high loop gain. Similarly, 7 of 19 of our patients reduced their OSA > 30% when we maintained SaO2 in the 95–98% range and 5 of the 7 also responded positively to the isocapnic treatment. However, unlike our isocapnic treatment, we were unable to predict treatment success with hyperoxia based on the patient's baseline plant or controller gains or CO2 reserve or Pcrit. Perhaps we might have increased predictive power of this treatment if we had also measured any change or lack thereof in controller and plant gains with the use of hyperoxia rather than depending strictly on our measurements of these characteristics under air breathing control conditions. We note that our isocapnia and especially the hypercapnia treatments were more effective in reducing AHI than hyperoxia in the same OSA patients and we speculate that this difference is attributable to 1) a greater reduction in plant gain with the isocapnic treatment than in controller gain with hyperoxia; and/or 2) a greater stimulatory role facilitating upper airway patency for the isocapnic and especially the hypercapnic treatments vs. the hyperoxic treatment, which may even have reduced central motor output to upper airway dilator muscles.
Conclusions
With respect to the effects of preventing transient hypocapnia on OSA, our findings are consistent with an important role for high chemoreflex gain, unstable central respiratory motor output, and narrowed CO2 reserve in both the pathogenesis and treatment of OSA in a significant number of patients with collapsible airways. At the same time these findings also show that not all OSA patients have increased chemoreceptor gain, and unstable central respiratory motor output as major influences underlying their cyclical OSA; accordingly, treatments aimed specifically at reducing controller and/or plant gain and thereby stabilizing central respiratory motor output are unlikely to be successful in the majority of severe OSA patients. Additional findings demonstrated that the use of continuous moderate hypercapnia was highly effective in the treatment of airway obstruction, apparently acting by both stabilizing central motor output as well as recruiting upper airway dilator musculature. Exactly how exogenous CO2 or, more likely, some pharmacological means of safely augmenting central respiratory motor output without increasing chemoreflex controller gain might be used in the treatment of OSA remains to be determined.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants 1RC-1-HL-099724-01 and HL-15469.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.X., D.F.P., and J.A.D. conception and design of research; A.X., D.F.P., M.C.T., Y.G., and J.E.F. performed experiments; A.X., M.C.T., Y.G., and J.E.F. analyzed data; A.X. and J.A.D. interpreted results of experiments; A.X. prepared figures; A.X. drafted manuscript; M.T., M.C.T., and J.A.D. edited and revised manuscript; J.A.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Ruth M. Benca, Paul E. Peppard, and Tim Juergens for kindly referring subjects for us, and Benjamin Dempsey and Tony Jacques for assistance with manuscript preparation. We also thank Dr. Michael Khoo who provided valuable suggestions for data interpretation.
The research work was performed at James B. Skatrud Pulmonary/Sleep Research Laboratory, VA Hospital, Madison, WI.
REFERENCES
- 1. Alex CG, Onal E, Lopata M. Upper airway occlusion during sleep in patients with Cheyne-Stokes respiration. Am Rev Respir Dis 133: 42– 45, 1986 [DOI] [PubMed] [Google Scholar]
- 2. Andreas S, Weidel K, Hagenah G, Heindl S. Treatment of Cheyne-Stokes respiration with nasal oxygen and carbon dioxide. Eur Respir J 12: 414– 419, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Asyali MH, Berry RB, Khoo MC. Assessment of closed-loop ventilatory stability in obstructive sleep apnea. IEEE Trans Biomed Eng 49: 206– 216, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Badr MS, Skatrud JB, Dempsey JA. Effect of chemoreceptor stimulation and inhibition on total pulmonary resistance in humans during NREM sleep. J Appl Physiol 76: 1682– 1692, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Badr MS, Skatrud JB, Simon PM, Dempsey JA. Effect of hypercapnia on total pulmonary resistance during wakefulness and during NREM sleep. Am Rev Respir Dis 144: 406– 414, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol 78: 1806– 1815, 1995 [DOI] [PubMed] [Google Scholar]
- 7. Beaumont M, Goldenberg F, Lejeune D, Marotte H, Harf A, Lofaso F. Effect of zolpidem on sleep and ventilatory patterns at simulated altitude of 4,000 meters. Am J Respir Crit Care Med 153: 1864– 1869, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Berssenbrugge A, Dempsey J, Iber C, Skatrud J, Wilson P. Mechanisms of hypoxia-induced periodic breathing during sleep in humans. J Physiol 343: 507– 524, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bowes G, Andrey SM, Kozar LF, Phillipson EA. Carotid chemoreceptor regulation of expiratory duration. J Appl Physiol 54: 1195– 1201, 1983 [DOI] [PubMed] [Google Scholar]
- 10. Cherniack NS, Longobardo GS. Mathematical models of periodic breathing and their usefulness in understanding cardiovascular and respiratory disorders. Exp Physiol 91: 295– 305, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Chow CM, Xi L, Smith CA, Saupe KW, Dempsey JA. A volume-dependent apneic threshold during NREM sleep in the dog. J Appl Physiol 76: 2315– 2325, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Cohn MA. Effects of zolpidem, codeine phosphate and placebo on respiration. A double-blind, crossover study in volunteers. Drug Saf 9: 312– 319, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol 90: 13– 24, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47– 112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edwards BA, Sands SA, Eckert DJ, White DP, Butler JP, Owens RL, Malhotra A, Wellman A. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol 590: 1199– 1211, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edwards BA, Sands SA, Feeney C, Skuza EM, Brodecky V, Wilkinson MH, Berger PJ. Continuous positive airway pressure reduces loop gain and resolves periodic central apneas in the lamb. Respir Physiol Neurobiol 168: 239– 249, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Francis DP, Willson K, Davies LC, Coats AJ, Piepoli M. Quantitative general theory for periodic breathing in chronic heart failure and its clinical implications. Circulation 102: 2214– 2221, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Franklin KA, Eriksson P, Sahlin C, Lundgren R. Reversal of central sleep apnea with oxygen. Chest 111: 163– 169, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Ghazanshahi SD, Khoo MC. Estimation of chemoreflex loop gain using pseudorandom binary CO2 stimulation. IEEE Trans Biomed Eng 44: 357– 366, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Giannoni A, Baruah R, Willson K, Mebrate Y, Mayet J, Emdin M, Hughes AD, Manisty CH, Francis DP. Real-time dynamic carbon dioxide administration: a novel treatment strategy for stabilization of periodic breathing with potential application to central sleep apnea. J Am Coll Cardiol 56: 1832– 1837, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Gilmartin G, McGeehan B, Vigneault K, Daly RW, Manento M, Weiss JW, Thomas RJ. Treatment of positive airway pressure treatment-associated respiratory instability with enhanced expiratory rebreathing space (EERS). J Clin Sleep Med 6: 529– 538, 2010 [PMC free article] [PubMed] [Google Scholar]
- 22. Hanly PJ, Millar TW, Steljes DG, Baert R, Frais MA, Kryger MH. The effect of oxygen on respiration and sleep in patients with congestive heart failure. Ann Intern Med 111: 777– 782, 1989 [DOI] [PubMed] [Google Scholar]
- 23. Haxhiu MA, van Lunteren E, Mitra J, Cherniack NS. Comparison of the response of diaphragm and upper airway dilating muscle activity in sleeping cats. Respir Physiol 70: 183– 193, 1987 [DOI] [PubMed] [Google Scholar]
- 24. Henke KG, Arias A, Skatrud JB, Dempsey JA. Inhibition of inspiratory muscle activity during sleep. Chemical and nonchemical influences. Am Rev Respir Dis 138: 8– 15, 1988 [DOI] [PubMed] [Google Scholar]
- 25. Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs. diaphragm muscle response to CO2 in rats. J Appl Physiol 92: 878– 887, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Hudgel DW, Chapman KR, Faulks C, Hendricks C. Changes in inspiratory muscle electrical activity and upper airway resistance during periodic breathing induced by hypoxia during sleep. Am Rev Respir Dis 135: 899– 906, 1987 [DOI] [PubMed] [Google Scholar]
- 27. Hudgel DW, Gordon EA, Thanakitcharu S, Bruce EN. Instability of ventilatory control in patients with obstructive sleep apnea. Am J Respir Crit Care Med 158: 1142– 1149, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Hudgel DW, Hendricks C, Dadley A. Alteration in obstructive apnea pattern induced by changes in oxygen- and carbon-dioxide-inspired concentrations. Am Rev Respir Dis 138: 16– 19, 1988 [DOI] [PubMed] [Google Scholar]
- 29. Iber C, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (1st ed.). Westchester, IL: American Academy of Sleep Medicine, 2007 [Google Scholar]
- 30. Iber C, Davies SF, Chapman RC, Mahowald MM. A possible mechanism for mixed apnea in obstructive sleep apnea. Chest 89: 800– 805, 1986 [DOI] [PubMed] [Google Scholar]
- 31. Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med 173: 234– 237, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Javaheri S, Ahmed M, Parker TJ, Brown CR. Effects of nasal O2 on sleep-related disordered breathing in ambulatory patients with stable heart failure. Sleep 22: 1101– 1106, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Jordan AS, White DP, Owens RL, Eckert DJ, Rahangdale S, Yim-Yeh S, Malhotra A. The effect of increased genioglossus activity and end-expiratory lung volume on pharyngeal collapse. J Appl Physiol 109: 469– 475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khayat RN, Xie A, Patel AK, Kaminski A, Skatrud JB. Cardiorespiratory effects of added dead space in patients with heart failure and central sleep apnea. Chest 123: 1551– 1560, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Khoo MC, Gottschalk A, Pack AI. Sleep-induced periodic breathing and apnea: a theoretical study. J Appl Physiol 70: 2014– 2024, 1991 [DOI] [PubMed] [Google Scholar]
- 36. Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol 53: 644– 659, 1982 [DOI] [PubMed] [Google Scholar]
- 37. Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, McGinley BM, Magnuson T, Schweitzer M, Smith PL, Patil SP. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol 104: 1618– 1624, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kohler M, Stradling JR. Pitfalls of clinical trials on pharmacological treatment for obstructive sleep apnoea: future directions. Expert Opin Investig Drugs 20: 1033– 1037, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Kuna S, Gillen J, Levine S. Effects of respiratory gases on the frequency and duration of obstructive apneic episodes in a patient with the sleep apnea-hypersomnolence syndrome. Respiration 43: 108– 113, 1982 [DOI] [PubMed] [Google Scholar]
- 40. Kuna ST, McCarthy MP, Smickley JS. Laryngeal response to passively induced hypocapnia during NREM sleep in normal adult humans. J Appl Physiol 75: 1088– 1096, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Lo YL, Jordan AS, Malhotra A, Wellman A, Heinzer RC, Schory K, Dover L, Fogel RB, White DP. Genioglossal muscle response to CO2 stimulation during NREM sleep. Sleep 29: 470– 477, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lorenzi-Filho G, Rankin F, Bies I, Douglas Bradley T. Effects of inhaled carbon dioxide and oxygen on Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med 159: 1490– 1498, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Maillard D, Thiercelin JF, Fuseau E, Rosenzweig P, Attali P. Effects of zolpidem versus diazepam and placebo on breathing control parameters in healthy human subjects. Int J Clin Pharmacol Res 12: 27– 35, 1992 [PubMed] [Google Scholar]
- 44. Manisty CH, Willson K, Wensel R, Whinnett ZI, Davies JE, Oldfield WL, Mayet J, Francis DP. Development of respiratory control instability in heart failure: a novel approach to dissect the pathophysiological mechanisms. J Physiol 577: 387– 401, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mateika JH, Millrood DL, Kim J, Rodriguez HP, Samara GJ. Response of human tongue protrudor and retractors to hypoxia and hypercapnia. Am J Respir Crit Care Med 160: 1976– 1982, 1999 [DOI] [PubMed] [Google Scholar]
- 46. McCann CC, Quera-Salva MA, Boudet J, Frisk M, Barthouil P, Borderies P, Meyer P. Effect of zolpidem during sleep on ventilation and cardiovascular variables in normal subjects. Fundam Clin Pharmacol 7: 305– 310, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Mebrate Y, Willson K, Manisty CH, Baruah R, Mayet J, Hughes AD, Parker KH, Francis DP. Dynamic CO2 therapy in periodic breathing: a modeling study to determine optimal timing and dosage regimes. J Appl Physiol 107: 696– 706, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meurice JC, Marc I, Series F. Influence of sleep on ventilatory and upper airway response to CO2 in normal subjects and patients with COPD. Am J Respir Crit Care Med 152: 1620– 1626, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Meza S, Giannouli E, Younes M. Control of breathing during sleep assessed by proportional assist ventilation. J Appl Physiol 84: 3– 12, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Carotid body denervation eliminates apnea in response to transient hypocapnia. J Appl Physiol 94: 155– 164, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. Am J Respir Crit Care Med 165: 1251– 1260, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol 61: 1438– 1443, 1986 [DOI] [PubMed] [Google Scholar]
- 53. Quera-Salva MA, McCann C, Boudet J, Frisk M, Borderies P, Meyer P. Effects of zolpidem on sleep architecture, night time ventilation, daytime vigilance and performance in heavy snorers. Br J Clin Pharmacol 37: 539– 543, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ravesloot MJ, de Vries N. Reliable calculation of the efficacy of non-surgical and surgical treatment of obstructive sleep apnea revisited. Sleep 34: 105– 110, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rice AJ, Nakayama HC, Haverkamp HC, Pegelow DF, Skatrud JB, Dempsey JA. Controlled versus assisted mechanical ventilation effects on respiratory motor output in sleeping humans. Am J Respir Crit Care Med 168: 92– 101, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Sakamoto T, Nakazawa Y, Hashizume Y, Tsutsumi Y, Mizuma H, Hirano T, Mukai M, Kotorii T. Effects of acetazolamide on the sleep apnea syndrome and its therapeutic mechanism. Psychiatry Clin Neurosci 49: 59– 64, 1995 [DOI] [PubMed] [Google Scholar]
- 57. Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med 181: 189– 193, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sands SA, Edwards BA, Kee K, Turton A, Skuza EM, Roebuck T, O'Driscoll DM, Hamilton GS, Naughton MT, Berger PJ. Loop gain as a means to predict a positive airway pressure suppression of Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med 184: 1067– 1075, 2011 [DOI] [PubMed] [Google Scholar]
- 59. Series F, Cormier Y, Desmeules M, La Forge J. Effects of respiratory drive on upper airways in sleep apnea patients and normal subjects. J Appl Physiol 67: 973– 979, 1989 [DOI] [PubMed] [Google Scholar]
- 60. Sharp JT, Druz WS, D'Souza V, Diamond E. Effect of metabolic acidosis upon sleep apnea. Chest 87: 619– 624, 1985 [DOI] [PubMed] [Google Scholar]
- 61. Skatrud JB, Dempsey JA. Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation. J Appl Physiol 55: 813– 822, 1983 [DOI] [PubMed] [Google Scholar]
- 62. Steens RD, Millar TW, Su X, Biberdorf D, Buckle P, Ahmed M, Kryger MH. Effect of inhaled 3% CO2 on Cheyne-Stokes respiration in congestive heart failure. Sleep 17: 61– 68, 1994 [DOI] [PubMed] [Google Scholar]
- 63. Szollosi I, Jones M, Morrell MJ, Helfet K, Coats AJ, Simonds AK. Effect of CO2 inhalation on central sleep apnea and arousals from sleep. Respiration 71: 493– 498, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Tojima H, Kunitomo F, Kimura H, Tatsumi K, Kuriyama T, Honda Y. Effects of acetazolamide in patients with the sleep apnoea syndrome. Thorax 43: 113– 119, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Verbraecken J, Willemen M, De Cock W, Wittesaele W, Govaert K, Van de Heyning P, De Backer W. Influence of longterm CPAP therapy on CO2 drive in patients with obstructive sleep apnea. Respir Physiol 123: 121– 130, 2000 [DOI] [PubMed] [Google Scholar]
- 66. Warner G, Skatrud JB, Dempsey JA. Effect of hypoxia-induced periodic breathing on upper airway obstruction during sleep. J Appl Physiol 62: 2201– 2211, 1987 [DOI] [PubMed] [Google Scholar]
- 67. Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, Gautam S, Owens RL, Malhotra A, White DP. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol 110: 1627– 1637, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz ES, Schory K, Edwards JK, White DP. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med 170: 1225– 1232, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol 162: 144– 151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. White DP, Zwillich CW, Pickett CK, Douglas NJ, Findley LJ, Weil JV. Central sleep apnea. Improvement with acetazolamide therapy. Arch Intern Med 142: 1816– 1819, 1982 [PubMed] [Google Scholar]
- 71. Whyte KF, Gould GA, Airlie MA, Shapiro CM, Douglas NJ. Role of protriptyline and acetazolamide in the sleep apnea/hypopnea syndrome. Sleep 11: 463– 472, 1988 [DOI] [PubMed] [Google Scholar]
- 72. Wilson CR, Satoh M, Skatrud JB, Dempsey JA. Non-chemical inhibition of respiratory motor output during mechanical ventilation in sleeping humans. J Physiol 518: 605– 618, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Xie A, Bedekar A, Skatrud JB, Teodorescu M, Gong Y, Dempsey JA. The heterogeneity of obstructive sleep apnea (predominant obstructive vs pure obstructive apnea). Sleep 34: 745– 750, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xie A, Skatrud JB, Puleo DS, Dempsey JA. Influence of arterial O2 on the susceptibility to posthyperventilation apnea during sleep. J Appl Physiol 100: 171– 177, 2006 [DOI] [PubMed] [Google Scholar]
- 75. Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med 165: 1245– 1250, 2002 [DOI] [PubMed] [Google Scholar]
- 76. Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med 168: 645– 658, 2003 [DOI] [PubMed] [Google Scholar]
- 77. Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 169: 623– 633, 2004 [DOI] [PubMed] [Google Scholar]
- 78. Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol 105: 1389– 1405, 2008 [DOI] [PubMed] [Google Scholar]
- 79. Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol 103: 1929– 1941, 2007. [DOI] [PubMed] [Google Scholar]