Abstract
Substantial mortality of in vitro manipulated porcine embryos is observed during peri-attachment development. Herein we describe our efforts to characterize the transcriptomes of embryonic disc (ED) and trophectoderm (TE) cells from porcine embryos derived from in vivo fertilization, in vitro fertilization (IVF), parthenogenetic oocyte activation (PA), and somatic cell nuclear transfer (SCNT) on days 10, 12, and 14 of gestation. The IVF, PA, and SCNT embryos were generated with in vitro matured oocytes and were cultured overnight in vitro before being transferred to recipient females. Sequencing of cDNA from the resulting embryonic samples was accomplished with the Genome Analyzer IIx platform from Illumina. Reads were aligned to a custom-built swine transcriptome. A generalized linear model was fit for ED and TE samples separately, accounting for embryo type, gestation day, and their interaction. Those genes with significant differences between embryo types were characterized in terms of gene ontologies and KEGG pathways. Transforming growth factor-β signaling was downregulated in the EDs of IVF embryos. In TE cells from IVF embryos, ubiquitin-mediated proteolysis and ErbB signaling were aberrantly regulated. Expression of genes involved in chromatin modification, gene silencing by RNA, and apoptosis was significantly disrupted in ED cells from SCNT embryos. In summary, we have used high-throughput sequencing technologies to compare gene expression profiles of various embryo types during peri-attachment development. We expect that these data will provide important insight into the root causes of (and possible opportunities for mitigation of) suboptimal development of embryos derived from assisted reproductive technologies.
Keywords: in vitro fertilization, parthenogenesis, somatic cell nuclear transfer, in vitro embryo production, Illumina, ART
in most mammalian species studied to date, only 50–70% of successfully fertilized oocytes will give rise to live, healthy offspring (reviewed in Refs. 20, 57, 79). Some embryos succumb to the effects of chromosomal abnormalities or faulty uterine environments, for example. But the underlying cause behind most early embryonic mortality is still poorly understood. Such inefficiency is concerning to human fertility clinical practitioners and to animal producers in agricultural settings as well. Embryos produced by assisted reproductive technologies (ART) are generally less fit and more prone to failure than in vivo produced embryos. It has been presupposed that oocyte maturation and embryo culture systems that are inadequate to promote proper development are primarily to blame, yet the precise mechanisms that contribute to deficiencies in developmental potential in in vitro manipulated embryos are unknown.
A large proportion of embryonic mortality in domestic mammals occurs before embryonic apposition and attachment to the uterus (reviewed in Refs. 19, 20, 79). Preattachment embryo growth includes cleavage-stage development and blastocyst formation and elongation. Experiments using artificially inseminated gilts demonstrate that early cleavage-stage development and blastocyst formation (until day 7 of development) in pigs is virtually 100% (30, 57). Therefore, the vast majority of early embryonic loss is likely to occur between day 7 and day 15–16, when embryonic attachment begins. Proper development of porcine embryos during this peri-attachment period involves dramatic morphological and structural as well as functional transformations, changes collectively referred to as “elongation,” as well as the emergence of two additional germ layers: endoderm and mesoderm. During this phase, the porcine blastocyst elongates from a one-millimeter sphere into a thread-like conceptus that can reach up to a meter in length (28, 29). Days 10–14 of gestation have been identified as a critical period of embryonic development: in vitro manipulated embryos demonstrate altered gross morphological and cytological characteristics in both embryonic disc (ED) and trophectoderm (TE) tissues when compared with in vivo produced embryos at similar stages of development (46). We surmised that ART-induced aberrations in global gene expression patterns would be evident during this period of elongation.
Recent advances in nucleic acid sequencing technologies have made it possible to undertake large-scale cDNA sequencing efforts to characterize relative gene expression patterns, even in extremely small and limiting samples. These so-called RNA-Seq experiments are characterized by the generation of millions of short sequencing “reads,” primarily by utilizing the sequencing platforms produced by Illumina (Genome Analyzer) or Applied Biosystems (SOLiD). We have applied these techniques to pre- and peri-attachment porcine embryo samples previously (see Refs. 8, 35, 59). Herein, we report our efforts to use high-throughput sequencing technologies to characterize gene expression patterns in ED and TE from day 10, day 12, and day 14 porcine embryos derived from artificial insemination [in vivo fertilization (IVV)], in vitro fertilization (IVF), somatic cell nuclear transfer (SCNT), and parthenogenetic oocyte activation (PA). We hypothesize that the in vitro gamete and embryo manipulations associated with these assisted reproductive technologies will induce enduring changes to gene expression patterns in peri-attachment stage embryos. Furthermore, we expect distinct patterns of gene disruption to occur in the different embryo types generated.
MATERIALS AND METHODS
All chemicals and other bioreagents were purchased from Sigma (St. Louis, MO), unless otherwise indicated.
Use and handling of animals were overseen and approved of by the Animal Care and Use Committee at the University of Missouri.
A concerted effort was made throughout this project to use as consistent a genetic background as possible: spermatozoa (IVV and IVF) and karyoplast donor cells (SCNT) were from half-sibling males; in vitro matured oocytes (IVF, SCNT, and PA) were purchased from the same commercial source (ART; Madison, WI), and all embryo recipients (IVV, IVF, SCNT, and PA) were bred and raised on the same swine farm facility at the University of Missouri (Columbia, MO). The IVV, IVF, and SCNT embryos were marked with an enhanced green fluorescent protein (eGFP) transgene derived from breeding stock produced and perpetuated at the University of Missouri (76).
Embryo Production and Sample Collection
IVV.
Embryos derived from artificial insemination (AI) served as controls for these experiments. Second- and third-cycle virgin gilts were artificially inseminated at 12 h and 24 h after first observed standing estrus with semen collected from a single, proven eGFP-transgenic boar according to standard industry husbandry practices.
IVF.
Semen from the same transgenic boar used for AI was frozen as per Wang et al. (72) and used for IVF as described elsewhere (1). Briefly, oocytes were fertilized for 5 h in a Tris-buffered fertilization medium at a concentration of 0.5 × 106 motile spermatozoa/ml and then washed twice before being placed into embryo culture medium.
SCNT.
The specific techniques utilized for SCNT are described elsewhere (40). Briefly, metaphase II (MII)-stage oocytes were enucleated by standard micromanipulation techniques. Karyoplast donors were selected from a mixed population of embryonic fibroblast cells expressing eGFP (76) and were placed into the perivitelline space of enucleated oocytes. Incorporation of the donor cell nuclei into the cytoplasm of the enucleated oocytes was accomplished by exposing cytoplast/karyoplast couplets to two 30 μs pulses of direct electrical current (1.2 kV/cm) in the presence of a calcium-rich fusion/activation medium (0.3 M mannitol, 1.0 mM CaCl2 · 2H2O, 0.1 mM MgCl2 · 6H2O and 0.5 mM HEPES; pH 7.3). Oocyte activation was accomplished by this same electrical stimulus.
PA.
Protocols for production of porcine parthenogenetic embryos have been published previously (34). MII-arrested oocytes were exposed to a brief electrical stimulus (see above) in the presence of a calcium-rich activation medium. These electrical pulses briefly cause voltage-dependent ion channels to open, allowing calcium influx. The resulting surge of free calcium ions serves to induce meiotic resumption and subsequent embryo development.
For IVF, SCNT, and PA production methods, embryos were produced and then cultured in PZM3 culture medium (82) in vitro overnight (15–18 h) at 39°C in 5% CO2 in air at saturating humidity until transfer into surrogate recipients. After overnight incubation, at least 100 embryos (range 100–159, average 136) were deposited into one oviduct of surrogate recipient females via surgical embryo transfer (ET). Occasionally, more embryos were produced in vitro than were needed for transfer. On these occasions (eight, three, and seven replicates for IVF, SCNT, and PA respectively), the superfluous embryos were cultured for 7 days in vitro under the conditions outlined above, and development to blastocyst was recorded. Fourteen gilts were used to generate AI embryos, and there were 12, 14, and 13 gilts that received embryos derived from IVF, SCNT, and PA, respectively. All embryo recipients were observed in standing estrus within the 24 h prior to ET. All embryo recipients were euthanized 10, 12, or 14 days after embryo transfer. We collected embryos by flushing the uterine horns with 30 ml of HEPES-buffered Tyrode's Lactate solution [see Lai and Prather (40) for formulation] supplemented with 0.1% (wt/vol) bovine serum albumin. ED and TE samples were collected from as many individual embryos as possible from each flush. Fine-gauge hypodermic needles were used to manually separate embryonic discs from surrounding tissue. It should be noted here that no effort was made to remove mesoderm or endoderm cells from the TE samples at any stage of embryo development, but for simplicity's sake, these samples will be referred to simply as TE throughout the rest of this paper. For spherical and ovoid embryos, all of the TE tissue that remained after removing the ED was harvested. For elongated, filamentous embryos, a small (∼5 mm) segment of the TE tissue was dissected from the embryo at a site ∼2 cm distal from the location of the embryonic disc. ED and TE samples were identified as having come from the same embryo, were snap-frozen in liquid nitrogen, and stored at −80°C until use.
RNA Extraction and cDNA Synthesis
ED samples from two embryos from each of three distinct recipient flushes were combined in preparation for RNA extraction. The paired TE samples from the embryos used for ED RNA collection were pooled in similar fashion. Total RNA was collected from the pooled samples using the AllPrep DNA/RNA Micro Kit (Qiagen, Gaithersburg, MD) according to the manufacturer's recommended protocol. First-strand cDNA synthesis was done using 3.5 μl of total RNA in each of three oligo dT-primed reverse transcription (RT) reactions performed for each sample using the SuperScript III First Strand Synthesis System (Invitrogen, Carlsbad, CA). The three RT reactions for each sample type were pooled together in an attempt to normalize any technical variation that might arise during the RT procedure. We used 15 μl of the pooled RT products in each of two second-strand cDNA synthesis reactions to generate double-stranded cDNA. Second-strand reactions were assembled into 150 μl reaction volumes, consisting of the following components: 15 μl of pooled first-strand RT cDNA product, 15 μl 10× buffer 2 (500 mM NaCl, 100 mM Tris·HCl, 100 mM MgCl2, 10 mM dithiothreitol) from New England Biolabs (Ipswich, MA), 1 μl RNase H (Invitrogen), 5 μl DNA Polymerase I (New England Biolabs), 4 μl 10 mM dNTP Mix (Promega, Madison, WI), 110 μl water. Second-strand synthesis reactions were incubated at 16°C for 4 h. Duplicate second-strand reactions were pooled together, and MinElute PCR purification columns (Qiagen) were used to purify the reactions. Double-stranded cDNA was eluted in 40 μl of 10 mM Tris·Cl (pH 8.5) and submitted to the University of Missouri Genomics Core for cDNA sequencing using the Genome Analyzer IIx (GAIIx) platform from Illumina (San Diego, CA). Prior to library preparation, cDNA was sheared to an average fragment length of 250 base pairs using the Bioruptor from Diagenode (Liege, Belgium) per technical parameters that had been established from previous empirical experimentation and observation (data not shown). We used 10 ng of fragmented double-stranded cDNA to prepare sequencing libraries according to protocols provided and validated by Illumina. Upon successful assembly of sequencing libraries, the samples were loaded at a concentration of 7 pM onto individual lanes of GAIIx flow cells and were sequenced per manufacturer-recommended cycling parameters. We sequenced 17 of the 24 samples on a single flow cell lane, while the remaining seven samples were sequenced on two flow cell lanes each, meaning that 31 lanes of data were generated from these 24 samples. These sequencing efforts resulted in millions of sequencing reads, 42 base pairs in length. Read count normalization and alignment were accomplished in a manner similar to what we have reported previously (35), with individual reads being aligned using the software program SOAP (42) to a custom swine transcriptome database (see Ref. 35 for details on database construction). Only reads aligning with two or fewer mismatches to exactly one database member were used in the final analysis.
Statistical Methods
A generalized linear model (6) was fit for the RNA-Seq counts for each discrete sequence within the custom transcriptomic database that had at least one read aligned. This model was fit for each cell type (ED or TE) separately and accounted for embryo type (with levels IVV, IVF, SCNT, PA), gestation days (with levels 10, 12, 14), and their interaction. The statistical analysis was performed by a custom routine written in R (70). All 24 factorial combinations of the three factors (cell type, embryo type, and gestation days) had at least one replicate in the experiment, while seven samples had two replicates, for a total sample size of 31. With additional resources, biological replication would have been preferable to technical, leading to increased statistical power (6, 33). While not optimal for statistical power, the design of this study does lend itself to an appropriate statistical analysis. The generalized linear model here used a Poisson distribution, which has been shown to be valid and most appropriate for technical replicates in RNA-Seq data (24, 45, 60). Across all genes, P values for overall embryo type and interaction effects were converted to q values (66) to control the false discovery rate (10) at 0.10. Post hoc tests between embryo types (but still in each cell type separately) were performed for genes with significant embryo type effects but nonsignificant interaction terms (52). The specific post hoc tests compared IVV vs. IVF (to test for an overall in vitro culture effect), SCNT vs. IVF (to test for an overall cloning effect), and PA vs. IVF (to test for an overall maternal chromosome only effect). Genes showing significance in any of these three post hoc tests (after Bonferroni correction within each gene's model) were characterized in terms of their associated Gene Ontology (GO) terms (5) and KEGG pathways (37). This test of overrepresentation/underrepresentation was performed by a conditional hypergeometric test (2, 9) as implemented in the GOstats package (23) for Bioconductor (31). P values for the GO terms and KEGG pathways were also converted to q values to control the false discovery rate.
In preparation for hierarchical clustering, normalized read counts were log(10)-transformed for all genes shown to be differentially expressed in at least one pairwise comparison between embryo types across all three time points evaluated. These log-transformed data points were then subjected to unsupervised bidirectional hierarchical clustering using the MultiExperiment Viewer (MeV) module of the TM4 Microarray Software Suite (63, 64). Euclidian distance and average linkage metrics were employed for cluster generation. Using the same log-transformed data points for all differentially expressed genes (DEGs), a principal component analysis of cell type, gestation day, and embryo production method was accomplished using the MeV module.
Quantitative PCR
The manufacturer's recommended protocol was used for quantitative PCR gene expression using the BioMark system from Fluidigm (South San Francisco, CA). This protocol incorporates the use of a DNA binding dye EvaGreen (Biotium, Hayward, CA) for analyzing expression of up to 48 genes for each of 48 samples on Fluidigm's 48.48 Dynamic Array Integrated Fluidic Circuits (IFC). A panel of 48 genes was selected for analysis by qPCR, in an effort to validate the overall consistency between RNA-Seq and qPCR. Genes were selected for qPCR analysis without regard for differential expression in the RNA-Seq experiment. The genes selected for qPCR analysis fell broadly into one of eight functional categories: housekeeping (EIF4A1, GAPDH, HSP90AA1, RPN1, TAF11), apoptosis (ATM, BCL2L1, CASP3, TP53, XIAP), epigenetic modifiers (ASH2L, DMAP1, DNMT1, DNMT3A, DNMT3B, EHMT2, EZH2, HDAC3, SIRT1), potentially imprinted (GNAS, GRB10, IGF2, IGF2R, NDN, NNAT, PEG10, UBE3A), maternal effect (BMP15, GDF9, MOS, NOBOX, ZAR1, ZP3), pluripotency (KLF4, LIN28, MYC, NANOG, POU5F1, SOX2), sexing (HPRT1, SRY), and trophoblast function (ASCL2, CYP17A1, ELF5, HAND1, HSD17B1, KRT8, TEAD4). Gene and PCR primer information for these genes are presented in Supplemental Table S1.1 Primer sets were validated previously (data not shown) and were determined to have similar amplification efficiencies: the coefficient of variation for amplification efficiency across all 48 assays was 0.0916, and only four of the 48 assays had amplification efficiencies that deviated >15% from optimum.
A specific target amplification (STA) was initially performed to enrich each sample prior to quantitative PCR thermal cycling for specific genes of interest. In preparation, a primer mix was prepared by pooling 1 μl of each primer pair (at 20 μM each, from Fluidigm) to form a 200 nM primer mix. For STA thermal cycling, each reaction was composed of 1.25 μl of this primer mix, 2.5 μl of the TaqMan PreAmp Master Mix (Applied Biosystems, Foster City, CA), and 1.25 μl of the cDNA. The reactions were activated at 95°C for 10 min and then amplified for 14 cycles (95°C for 15 s then 60°C for 4 min). Following STA thermal cycling, each reaction was treated with Exonuclease I (ExoI, New England Biolabs) to remove any unincorporated primers. The Exonuclease reaction solution was made using 1.4 μl water, 0.2 μl ExoI Reaction Buffer, and 0.4 μl ExoI for each reaction. Two microliters of ExoI reaction solution was added to every STA reaction and then placed in a thermal cycler at 37°C for 30 min to allow the digest to run to completion. The reactions were then inactivated at 80°C for 15 min. Each reaction was then diluted by adding 18 μl of water to the 7 μl reaction (volume of STA reaction + ExoI) for a total volume of 25 μl. Reactions were then stored at −20°C until further use.
In preparation for running the Fluidigm chip on the BioMark, a sample Pre-Mix solution was applied to the Fluidigm 48.48 Dynamic Array IFC. This solution consisted of 2.5 μl of the 2× TaqMan Gene Expression Master Mix (Applied Biosystems), 0.25 μl of 20× DNA Binding Dye Sample Loading Reagent (Fluidigm), 0.25 μl EvaGreen DNA Binding Dye (Biotium), and 2 μl of the diluted ExoI-treated STA sample. Each sample mix solution was vortexed for 20 s and centrifuged for 30 s. The assay (primer) mix solution was then made for every primer set by using 2.5 μl of the 2× Assay Loading Reagent (Fluidigm), 0.25 μl water, and 2.25 μl of each 20 μM Forward and Reverse Primer Mix. All assay mixes were vortexed for 20 s and centrifuged for 30 s. The IFC chip was primed by injecting 300 μl of control line fluid into each accumulator on the chip and then allowing the IFC Controller MX machine to run the Primer (113x) script. When priming had finished, 5 μl of each assay and 5 μl of each sample were pipetted into their respective inlets on the chip and the chip was returned to the IFC Controller for chip loading. The chip was then placed in the BioMark thermal cycler for quantitative PCR. Cycling parameters included a 5 min initial enzyme activation step, followed by 35 denaturation/extension cycles (95°C for 15 s followed by 60°C for 60 s), and a 3 min final extension cycle. A melt curve analysis was performed following PCR amplification to assess the quality of each amplicon. Quantitative PCR reactions were performed in triplicate for each sample type/primer set combination.
Data was analyzed via the Fluidigm Real-Time PCR Analysis Software. This software uses the CT value determined through thermal cycling to quantitate the ΔCT value by normalizing through a selected housekeeping gene (HSP90AA1). This software also assessed the melting curve to determine the quality of each reaction. We then calculated ΔΔCT values by subtracting the ΔCT of each experimental sample from the ΔCt of a calibrator sample (universal pig reference cDNA sample; Ref. 75) for each gene.
RESULTS
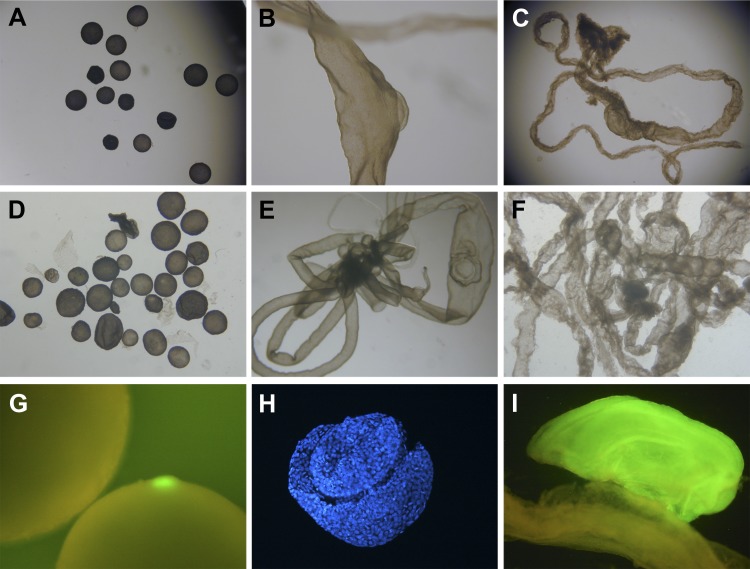
When more in vitro manipulated embryos were produced than were required for embryo transfer, those supernumerary embryos were maintained in culture in the laboratory, to assess development in vitro. Embryos derived from PA were the most successful at developing in vitro, with 30% (87/290, 7 replicates) of activated oocytes developing to blastocyst stage by day 7. Approximately 15.3% (71/464, 8 replicates) of IVF embryos developed to the blastocyst stage. The SCNT embryos were the least successful at developing in vitro, with 11.8% (10/85, 3 replicates) of activated couplets developing into blastocysts by day 7. While in vitro developmental potential may have varied somewhat between embryo production technique, no obvious differences were noted in the ability of the distinct embryo types to develop successfully after embryo transfer to the prescribed collection days: only seven of the 53 gilts euthanized for embryo collections were open or failed to provide sufficient embryo numbers at the time of flushing (3/14 IVV, 1/12 IVF, 3/14 SCNT, and 0/13 PA). Absolute embryo numbers recovered from flushes were not determined, because the day 12 and day 14 embryos were flushed as filamentous masses that were impossible to untangle. No obvious discrepancies in embryo recovery rates were noted between embryo production types. Embryos derived from SCNT and PA occasionally (but not consistently) appeared to be slightly retarded in their development (spherical or ovoid embryos on day 14; e.g., Ref. 14) compared with IVV and IVF embryos for a given collection day: Of embryos selected for sampling, there was an 11.3% incidence (from 2/4 litters) of spherical/ovoid day 14 embryos, and a 13.3% incidence (from 3/4 litters) of ovoid only day 14 embryos for SCNT and PA embryo types, respectively, whereas no spherical or ovoid embryos were observed in any IVF or IVV day 14 embryo flushes. Only samples at “normal” developmental stages were selected for downstream molecular analyses. Representative images of day 10, day 12, and day 14 embryos derived from IVV, IVF, SCNT, and PA can be seen in Fig. 1.
Fig. 1.
Porcine embryos from days 10, 12, or 14 of gestation. Representative images of embryos flushed from reproductive tracts of pregnant gilts. A, D, G: embryos flushed from gilts on day 10 after embryo transfer; B, E, H: embryos flushed on day 12; C, F, I: embryos flushed on day 14. A, B, C: in vivo-derived embryos on days 10, 12, and 14 of gestation, respectively. D: day 10 parthenogenetic embryos. E: day 12 embryo derived from in vitro fertilization (IVF). F: day 14 embryo derived from somatic cell nuclear transfer (SCNT). G: fluorescence micrograph showing 2 day 10 embryos generated by artificial insemination with semen from a boar hemizygous for an enhanced green fluorescence transgene. Note the bright green fluorescence emanating from the embryonic disc (ED). The ED from the second embryo is not in view. H: ED sample dissected from a day 12 SCNT embryo and stained with Hoechst 33342 to show nuclei of cells. I: highly fluorescent ED sample from a day 14 IVF embryo sitting in close apposition to its corresponding trophectoderm (TE) sample, which displays much lower levels of transgene expression. Fertilization was performed with spermatozoa from the same boar utilized to generate the embryos in G.
Illumina sequencing was effective at producing large numbers of high-quality sequencing reads from all samples submitted. It is significant to note that, on average, 73% of the reads generated were culled before alignment, because of low quality (5.4% of total) or high homology to endogenous “contaminants” such as 18S ribosomal RNA, mitochondrial DNA, etc. (67.8% of total reads). Even after sustaining the loss of these reads, 4.82 × 107 reads remained that aligned with two or fewer mismatches to the putative transcript sequences within our custom swine transcriptome database (an average of 1.55 × 106 reads per sample). Of those reads that aligned to the transcripts in the database, however, ∼37.5% matched more than one member of the database with high homology and so were not included in further analyses. Overall, from the 31 sequencing lanes run, 30,061,950 reads, an average of 969,740 reads per lane, aligned to exactly one member of the database and were used in the downstream statistical analyses. Table 1 provides summary data for these alignment statistics for ED and TE samples, respectively. Supplemental Table S2 provides a more thorough documentation of the trimming, purging, and alignment statistics for all samples combined. It should be noted that alignment statistics were extremely low for one sample in particular (10-PA-ED), with only ∼1.5% of the total reads generated for that sample aligning to the database, and <1% aligning uniquely.
Table 1.
Short read alignment summary
| Sample | Reads Aligned Uniquely | % |
|---|---|---|
| 10-IVV-ED | 710,646 | 13.8 |
| 10-IVV-TE | 737,269 | 24.5 |
| 10-IVF-ED | 673,960 | 8.6 |
| 10-IVF-TE | 1,590,733 | 41.1 |
| 10-SCNT-ED | 870,343 | 21.2 |
| 10-SCNT-TE | 889,254 | 34.1 |
| 10-PA-ED | 195,839 | 0.9 |
| 10-PA-TE | 1,738,151 | 32.5 |
| 12-IVV-ED | 2,179,666 | 36.4 |
| 12-IVV-TE | 1,420,081 | 42.9 |
| 12-IVF-ED | 1,210,916 | 32.9 |
| 12-IVF-TE | 1,646,786 | 42.2 |
| 12-SCNT-ED | 1,836,592 | 17.8 |
| 12-SCNT-TE | 870,940 | 30.7 |
| 12-PA-ED | 2,817,828 | 31.3 |
| 12-PA-TE | 1,378,641 | 18.6 |
| 14-IVV-ED | 764,270 | 25.9 |
| 14-IVV-TE | 1,107,690 | 35.3 |
| 14-IVF-ED | 1,573,189 | 38.9 |
| 14-IVF-TE | 2,073,458 | 26.7 |
| 14-SCNT-ED | 1,301,997 | 28.3 |
| 14-SCNT-TE | 1,197,472 | 39.0 |
| 14-PA-ED | 820,979 | 30.9 |
| 14-PA-TE | 455,250 | 17.7 |
| Maximum | 2,817,828 | 42.9 |
| Minimum | 195,839 | 0.9 |
| Average | 1,252,581 | 28.0 |
Summary of alignment statistics across all lanes of data for all samples analyzed. Presented in the “Reads Aligned Uniquely” column are the total numbers of reads (summed across replicates, if applicable) that aligned with 2 or fewer mismatches to exactly 1 database member. The data in the “%” column show what percentage of the total number of reads (after purging of endogenous “contaminants”) aligned uniquely to the custom database for each sample. Within the “sample” column, “10,” “12,” and “14” refer to embryos collected on gestational days 10, 12, and 14, respectively. IVV, in vivo fertilized; IVF, in vitro fertilized; SCNT, somatic cell nuclear transfer; PA, parthenogenetic activation; ED, embryonic disc; TE, trophectoderm. For more detailed information regarding the derivation of these summary statistics, readers are referred to Supplemental Table S2.
A spreadsheet containing the full data set of all normalized read counts for all samples is available as supplemental data, hosted at the authors' institutional websites (see endnote for URL). Interested parties are invited to download the data set for full access to the concrete data associated with this sequencing effort.
Alignment of sequencing reads from all 31 lanes of data to the custom swine transcriptome database resulted in at least one read aligned to over half of the total database members (41,693/83,126 = 52.56%). Vertebrate RefSeq annotation was unavailable for 9,339 of those, and Human RefSeq annotation was not applicable for 20,174 of the 41,693 members represented by our sequencing effort. After those database members with unknown annotation and removing duplicates based on identical RefSeq IDs had been accounted for, sequencing reads from the current effort aligned to database members representing 14,455 unique human RefSeq members with annotation, and 21,068 unique vertebrate RefSeq members with annotation. It should be emphasized, however, that only 14,447 (34.7%) of the 41,693 transcripts detected had an average read count (across all 31 sequencing lanes) of 10 reads or more, and more than one-third (n = 14,248; 34.2%) of the total transcripts detected were represented by an average of fewer than one read. A summary of the statistical pairwise comparisons of ED and TE tissues between each embryo type and its most relevant control sample is presented in Table 2. There were 2,677 transcripts with significantly different read counts in at least one pairwise comparison between embryo types across all gestation days in either ED or TE. There was some overlap of DEGs between comparison groups. The overlap of differential gene expression between embryo types is depicted using area-proportional Venn diagrams (78) in Supplemental Fig. S1 (see endnote for URL). The 2,677 DEGs could be condensed to 1,908 unique members that were differentially expressed in ED or TE of different embryo types across all gestation days. Of the 1,908 unique genes, 1,734 (90.9%) had human RefSeq annotation, and 1,790 (93.8%) had vertebrate RefSeq annotation.
Table 2.
Summary of statistical and gene set enrichment analyses
| Tissue | Up In | Versus | Genes | BP | MF | CC | KEGG |
|---|---|---|---|---|---|---|---|
| ED | IVV | IVF | 165 | 0 | 0 | 0 | 1 |
| IVF | IVV | 140 | 289 | 42 | 24 | 0 | |
| ED | SCNT | IVF | 124 | 0 | 0 | 0 | 0 |
| IVF | SCNT | 290 | 206 | 15 | 26 | 0 | |
| ED | PA | IVF | 150 | 143 | 4 | 22 | 0 |
| IVF | PA | 54 | 113 | 130 | 14 | 0 | |
| TE | IVV | IVF | 185 | 0 | 0 | 13 | 0 |
| IVF | IVV | 349 | 46 | 18 | 36 | 9 | |
| TE | SCNT | IVF | 142 | 311 | 0 | 8 | 1 |
| IVF | SCNT | 442 | 11 | 19 | 20 | 0 | |
| TE | PA | IVF | 167 | 124 | 70 | 15 | 1 |
| IVF | PA | 469 | 1 | 0 | 45 | 1 |
Summary of the numbers of “genes”' (database members), Gene Ontology (GO) terms, and signaling pathways demonstrated to be significantly enriched in each embryo type, across all gestation days, relative to its most appropriate control sample. BP, GO:Biological Process; MF, GO:Molecular Function; CC, GO:Cellular Component; KEGG, Kyoto Encyclopedia of Genes and Genomes signaling pathways. For example, 140 genes were upregulated in IVF ED samples relative to ED samples from IVV embryos, and a gene set enrichment analysis showed that there were 289 “Biological Process” terms, 42 “Molecular Function” terms, and 24 “Cellular Component” terms enriched for in IVF vs. IVV ED samples. Using the KEGG database, we show no cellular signaling pathways to be significantly affected.
Functional descriptions of the differentially expressed transcripts between embryo types are summarized in Tables 2–4. Presented in Table 2 are the numbers of GO categories or KEGG pathways that were significantly enriched between each embryo type and its appropriate control sample. Table 3 and Table 4 contain examples of significantly enriched GO terms and signaling pathways especially relevant to early embryo development in ED and TE, respectively. The full data set, including normalized read counts and sequence information for each transcribed region, complete lists of all differentially expressed transcripts and full results of gene set enrichment analyses for the pairwise comparisons between embryo types, can be found in the supporting information associated with this manuscript (see endnote for URL).
Table 3.
Gene set enrichment analysis results: ED
| Term/Pathway | Up In | Compared With | Database | Sig. Level |
|---|---|---|---|---|
| Chromatin modification | IVF | SCNT | GO:BP | 0.00005 |
| Nucleocytoplasmic transport | PA | IVF | GO:BP | 0.002 |
| mRNA metabolic process | PA | IVF | GO:BP | 0.002 |
| Gene silencing by RNA | IVF | SCNT | GO:BP | 0.004 |
| Epigenetic regulation of gene expression | IVF | SCNT | GO:BP | 0.007 |
| Dosage compensation by X-inactivation | PA | IVF | GO:BP | 0.008 |
| TGFB signaling | IVV | IVF | KEGG | 0.00999 |
| JNK cascade | IVF | IVV | GO:BP | 0.02 |
| Histone methyltransferase activity | IVF | IVV | GO:MF | 0.039 |
| MAPKKK cascade | IVF | PA | GO:BP | 0.04 |
| BMP signaling pathway | IVV | IVF | GO:BP | 0.09 |
GO terms and KEGG Pathways enriched in ED for each embryo type relative to its most appropriate control. Sig. Level is the q value or false discovery rate-adjusted P value for the term or pathway.
Table 4.
Gene set enrichment analysis results: TE
| Term/Pathway | Up In | Compared With | Database | Sig. Level |
|---|---|---|---|---|
| Protein processing in endoplasmic reticulum | IVF | PA | KEGG | 0.0004 |
| Protein catabolic process | IVF | IVV | GO:BP | 0.002 |
| Cellular catabolic process | SCNT | IVF | GO:BP | 0.007 |
| Proteasomal ubiquitin-dependent protein catabolic process | SCNT | IVF | GO:BP | 0.0077 |
| Pyrimidine metabolism | SCNT | IVF | KEGG | 0.018 |
| Chromatin modification | IVF | IVV | GO:BP | 0.024 |
| Neurotrophin signaling | PA | IVF | KEGG | 0.025 |
| ErbB signaling pathway | IVF | IVV | KEGG | 0.03 |
| Gene silencing by RNA | PA | IVF | GO:BP | 0.03 |
| Ubiquitin mediated proteolysis | IVF | IVV | KEGG | 0.03 |
| Germ cell development | PA | IVF | GO:BP | 0.031 |
| mRNA 3′-end processing | IVF | SCNT | GO:BP | 0.039 |
| SAPK signaling cascade | PA | IVF | GO:BP | 0.042 |
| N-glycan biosynthesis | IVV | IVF | KEGG | 0.059 |
GO terms and KEGG Pathways enriched in TE for each embryo type relative to its most appropriate control.
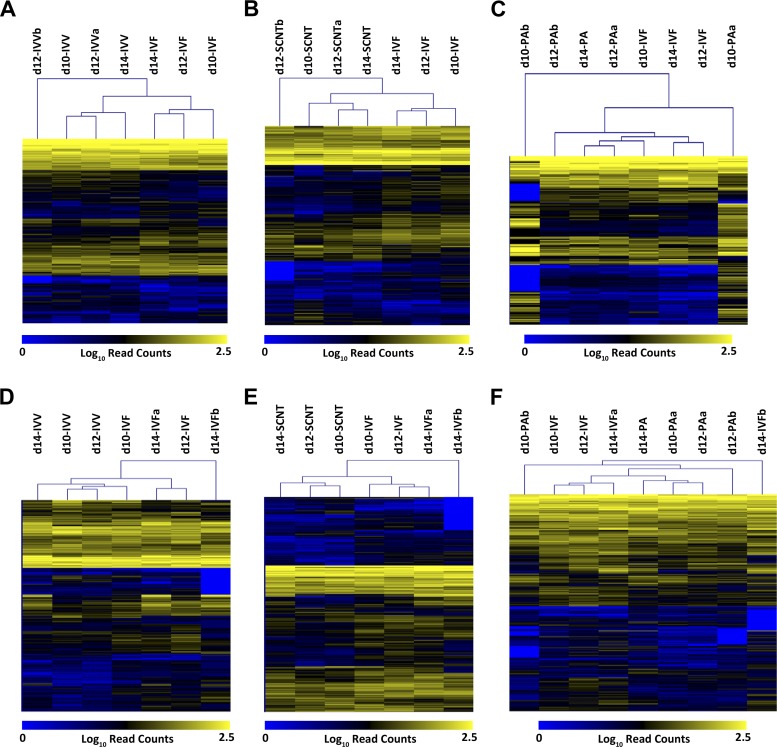
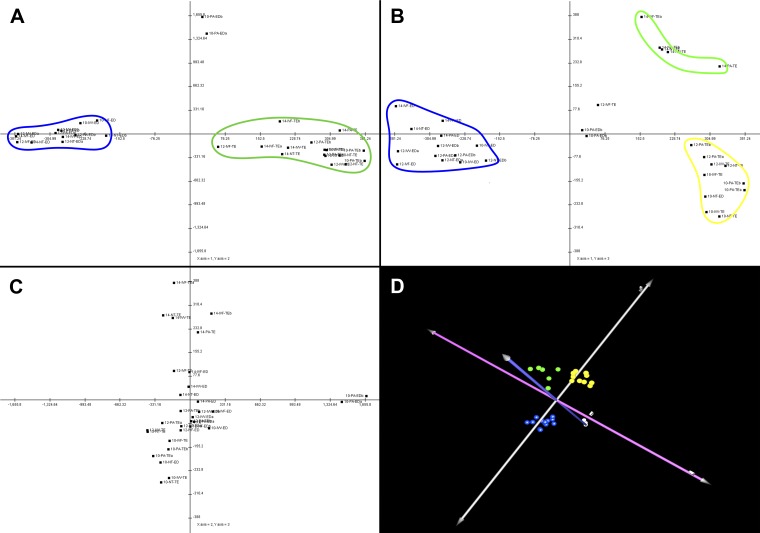
Unsupervised bidirectional hierarchical clustering of differentially expressed transcripts for each pairwise comparison undertaken shows clear and consistent separation of samples by embryo type (see Fig. 2). Principal component analysis of all 1,908 differentially expressed transcripts for all samples concurrently, however, showed only the clear separation of samples by tissue type (Fig. 3). No separation by day or by embryo type was readily apparent for the ED samples, although some separation by day was apparent in TE samples, irrespective of embryo type.
Fig. 2.
Heat maps showing relative expression levels of genes differentially expressed between embryo types. For each pairwise comparison between sample types, normalized read counts for the transcripts shown to be differentially expressed across all gestation days were log transformed and subjected to unsupervised bidirectional hierarchical clustering. Within the heat map proper, yellow indicates a high number of aligned reads (high expression), blue indicates very low numbers of reads (low expression), and grey indicates zero reads, or missing values. The dendrograms above the heat maps illustrate the calculated relationships between samples and genes, respectively. A: in vivo fertilization (IVV) vs. IVF ED; B: IVF vs. SCNT ED; C: IVF vs. parthenogenetic oocyte activation (PA) ED; D: IVV vs. IVF TE; E: IVF vs. SCNT TE; F: IVF vs. PA TE.
Fig. 3.
Principal component analysis. Principal component analysis (PCA) of the 31 sequencing lanes was undertaken using all 1,908 differentially expressed genes as data points. The analysis shows clear separation by tissue type and some separation of TE by day of gestation. No clear distinction of samples by embryo production method was noted. A: samples plotted on PC1 (x-axis) vs. PC2 (y-axis). The blue line surrounds all the ED samples (except for the 2 day 10 ED samples from parthenogenetic embryos, which were consistent outliers in this experiment due to poor sequencing); the green line encircles the trophoblast (TE) samples. B: samples plotted on PC1 (x-axis) vs. PC3 (y-axis). The blue line surrounds the ED samples again. The green line surrounds the day 14 TE samples, and the yellow line surrounds the day 10 and day 12 TE samples (except for the day 12 TE sample from IVF embryos, which was an outlier on this plot). C: PC2 (x-axis) vs. PC3 (y-axis). This principal component was clearly driven by the differences between the day 10 PA ED samples (which didn't sequence well) and all the rest of the samples; no further clustering was noted on this plot. D: 3-dimensional rendering of the PCA. The axes have been rotated to highlight the separation of the distinct clusters. Blue spheres represent the ED samples; the green spheres represent the “late” TE samples, and the yellow spheres represent the “early” TE samples. Principal components 1, 2, and 3 are represented by the silver, blue, and pink axes, respectively.
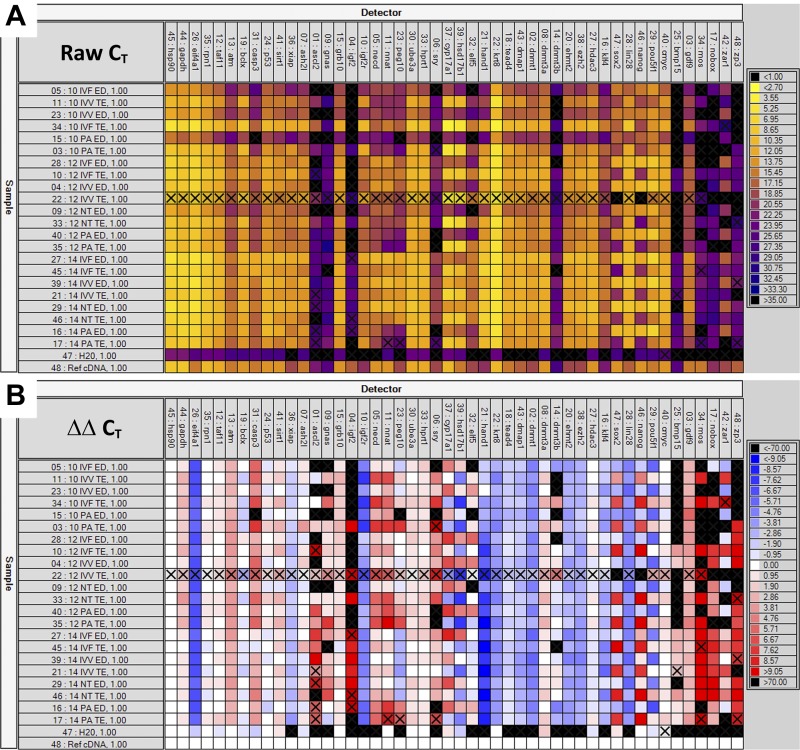
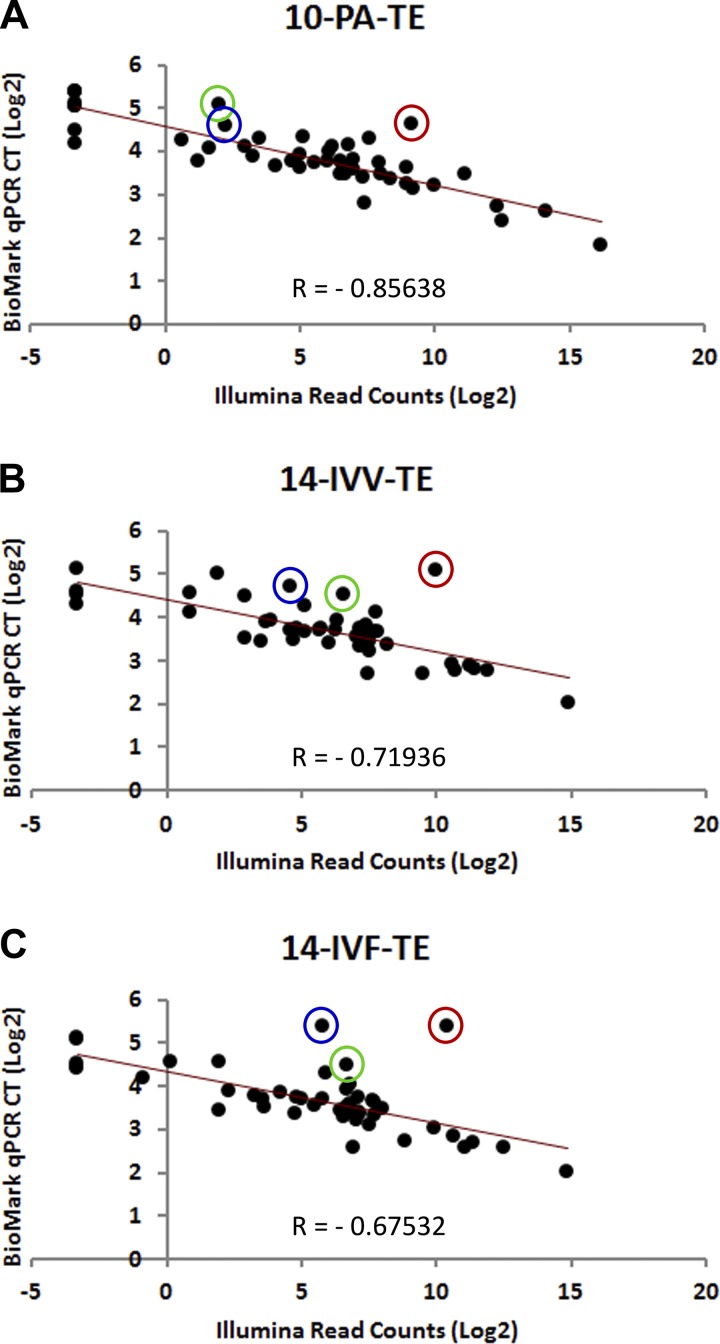
Quantitative PCR analysis of 48 genes using the microfluidics-driven BioMark system from Fluidigm yielded results that were consistent with the sequencing data. Images of representative quantitative PCR data are presented in Fig. 4, and the summarized data are presented as Supplemental Tables S3, S4, and S5 (for ED, TE, and ED/TE ratio, respectively). The coefficients of correlation between the number of sequencing reads and the CT values for all 48 genes within a tissue sample averaged −0.722 and ranged from −0.452 to −0.856. [The least significant correlation value (−0.452) corresponds to the 10-PA-ED sample noted above as being represented by a very low number of aligned reads. If this outlier were removed from consideration, the average correlation coefficient would be −0.735, with the least significant correlation being −0.675.] Scatter plots depicting the inverse relationship between read counts (Illumina) and CT values (BioMark) of representative samples are presented in Fig. 5.
Fig. 4.
Heat map depiction of quantitative PCR (qPCR) data. qPCR was performed with the BioMark system from Fluidigm. Genes are listed across the top of each panel and are grouped by purported function. Samples are listed vertically along the left side of the images. Color code keys are found on the right side of each respective panel. A: raw CT values for all 48 genes in all 22 experimental samples, plus a universal reference cDNA sample and a negative control sample. Lower CT values (higher expression) are brighter colors, while higher CT values (lower expression) are darker colors. B: ΔΔCT values for all experimental samples and assays; the universal reference cDNA was used as the calibrator sample, and HSP90 was used as the reference housekeeping gene. More intense blues represent higher normalized expression levels relative to the universal reference; more intense reds indicate lower expression relative to the reference sample. Only the raw CT values were used in validating the sequencing data. The ΔΔCT values serve to illustrate the dramatic variation that exists, especially between tissue types, but also between different embryo types and across gestation days.
Fig. 5.
Scatter plots depicting the correlations between qPCR and Illumina sequencing data. Triplicate qPCR reactions were run for 48 genes of interest. The average CT values for the three reactions for each gene/sample combination were log(2)-transformed and plotted against log(2)-transformed normalized read counts from the Illumina sequencing data. Presented here are 3 representative plots. A: the sample with the best correlation between qPCR and sequencing data (10-PA-TE). B: the scatter plot for a sample with a correlation coefficient very similar to the average correlation coefficient across all samples (14-IVV-TE). C: one of the worst correlations between qPCR and sequencing data (14-IVF-TE). The negative correlation is expected, as higher read counts (higher gene expression) correspond with lower CT values. The 3 circled points represent 3 qPCR assays (red, DNMT3B; blue, GNAS; green, IGF2) that showed consistently poor correlation between the qPCR data and the sequencing data, as discussed elsewhere.
DISCUSSION
To our knowledge, this is the first description of global gene expression patterns in peri-attachment porcine embryos generated by ART. Additionally, this effort is a further development and demonstration of the utility of high-throughput sequencing technologies in carrying out genome-scale experimentation in nontraditional model species.
With the advent of so-called “next-generation” sequencing technologies, it has become possible to generate large numbers of short nucleic acid sequencing reads that relate in identity and abundance to the population of nucleic acids subjected to sequencing. Initially, these technologies were used primarily for whole genome resequencing, characterization of allele frequencies, and identification of single nucleotide polymorphisms. Mortazavi et al. (50), Marioni et al. (45), and Rosenkranz et al. (62) were among the first to pioneer the utilization of high-throughput short-read sequencing technologies for transcriptional profiling purposes. Today, RNA-Seq is finding wider and wider application, with potential advantages and some unique challenges compared with other technologies (i.e., solid-state arrays), which have been reviewed elsewhere (44, 54, 73). It should be noted that for the research presented herein, the number of reads aligned per sample type is somewhat lower than that recommended for RNA-Seq “best practices,” as put forward by the Encyclopedia of DNA Elements Consortium (21). In no way does this invalidate the data presented here, as these experiments should provide valuable insight into poorly understood developmental phenomena. But it should not be inferred that this dataset is definitive in its description of time-, tissue-, and treatment-associated changes in gene expression patterns during peri-implantation porcine embryo development, and interpretation of expression profiles and GO analyses (and the discussion of such in the paragraphs that follow) should be undertaken with circumspection.
The primary purpose of this research was to test the hypothesis that assisted reproductive technologies could have profound effects on patterns of gene expression up to and including the period of peri-attachment development in porcine embryos. The in vitro manipulations used in these experiments were limited to in vitro oocyte maturation, embryo production, and a short period of in vitro embryo culture before transfer into surrogate recipient females. Comparisons in patterns of relative gene expression between embryo production methods were performed in a pair-wise manner, comparing each experimental method to its most appropriate control: IVF to IVV, SCNT to IVF, and PA to IVF as well. The first of these pairwise comparisons (IVV vs. IVF) allowed for a direct evaluation of genes responsive only to the in vitro manipulations, with the embryo production method (oocyte fertilized by spermatozoon) held constant. The remaining comparisons (SCNT vs. IVF and PA vs. IVF) revealed those genes and pathways that may be altered in response to the different embryo production methods without the potentially confounding effects of those redundant genes altered by in vitro culture.
The potential local and/or systemic effects of surgical embryo transfer on uterine physiology and embryo development are acknowledged. And, although there were no obvious suggestions that some or all of the changes in gene expression between IVV and IVF embryos were in direct response to surgery-induced maternal immunomodulation, that possibility cannot be entirely discounted. These effects were controlled for in the other pairwise comparisons, however, as all of the surrogates for in vitro manipulated embryos shared a common surgical insult upon embryo transfer.
The observation that many transcripts (305 and 534 in ED and TE, respectively) were shown to be significantly altered in embryos fertilized in vitro- compared with in vivo-fertilized embryos was somewhat unexpected, since previous efforts from our laboratory using a microarray approach yielded significantly fewer DEGs (n = 30) between in vivo- and in vitro-fertilized embryos at the blastocyst stage (77). Significant differences in the experimental platforms, statistical analyses, and embryo stages might all be invoked as reasons behind this seeming discrepancy. In support of the suggestion that the experimental platform can have profound influences on experimental results even in very similarly conceived experimental designs, Miles et al. (48) used serial analysis of gene expression to assess relative transcript abundance in porcine blastocysts and found >900 transcripts that were putatively differentially expressed in embryos generated in vitro vs. in vivo. Also, as the genes presented herein as differentially expressed are only those that were shown to be different across all gestation stages, it is highly unlikely that asynchrony between embryo types is responsible for the high number of aberrantly expressed genes. Thus, the larger numbers of DEGs in this report are likely reflective of the relative developmental complexity of peri-attachment embryos compared with blastocyst-stage embryos, as well as the exquisite sensitivity and bias-free nature of the Illumina sequencing platform for transcriptome profiling (i.e., not limited to features on the array).
One pathway shown to be significantly altered in in vitro fertilized embryos was the transforming growth factor (TGF)-β signaling pathway. This pathway is essential for development and patterning during embryogenesis in vertebrates and invertebrates alike (reviewed in Ref. 81). TGF-β signaling has recently been implicated in mediating the interaction of porcine conceptuses with the uterine endometrium at both early and late stages of embryonic attachment (47). Interestingly, though, in our data, the suppression of TGF-β signaling in IVF embryos was observed primarily in the ED samples, and not the TE samples. When evaluated at the pathway level, it is the bone morphogenetic protein (BMP) arm of the TGF-β signaling pathway that is relatively underexpressed in IVF embryos. In fact, every level of the BMP signaling cascade (from the receptor level to the DNA transcription level) is represented in the list of genes expressed at consistently higher levels in ED samples from IVV embryos than from IVF embryos. The BMP signaling family is important in body axis determination, limb development, and organogenesis (reviewed in Refs. 38, 71). It is tempting to speculate that depressed BMP signaling at crucial developmental windows could, at least in part, explain the relatively lower viability rates of in vitro manipulated embryos. Of course, further validation of this hypothesis is necessary.
TE samples from IVF embryos are characterized by an upregulation of the cellular machinery involved in ubiquitin-mediated proteolysis, which may be symptomatic of protein synthesis and/or processing defects, although this too requires further confirmation. Also of interest is the observation that the TE of IVF embryos demonstrated a significant upregulation of components of the ErbB signaling pathway relative to in vivo fertilized embryos. Although none of the canonical ErbB receptors (ErbB1–4) were upregulated in IVF embryos, several members of the signaling cascade(s) downstream of the receptors were upregulated, including STAT5B, PAK1, PAK2, SOS1, and mTOR. It is yet too early to understand the exact consequences of hyperactive ErbB signaling in porcine peri-attachment IVF embryos, but it is interesting to note that significant perturbations in placental structure and function are observed in mice that overexpress EGFR, the founding member of the ErbB receptor family (17). Specifically, placental weights from pups heterozygous or homozygous for a hypermorphic EGFR (ErbB1) allele were from 17–55% greater than those from control pups from the same litters. In this mouse model, expression levels of a number of trophoblast-specific genes were altered as well as a consequence of altered ErbB signaling dynamics. Placental hyperplasia and fetal overgrowth have been commonly observed in pregnancies resulting from in vitro manipulated embryos in a number of species (25, 41, 65, 80, 83). To our knowledge, this is the first report of aberrant ErbB signaling in trophoblast cells of in vitro manipulated embryos from any species. It remains to be seen, then, whether this signaling pathway is affected in trophoblast cells of other species at comparable developmental time points and whether or not these signaling defects could be at least in part responsible for the altered placental dynamics observed in in vitro manipulated embryos in pigs as well as other model species.
GO analysis results from the SCNT/IVF (ED) comparison reveal three primary themes disrupted by SCNT: epigenetic control of gene expression, gene silencing by microRNAs, and apoptosis. It comes as no surprise that epigenetic modification of the genome is disrupted in nuclear transfer embryos; this phenomenon has been extensively reviewed elsewhere (3, 18, 51, 84). It is somewhat surprising, however, that genes involved in epigenetic processes were overwhelmingly downregulated in embryos derived from SCNT. If the epigenome was significantly misprogrammed after nuclear transfer, it would not be unreasonable to expect to see an upregulation of the epigenetic machinery in an attempt to correct the faulty programming. Instead, we observed widespread suppression of the genes that control proper epigenome constitution in SCNT embryos. An alternative explanation for this observation is that the epigenetic machinery of the incipient SCNT embryos was somehow inherently dysfunctional, resulting in faulty embryo reprogramming and poor SCNT success rates. Whatever the explanation, widespread downregulation of genes involved in epigenetic control of gene expression has also been observed in cloned bovine embryos (61). The potential cause-and-effect relationships between faulty genome programming and the epigenetic machinery of the oocyte and early embryo demand further investigations, as additional insight into the causes of and potential strategies for mitigation of faulty epigenetic reprogramming in SCNT embryos is sorely needed.
Very little information is available regarding the status of the cellular machinery responsible for gene silencing by microRNAs in cloned embryos. Cui et al. (16) report aberrant reprogramming of a very limited number of specific microRNAs in cloned mouse embryos, and the same group showed that a histone deacetylase inhibitor (Trichostatin A) can improve the reprogramming of microRNA genes in addition to protein-coding genes in cloned embryos (15). One recent report suggests that microRNA reprogramming after SCNT is incomplete and inconsistent in elongating bovine embryos (13). Our data support the notion that microRNA-mediated gene silencing may be significantly disrupted in nuclear transfer embryos compared with IVF controls.
An increase in the incidence of apoptosis in SCNT embryos cultured in vitro has been reported previously (22, 32, 43). Our results suggest that increased programmed cell death may not be unique to SCNT embryos maintained in an in vitro culture environment but, rather, is associated with the SCNT process regardless of culture environment. Indeed, it is likely that the cellular stress imposed by widespread gene misregulation (via epigenetic and/or microRNA pathways) would lead to apoptosis in embryos, as this phenomenon has been reported in other model systems (see Refs. 7, 27, 36, 58), including preimplantation-stage bovine embryos (53).
It is interesting to note that even though widespread misregulation of gene expression was also observed in trophoblast tissue from SCNT embryos (as in the ED), neither the same genes nor the same types of genes were shown to be aberrantly expressed in the distinct cell types. Deficiencies in control over gene expression (ED) are instead replaced by dysfunctional metabolic/catabolic pathways and subcellular organizational defects in trophoblast tissue. These observations fit nicely with data previously reported by our laboratory that details gross cytological and histological defects in trophoblast tissue of elongating nuclear transfer embryos (46). It is as yet unclear why, in SCNT embryos, trophoblast cells and cells of the ED have developed such distinct responses to the shared insult of faulty genomic reprogramming during early cleavage-stage development. Whatever the reason, the consequences are clear: placental defects are a key factor in the low embryonic, fetal, and neonatal survival rates after SCNT in all species studied to date (reviewed in Refs. 4, 14, 56). Further insight into this apparent discrepancy between ED and TE regarding epigenomic reprogramming will be crucial to improving the poor performance of SCNT embryos.
Embryos derived from PA were included as a part of this study in an attempt to shed additional light on the phenomenon of genomic imprinting and how faulty imprinting can impact preattachment development. Parthenogenetic embryos are the result of artificially activated oocytes, without any contribution (genetic or otherwise) from spermatozoa. Mammalian parthenogenotes created in this manner are incapable of developing to term, largely as a consequence of improper genomic imprinting (reviewed in Refs. 11, 55). [Parthenogenetic mice have been born that survived into adulthood, but significant genetic engineering to imprinted regions of the genome was necessary to allow this to happen (39).] We hypothesized that a subset of imprinted genes (those expressed exclusively, or preferentially, from the paternally derived allele) would be consistently absent or underexpressed when comparing parthenogenotes against IVF embryos. Indeed, when we compared the ED read counts from parthenogenetic samples against those from IVF samples, eight genes (PLAGL1, DIRAS3, SNRPN, NNAT, PEG10, NDN, TMSB4X, KBTBD6) showed a read-count ratio (PA/IVF) of <0.1 for at least two of the three gestation days examined; six of the eight have been previously shown to be preferentially expressed from the paternal allele in one or more mammalian species (in italics above; Ref. 49). For TE samples, 14 genes (PEG10, MEST, AVP, TMEM22, IGFBP7, BGN, APLNR, EHD2, NRN1, CTSL1, SLC22A7, TMEM119, CLEC10A, TMSL3) were found that fit that same criteria, yet only two of those were known imprinted genes (in italics above; Ref. 49). Differential gene expression between PA and IVF samples does not automatically qualify a gene as being imprinted. Nevertheless, these are intriguing candidates that we are aggressively investigating at the current time. It has been demonstrated that IVF and SCNT embryos alike are plagued by ART-induced defects in imprinted gene expression (see Refs. 12, 26, 67–69, 74). We anticipate that a more complete understanding of genomic imprinting and the role it plays in early embryo development may inform future research regarding the treatment of embryos generated using ART.
The correlations between the quantitative PCR data (CT values) and the sequencing data (read counts) were compelling. We observed a very few conspicuous discrepancies (DNMT3B, GNAS, IGF2, e.g.) that were likely due to faulty quantitative PCR assays, as the CT values for these three genes were consistently noted as outliers. Removal of these three genes from the analysis boosted the average correlation coefficient between CT values and read counts from −0.722 to −0.861. We attribute the remaining minor discordance between CT values and read counts to the conservative approach we took to aligning reads to our database. Reads were accepted as aligned only if they mapped to exactly one member of our database, thus expressed genes with closely related family members or common and highly conserved motifs may be underrepresented in the read count data but accounted for accurately via quantitative PCR. Notably absent from the quantitative PCR experiments were the day 10 nuclear transfer samples. These were our least abundant samples, and after sequencing, not enough cDNA was left for quantitative PCR analysis.
In summary, this was, to our knowledge, the first large-scale effort to describe differences in global gene expression patterns between peri-attachment pig conceptuses produced using different ART. We have been successful at identifying specific biological processes and signaling pathways that may be impinged upon due to in vitro culture, somatic cell nuclear reprogramming, and parthenogenesis. We have identified a number of new putatively imprinted genes that merit additional consideration. In addition, we have further refined our technical and statistical approaches to global gene expression profiling by high-throughput sequencing. We validated our data with medium-throughput quantitative PCR using the BioMark system from Fluidigm. Significant embryonic losses are incurred in ART embryos at or around the time of embryonic attachment. These data will serve as an invaluable resource to those with an interest in discovering the cause(s) of and strategies for mitigation of early embryonic loss in swine embryos generated by ART.
GRANTS
This work was funded primarily by the National Center for Research Resources Grant R01 RR-013438 and Food for the 21st Century at the University of Missouri (R. S. Prather) and through Utah Agricultural Experiment Station program projects UTA00151 and UTA01062 for S. C. Isom and J. R. Stevens, respectively.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ENDNOTE
At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found at the institutional website of author S. C. Isom, which at the time of publication is: https://advs.usu.edu/htm/faculty-staff/memberID=4042. These materials are not a part of this manuscript and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the website address, or for any links to or from it.
AUTHOR CONTRIBUTIONS
SCI was responsible for experimental design, sample collection, data generation and analysis, and manuscript preparation; JRS developed and implemented the statistical models for read count analysis; RL performed the manipulations for somatic cell nuclear transfer; WGS oversaw the bioinformatics aspects of this project (short read trimming, parsing, and alignment); LC carried out the quantitative PCR; LDS performed embryo transfers, and assisted with sample collections and in vitro embryo culture; CNM performed the surgical embryo transfers; RSP paid for, directed, and helped to design the experiments detailed herein.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge August Rieke, Melissa Samuel, Eric Walters, and David Wax for invaluable contributions to the extensive animal husbandry efforts required for this project; Kristin Whitworth for molecular biology assistance; and Fluidigm for providing assays for RNA-Seq validation with the BioMark qPCR system.
Current address for R. Li: Center of Metabolic Disease Research, Nanjing Medical University, 140 Han Zhong Rd., Nanjing 210029, China.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Abeydeera LR, Day BN. In vitro penetration of pig oocytes in a modified Tris-buffered medium: effect of BSA, caffeine and calcium. Theriogenology 48: 537–544, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Alexa A, Rahnenfuhrer J, Lengauer T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22: 1600–1607, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Armstrong L, Lako M, Dean W, Stojkovic M. Epigenetic modification is central to genome reprogramming in somatic cell nuclear transfer. Stem Cells 24: 805–814, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Arnold DR, Fortier AL, Lefebvre R, Miglino MA, Pfarrer C, Smith LC. Placental insufficiencies in cloned animals - a workshop report. Placenta 29, Suppl A: S108–S110, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25: 25–29, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Auer PL, Doerge RW. Statistical design and analysis of RNA sequencing data. Genetics 185: 405–416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balasubramanyam K, Altaf M, Varier RA, Swaminathan V, Ravindran A, Sadhale PP, Kundu TK. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J Biol Chem 279: 33716–33726, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Bauer BK, Isom SC, Spate LD, Whitworth KM, Spollen WG, Blake SM, Springer GK, Murphy CN, Prather RS. Transcriptional profiling by deep sequencing identifies differences in mRNA transcript abundance in in vivo-derived versus in vitro-cultured porcine blastocyst stage embryos. Biol Reprod 83: 791–798, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Beissbarth T, Speed TP. GOstat: find statistically overrepresented Gene Ontologies within a group of genes. Bioinformatics 20: 1464–1465, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 57: 289–300, 1995 [Google Scholar]
- 11. Brevini TA, Pennarossa G, Vanelli A, Maffei S, Gandolfi F. Parthenogenesis in non-rodent species: developmental competence and differentiation plasticity. Theriogenology 77: 766–772, 2012 [DOI] [PubMed] [Google Scholar]
- 12. Calle A, Fernandez-Gonzalez R, Ramos-Ibeas P, Laguna-Barraza R, Perez-Cerezales S, Bermejo-Alvarez P, Ramirez MA, Gutierrez-Adan A. Long-term and transgenerational effects of in vitro culture on mouse embryos. Theriogenology 77: 785–793, 2012 [DOI] [PubMed] [Google Scholar]
- 13. Castro FO, Sharbati S, Rodriguez-Alvarez LL, Cox JF, Hultschig C, Einspanier R. MicroRNA expression profiling of elongated cloned and in vitro-fertilized bovine embryos. Theriogenology 73: 71–85, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Chavatte-Palmer P, Camous S, Jammes H, Le Cleac'h N, Guillomot M, Lee RS. Review: Placental perturbations induce the developmental abnormalities often observed in bovine somatic cell nuclear transfer. Placenta 33, Suppl: S99–S104, 2012 [DOI] [PubMed] [Google Scholar]
- 15. Cui XS, Xu YN, Shen XH, Zhang LQ, Zhang JB, Kim NH. Trichostatin A modulates apoptotic-related gene expression and improves embryo viability in cloned bovine embryos. Cell Reprogram 13: 179–189, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Cui XS, Zhang DX, Ko YG, Kim NH. Aberrant epigenetic reprogramming of imprinted microRNA-127 and Rtl1 in cloned mouse embryos. Biochem Biophys Res Commun 379: 390–394, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Dackor J, Li M, Threadgill DW. Placental overgrowth and fertility defects in mice with a hypermorphic allele of epidermal growth factor receptor. Mamm Genome 20: 339–349, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dinnyes A, Tian XC, Yang X. Epigenetic regulation of foetal development in nuclear transfer animal models. Reprod Domest Anim 43, Suppl 2: 302–309, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Diskin MG, Morris DG. Embryonic and early foetal losses in cattle and other ruminants. Reprod Domest Anim 43, Suppl 2: 260–267, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Diskin MG, Parr MH, Morris DG. Embryo death in cattle: an update. Reprod Fertil Dev 24: 244–251, 2011 [DOI] [PubMed] [Google Scholar]
- 21. ENCODE Consortium Standards, Guidelines and Best Practices for RNA-Seq v1.0. National Human Genome Research Institute; Available at: http://www.encodeproject.org/ENCODE/protocols/dataStandards/ENCODE_RNAseq_Standards_V1.0.pdf [Google Scholar]
- 22. Fahrudin M, Otoi T, Karja NW, Mori M, Murakami M, Suzuki T. Analysis of DNA fragmentation in bovine somatic nuclear transfer embryos using TUNEL. Reproduction 124: 813–819, 2002 [PubMed] [Google Scholar]
- 23. Falcon S, Gentleman R. Using GOstats to test gene lists for GO term association. Bioinformatics 23: 257–258, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Fang Z, Cui X. Design and validation issues in RNA-seq experiments. Brief Bioinform 12: 280–287, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Farin PW, Piedrahita JA, Farin CE. Errors in development of fetuses and placentas from in vitro-produced bovine embryos. Theriogenology 65: 178–191, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Fernandez-Gonzalez R, Ramirez MA, Bilbao A, De Fonseca FR, Gutierrez-Adan A. Suboptimal in vitro culture conditions: an epigenetic origin of long-term health effects. Mol Reprod Dev 74: 1149–1156, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Gartel AL. Transcriptional inhibitors, p53 and apoptosis. Biochim Biophys Acta 1786: 83–86, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Geisert RD, Brookbank JW, Roberts RM, Bazer FW. Establishment of pregnancy in the pig: II. Cellular remodeling of the porcine blastocyst during elongation on day 12 of pregnancy. Biol Reprod 27: 941–955, 1982 [DOI] [PubMed] [Google Scholar]
- 29. Geisert RD, Renegar RH, Thatcher WW, Roberts RM, Bazer FW. Establishment of pregnancy in the pig: I. Interrelationships between preimplantation development of the pig blastocyst and uterine endometrial secretions. Biol Reprod 27: 925–939, 1982 [DOI] [PubMed] [Google Scholar]
- 30. Geisert RD, Schmitt RAM. Early embryonic survival in the pig: can it be improved? J Anim Sci 80: E54–E65, 2002 [Google Scholar]
- 31. Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hao Y, Lai L, Mao J, Im GS, Bonk A, Prather RS. Apoptosis and in vitro development of preimplantation porcine embryos derived in vitro or by nuclear transfer. Biol Reprod 69: 501–507, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Illumina I. The Power of Replicates (Technical Note). 2010 [Google Scholar]
- 34. Isom SC, Prather RS, Rucker EB., 3rd Heat stress-induced apoptosis in porcine in vitro fertilized and parthenogenetic preimplantation-stage embryos. Mol Reprod Dev 74: 574–581, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Isom SC, Spollen WG, Blake SM, Bauer BK, Springer GK, Prather RS. Transcriptional profiling of day 12 porcine embryonic disc and trophectoderm samples using ultra-deep sequencing technologies. Mol Reprod Dev 77: 812–819, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Jackson-Grusby L, Beard C, Possemato R, Tudor M, Fambrough D, Csankovszki G, Dausman J, Lee P, Wilson C, Lander E, Jaenisch R. Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nat Genet 27: 31–39, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucl Acids Res 28: 27–30, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev 16: 265–278, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Kono T, Obata Y, Wu Q, Niwa K, Ono Y, Yamamoto Y, Park ES, Seo JS, Ogawa H. Birth of parthenogenetic mice that can develop to adulthood. Nature 428: 860–864, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Lai L, Prather RS. Production of cloned pigs by using somatic cells as donors. Cloning Stem Cells 5: 233–241, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Lazzari G, Wrenzycki C, Herrmann D, Duchi R, Kruip T, Niemann H, Galli C. Cellular and molecular deviations in bovine in vitro-produced embryos are related to the large offspring syndrome. Biol Reprod 67: 767–775, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Li R, Li Y, Kristiansen K, Wang J. SOAP: short oligonucleotide alignment program. Bioinformatics 24: 713–714, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Liu SZ, Yao LJ, Jiang MX, Lei ZL, Zhang LS, Zhang YL, Sun QY, Zheng YL, Song XF, Chen DY. Apoptosis in rabbit embryos produced by fertilization or nuclear transfer with fibroblasts and cumulus cells. Reproduction 130: 359–366, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Marguerat S, Bahler J. RNA-seq: from technology to biology. Cell Mol Life Sci 67: 569–579, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Marioni JC, Mason CE, Mane SM, Stephens M, Gilad Y. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res 18: 1509–1517, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Martin L, Besch-Williford C, Lai L, Cheong HT, Im GS, Park KW, Murphy C, Hao Y, Ellersieck MR, Keisler DH, Schatten H, Green JA, Prather RS. Morphologic and histologic comparisons between in vivo and nuclear transfer derived porcine embryos. Mol Reprod Dev 74: 952–960, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Massuto DA, Kneese EC, Johnson GA, Burghardt RC, Hooper RN, Ing NH, Jaeger LA. Transforming growth factor beta (TGFB) signaling is activated during porcine implantation: proposed role for latency-associated peptide interactions with integrins at the conceptus-maternal interface. Reproduction 139: 465–478, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Miles JR, Blomberg LA, Krisher RL, Everts RE, Sonstegard TS, Van Tassell CP, Zuelke KA. Comparative transcriptome analysis of in vivo- and in vitro-produced porcine blastocysts by small amplified RNA-serial analysis of gene expression (SAR-SAGE). Mol Reprod Dev 75: 976–988, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Morison IM, Ramsay JP, Spencer HG. A census of mammalian imprinting. Trends Genet 21: 457–465, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Meth 5: 621–628, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Niemann H, Tian XC, King WA, Lee RS. Epigenetic reprogramming in embryonic and foetal development upon somatic cell nuclear transfer cloning. Reproduction 135: 151–163, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Oehlert GW. A First Course in Design and Analysis of Experiments. New York: W. H. Freeman, 2000 [Google Scholar]
- 53. Oliveira CS, Saraiva NZ, de Souza MM, Tetzner TA, de Lima MR, Garcia JM. Effects of histone hyperacetylation on the preimplantation development of male and female bovine embryos. Reprod Fertil Dev 22: 1041–1048, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Ozsolak F, Milos PM. RNA sequencing: advances, challenges and opportunities. Nat Rev Genet 12: 87–98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paffoni A, Brevini TA, Gandolfi F, Ragni G. Parthenogenetic activation: biology and applications in the ART laboratory. Placenta 29, Suppl B: 121–125, 2008 [DOI] [PubMed] [Google Scholar]
- 56. Palmieri C, Loi P, Ptak G, Della Salda L. Review paper: a review of the pathology of abnormal placentae of somatic cell nuclear transfer clone pregnancies in cattle, sheep, and mice. Vet Pathol 45: 865–880, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Pope WF. Embryonic mortality in swine. In: Embryonic Mortality in Domestic Species, edited by Zavy MT, Geisert RD. Boca Raton, FL: CRC, 1994, p. 53–77 [Google Scholar]
- 58. Radhakrishnan SK, Gartel AL. A novel transcriptional inhibitor induces apoptosis in tumor cells and exhibits antiangiogenic activity. Cancer Res 66: 3264–3270, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Redel BK, Brown AN, Spate LD, Whitworth KM, Green JA, Prather RS. Glycolysis in preimplantation development is partially controlled by the Warburg Effect. Mol Reprod Dev 79: 262–271, 2012 [DOI] [PubMed] [Google Scholar]
- 60. Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol 11: R25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rodriguez-Osorio N, Wang Z, Kasinathan P, Page GP, Robl JM, Memili E. Transcriptional reprogramming of gene expression in bovine somatic cell chromatin transfer embryos. BMC Genomics 10: 190, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rosenkranz R, Borodina T, Lehrach H, Himmelbauer H. Characterizing the mouse ES cell transcriptome with Illumina sequencing. Genomics 92: 187–194, 2008 [DOI] [PubMed] [Google Scholar]
- 63. Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J. TM4 microarray software suite. Meth Enzymol 411: 134–193, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34: 374–378, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Sinclair KD, Young LE, Wilmut I, McEvoy TG. In-utero overgrowth in ruminants following embryo culture: lessons from mice and a warning to men. Hum Reprod 15 Suppl 5: 68–86, 2000 [DOI] [PubMed] [Google Scholar]
- 66. Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100: 9440–9445, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Su H, Li D, Hou X, Tan B, Hu J, Zhang C, Dai Y, Li N, Li S. Molecular structure of bovine Gtl2 gene and DNA methylation status of Dlk1-Gtl2 imprinted domain in cloned bovines. Anim Reprod Sci 127: 23–30, 2011 [DOI] [PubMed] [Google Scholar]
- 68. Su JM, Yang B, Wang YS, Li YY, Xiong XR, Wang LJ, Guo ZK, Zhang Y. Expression and methylation status of imprinted genes in placentas of deceased and live cloned transgenic calves. Theriogenology 75: 1346–1359, 2011 [DOI] [PubMed] [Google Scholar]
- 69. Suzuki J, Jr, Therrien J, Filion F, Lefebvre R, Goff AK, Smith LC. In vitro culture and somatic cell nuclear transfer affect imprinting of SNRPN gene in pre- and post-implantation stages of development in cattle. BMC Dev Biol 9: 9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2011 [Google Scholar]
- 71. Wagner TU. Bone morphogenetic protein signaling in stem cells–one signal, many consequences. FEBS J 274: 2968–2976, 2007 [DOI] [PubMed] [Google Scholar]
- 72. Wang WH, Niwa K, Okuda K. In-vitro penetration of pig oocytes matured in culture by frozen-thawed ejaculated spermatozoa. J Reprod Fertil 93: 491–496, 1991 [DOI] [PubMed] [Google Scholar]
- 73. Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10: 57–63, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wei Y, Zhu J, Huan Y, Liu Z, Yang C, Zhang X, Mu Y, Xia P. Aberrant expression and methylation status of putatively imprinted genes in placenta of cloned piglets. Cell Reprogram 12: 213–222, 2010 [DOI] [PubMed] [Google Scholar]
- 75. Whitworth KM, Agca C, Kim JG, Patel RV, Springer GK, Bivens NJ, Forrester LJ, Mathialagan N, Green JA, Prather RS. Transcriptional profiling of pig embryogenesis by using a 15-K member unigene set specific for pig reproductive tissues and embryos. Biol Reprod 72: 1437–1451, 2005 [DOI] [PubMed] [Google Scholar]
- 76. Whitworth KM, Li R, Spate LD, Wax DM, Rieke A, Whyte JJ, Manandhar G, Sutovsky M, Green JA, Sutovsky P, Prather RS. Method of oocyte activation affects cloning efficiency in pigs. Mol Reprod Dev 76: 490–500, 2009 [DOI] [PubMed] [Google Scholar]
- 77. Whitworth KM, Spate LD, Li R, Rieke A, Sutovsky P, Green JA, Prather RS. Activation method does not alter abnormal placental gene expression and development in cloned pigs. Mol Reprod Dev 77: 1016–1030, 2010 [DOI] [PubMed] [Google Scholar]
- 78. Wilkinson L. Exact and approximate area-proportional circular Venn and Euler diagrams. IEEE Trans Vis Comput Graph 18: 321–331, 2012 [DOI] [PubMed] [Google Scholar]
- 79. Wilmut I, Sales DI, Ashworth CJ. Maternal and embryonic factors associated with prenatal loss in mammals. J Reprod Fertil 76: 851–864, 1986 [DOI] [PubMed] [Google Scholar]
- 80. Wrenzycki C, Herrmann D, Lucas-Hahn A, Lemme E, Korsawe K, Niemann H. Gene expression patterns in in vitro-produced and somatic nuclear transfer-derived preimplantation bovine embryos: relationship to the large offspring syndrome? Anim Reprod Sci 82–83: 593–603, 2004 [DOI] [PubMed] [Google Scholar]
- 81. Wu MY, Hill CS. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev Cell 16: 329–343, 2009 [DOI] [PubMed] [Google Scholar]
- 82. Yoshioka K, Suzuki C, Tanaka A, Anas IM, Iwamura S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol Reprod 66: 112–119, 2002 [DOI] [PubMed] [Google Scholar]
- 83. Young LE, Sinclair KD, Wilmut I. Large offspring syndrome in cattle and sheep. Rev Reprod 3: 155–163, 1998 [DOI] [PubMed] [Google Scholar]
- 84. Zhao J, Whyte J, Prather RS. Effect of epigenetic regulation during swine embryogenesis and on cloning by nuclear transfer. Cell Tiss Res 341: 13–21, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.