Abstract
Overconsumption of a high-fat diet promotes weight gain that can result in obesity and associated comorbidities, including Type 2 diabetes mellitus. Consumption of a high-fat diet also alters gut-brain communication. Glucagon-like peptide 1 (GLP-1) is an important gastrointestinal signal that modulates both short- and long-term energy balance and is integral in maintenance of glucose homeostasis. In the current study, we investigated whether high-fat diets (40% or 81% kcal from fat) modulated the ability of the GLP-1 receptor (GLP-1r) agonists exendin-4 (Ex4) and liraglutide to reduce food intake and body weight. We observed that rats maintained on high-fat diets had a delayed acute anorexic response to peripheral administration of Ex4 or liraglutide compared with low-fat diet-fed rats (17% kcal from fat). However, once suppression of food intake in response to Ex4 or liraglutide started, the effect persisted for a longer time in the high-fat diet-fed rats compared with low-fat diet-fed rats. In contrast, centrally administered Ex4 suppressed food intake similarly between high-fat diet-fed and low-fat diet-fed rats. Chronic consumption of a high-fat diet did not change the pharmacokinetics of Ex4 but increased intestinal Glp1r expression and decreased hindbrain Glp1r expression. Taken together, these findings demonstrate that dietary composition alters the temporal profile of the anorectic response to exogenous GLP-1r agonists.
Keywords: glucagon-like peptide 1, food intake, liraglutide, exendin-4, obesity
obesity results from an imbalance between energy intake and energy expenditure. To maintain energy balance, continuous communication between peripheral tissues and the central nervous system (CNS) is necessary, and a crucial part of this communication is the gut-brain axis (9). The gut-brain axis comprises neural, hormonal, and nutritional signals generated prior to, during, and following caloric intake and that prepare the gastrointestinal (GI) system for caloric influx and mediate subsequent central responses to control ingestion. The overall result is the initiation of a series of compensatory mechanisms to regulate nutrient disposal and energy expenditure, contributing to the homeostasis of energy balance (45).
Glucagon-like peptide 1 (GLP-1), a product of the preproglucagon gene Gcg (34), is an important gastrointestinal (GI) signal that modulates both short- and long-term energy balance and is integral in maintenance of glucose homeostasis (7). GLP-1 is produced in intestinal L-cells and in neurons of the nucleus of the solitary tract (NTS) (39, 49). Peripheral GLP-1 acts as a gut-derived satiety signal, modulating energy balance on a short-term, meal-to-meal basis, whereas it also interacts with leptin, a known regulator of long-term energy balance (7). Further, NTS neurons are necessary for long-term energy balance, as when the expression of Gcg is down-regulated specifically in the hindbrain using a lentiviral approach, body weight and adiposity are significantly elevated (6). After nutrient ingestion, peripheral GLP-1 is secreted from the intestine, and one of its major functions is to augment glucose-stimulated insulin secretion. This has prompted development of GLP-1-receptor (GLP-1r) agonists as treatments of Type 2 diabetes mellitus (T2D) (41). Endogenous circulating active GLP-1 is rapidly degraded by dipeptidyl peptidase-4 (DDP-4) to a shorter, inactive peptide (13, 30, 38), and GLP-1 analogs resistant to DPP-4 have consequently been developed. These include exendin-4 (Ex4), a synthetic long-acting GLP-1r agonist (21, 54) that is injected twice daily to treat T2D in humans, and an acylated albumin-bound GLP-1 analog, liraglutide, which is injected once daily (1, 14, 16). Both Ex4 (Byetta) and liraglutide (Victoza) were approved by the U.S. Food and Drug Administration as pharmacotherapies to improve glycemic control in T2D patients. In rodents, when administered peripherally or centrally, both Ex4 and liraglutide potently inhibit food intake and induce body mass loss (5, 7, 35, 42, 43). In humans, GLP-1r agonists also induce weight loss (4, 11, 19, 41), suggesting that GLP-1r-agonism can also be viewed as a potential weight-loss therapy.
Consumption of a high-fat diet alters gut-brain communication and attenuates subsequent satiation signaling (17, 18, 37). This decreased sensitivity to nutrients could result from changed levels of intestinal peptides, altered triggering of GI feedback signals, or desensitized receptor activation. For example, high-fat diet-fed rodents have decreased plasma active GLP-1 levels (3) and impaired anorectic responses to Ex4 (53); however, food intake was monitored only up to 24 h following Ex4 administration (53). Finally, another study reported a longer latency for liraglutide-mediated food intake suppression in high-fat/sucrose-fed diet-induced obese rats vs. chow-fed, nonobese rats (22). Currently, it is unknown whether consumption of a high-fat diet affects the efficacy of GLP-1r agonists to decrease food intake over a longer period (longer than 24 h). Therefore, we compared the ability of Ex4 and liraglutide to reduce food intake and body mass in rats maintained on a low-fat diet (17% kcal from fat), a high-fat diet (40% kcal from fat), or a very high-fat diet (81% kcal from fat).
MATERIALS AND METHODS
Ethics
The University of Cincinnati Institutional Animal Care and Use Committee approved all procedures for animal use.
Rats, Housing, and Diets
Naïve male Long-Evans rats (Harlan Laboratories, Indianapolis, IN) were individually housed and maintained on a 12:12-h light-dark cycle (lights off at 1400) at 25°C and 50–60% humidity in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facilities of the Metabolic Diseases Institute of the University of Cincinnati. All rats had ad libitum access to water and one of the following diets: a low-fat pelleted diet (LFD; LM-485 no. 7012, 3.1 kcal/g, 25%, 58%, and 17% kcal from protein, carbohydrate, and fat, respectively; Harlan Teklad, Madison, WI), a pelleted diet containing 40% fat (MFD; D03082706, 4.54 kcal/g, 15%, 46%, and 40% kcal from protein, carbohydrate, and fat, respectively; Open Source Diets, New Brunswick, NJ), or a paste diet containing 81% fat (HFD; Dyet 100959, 6.04 kcal/g, 14%, 5%, and 81% kcal from protein, carbohydrate, and fat, respectively; Dyets, Bethlehem, PA; also see Table 1). All rats had access to home-cage enrichment (red rat retreat; Bioserve Biotechnologies, Beltsville, MD).
Table 1.
Macronutrient composition, energy density, and fatty acid composition of the LFD, MFD, and HFD diets used in these studies
| % kcal From |
% Fatty Acids From |
||||||
|---|---|---|---|---|---|---|---|
| Diet | Protein | Carbohydrate | Fat | kcal/g | Sat. | Mono-un. | Poly-un. |
| LFD | 25 | 58 | 17 | 3.10 | 16 | 26 | 58 |
| MFD | 15 | 46 | 40 | 4.54 | 62 | 31 | 7 |
| HFD | 14 | 5 | 81 | 6.04 | 29 | 58 | 13 |
Sat., saturated; Mono-un., monounsaturated; Poly-un., polyunsaturated.
Peptides
Ex4 was obtained from American Peptide (Sunnyvale, CA), and liraglutide was a generous gift from Novo Nordisk (Bagsvaerd, Denmark). Ex4 and liraglutide were dissolved in sterile 0.9% saline and PBS, respectively, and administered intraperitoneally in a volume of 1 ml/kg.
Body Composition
Fat mass and lean mass were measured in conscious rats using an EchoMRI analyzer (Echo Medical Systems, Houston, TX) during week 6 (Ex4 dose-response study), week 13 (Ex4 flat-dose study), and week 16 (liraglutide study).
Cohort 1
Effect of high-fat diet on Ex4- or liraglutide-induced hypophagia.
Before the start of the experiment, all rats were handled daily and habituated to intraperitoneal injection of 0.5 ml saline and measurement of food intake throughout the dark phase on three occasions. On the first day of the experiment, after 5 wk of maintenance on their designated diet, all rats were weighed, and food was removed 2 h before the onset of the dark. Ex4 (3, 10, or 33 μg/kg) or saline (1 ml/kg) was injected intraperitoneally 1 h before the start of the dark. At the onset of dark, preweighed food was returned to all rats. Each test session was separated by seven nontest days (experiments performed when rats were 5, 6, and 7 wk on the diet). The same strategy was used in administering a flat dose of Ex4 (15 μg·0.5 ml−1·rat−1) or saline (0.5 ml) when rats had been on their respective diet for 13 wk, and liraglutide (300 μg/kg) or saline (1 ml/kg) when rats had been on their diet for 16 wk. Food intake was measured 2, 4, 24, 48 h postinjection (Ex4 dose-response study), or 2, 4, 24, 48, 72, and 96 h postinjection (Ex4 flat dose and liraglutide studies). Body mass was measured 24 and 48 h postinjection (Ex4 dose-response study), or 24 h postinjection (Ex4 flat dose and liraglutide studies).
Cohort 2
Third cerebroventricular cannulation.
For the third cerebroventricular (I3VT) experiment, rats were outfitted with a cannula (Plastics One, Roanoke, VA) directed toward the I3VT, and correct placement was confirmed as previously described (8). Briefly, rats were anesthetized with intraperitoneal ketamine (70 mg/kg) and xylazine (6 mg/kg). Rats were shaved and positioned with the skull horizontal in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA). After a small hole was drilled through the skull at a position 2.2 mm posterior to bregma, on the midline, the sagittal sinus was displaced laterally, and a stainless-steel guide cannula (Plastics One) was lowered 7.5 mm ventral to the dura. The cannula was fixed with dental acrylic anchored to the scull with four screws, and an obturator was inserted that extended 0.5 mm below the cannula. The surgery was performed using sterile techniques. Five days prior to food intake experiments, correct placement and viability of the cannula were confirmed by behavioral means using 10 ng of ANG II (American Peptides, Sunnyvale, CA) in 1 μl of saline. Rats consuming at least 5 ml of water within 30 min were considered to have a viable cannula.
Effect of high-fat diet on the action of I3VT Ex4 to induce anorexia.
Rats were maintained on either LFD or HFD for 4 wk before I3VT cannulation. Rats were allowed to recover for 1 wk before the start of the experiment. Before the start of the experiment, all rats were handled daily and habituated to I3VT injection of 1 μl of saline and measurement of food intake throughout the dark phase on two occasions. On the first day of the experiment, after 6 wk of maintenance on their designated diet, all rats were weighed, and food was removed 2 h before the onset of the dark. Ex4 (0.1 μg/1 μl) or saline (1 μl) was injected I3VT 1 h before the start of the dark. At the onset of the dark, preweighed food was returned to all rats. Food intake was measured 2, 4, and 24 h postinjection.
Cohort 3
Effect of high-fat diet on Ex4 pharmacokinetics.
On the first day of the experiment, after 11 wk of maintenance on their designated diet, all rats were weighed, and food was removed 2 h before the onset of the dark. Intraperitoneal Ex4 (33 μg) was injected 30 min before dark onset, and at the onset of dark, preweighed food was returned to all rats. Tail-vein blood samples were collected before injection (baseline) and 2, 4, and 24 h after injection. Serum samples were obtained by centrifugation and stored at −80°C until required for assessment of Ex4 using a commercially available EIA kit (Bachem, Torrance, CA).
Tissue isolation.
After 24 wk on the designated diets, 3-h fasted rats were killed during the first half of the light phase using CO2 asphyxiation followed by decapitation. The whole hypothalamus, an intestinal sample just proximal of the ileocecal valve (distal ileum), the NTS at the level of the area postrema, and the left inferior nodose ganglion of the vagus nerve were isolated. The hypothalamus and NTS samples were cut symmetrically before freezing. All samples were frozen in liquid nitrogen-cooled isopentane as fast as possible and stored at −80°C until further analyses.
Assessment of Glp1r and Gcg mRNAs.
RNA was isolated using Agencourt RNAdvance tissue kit (Beckman Coulter, Brea, CA), and genomic DNA was eliminated by DNaseI treatment using an RNase-free DNase set (Qiagen, Valencia, CA). RNA quantity and quality were assessed using a NanoVue (GE Healthcare, Piscataway, NJ). cDNA was generated by reverse transcriptase using iScript (Bio-Rad, Hercules, CA), and diluted in MQ (1:6). cDNA was generated simultaneously for all samples to minimize experimental variations. Glp1r and Gcg were amplified from 1 μg of reverse-transcribed total RNA using TaqMan Gene Expression Master Mix (Applied Biosystems, Carlsbad, CA) with Glp1r and Gcg sense and antisense primers, and a dual-labeled probe (5′FAM, 3′-TAMRA) (Applied Biosystems; assay on demand Rn00562406_m1 and Rn00562293_m1, respectively). Samples were run in duplicate, and mRNA expression was normalized to the L32 housekeeping gene. Calculations were performed by a comparative method (2.0−ΔΔCt), taking the efficiency of the PCR into account (2.0). Quantitative PCR was performed on an ABI PRISM 7900 HT Sequence Detection System (Applied Biosystems).
Total GLP-1 protein analysis.
Peptides were extracted from frozen tissue samples by homogenization in acetic acid using a boiling water bath and centrifuged to pellet cellular debris (48). Subsequently, the supernatant was air-dried overnight and dissolved in 1.5 ml MQ water. Total GLP-1 in tissue extracts was measured using a commercially available immunometric assay kit (MesoScale Discovery, Gaithersburg, MD), as instructed by the manufacturer. Total proteins were measured using a commercially available Pierce BCA protein assay kit (Thermo Scientific, Florence, KY).
Data Analysis
Data for each respective study were analyzed separately and displayed as means ± SE. For all experiments, single comparisons between means were analyzed by unpaired Student's t-test, whereas multiple comparisons between means were analyzed using one-, two-, or three-way ANOVA, with repeated measures where applicable. If appropriate, post hoc analyses were made using Tukey's honestly significant difference test with P < 0.05 accepted as statistically different. Analyses were made using Statistica 10 (StatSoft, Tulsa, OK).
RESULTS
Effect of High-Fat Diet on Ex4-Induced Hypophagia
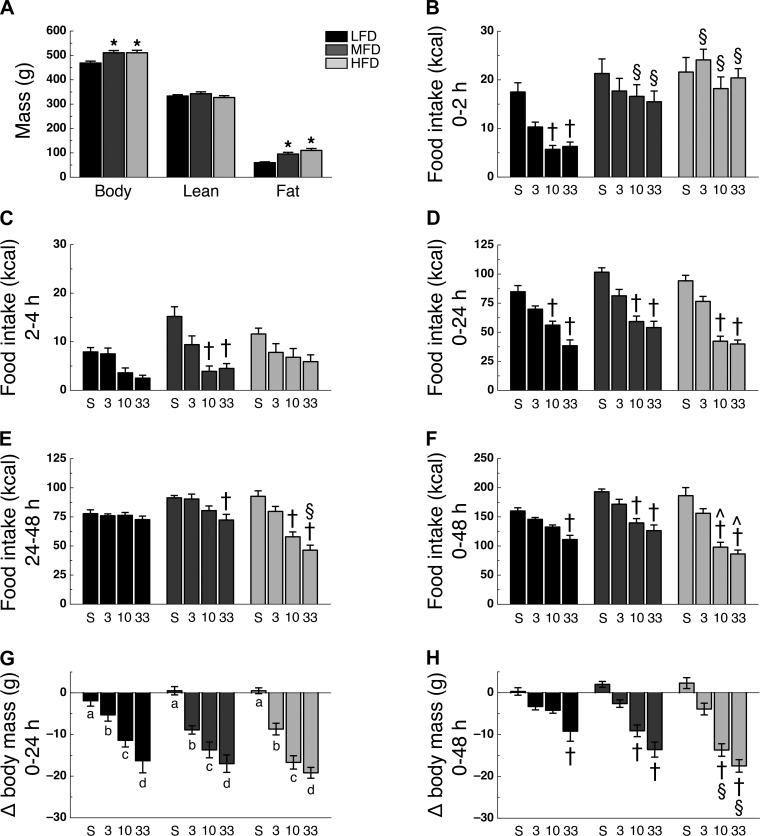
To study the effect of high-fat diet maintenance on GLP-1r agonist-mediated hypophagia, we opted to use two different high-fat diets (Table 1). After 6 wk on their designated diet, MFD and HFD rats had greater body mass (F2,38 = 7.46, P < 0.05) and fat mass (F2,38 = 16.71, P < 0.05) than LFD rats, with no significant difference between MFD and HFD rats (Fig. 1A). Lean mass was similar among all groups (Fig. 1A). Analysis of food intake during the 0–2- and 2–4-h intervals following Ex4 administration revealed a time × diet × drug interaction (F6,111 = 2.26; P < 0.05). During the 0–2-h interval, LFD rats treated with 10 or 33 μg/kg Ex4 had reduced food intake compared with saline-treated controls (P < 0.05; Fig. 1B). However, food intake at this same dose of Ex4 was significantly higher in MFD and HFD rats during this time interval (Fig. 1B). During the 2–4-h interval, treatment with 10 or 33 μg/kg Ex4 reduced food intake in MFD rats compared with saline-treated controls (P < 0.05; Fig. 1C). Analysis of food intake during the 0–24- and 24–48-h intervals following Ex4 administration revealed a time × diet × drug interaction (F6,111 = 3.05, P < 0.05). Post hoc analysis revealed that during the 0–24-h interval, 10 or 33 μg/kg Ex4 treatment significantly reduced food intake compared with saline-treated controls in all diets (P < 0.05; Fig. 1D). However, during the 24–48-h interval, food intake was no longer significantly reduced in LFD rats at any dose of Ex4 but remained suppressed in 33 μg/kg Ex4-treated MFD and 10 and 33 μg/kg Ex4-treated HFD rats (P < 0.05; Fig. 1E). Analysis of food intake during the 0–48-h interval following Ex4 administration revealed a diet × drug interaction (F6,111 = 3.66; P < 0.05). Food intake was decreased in 33 μg/kg Ex4-treated LFD rats, 10 and 33 μg/kg Ex4-treated MFD rats, and 10 and 33 μg/kg Ex4-treated HFD rats compared with their saline-treated controls (Fig. 1F). The reduction in food intake was significantly greater in the 10 and 33 μg/kg Ex4-treated HFD rats than MFD rats treated with a similar dose (P < 0.05; Fig. 1F). Ex4 treatment lowered body mass over 24 h following Ex4 administration (main effect of drug, F3,111 = 62.35; P < 0.05), with all Ex4-treated rats losing more mass than saline-treated controls in a dose-dependent manner and independent of diet (P < 0.05; Fig. 1G). Forty-eight hours following Ex4 administration, changes in body mass revealed a diet × drug interaction (F6,111 = 4.08; P < 0.05), with 33 μg/kg Ex4-treated LFD rats, 10 and 33 μg/kg Ex4-treated MFD rats, and 10 and 33 μg/kg Ex4-treated HFD rats having lost more body mass than their respective saline-treated controls (P < 0.05; Fig. 1H). Moreover, the 10- or 33 μg/kg Ex4-treated HFD rats had lost significantly more body mass than LFD rats treated with a similar dose (P < 0.05; Fig. 1H).
Fig. 1.
High-fat diet alters time profile of IP Ex4-induced hypophagia. A: body mass (left), lean mass (middle), and fat mass (right). Caloric intake during the 0–2-h (B), 2–4-h (C), 0–24-h (D), 24–48-h (E), and 0–48-h (F) intervals following intraperitoneal administration of saline (S), 3 μg/kg Ex4 (3), 10 μg/kg Ex4 (10), or 33 μg/kg Ex4 (33). Body mass change during the 0–24-h (G) and 0–48-h (H) intervals following Ex4 administration. Rats were on their respective diet for 6 wk (n = 9–10 per group). *P < 0.05 vs. LFD; †P < 0.05 vs. saline treatment within same diet; §P < 0.05 vs. similar Ex4 dose LFD; ^P < 0.05 vs. similar Ex4 dose MFD; a,b,c,dP < 0.05, different letters indicate significant differences between dosage groups.
In the above-mentioned experiment Ex4 was dosed per body mass. As MFD and HFD rats were heavier at the onset of the experiment, we also administered Ex4 using a flat dose (15 μg/rat) to exclude any effect of dosing strategy. Similar to the dose-response study, administration of 15 μg of Ex4 demonstrated a delayed onset and prolonged hypophagic effect in MFD and HFD rats (Fig. 2, A–E).
Fig. 2.
High-fat diet alters time profile of intraperitoneal Ex4 flat dose-induced hypophagia. A: body mass (left), lean mass (middle), and fat mass (right). Caloric intake during the 0–2-h (B), 2–4-h (C), 0–24-h, 24–48-h, 48–72-h, and 72–96-h intervals (D) following intraperitoneal administration of saline (S) or 15 μg Ex4 (Ex4). E: body mass change during the 0–24-h interval following Ex4 administration. Rats were on their respective diet for 13 wk (n = 13 or 14/group). *P < 0.05 vs. LFD; a,bP < 0.05, different letters indicate significant differences between treatment groups; †P < 0.05 vs. saline treatment within same diet; §P < 0.05 vs. Ex4 treatment LFD.
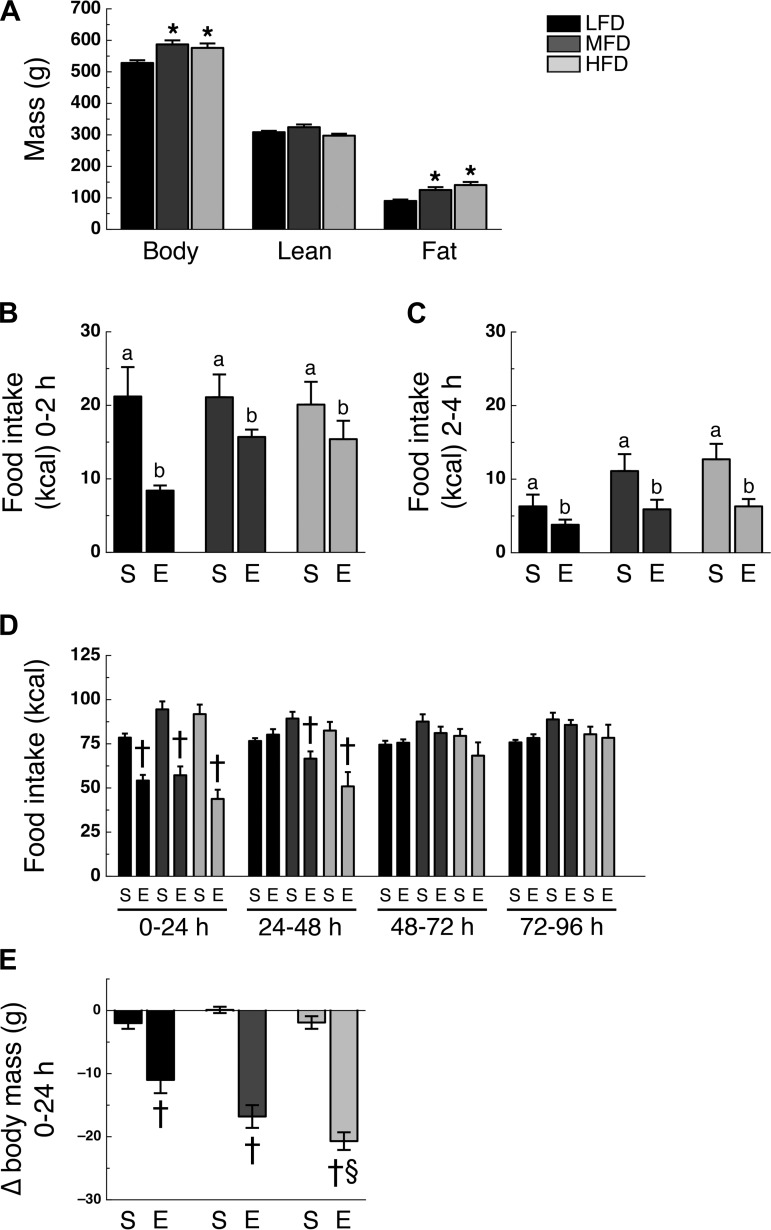
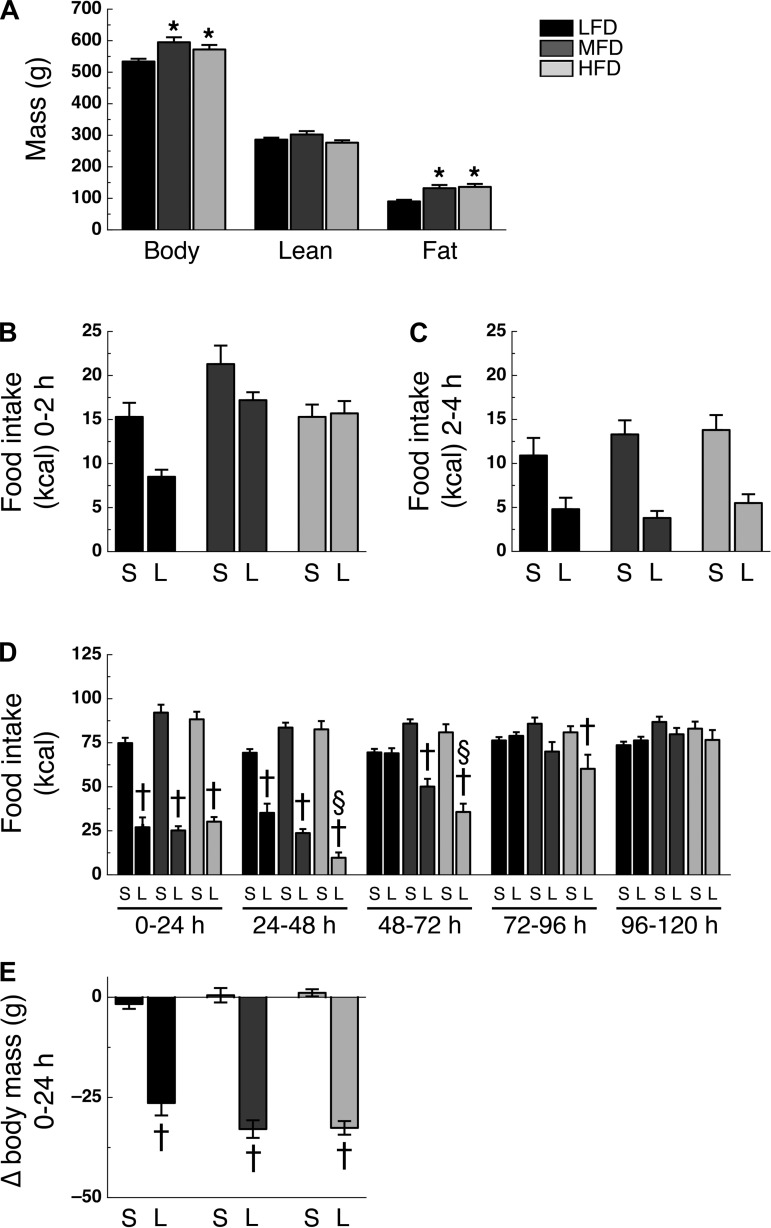
Effect of High-Fat Diet on Liraglutide-Induced Hypophagia
After 16 wk on their designated diet, MFD and HFD rats had greater body mass (F2,38 = 5.47; P < 0.05) and fat mass (F2,38 = 9.11; P < 0.05) than LFD rats, with no significant difference between MFD and HFD rats (Fig. 3A). Lean mass was similar among groups (Fig. 3A). Analysis of food intake during the 0–2- and 2–4-h intervals after liraglutide administration revealed no significant time × diet × drug interaction (F2,76 = 1.86; P > 0.05; Fig. 3, B and C). However, analysis of food intake during the 0–24-h, 24–48-h, 48–72-h, 72–96-h, and 96–120-h intervals after intraperitoneal administration of liraglutide did reveal a time × diet × drug interaction (F8,304 = 4.83; P < 0.05). During the 0–24-h and 24–48-h intervals, liraglutide-treated LFD, MFD, and HFD rats ate less than their saline-treated controls (P < 0.05; Fig. 3D). However, during the 48–72-h intervals, food intake was not significantly different between liraglutide- and saline-treated LFD rats, but was still significantly less in liraglutide-treated MFD and HFD rats compared with their saline-treated controls (P < 0.05), whereas the HFD rats were the only rats to maintain a significantly lower food intake compared with controls during the 72–96-h interval (Fig. 3D). During the 96–120-h interval, food intake was no longer significantly suppressed in LFD, MFD, or HFD rats (Fig. 3D). During the 24–48-h and 48–72-h intervals, liraglutide-treated HFD rats ate less than liraglutide-treated LFD rats (P < 0.05; Fig. 3D). Liraglutide treatment significantly lowered body mass 24 h following administration (diet × drug interaction; F2,76 = 3.46; P < 0.05), with all liraglutide-treated rats losing more mass than their saline-treated rats (P < 0.05), but no statistical difference was found between the liraglutide-treated groups (Fig. 3E).
Fig. 3.
High-fat diet alters time profile of intraperitoneal liraglutide-induced hypophagia. A: body mass (left), lean mass (middle), and fat mass (right). Caloric intake during the 0–2-h (B), 2–4-h (C), 0–24-h, 24–48-h, 48–72-h, 72–96-h, and 96–120-h (D) intervals following intraperitoneal administration of either saline (S) or 300 μg/kg liraglutide (L). E: body mass change during the 0–24-h interval following intraperitoneal administration of saline (S) or liraglutide (L). Rats were on their respective diet for 16 wk (n = 13 or 14/group). *P < 0.05 vs. LFD; †P < 0.05 vs. saline treatment within same diet; §P < 0.05 vs. liraglutide treatment LFD.
At the start of the liraglutide study, MFD and HFD rats had greater body mass and fat mass than LFD rats (Fig. 3A), suggesting that the obese state itself, as opposed to the chronic exposure to a high-fat diet, could mediate the duration of GLP-1r-mediated hypophagia. Therefore, we analyzed data from a subset of LFD, MFD, and HFD rats that had similar body (546 ± 20 g, 543 ± 21 g, and 536 ± 25 g, respectively, P > 0.05) and fat-mass (99 ± 6 g, 106 ± 10 g, and 130 ± 16 g, respectively, P > 0.05; n = 5 per group) from the above-mentioned experiment. Additional analyses of food intake during the 48–72-h interval following liraglutide administration with these body mass- and fat mass-matched LFD, MFD, and HFD rats revealed significant anorectic effects in the liraglutide-treated MFD and HFD rats compared with saline-treated control rats (43 ± 5% and 54 ± 7% suppression of food intake, respectively), whereas this effect was absent in the LFD group (4 ± 7% suppression of food intake; data not shown).
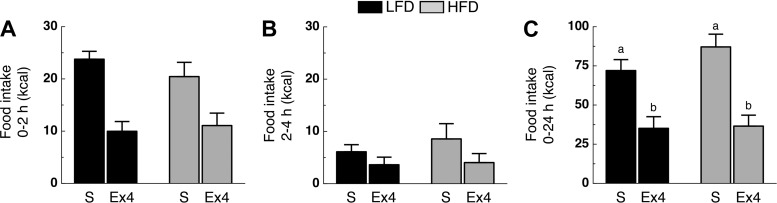
Effect of High-Fat Diet on I3VT Ex4-Induced Hypophagia
LFD and HFD rats were injected with saline or Ex4 (0.1 μg, I3VT), and food intake was monitored after 2, 4, and 24 h. Analysis of food intake during the 0–2- and 2–4-h intervals after Ex4 administration revealed no time × diet × drug interaction (F1,20 = 0.98, P > 0.05; Fig. 4, A and B). During the 0–24-h interval (main effect of drug; F1,20 = 51.07; P < 0.05), Ex4-treated rats ate less than saline-treated controls independent of diet (P < 0.05; Fig. 4C).
Fig. 4.
High-fat diet does not alter time profile of I3VT Ex4-induced hypophagia. Caloric intake during the 0–2-h (A), 2–4-h (B), and 0–24-h (C) intervals following I3VT administration of either saline (S) or 0.1 μg Ex4 (Ex4). Rats were on their respective diet for 6 wk (n = 4–6/group). a,bP < 0.05, different letters indicate significant differences between treatment groups.
Effect of High-Fat Diet on Ex4 Pharmacokinetics
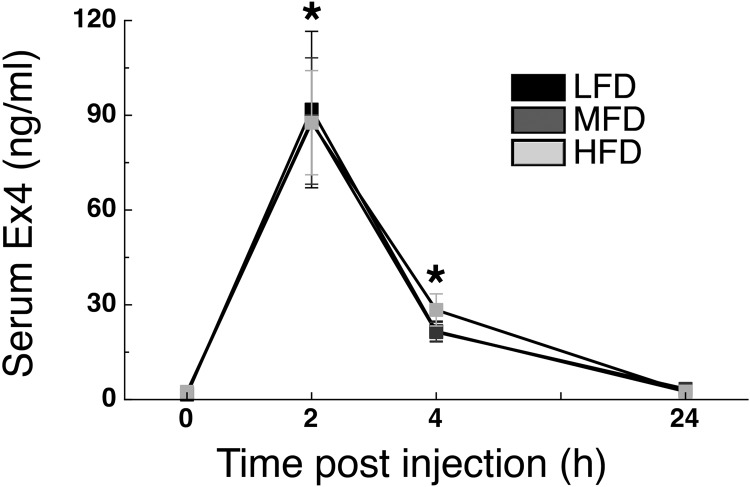
Because Ex4 had a delayed but longer-lasting hypophagic effect in MFD and HFD rats compared with what occurred in LFD rats, we analyzed serum Ex4 levels 2, 4, and 24 h after administration of exogenous Ex4 in rats that had been on their designated diet for 11 wk. Serum Ex4 levels were significantly increased in all diets at 2 and 4 h following Ex4 administration (P < 0.05) and returned to baseline levels 24 h after intraperitoneal Ex4 injection (main effect of time; F3,168 = 52.87; P < 0.05; Fig. 5).
Fig. 5.
High-fat diet does not alter Ex4 pharmacokinetics. Serum Ex4 levels before (0) and following (2, 4, and 24 h) intraperitoneal administration of 33 μg/kg Ex4. Rats were on their respective diet for 11 wk (n = 19–20/group). *P < 0.05 vs. 0-h time point.
Effect of High-Fat Diet on Glp1r and Gcg Expression
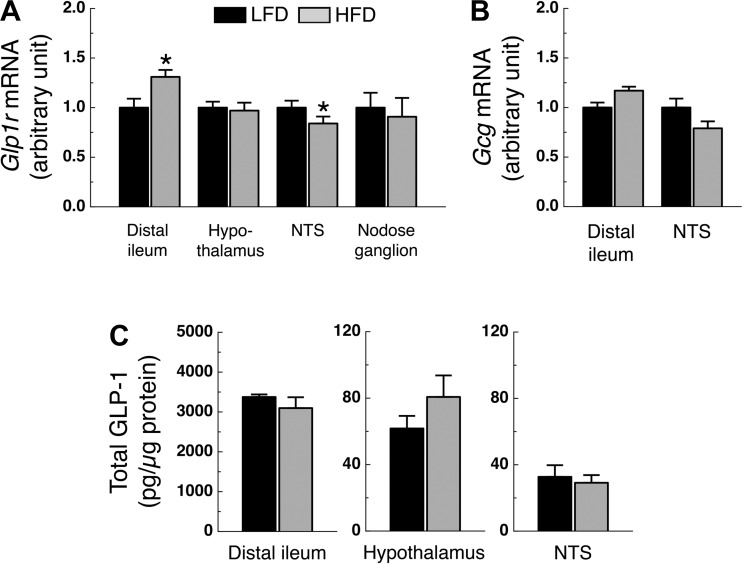
Glp1r expression in the distal ileum and NTS of HFD rats was significantly higher and lower, respectively, compared with levels in LFD rats (P < 0.05; Fig. 6A). However, Glp1r expression in the hypothalamus and nodose ganglion did not differ significantly among groups (Fig. 6A). Ileal and NTS Gcg expression had an increased and decreased trend in HFD rats compared with LFD rats (P = 0.08 and P = 0.09, respectively; Fig. 6B). Ileal, hypothalamic, and NTS total GLP-1 protein levels did not differ between LFD and HFD rats (Fig. 6C).
Fig. 6.
Glp1r and Gcg gene expression and GLP-1 protein levels. A: Glp1r expression in whole hypothalamus, NTS, distal ileum (n = 10–14/group), and nodose ganglion (n = 5–6/group). B: Gcg expression in NTS and distal ileum (n = 10–14/group). C: total GLP-1 levels in hypothalamus, NTS, and distal ileum (n = 7–14/group). Rats were on their respective diet for 24 wk. *P < 0.05 vs. LFD.
DISCUSSION
The present data demonstrate that diets high in fat content change the temporal profile of hypophagia induced by exogenous long-acting GLP-1r agonists and that this is manifested by a delayed onset but longer-lasting anorexic action. In fact, the anorexic effect of a single administration of liraglutide was still present during the fourth day after administration in rats on a high-fat diet. The delayed onset occurs only following peripheral administration of GLP-1r agonists, and the longer-lasting hypophagia is not due to decreased clearance of the drugs and, thus, may relate to changes in tissue-specific GLP-1r function. These findings suggest that dietary composition impacts the anorectic actions of peripherally administered GLP-1r agonists.
Decreased breakdown of Ex4 or liraglutide and subsequent prolonged activation of GLP-1r could be a possible explanation for the longer-lasting anorexic actions of Ex4 or liraglutide observed in this study. Because Ex4 and liraglutide have greater stability in the circulation than GLP-1 (7), we hypothesized that physiological changes resulting from chronic consumption of a high-fat diet enhanced their stability even further. However, serum Ex4 levels did not differ among LFD, MFD, and HFD rats, suggesting that differences in peptide clearance were not responsible for the observed differences in food intake suppression. Although there were no differences in serum Ex4 at 2 h postinjection, it is possible that up to that point, serum Ex4 may have been reduced, contributing to a delay in Ex4-induced hypophagia. However, Ex4 levels were not elevated at later time points. Thus, our data suggest that absorption, and subsequent delayed release of Ex4, by increased amounts of white adipose tissue in MFD and HFD rats is not involved in mediating the longer-lasting action of GLP-1r-mediated hypophagia during high-fat diet maintenance.
During the food intake studies, MFD and HFD rats had higher body mass than LFD rats, suggesting that an obese state itself could mediate the changed time course of GLP-1r-induced hypophagia. However, additional analyses of food intake during the third day following liraglutide administration using a subset of LFD, MFD, and HFD rats that were matched for body and fat mass suggest that the prolonged duration of hypophagia induced by exogenous long-acting GLP-1r agonists is independent of body and fat mass.
CNS GLP-1rs, most notably in the hypothalamic paraventricular nucleus (PVN) and the hindbrain, are implicated in the ability of GLP-1r agonists to decrease food intake (2, 7, 12, 15, 20, 24, 36, 39, 44, 50). However, pharmacological ablation of afferent C-type neural fibers using a capsaicin approach or a subdiaphragmatic vagal deafferentation approach partially blocks the ability of peripheral Ex4 to reduce food intake (27, 33, 46). This suggests that GLP-1r on sensory afferent neurons is at least important for the short-term anorectic function of GLP-1r agonists. In the present study, chronic HFD consumption decreased NTS but not nodose ganglion or hypothalamic Glp1r expression. In addition, central administration of Ex4 had similar anorectic potency in LFD and HFD rats. This indicates that during HFD-feeding, hypothalamic GLP-1r function appears normally responsive to Ex4 and suggests that functional changes are related but may not be limited to the level of the hindbrain. As the NTS is a relay for peripherally originating neuronal signals to be forwarded to higher brain centers, decreased NTS Glp1r expression might be associated with a reduced efficacy of Ex4 to lower food intake shortly after administration; however, this does not explain the longer-lasting effects. Thus, a speculative but plausible model is that the short-term effects of peripheral Ex4 or liraglutide are mediated through visceral afferent nerve signaling, while the longer-term effects result from movement of the agonists into the brain, where they can interact with central GLP-1r directly. Consistent with this idea, one study found that subdiaphragmatic vagotomy shifted the dose response curve of Ex4 and liraglutide to the right meaning that higher doses were necessary to get the same anorectic effects (27). Another study found that subdiaphragmatic vagotomy impairs early, but not later satiating effects of Ex4 (33). Further research is necessary to determine whether high-fat diets impair the ability of these long-acting agonists to directly activate vagal afferent neurons.
While these long-acting agonists can act via a GLP-1r-dependent sensory afferent pathway, they can rapidly cross the blood-brain barrier (BBB) (25, 29) and, therefore, have direct actions on the CNS that are independent of their actions on vagal afferents. Central resistance to the anorexic action of both leptin and insulin, both important regulators of long-term energy balance, has been hypothesized to be at least partially explained by reduced transport of leptin and insulin across the BBB during high-fat diet-induced and genetic obesity (10, 26). Thus, maintenance on a high-fat diet (and possibly the consequent obese state) might compromise the transport of Ex4 or liraglutide into the brain, although this was not directly tested here.
In rodents, activation of GLP-1r is accompanied by malaise and visceral illness, either assessed using a conditioned taste aversion paradigm or a pica response paradigm (i.e., the consumption of a nonnutritive substance) (28, 31, 47). Subdiaphragmatic vagotomy did not blunt the ability of peripheral Ex4 to induce nausea (28), indicating that the vagus nerve is not necessary for this ability. Thus, although not directly tested here, it is possible that high-fat diet maintenance induces stronger malaise side effects than low-fat diet maintenance, and future experiments are warranted to investigate this in more detail.
It has to be noted that, as indicated in Table 1, the fatty acid profile differs substantially between the high-fat diets used. In general, we observed similar efficacy of Ex4 and liraglutide to suppress food intake in MFD and HFD rats. However, during the 0–48-h interval, food intake was suppressed to a greater extent in 10 or 33 μg/kg Ex4-treated HFD rats than MFD rats treated with a similar dose (Fig. 1F). This suggests that different classes of fatty acids might have differential effects on GLP-1r-signaling during long-term high-fat diet consumption; however, this needs to be investigated in the future.
While it is easier to speculate as to why there was a delay in GLP-1r-agonist action, it is difficult to understand the longer-lasting effects. GLP-1r-mediated suppression of food intake and body mass, at least in the NTS, is dependent on an increase in cAMP-dependent PKA activity and an inhibition of AMPK activity (23). Leptin signaling is mediated, in part, by these same intracellular pathways, and consumption of a high-fat diet induces leptin resistance within days (40, 51). Furthermore, leptin and GLP-1 interact to suppress food intake (52). Although not directly tested here, it is likely that the MFD and HFD rats are leptin-resistant after 6 wk on the high-fat diet, which is when the rats were tested for the first time (51). Thus, the temporal profile of GLP-1r action during chronic high-fat diet consumption might be affected by both “initial” central leptin resistance and down-regulation of hindbrain Glp1r. However, as GLP-1rs are activated in higher brain centers (i.e., the PVN and reward-related brain areas) at a later stage, some of the central leptin resistance could potentially be overcome leading to a more prolonged suppression of food intake.
Knauf et al. (32) reported that 2-wk consumption of a high-fat diet increased brain stem Gcg mRNA levels in mice. Furthermore, our laboratory has observed a positive correlation between body weight and NTS Gcg expression after 4 wk on a high-fat diet (6). However, in the present study, NTS Gcg expression did not differ between LFD and HFD rats, nor did we observe a correlation between body mass and NTS Gcg expression within any group (data not shown). This might be explained by differences in diet exposure, as Gcg expression was analyzed after 20 wk of HFD consumption.
In conclusion, chronic consumption of a high-fat diet in rats delayed the onset but also prolonged the action of GLP-1r-mediated depression of food intake. The molecular mechanisms that lead to this effect remain to be determined. GLP-1r-based therapies are currently being used to treat T2D and overweight in humans (41). Although it remains to be seen whether our rodent data can be translated to the human situation, our data suggest that although diets high in fat content alter the temporal profile of GLP-1r-mediated hypophagia, in the end this alteration may potentially serve to benefit the weight-loss effects of the drug.
GRANTS
This work was supported by grants from National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (DK-017844 to S. C. Woods; DK-54080, DK-82480, DK-54890, and DK-056863 to R. J. Seeley; DK-082480 to D. A. Sandoval). J. D. Mul is supported by an American Society for Metabolic and Bariatric Surgery grant. D. P. Begg is supported by an National Health and Medical Research Council of Australia Early Career Fellowship (1013264).
DISCLOSURES
J. D. Mul is a paid speaker for Taconic. D. A. D'Alessio does consulting for Amylin, Lilly, Novo Nordisk and Takeda, research support from Ethicon Endosurgery and MannKind, and receives support for lectures from Merck. R. J. Seeley currently has stock/stock options with Zafgen and consults for Zafgen, Eli Lilly, and Ethicon Endo-Surgery. D. A. S'Alessio receives grant support from Ethicon Endo-Surgery and Novo Nordisk. The remaining authors disclose no conflicts.
AUTHOR CONTRIBUTIONS
Author contributions: J.D.M., D.P.B., J.G.B., and D.A.S. conception and design of research; J.D.M., D.P.B., J.G.B., B.L., and E.K.M. performed experiments; J.D.M., D.P.B., and J.G.B. analyzed data; J.D.M., D.P.B., J.G.B., D.A.D., S.C.W., R.J.S., and D.A.S. interpreted results of experiments; J.D.M. prepared figures; J.D.M. drafted manuscript; J.D.M., D.P.B., J.G.B., D.A.D., S.C.W., R.J.S., and D.A.S. edited and revised manuscript; J.D.M., D.P.B., J.G.B., B.L., E.K.M., D.A.D., S.C.W., R.J.S., and D.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We would like to thank members of the Seeley Laboratory for helpful discussions, M. Miller for help with animal handling, A. P. Chambers, H. Hoppert, and A. Lewis for help with tissue isolation, and E. Smith and B. Reedy for help with the Ex4 EIA and total GLP-1 analysis.
REFERENCES
- 1.Agerso H, Jensen LB, Elbrond B, Rolan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia 45: 195–202, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153: 647–658, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes 52: 252–259, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374: 1606–1616, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Barrera JG, D'Alessio DA, Drucker DJ, Woods SC, Seeley RJ. Differences in the central anorectic effects of glucagon-like peptide-1 and exendin-4 in rats. Diabetes 58: 2820–2827, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 31: 3904–3913, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrera JG, Sandoval DA, D'Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol 7: 507–516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Begg DP, Mul JD, Liu M, Reedy BM, D'Alessio DA, Seeley RJ, Woods SC. Reversal of diet-induced obesity increases insulin transport into cerebrospinal fluid and restores sensitivity to the anorexic action of central insulin in male rats. Endocrinology 154: 1047–1054, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 20 Suppl 1: 64–72, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burguera B, Couce ME, Curran GL, Jensen MD, Lloyd RV, Cleary MP, Poduslo JF. Obesity is associated with a decreased leptin transport across the blood-brain barrier in rats. Diabetes 49: 1219–1223, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374: 39–47, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Campos RV, Lee YC, Drucker DJ. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology 134: 2156–2164, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 44: 1126–1131, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V, Rungby J, Landau BR, Schmitz O. One week's treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes 53: 1187–1194, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 32: 4812–4820, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drucker DJ, Dritselis A, Kirkpatrick P. Liraglutide. Nat Rev Drug Discov 9: 267–268, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Duca FA, Swartz TD, Sakar Y, Covasa M. Decreased intestinal nutrient response in diet-induced obese rats: role of gut peptides and nutrient receptors. Int J Obes (Lond) 37: 375–381, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes Res 11: 845–851, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Freeman JS. Optimizing outcomes for GLP-1 agonists. J Am Osteopath Assoc 111: eS15–eS20, 2011 [PubMed] [Google Scholar]
- 20.Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 7: 2294–2300, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Greig NH, Holloway HW, De Ore KA, Jani D, Wang Y, Zhou J, Garant MJ, Egan JM. Once daily injection of exendin-4 to diabetic mice achieves long-term beneficial effects on blood glucose concentrations. Diabetologia 42: 45–50, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Hayes MR, Kanoski SE, Alhadeff AL, Grill HJ. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity (Silver Spring) 19: 1342–1349, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 13: 320–330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149: 4059–4068, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter K, Holscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood-brain barrier and enhance neurogenesis. BMC Neurosci 13: 33, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiyala K, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes 49: 1525–1533, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152: 3103–3112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 62: 1916–1927, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord 27: 313–318, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 136: 3585–3596, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22: 10470–10476, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knauf C, Cani PD, Ait-Belgnaoui A, Benani A, Dray C, Cabou C, Colom A, Uldry M, Rastrelli S, Sabatier E, Godet N, Waget A, Penicaud L, Valet P, Burcelin R. Brain glucagon-like peptide 1 signaling controls the onset of high-fat diet-induced insulin resistance and reduces energy expenditure. Endocrinology 149: 4768–4777, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Labouesse MA, Stadlbauer U, Weber E, Arnold M, Langhans W, Pacheco-Lopez G. Vagal afferents mediate early satiation and prevent flavour avoidance learning in response to intraperitoneally infused exendin-4. J Neuroendocrinol 24: 1505–1516, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Lopez LC, Frazier ML, Su CJ, Kumar A, Saunders GF. Mammalian pancreatic preproglucagon contains three glucagon-related peptides. Proc Natl Acad Sci USA 80: 5485–5489, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA, Roan JL, Vu C, Laugero KD, Parkes DG, Young AA. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond) 30: 1332–1340, 2006 [DOI] [PubMed] [Google Scholar]
- 36.McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol Regul Integr Comp Physiol 274: R23–R29, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, Sakai RR. Acute exposure to a high-fat diet alters meal patterns and body composition. Physiol Behav 99: 33–39, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem 214: 829–835, 1993 [DOI] [PubMed] [Google Scholar]
- 39.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403: 261–280, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415: 339–343, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Peters A. Incretin-based therapies: review of current clinical trial data. Am J Med 123: S28–S37, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes 56: 8–15, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Rodriquez de Fonseca F, Navarro M, Alvarez E, Roncero I, Chowen JA, Maestre O, Gomez R, Munoz RM, Eng J, Blazquez E. Peripheral versus central effects of glucagon-like peptide-1 receptor agonists on satiety and body weight loss in Zucker obese rats. Metabolism 49: 709–717, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V. Peptides that regulate food intake: glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol 284: R1427–R1435, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146: 3748–3756, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, Bernstein IL, Seeley RJ. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol Regul Integr Comp Physiol 272: R726–R730, 1997 [DOI] [PubMed] [Google Scholar]
- 48.van Delft J, Uttenthal LO, Hermida OG, Fontela T, Ghiglione M. Identification of amidated forms of GLP-1 in rat tissues using a highly sensitive radioimmunoassay. Regul Pept 70: 191–198, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Varndell IM, Bishop AE, Sikri KL, Uttenthal LO, Bloom SR, Polak JM. Localization of glucagon-like peptide (GLP) immunoreactants in human gut and pancreas using light and electron microscopic immunocytochemistry. J Histochem Cytochem 33: 1080–1086, 1985 [DOI] [PubMed] [Google Scholar]
- 50.Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res 1149: 118–126, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L. Overfeeding rapidly induces leptin and insulin resistance. Diabetes 50: 2786–2791, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes 55: 3387–3393, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Williams DL, Hyvarinen N, Lilly N, Kay K, Dossat A, Parise E, Torregrossa AM. Maintenance on a high-fat diet impairs the anorexic response to glucagon-like-peptide-1 receptor activation. Physiol Behav 103: 557–564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young AA, Gedulin BR, Bhavsar S, Bodkin N, Jodka C, Hansen B, Denaro M. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 48: 1026–1034, 1999 [DOI] [PubMed] [Google Scholar]